Unlocking the Industrial Potential of Cambuci Peel: A Sustainable Approach Based on Its Physicochemical Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Sample Characterization
2.2.1. Physical Parameters
2.2.2. Chemical Parameters
2.2.3. Chromatographic Compound Identification
2.2.4. Thermal Parameters
2.2.5. Morphological and Structural Parameters
2.3. Statistical Analysis
3. Results
3.1. Sample Characterization
3.1.1. Physical Parameters
3.1.2. Chemical Parameters
3.1.3. Chromatographic Compound Identification
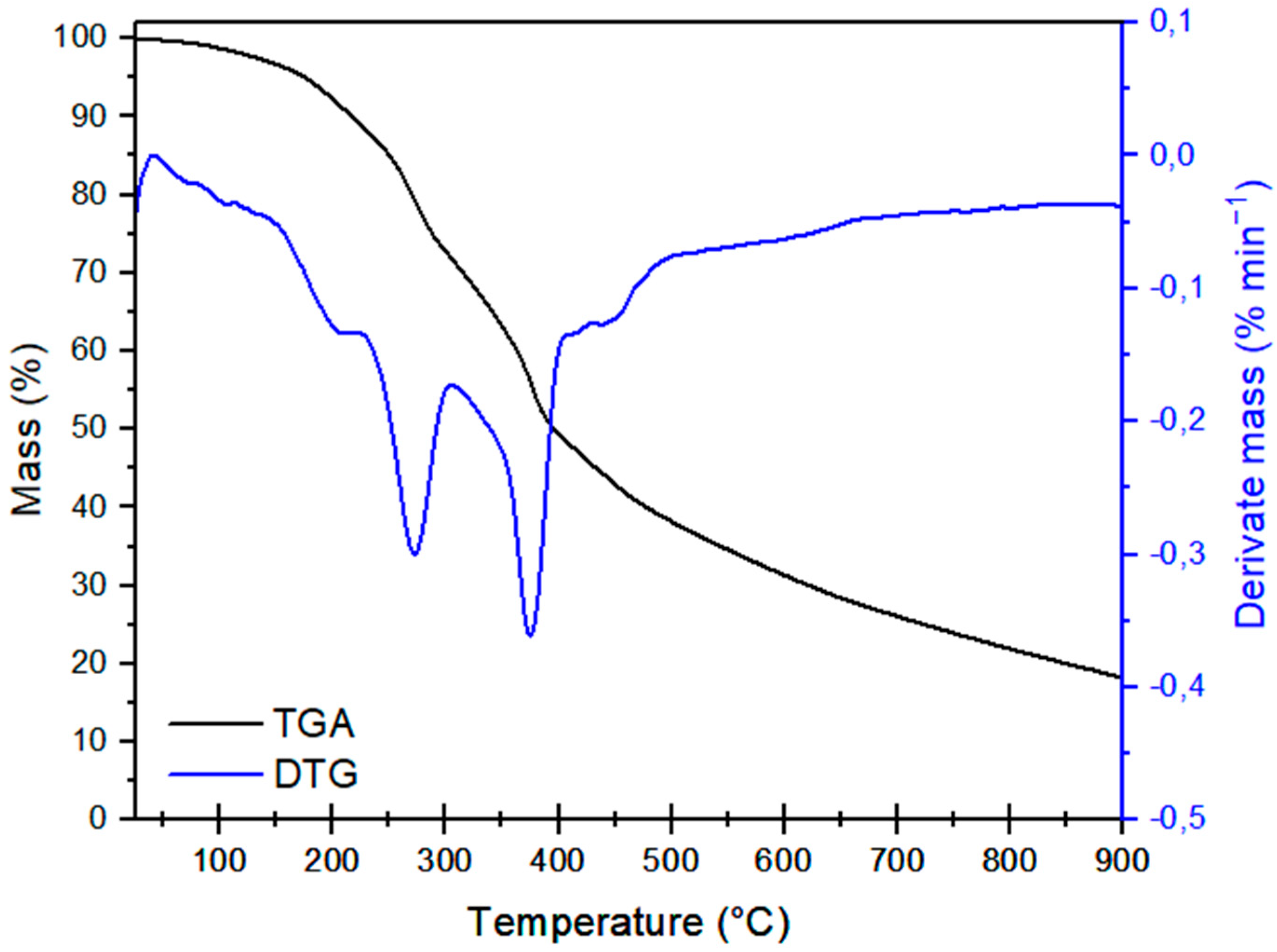
3.1.4. Thermal Parameters
3.1.5. Morphological and Structural Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Carvalho, A.P.A.; Conte-Junior, C.A. Health Benefits of Phytochemicals from Brazilian Native Foods and Plants: Antioxidant, Antimicrobial, Anti-Cancer, and Risk Factors of Metabolic/Endocrine Disorders Control. Trends Food Sci. Technol. 2021, 111, 534–548. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How Diverse Is the Chemistry and Plant Origin of Brazilian Propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Yamada, L.T.; Fagg, C.W.; Brandão, M.G.L. Native Foods from Brazilian Biodiversity as a Source of Bioactive Compounds. Food Res. Int. 2012, 48, 170–179. [Google Scholar] [CrossRef]
- da Veiga Correia, V.T.; da Silva, P.R.; Ribeiro, C.M.S.; Ramos, A.L.C.C.; Mazzinghy, A.C.d.C.; Silva, V.D.M.; Júnior, A.H.O.; Nunes, B.V.; Vieira, A.L.S.; Ribeiro, L.V.; et al. An Integrative Review on the Main Flavonoids Found in Some Species of the Myrtaceae Family: Phytochemical Characterization, Health Benefits and Development of Products. Plants 2022, 11, 2796. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; Neri-Numa, I.A.; de Araújo, F.F.; Pastore, G.M. A Critical Review of Some Fruit Trees from the Myrtaceae Family as Promising Sources for Food Applications with Functional Claims. Food Chem. 2020, 306, 125630. [Google Scholar] [CrossRef]
- Saber, F.R.; Munekata, P.E.S.; Rizwan, K.; El-Nashar, H.A.S.; Fahmy, N.M.; Aly, S.H.; El-Shazly, M.; Bouyahya, A.; Lorenzo, J.M. Family Myrtaceae: The Treasure Hidden in the Complex/Diverse Composition. Crit. Rev. Food Sci. Nutr. 2023, 64, 6737–6755. [Google Scholar] [CrossRef]
- Pereira, M.C.; Steffens, R.S.; Jablonski, A.; Hertz, P.F.; de O Rios, A.; Vizzotto, M.; Flôres, S.H. Characterization and Antioxidant Potential of Brazilian Fruits from the Myrtaceae Family. J. Agric. Food Chem. 2012, 60, 3061–3067. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Pessoa, É.V.M.; Rodrigues, L.; Rossi, R.; Moura, M.H.C.; dos Santos-Donado, P.R.; Castro, É.; Festuccia, W.T.; Genovese, M.I. Polyphenols of Cambuci (Campomanesia Phaea (O. Berg.)) Fruit Ameliorate Insulin Resistance and Hepatic Steatosis in Obese Mice. Food Chem. 2021, 340, 128169. [Google Scholar] [CrossRef]
