Tundish Deskulling Waste as a Source of MgO for Producing Magnesium Phosphate Cement-Based Mortars: Advancing Sustainable Construction Materials
Abstract
1. Introduction
- (A)
- Dissolution of MgO particles: in the initial step, MgO particles react with water, dissolving into Mg2+ and 2 OH− ions.
- (B)
- Formation of aquosols: cations can react with water to form positively charged species, such as [Mg ← OH2]2+(aq).
- (C)
- Acid–base reaction and condensation: in this step, the magnesium phosphate forms and subsequently condensates to produce the initial particle aggregates.
- (D)
- Gel formation and percolation: the newly formed salt particles interact to create a network, which subsequently leads to the formation of a gel.
- (E)
- Crystallization: the formation of the gel increases saturation, leading to the creation of crystals.
2. Materials and Methods
2.1. Materials
2.2. TUN Characterization
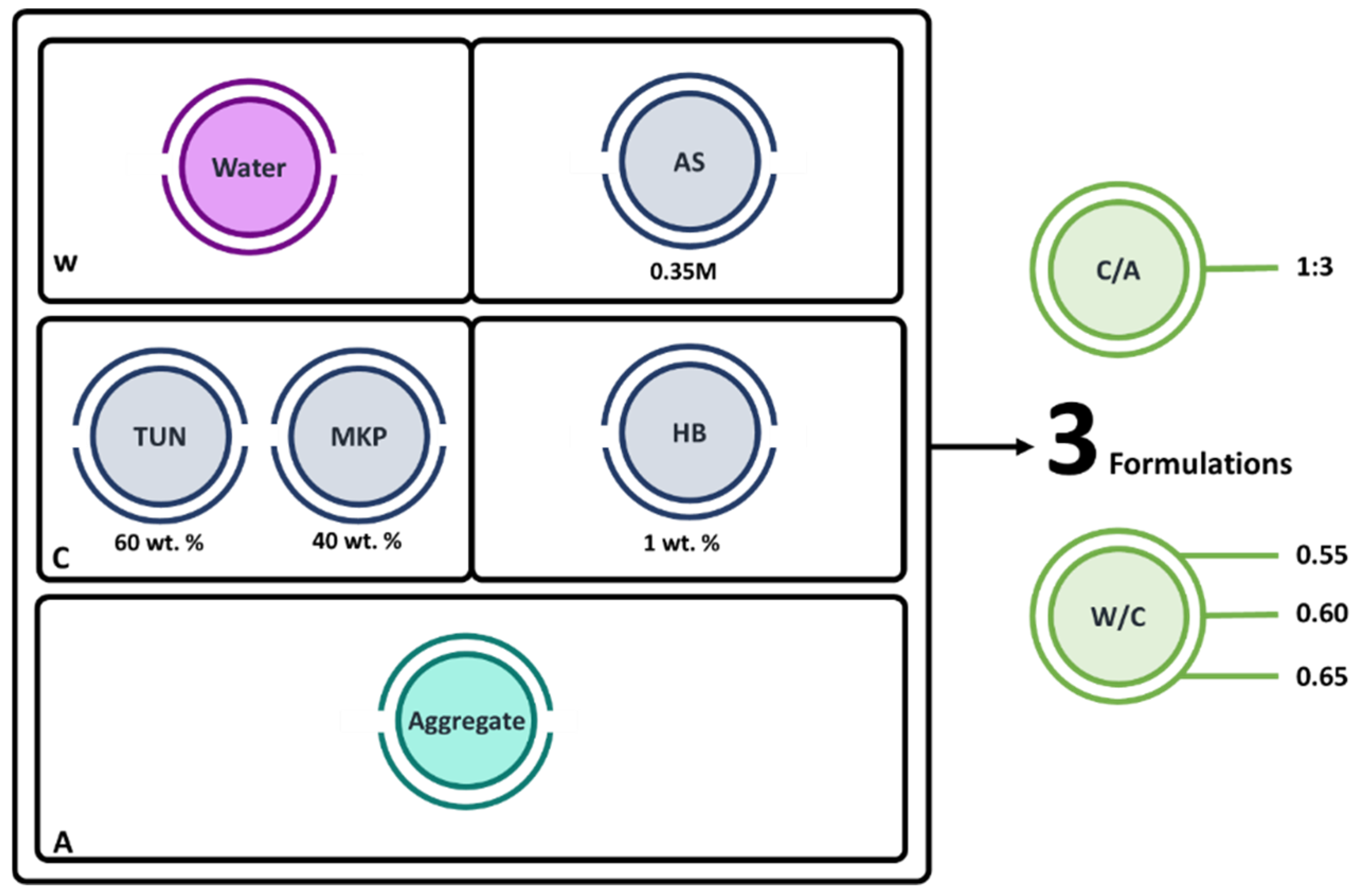
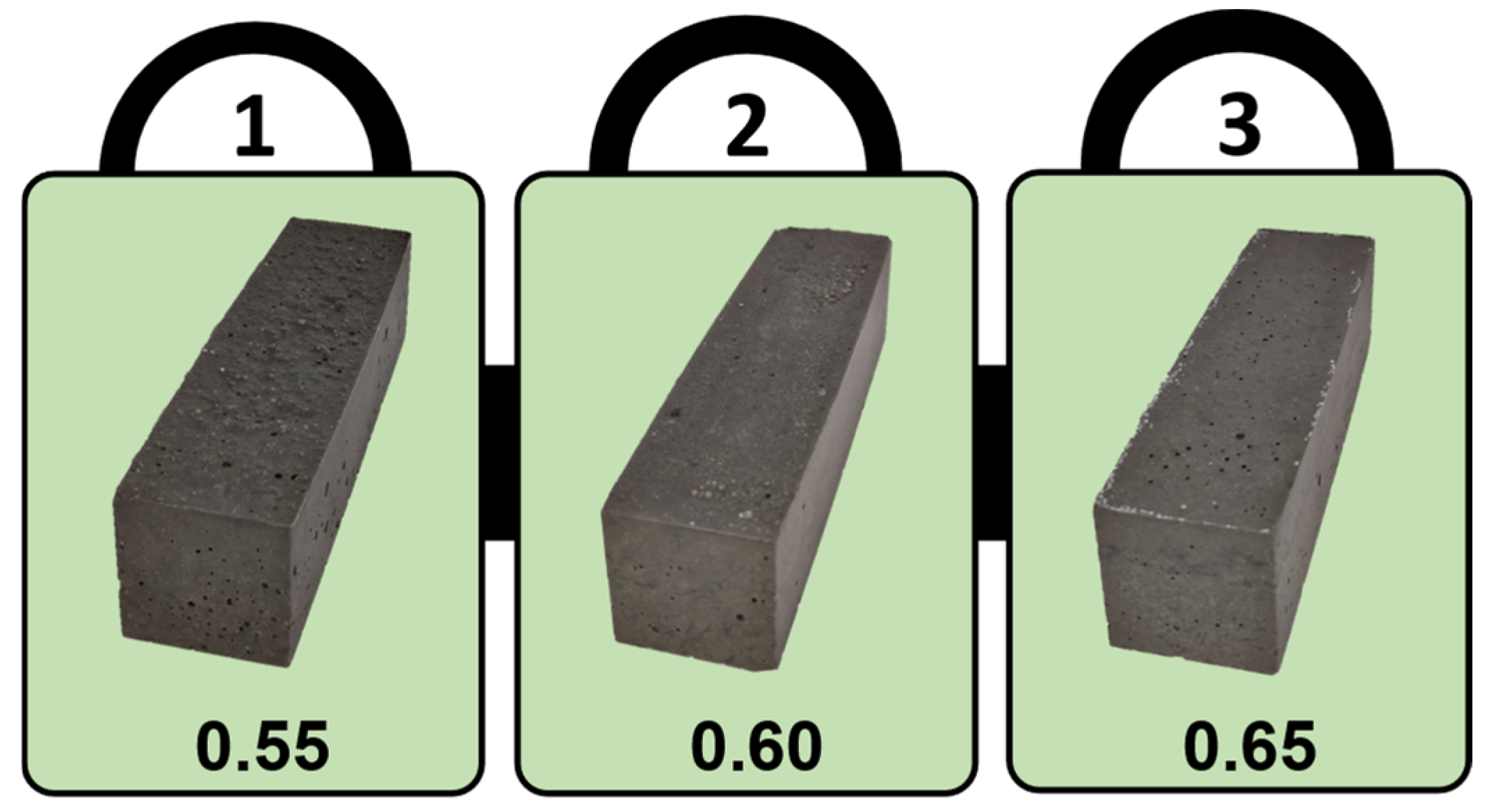
2.3. Samples Preparation
2.4. Characterization of the MPC-TUN Mortars Formulation
3. Results and Discussion
3.1. Slump Test