- Spricigo, P.C.; Correia, B.S.B.; Borba, K.R.; Taver, I.B.; Machado, G.D.O.; Wilhelms, R.Z.; Queiroz Junior, L.H.K.; Jacomino, A.P.; Colnago, L.A. Classical Food Quality Attributes and the Metabolic Profile of Cambuci, a Native Brazilian Atlantic Rainforest Fruit. Molecules 2021, 26, 3613. [Google Scholar] [CrossRef]
- Tokairin, T.D.O.; Neto, H.B.; Jacomino, A.P. Cambuci—Campomanesia Phaea (O. Berg.) Landrum. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., Sousa de Brito, E., Eds.; Academic Press: London, UK, 2018; pp. 91–95. [Google Scholar]
- Tokairin, T.D.O.; Silva, A.P.G.D.; Spricigo, P.C.; Alencar, S.M.D.; Jacomino, A.P. Cambuci: A Native Fruit from the Brazilian Atlantic Forest Showed Nutraceutical Characteristics. Rev. Bras. Frutic. 2018, 40, e-0666. [Google Scholar] [CrossRef]
- Paes, M.S.; Pessoa Filho, P.D.A.; Tadini, C.C. Sorption Properties of Cambuci (Campomanesia Phaea O. Berg) Untreated and Pre-Treated with Sorbitol as Osmotic Solute. LWT 2021, 139, 110569. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Belchior, T.; Festuccia, W.T.; Genovese, M.I. Phenolic Compounds from Cambuci (Campomanesia Phaea O. Berg) Fruit Attenuate Glucose Intolerance and Adipose Tissue Inflammation Induced by a High-Fat, High-Sucrose Diet. Food Res. Int. 2015, 69, 170–178. [Google Scholar] [CrossRef]
- Vallilo, M.I.; Garbelotti, M.L.; Oliveira, E.D.; Lamardo, L.C.A. Características Físicas e Químicas Dos Frutos Do Cambucizeiro (Campomanesia Phaea). Rev. Bras. Frutic. 2005, 27, 241–244. [Google Scholar] [CrossRef]
- Taver, I.B.; Spricigo, P.C.; Neto, H.B.; de Alencar, S.M.; Massarioli, A.P.; Jacomino, A.P. Bioactive Compounds and In Vitro Antioxidant Capacity of Cambuci and Uvaia: An Extensive Description of Little-Known Fruits from the Myrtaceae Family with High Consumption Potential. Foods 2022, 11, 2612. [Google Scholar] [CrossRef]
- Rojas, M.L.; Gomes, B.D.O.; Carvalho, G.R.; Santos, K.C.; Guedes, J.S.; Bitencourt, B.S.; Augusto, P.E.D. Convective Drying of Cambuci, a Native Fruit from the Brazilian Atlantic Forest: Effect of Pretreatments with Ethanol and Freezing. J. Food Process Eng. 2021, 44, e13822. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Moura, M.H.C.; de Araujo, R.L.; de Lima Santiago, G.; de Moraes Barros, H.R.; Genovese, M.I. Polyphenols from Brazilian Native Myrtaceae Fruits and Their Potential Health Benefits against Obesity and Its Associated Complications. Curr. Opin. Food Sci. 2018, 19, 42–49. [Google Scholar] [CrossRef]
- FAO. Food Wastage Footprint: Full-Cost Accounting; FAO: Rome, Italy, 2014. [Google Scholar]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2011. [Google Scholar]
- Chauhan, C.; Dhir, A.; Akram, M.U.; Salo, J. Food Loss and Waste in Food Supply Chains. A Systematic Literature Review and Framework Development Approach. J. Clean. Prod. 2021, 295, 126438. [Google Scholar] [CrossRef]