3.2. Apparent Fresh Density
3.3. Apparent Density
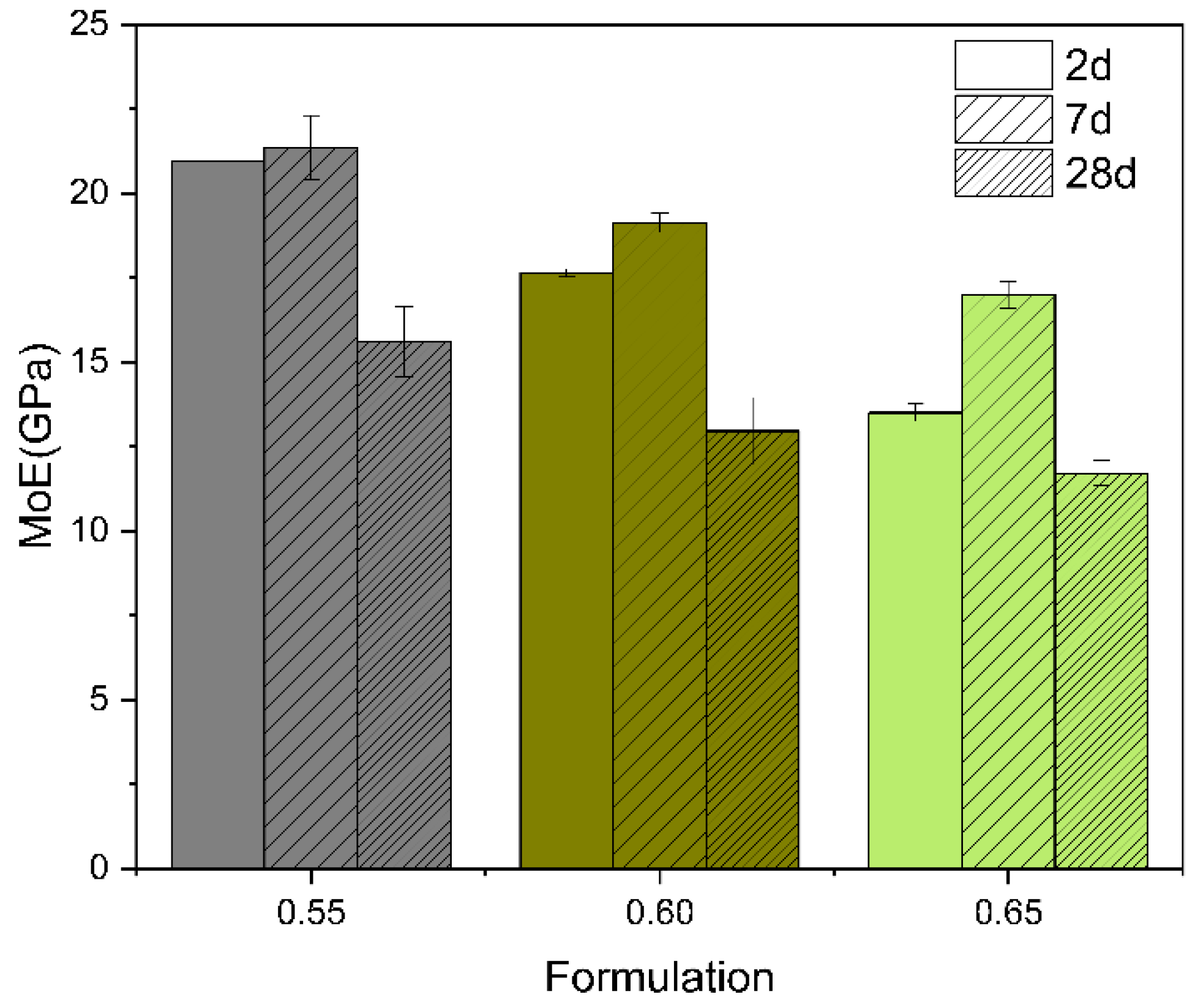
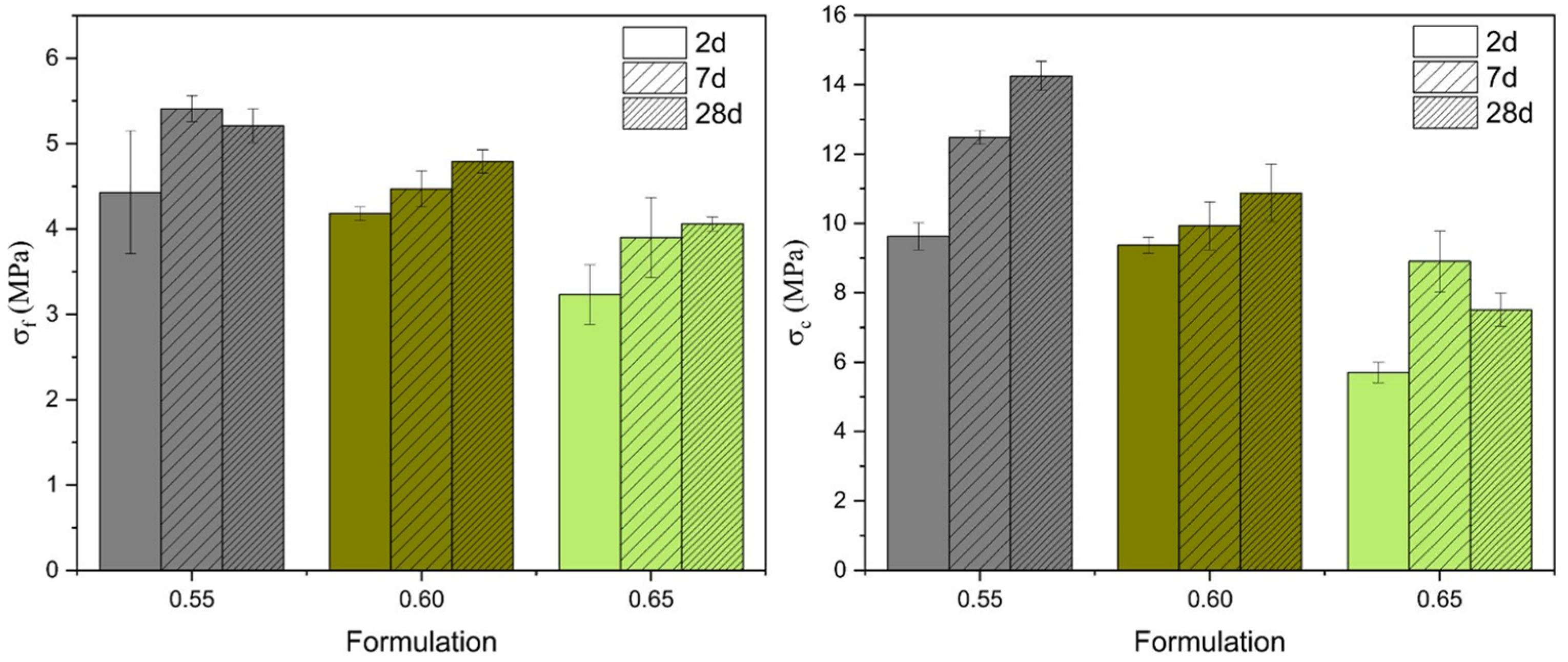
3.4. Modulus of Elasticity, Flexural Strength, and Compressive Strength
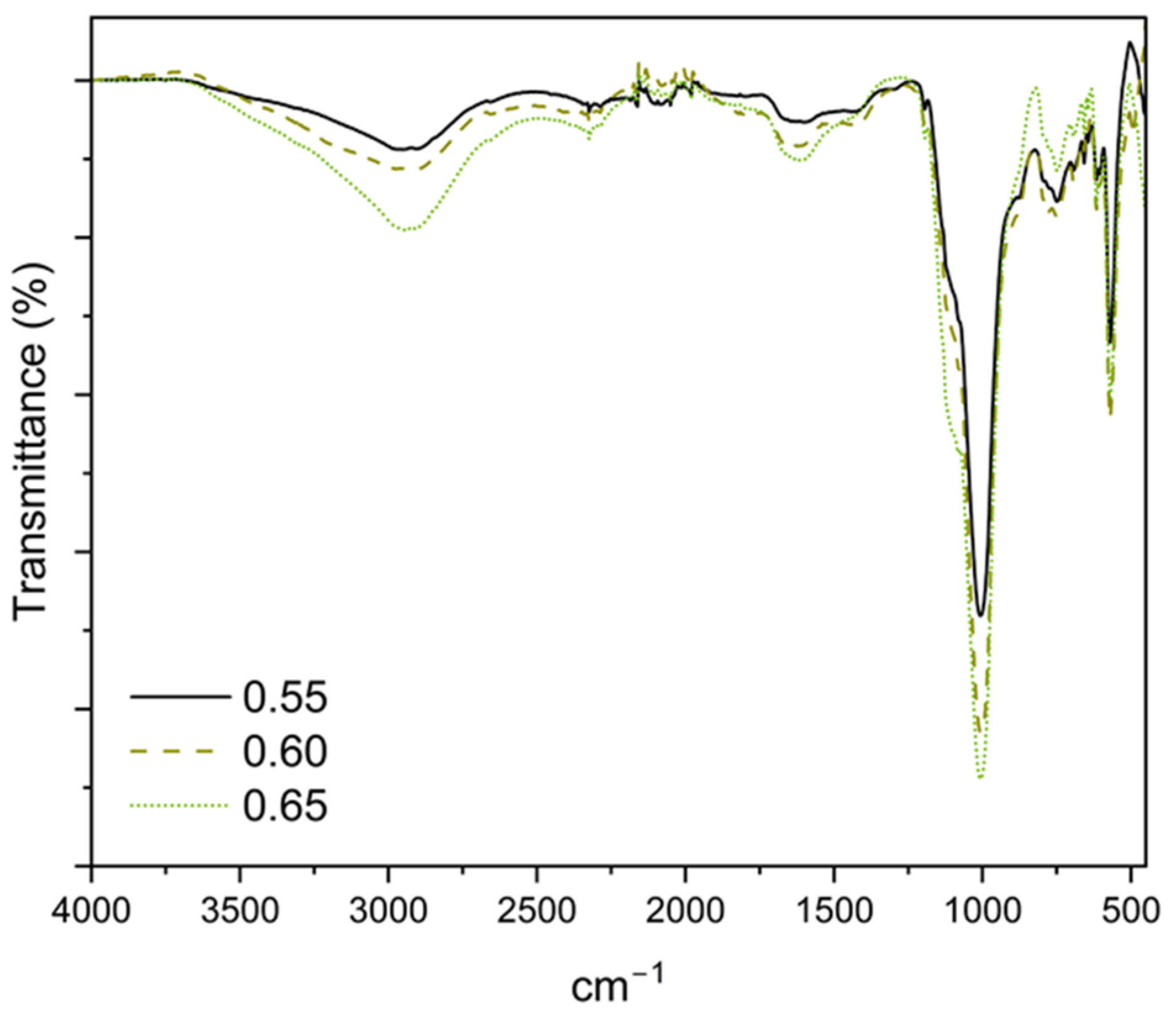