- Ishangulyyev, R.; Kim, S.; Lee, S. Understanding Food Loss and Waste—Why Are We Losing and Wasting Food? Foods 2019, 8, 297. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Stangherlin, I.D.C. Upcycled By-Product Use in Agri-Food Systems from a Consumer Perspective: A Review of What We Know, and What Is Missing. Technol. Forecast. Soc. Change 2021, 168, 120749. [Google Scholar] [CrossRef]
- Jackowski, M.; Niedźwiecki, Ł.; Jagiełło, K.; Uchańska, O.; Trusek, A. Brewer’s Spent Grains—Valuable Beer Industry By-Product. Biomolecules 2020, 10, 1669. [Google Scholar] [CrossRef]
- Teixeira, J.D.; Soares Mateus, A.R.; Sanchez, C.; Parpot, P.; Almeida, C.; Sanches Silva, A. Antioxidant Capacity and Phenolics Profile of Portuguese Traditional Cultivars of Apples and Pears and Their By-Products: On the Way to Newer Applications. Foods 2023, 12, 1537. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Colpini, L.M.S. All-around Characterization of Brewers’ Spent Grain. Eur. Food Res. Technol. 2021, 247, 3013–3021. [Google Scholar] [CrossRef]
- Masala, V.; Jokić, S.; Aladić, K.; Molnar, M.; Tuberoso, C.I.G. Exploring Phenolic Compounds Extraction from Saffron (C. sativus) Floral By-Products Using Ultrasound-Assisted Extraction, Deep Eutectic Solvent Extraction, and Subcritical Water Extraction. Molecules 2024, 29, 2600. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemistry, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Soest, P.J. Van Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. AOAC Int. 1990, 73, 491–497. [Google Scholar] [CrossRef]
- Nelson, N. A Photometric Adaptation of the Somogyi Method for the Determination of Glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Barroso, T.; Sganzerla, W.; Rosa, R.; Castro, L.; Maciel-Silva, F.; Rostagno, M.; Forster-Carneiro, T. Semi-Continuous Flow-through Hydrothermal Pretreatment for the Recovery of Bioproducts from Jabuticaba (Myrciaria Cauliflora) Agro-Industrial by-Product. Food Res. Int. 2022, 158, 111547. [Google Scholar] [CrossRef]
- Jimenez Moreno, J.A.; de Freitas Marinho, L.; Sanches Contieri, L.; Barroso, T.L.C.T.; Rostagno, M.A.; Forster Carneiro, T. Obtaining Extracts and Hydrolysates from Cambuci Peel Through Subcritical Water: An In-Line Detection Approach. Food Bioproc. Tech. 2024, 17, 4960–4979. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of Ecological Factors on the Antioxidant Potential and Total Phenol Content of Scrophularia Striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef]
- Dias, P.G.I.; Sajiwanie, J.W.A.; Rathnayaka, R.M.U.S.K. Chemical Composition, Physicochemical and Technological Properties of Selected Fruit Peels as a Potential Food Source. Int. J. Fruit Sci. 2020, 20, S240–S251. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical Characteristics of Biomass Ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Pour, F.H.; Makkawi, Y.T. A Review of Post-Consumption Food Waste Management and Its Potentials for Biofuel Production. Energy Rep. 2021, 7, 7759–7784. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.; Wu, G.; Zhan, X. Impact of Total Solids Content on Anaerobic Co-Digestion of Pig Manure and Food Waste: Insights into Shifting of the Methanogenic Pathway. Waste Manag. 2020, 114, 96–106. [Google Scholar] [CrossRef]