3.5. Fourier-Transformed Infrared Spectroscopy in Attenuated Total Reflectance (FTIR-ATR)
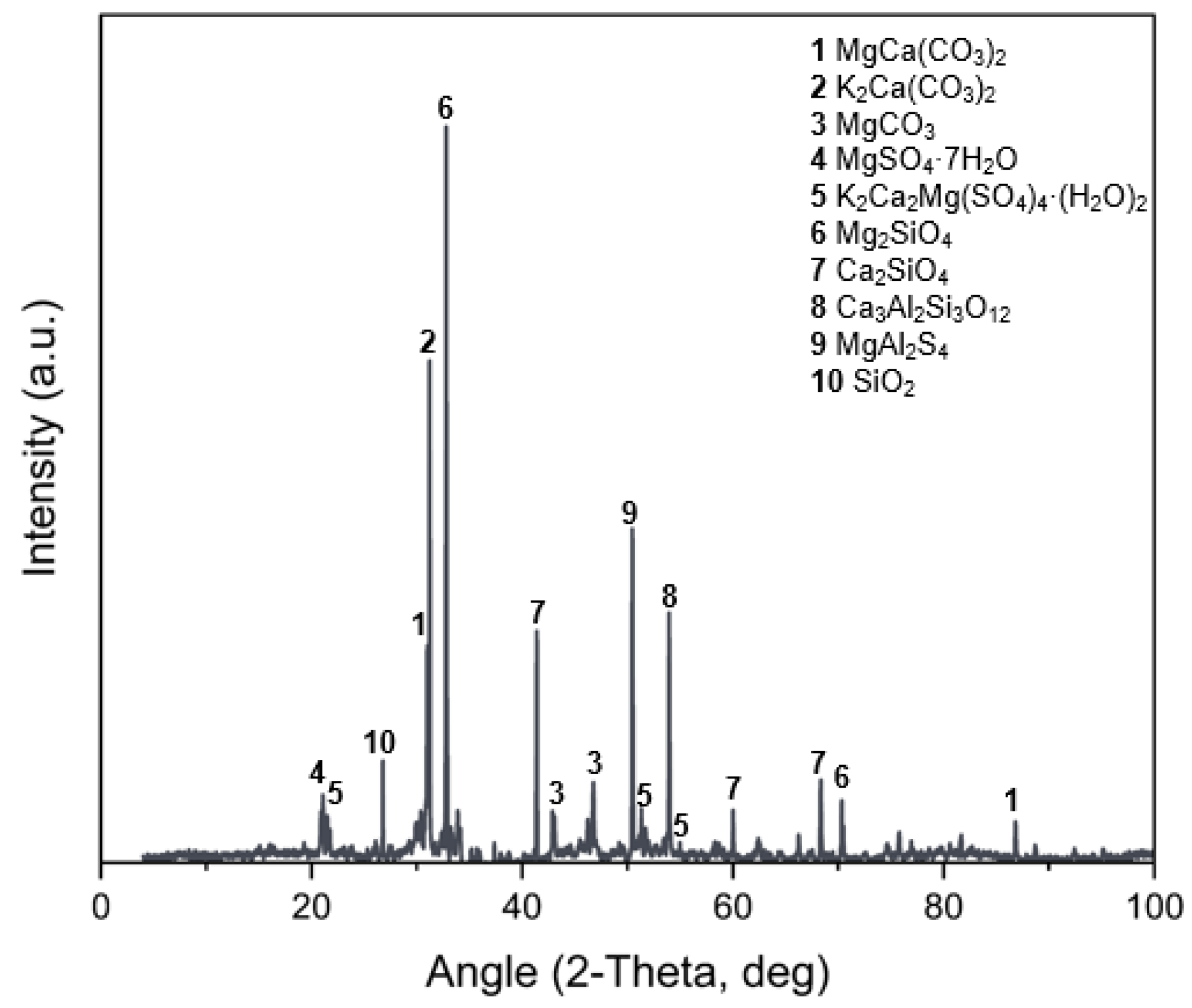
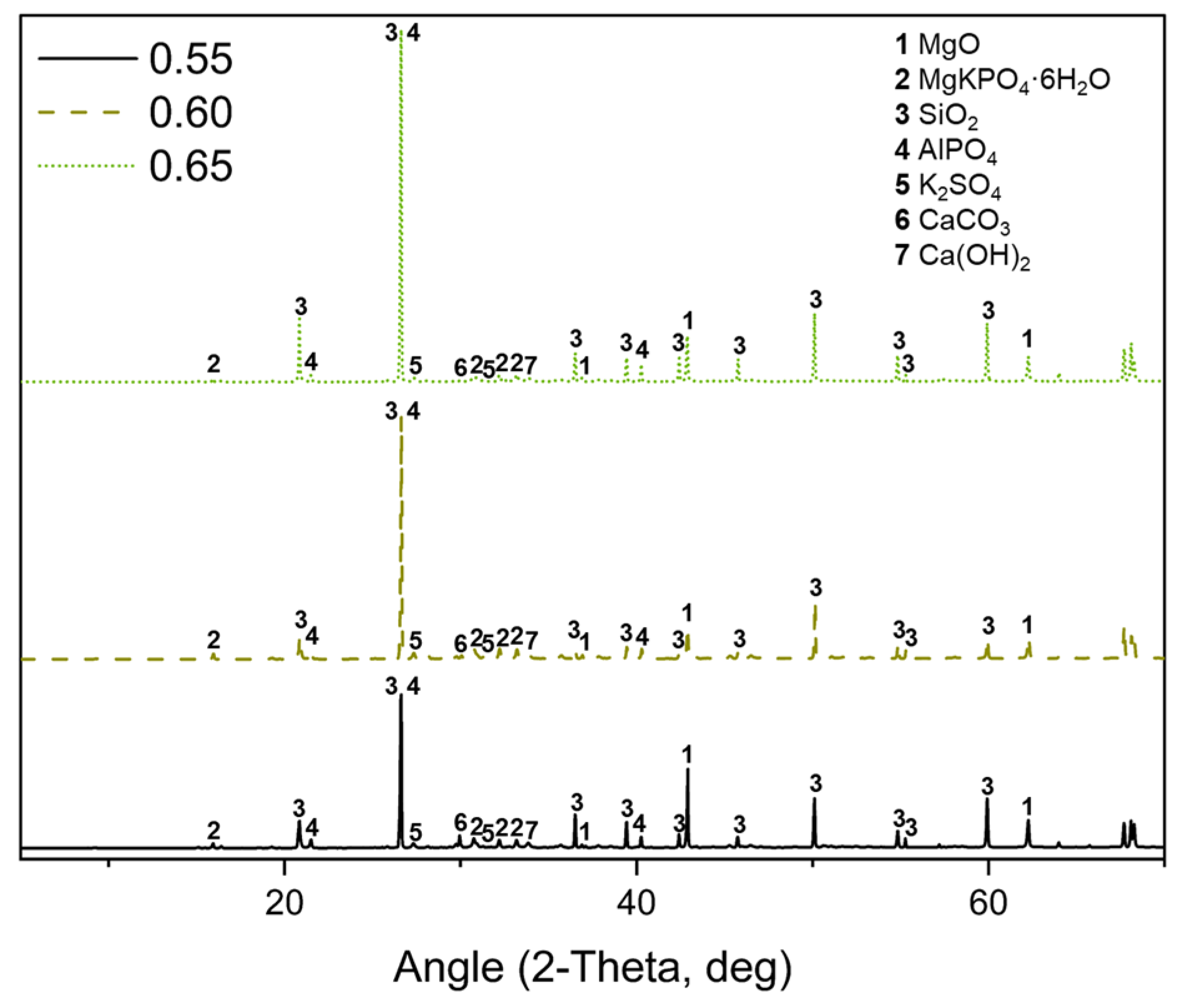
3.6. X-Ray Diffraction (XRD)