- Fröner-Lacerda, L.R.R.; Lacerda, V.F.; Ampese, L.C.; Ziero, H.D.D.; Pérez, M.; Forster-Carneiro, T. The Assessment of the Operational Performance of a Dry Anaerobic Reactor of Cambuci Husks to Bioenergy Potential and Biorefinery Integration. Bioenergy Res. 2024, 17, 1375–1385. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic Digestion of Food Waste—Challenges and Opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef]
- Jacqueline, P.J.; Velvizhi, G. Substantial Physicochemical Pretreatment and Rapid Delignification of Lignocellulosic Banana, Pineapple and Papaya Fruit Peels: A Study on Physical-Chemical Characterization. Sustain. Chem. Pharm. 2024, 37, 101347. [Google Scholar] [CrossRef]
- Becerra, L.D.; Quintanilla-Carvajal, M.X.; Escobar, S.; Ruiz, R.Y. Correlation between Color Parameters and Bioactive Compound Content during Cocoa Seed Transformation under Controlled Process Conditions. Food Biosci. 2023, 53, 102526. [Google Scholar] [CrossRef]
- Filgueiras Rebelo de Matos, M.; Quênia Muniz Bezerra, P.; Conceição Argôlo Correia, L.; Nunes Viola, D.; de Oliveira Rios, A.; Izabel Druzian, J.; Larroza Nunes, I. Innovative Methodological Approach Using CIELab and Dye Screening for Chemometric Classification and HPLC for the Confirmation of Dyes in Cassava Flour: A Contribution to Product Quality Control. Food Chem. 2021, 365, 130446. [Google Scholar] [CrossRef]
- Waqas, M.; Nizami, A.S.; Aburiazaiza, A.S.; Barakat, M.A.; Rashid, M.I.; Ismail, I.M.I. Optimizing the Process of Food Waste Compost and Valorizing Its Applications: A Case Study of Saudi Arabia. J. Clean. Prod. 2018, 176, 426–438. [Google Scholar] [CrossRef]
- Loubet Filho, P.S.; Baseggio, A.M.; Vuolo, M.M.; Reguengo, L.M.; Telles Biasoto, A.C.; Correa, L.C.; Junior, S.B.; Alves Cagnon, V.H.; Betim Cazarin, C.B.; Maróstica Júnior, M.R. Gut Microbiota Modulation by Jabuticaba Peel and Its Effect on Glucose Metabolism via Inflammatory Signaling. Curr. Res. Food Sci. 2022, 5, 382–391. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of Mango, Apple and Banana Fruit Peels as Prebiotics and Functional Ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Del Sánchez-Camargo, A.P.; Gutiérrez, L.-F.; Vargas, S.M.; Martinez-Correa, H.A.; Parada-Alfonso, F.; Narváez-Cuenca, C.-E. Valorisation of Mango Peel: Proximate Composition, Supercritical Fluid Extraction of Carotenoids, and Application as an Antioxidant Additive for an Edible Oil. J. Supercrit. Fluids 2019, 152, 104574. [Google Scholar] [CrossRef]
- Ribeiro, L.D.O.; Viana, E.D.S.; Godoy, R.L.D.O.; Freitas, S.C.D.; Freitas, S.P.; Matta, V.M.D. Nutrients and Bioactive Compounds of Pulp, Peel and Seed from Umbu Fruit. Ciência Rural 2019, 49, e20180806. [Google Scholar] [CrossRef]
- Du, M.; Zhu, Y.; Nan, H.; Zhou, Y.; Pan, X. Regulation of Sugar Metabolism in Fruits. Sci. Hortic. 2024, 326, 112712. [Google Scholar] [CrossRef]
- Hussain, T.; Kalhoro, D.H.; Yin, Y. Identification of Nutritional Composition and Antioxidant Activities of Fruit Peels as a Potential Source of Nutraceuticals. Front. Nutr. 2023, 9, 1065698. [Google Scholar] [CrossRef]
- Aniyikaiye, T.; Oluseyi, T.; Odiyo, J.; Edokpayi, J. Physico-Chemical Analysis of Wastewater Discharge from Selected Paint Industries in Lagos, Nigeria. Int. J. Environ. Res. Public Health 2019, 16, 1235. [Google Scholar] [CrossRef]
- Benny, N.; Shams, R.; Dash, K.K.; Pandey, V.K.; Bashir, O. Recent Trends in Utilization of Citrus Fruits in Production of Eco-Enzyme. J. Agric. Food Res. 2023, 13, 100657. [Google Scholar] [CrossRef]
- Blejan, A.M.; Nour, V.; Păcularu-Burada, B.; Popescu, S.M. Wild Bilberry, Blackcurrant, and Blackberry by-Products as a Source of Nutritional and Bioactive Compounds. Int. J. Food Prop. 2023, 26, 1579–1595. [Google Scholar] [CrossRef]
- Pérez-Chabela, M.D.L.; Cebollón-Juárez, A.; Bpsquez-Molina, E.; Totosaus, A. Mango Peel Flour and Potato Peel Flour as Bioactive Ingredients in the Formulation of Functional Yogurt. Food Sci. Technol. 2022, 42, e38220. [Google Scholar] [CrossRef]
- O’Shea, N.; Ktenioudaki, A.; Smyth, T.P.; McLoughlin, P.; Doran, L.; Auty, M.A.E.; Arendt, E.; Gallagher, E. Physicochemical Assessment of Two Fruit By-Products as Functional Ingredients: Apple and Orange Pomace. J. Food Eng. 2015, 153, 89–95. [Google Scholar] [CrossRef]
- Mala, T.; Piayura, S.; Itthivadhanapong, P. Characterization of Dried Pineapple (Ananas comosus L.) Peel Powder and Its Application as a Novel Functional Food Ingredient in Cracker Product. Future Foods 2024, 9, 100322. [Google Scholar] [CrossRef]
- Widiarsih, S.; Nagel, M.; Börner, A.; Feussner, K.; Feussner, I.; Möllers, C. Inheritance of Seed Quality and Seed Germination in Two Doubled Haploid Populations of Oilseed Rape Segregating for Acid Detergent Lignin (ADL) Content. Euphytica 2021, 217, 161. [Google Scholar] [CrossRef]
- Miranda-Romero, L.A.; Tirado-González, D.N.; Tirado-Estrada, G.; Améndola-Massiotti, R.; Sandoval-González, L.; Ramírez-Valverde, R.; Salem, A.Z. Quantifying Non-fibrous Carbohydrates, Acid Detergent Fiber and Cellulose of Forage through an in Vitro Gas Production Technique. J. Sci. Food Agric. 2020, 100, 3099–3110. [Google Scholar] [CrossRef]
- Grizotto, R.K.; Siqueira, G.R.; Campos, A.F.; Modesto, R.T.; Resende, F.D. de Fermentative Parameters and Aerobic Stability of Orange Peel Silage with Pelleted Citrus Pulp. Rev. Bras. Zootec. 2020, 49, e20190265. [Google Scholar] [CrossRef]
- Youssef, M. Utilization of Some Citrus Peels in Formulating Functional Cookies and Pasta. Alex. J. Food Sci. Technol. 2023, 21, 21–32. [Google Scholar] [CrossRef]
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Papakyriakopoulou, P.; Velidakis, N.; Khattab, E.; Valsami, G.; Korakianitis, I.; Kadoglou, N.P. Potential Pharmaceutical Applications of Quercetin in Cardiovascular Diseases. Pharmaceuticals 2022, 15, 1019. [Google Scholar] [CrossRef]
- Castro, L.E.N.; Sganzerla, W.G.; Barroso, T.L.C.T.; Maciel-Silva, F.W.; Colpini, L.M.S.; Bittencourt, P.R.S.; Rostagno, M.A.; Forster-Carneiro, T. Improving the Semi-Continuous Flow-through Subcritical Water Hydrolysis of Grape Pomace (Vitis Vinifera L.) by PH and Temperature Control. J. Supercrit. Fluids 2023, 196, 105894. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Baseggio, A.M.; Torres-Mayanga, P.C.; Ávila, P.F.; Tompsett, G.A.; Marostica, M.; Goldbeck, R.; Timko, M.T.; Rostagno, M.; Martinez, J.; et al. Sequential Subcritical Water Process Applied to Orange Peel for the Recovery Flavanones and Sugars. J. Supercrit. Fluids 2020, 160, 104789. [Google Scholar] [CrossRef]
- Volli, V.; Gollakota, A.R.K.; Shu, C.-M. Comparative Studies on Thermochemical Behavior and Kinetics of Lignocellulosic Biomass Residues Using TG-FTIR and Py-GC/MS. Sci. Total Environ. 2021, 792, 148392. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Domenico, M.D.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Investigation on Prospective Bioenergy from Pyrolysis of Butia Seed Waste Using TGA-FTIR: Assessment of Kinetic Triplet, Thermodynamic Parameters and Evolved Volatiles. Renew. Energy 2022, 191, 238–250. [Google Scholar] [CrossRef]
- Dubey, S.; Kumar, R.; KumarMondal, M. Pyrolysis Kinetics and Thermodynamics of Pomegranate Peel UsingTG/DTG Analysis. Biomass Convers. Biorefin. 2024, 14, 12411–12425. [Google Scholar] [CrossRef]
- Melikoglu, M.; Ozdemir, M.; Ates, M. Pyrolysis Kinetics, Physicochemical Characteristics and Thermal Decomposition Behavior of Agricultural Wastes Using Thermogravimetric Analysis. Energy Nexus 2023, 11, 100231. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Prasad, N.; Kapoor, A.; Arya, A.K.; Pal, D.B. Sustainable Valorization of Cascabela Thevetia Fruit Peel and Seed Waste Biomass: Characterization and Thermo-Kinetic Analysis. Biomass Convers. Biorefin. 2023, 1–13. [Google Scholar] [CrossRef]
- Hanan Taharuddin, N.; Jumaidin, R.; Ridzuan Mansor, M.; Asyadi Md Yusof, F.; Hanim Alamjuri, R. Characterization of Potential Cellulose from Hylocereus Polyrhizus (Dragon Fruit) Peel: A Study on Physicochemical and Thermal Properties. J. Renew. Mater. 2023, 11, 131–145. [Google Scholar] [CrossRef]
- Gan, L.; Guo, H.; Xiao, Z.; Jia, Z.; Yang, H.; Sheng, D.; Pan, H.; Xu, W.; Wang, Y. Dyeing and Characterization of Cellulose Powder Developed from Waste Cotton. Polymers 2019, 11, 1982. [Google Scholar] [CrossRef]
- Weyhrich, C.W.; Petrova, S.P.; Edgar, K.J.; Long, T.E. Renewed Interest in Biopolymer Composites: Incorporation of Renewable, Plant-Sourced Fibers. Green. Chem. 2023, 25, 106–129. [Google Scholar] [CrossRef]
- Stabrauskiene, J.; Pudziuvelyte, L.; Bernatoniene, J. Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x Paradisi L. Peels. Pharmaceuticals 2024, 17, 596. [Google Scholar] [CrossRef]
- Cebi, N.; Arici, M.; Sagdic, O. The Famous Turkish Rose Essential Oil: Characterization and Authenticity Monitoring by FTIR, Raman and GC–MS Techniques Combined with Chemometrics. Food Chem. 2021, 354, 129495. [Google Scholar] [CrossRef]
- Kalantari, H.; Turner, R.J. Structural and Antimicrobial Properties of Synthesized Gold Nanoparticles Using Biological and Chemical Approaches. Front. Chem. 2024, 12, 1482102. [Google Scholar] [CrossRef]
- Selvaraju, R.; Sakuntala, P.; Jaleeli, K.A. GC–MS and FTIR Analysis of Chemical Compounds in Ocimum Gratissimum Plant. Biophysics 2021, 66, 401–408. [Google Scholar] [CrossRef]
- Osanloo, M.; Firoozian, S.; Zarenezhad, E.; Montaseri, Z.; Satvati, S. A Nanoliposomal Gel Containing Cinnamomum Zeylanicum Essential Oil with Effective Repellent against the Main Malaria Vector Anopheles Stephensi. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 1–6. [Google Scholar] [CrossRef]
- Lin, L.; E, Y.; Sun, Q.; Chen, Y.; Dai, W.; Bao, Z.; Niu, W.; Meng, J. Analysis of the Pyrolysis Kinetics, Reaction Mechanisms, and By-Products of Rice Husk and Rice Straw via TG-FTIR and Py-GC/MS. Molecules 2024, 30, 10. [Google Scholar] [CrossRef]
- Chun Tong, Y.; Yun Wang, Q.; Feng, M.; Ling He, Q.; Liu, Y. Preparing an ATP/P (N-BuMA-St)/Fe3O4 Oil-Absorbing Resin Composite via a Double Pickering Emulsion Template Method and Elucidation of Its Properties. Chempluschem 2022, 87, e202200100. [Google Scholar] [CrossRef]
- Kozhakhmetova, M.; Akimbekov, N.; Digel, I.; Tastambek, K. Evaluating the Low-Rank Coal Degradation Efficiency Bioaugmented with Activated Sludge. Sci. Rep. 2024, 14, 14827. [Google Scholar] [CrossRef]
- Arif, M.; Li, Y.; El-Dalatony, M.M.; Zhang, C.; Li, X.; Salama, E.-S. A Complete Characterization of Microalgal Biomass through FTIR/TGA/CHNS Analysis: An Approach for Biofuel Generation and Nutrients Removal. Renew. Energy 2021, 163, 1973–1982. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of Their Candidacy for Alternate Renewable Fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Singh, Y.D.; Mahanta, P.; Bora, U. Comprehensive Characterization of Lignocellulosic Biomass through Proximate, Ultimate and Compositional Analysis for Bioenergy Production. Renew. Energy 2017, 103, 490–500. [Google Scholar] [CrossRef]
- Shiue, A.; Chin, K.-Y.; Yin, M.-J.; Cheng, C.-Y.; Chang, S.-M.; Leggett, G. Poly (Allylamine)–Based Amine Blends for Separation of Carbon Dioxide in the Indoor Environment. Optik 2023, 284, 170973. [Google Scholar] [CrossRef]




| Parameter | Experimental Value |
|---|---|
| Moisture content (%) | 9.41 ± 1.69 |
| Ash (%) | 3.18 ± 0.41 |
| Total solids (%) | 90.59 ± 1.69 |
| Volatile solids (%) | 87.41 ± 1.69 |
| L* | 24.44 ± 0.37 |
| a* | 8.39 ± 0.12 |
| b* | 40.12 ± 0.37 |
| Parameter | Cambuci Peel |
|---|---|
| Titratable acidity, g CA/100 g (d.w.) | 0.1443 ± 0.0055 |
| Fat content, g/100 g (d.w.) | 7.27 ± 0.74 |
| Reducing sugar (mg g−1) | 108.22 ± 3.71 |
| Non-reducing sugar (mg g−1) | 30.58 ± 3.16 |
| Crude fiber (%) | 11.95 ± 0.30 |
| Neutral detergent fiber (%) | 36.65 ± 0.19 |
| Acid detergent fiber (%) | 18.91 ± 0.05 |
| Cellulose (%) | 11.47 ± 0.10 |
| Lignin (%) | 7.44 ± 0.06 |
| Hemicellulose (%) | 17.74 ± 0.20 |
| Retention Time (min) | Name | m/z | Lamba (λ) | Fraction |
|---|---|---|---|---|
| 1.0 | Gallic acid | 169.10 [M-H]- | 273 | A, B |
| 2.2 | Bis-HHDP-hexoside (Pedunculagin) | 783.29 [M-H]- | 258 | A, B |
| 3.3 | Tetragalloyl hexoside | 787.05 [M-H]- | 277 | B |
| 3.4 | Ellagic acid | 301.06 [M-H]- | 253, 356 | A, B |
| 3.6 | Quercetin pentoside | 433.22 [M-H]- | 255, 356 | A, B |
| 3.9 | Quercetin-O-(O-galloyl)-pentoside | 585.24 [M-H]- | 354, 346 | B |
| 5.6 | Quercetin rhamnose | 447.20 [M-H]- | 265, 348 | A, B |
| 10.9 | Methyl ellagic acid sulfate | 397.14 [M+H]+ | 259 | A, B, C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez Moreno, J.A.; Linhares Cruz Tabosa Barroso, T.; Nochi Castro, L.E.; Saragiotto Colpini, L.M.; Sanchez Bragagnolo, F.; Rostagno, M.A.; Forster Carneiro, T. Unlocking the Industrial Potential of Cambuci Peel: A Sustainable Approach Based on Its Physicochemical Profile. Resources 2025, 14, 109. https://doi.org/10.3390/resources14070109
Jimenez Moreno JA, Linhares Cruz Tabosa Barroso T, Nochi Castro LE, Saragiotto Colpini LM, Sanchez Bragagnolo F, Rostagno MA, Forster Carneiro T. Unlocking the Industrial Potential of Cambuci Peel: A Sustainable Approach Based on Its Physicochemical Profile. Resources. 2025; 14(7):109. https://doi.org/10.3390/resources14070109
Chicago/Turabian StyleJimenez Moreno, Juver Andrey, Tiago Linhares Cruz Tabosa Barroso, Luiz Eduardo Nochi Castro, Leda Maria Saragiotto Colpini, Felipe Sanchez Bragagnolo, Mauricio Ariel Rostagno, and Tânia Forster Carneiro. 2025. "Unlocking the Industrial Potential of Cambuci Peel: A Sustainable Approach Based on Its Physicochemical Profile" Resources 14, no. 7: 109. https://doi.org/10.3390/resources14070109
APA StyleJimenez Moreno, J. A., Linhares Cruz Tabosa Barroso, T., Nochi Castro, L. E., Saragiotto Colpini, L. M., Sanchez Bragagnolo, F., Rostagno, M. A., & Forster Carneiro, T. (2025). Unlocking the Industrial Potential of Cambuci Peel: A Sustainable Approach Based on Its Physicochemical Profile. Resources, 14(7), 109. https://doi.org/10.3390/resources14070109