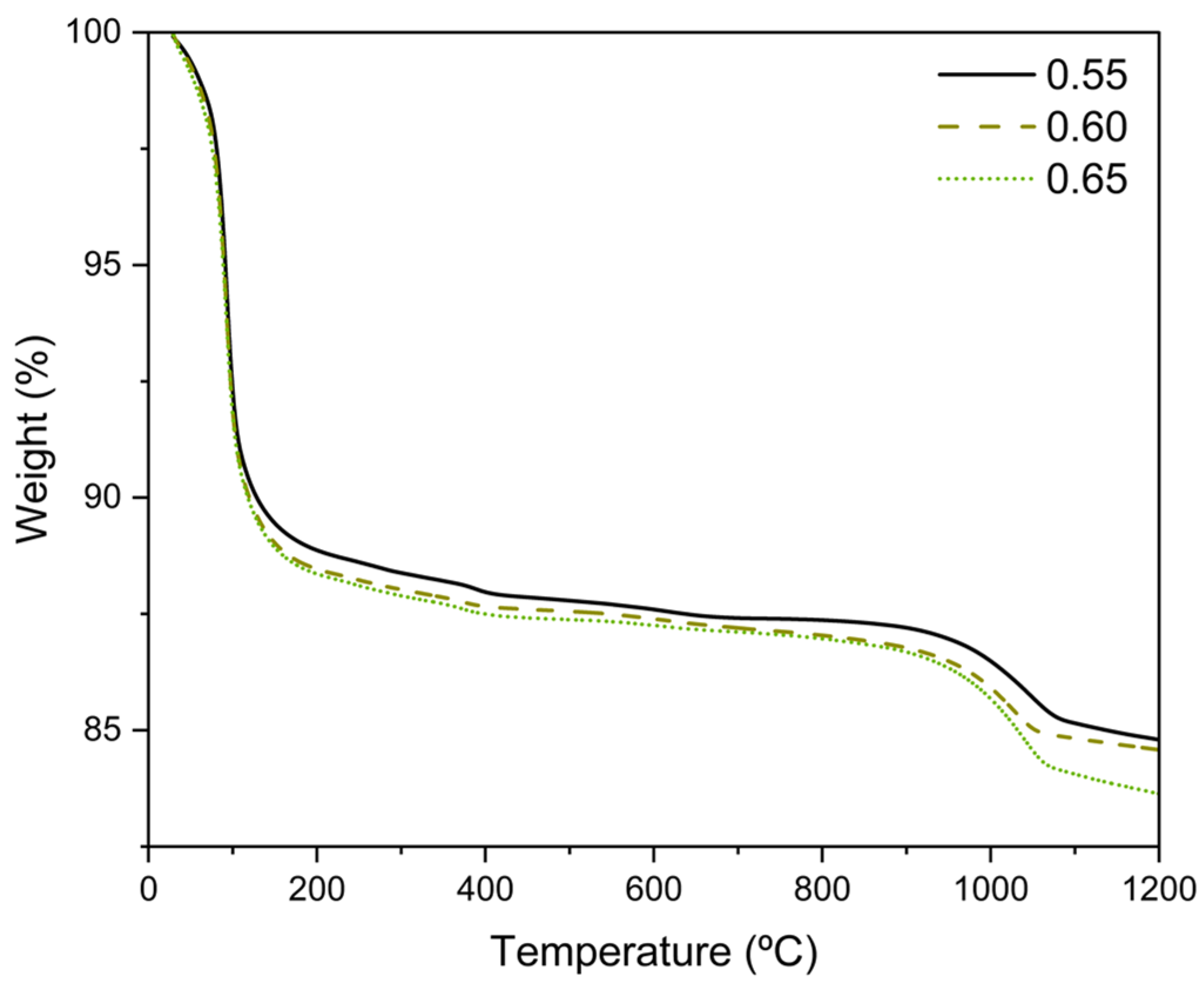
3.7. Thermogravimetric Analysis (TG)
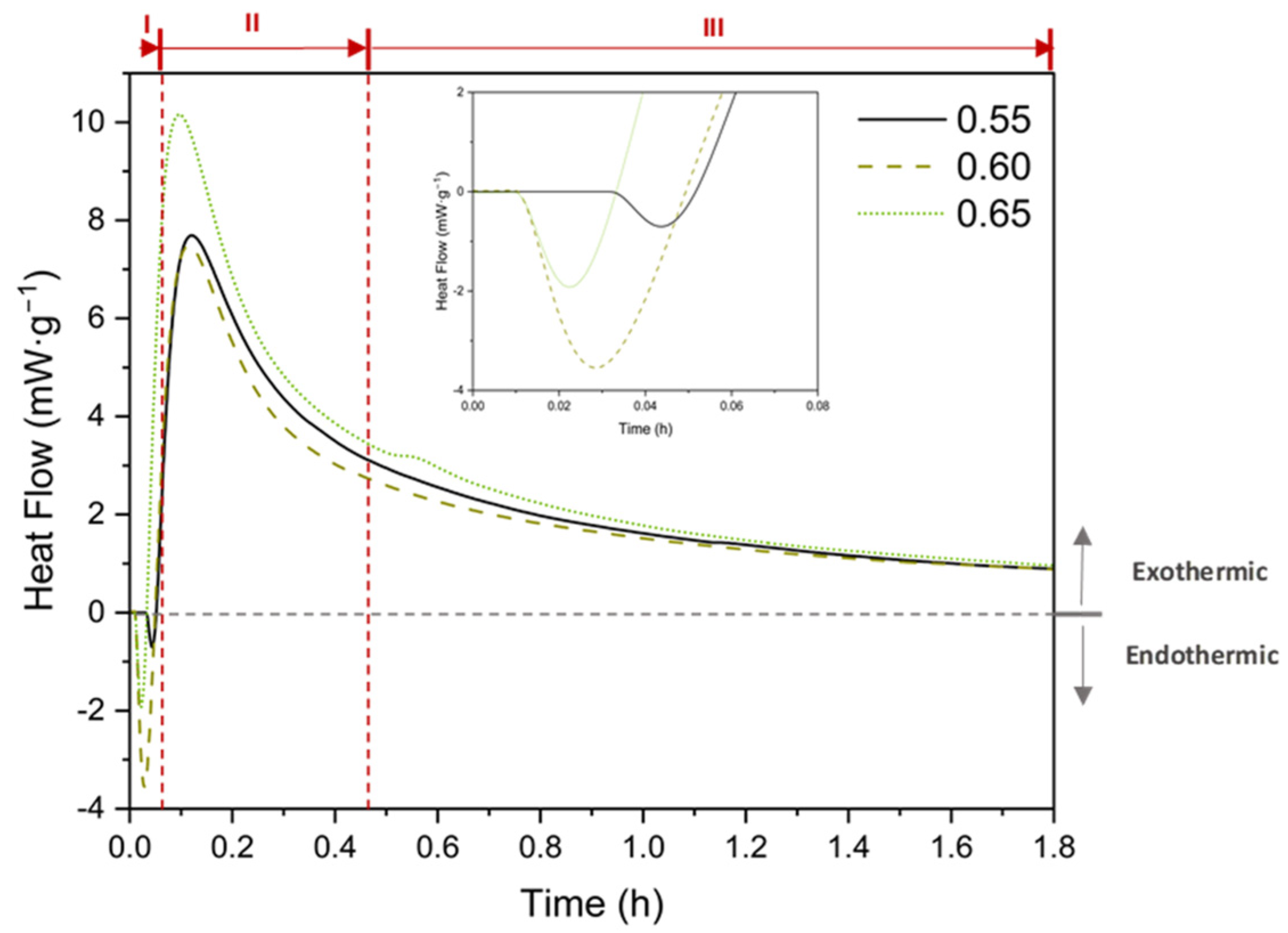
3.8. Isothermal Conduction Calorimetry (ICC)
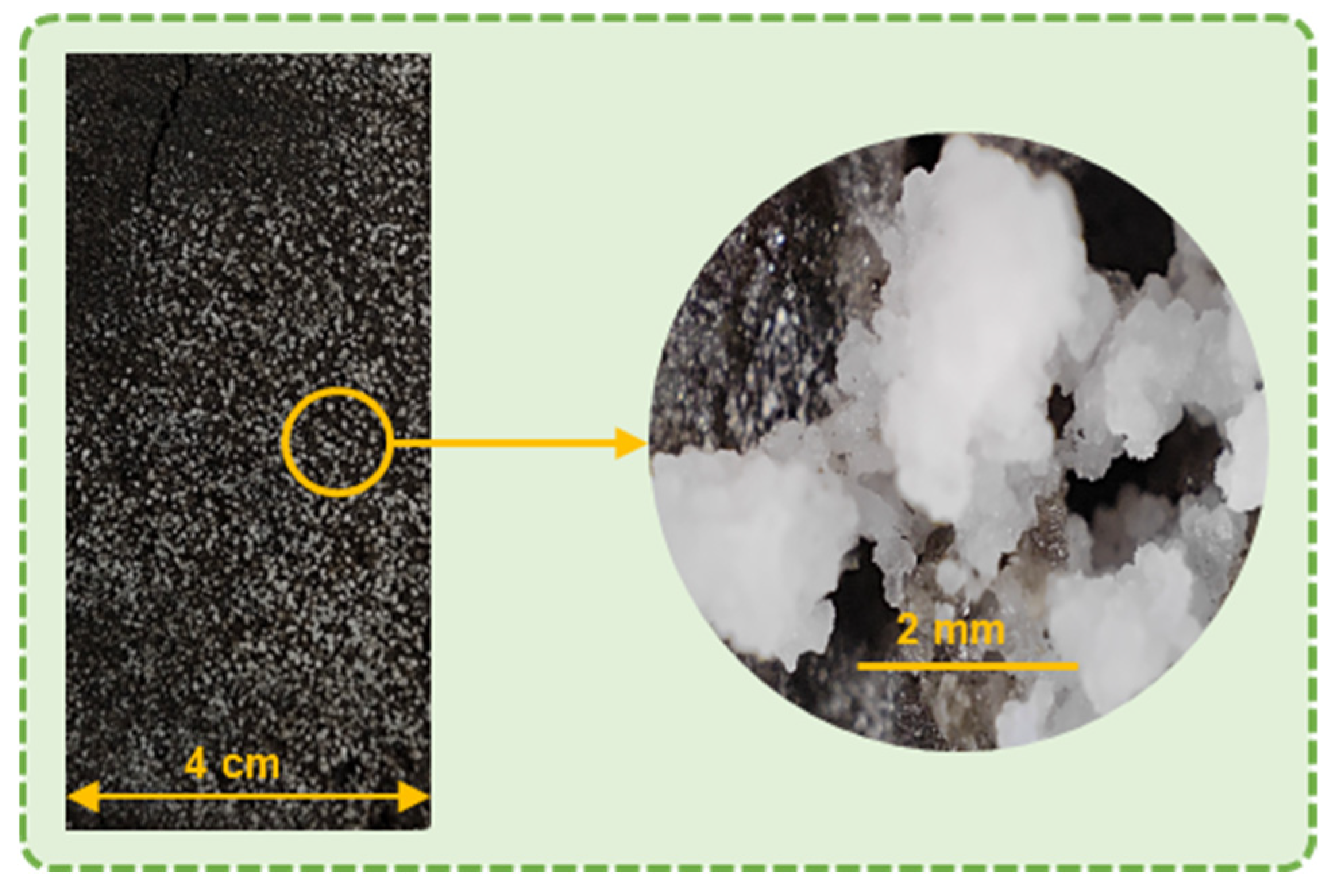
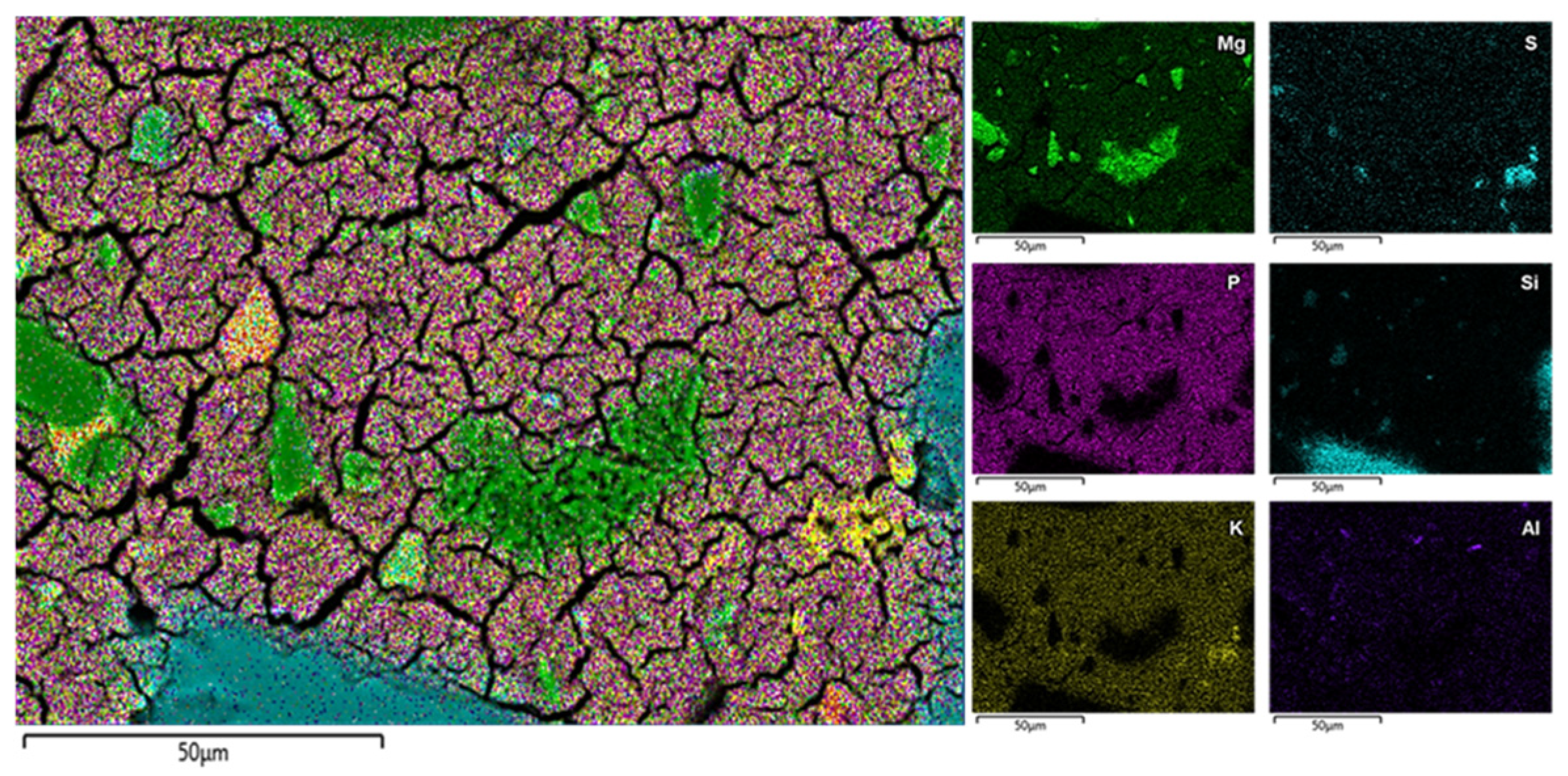
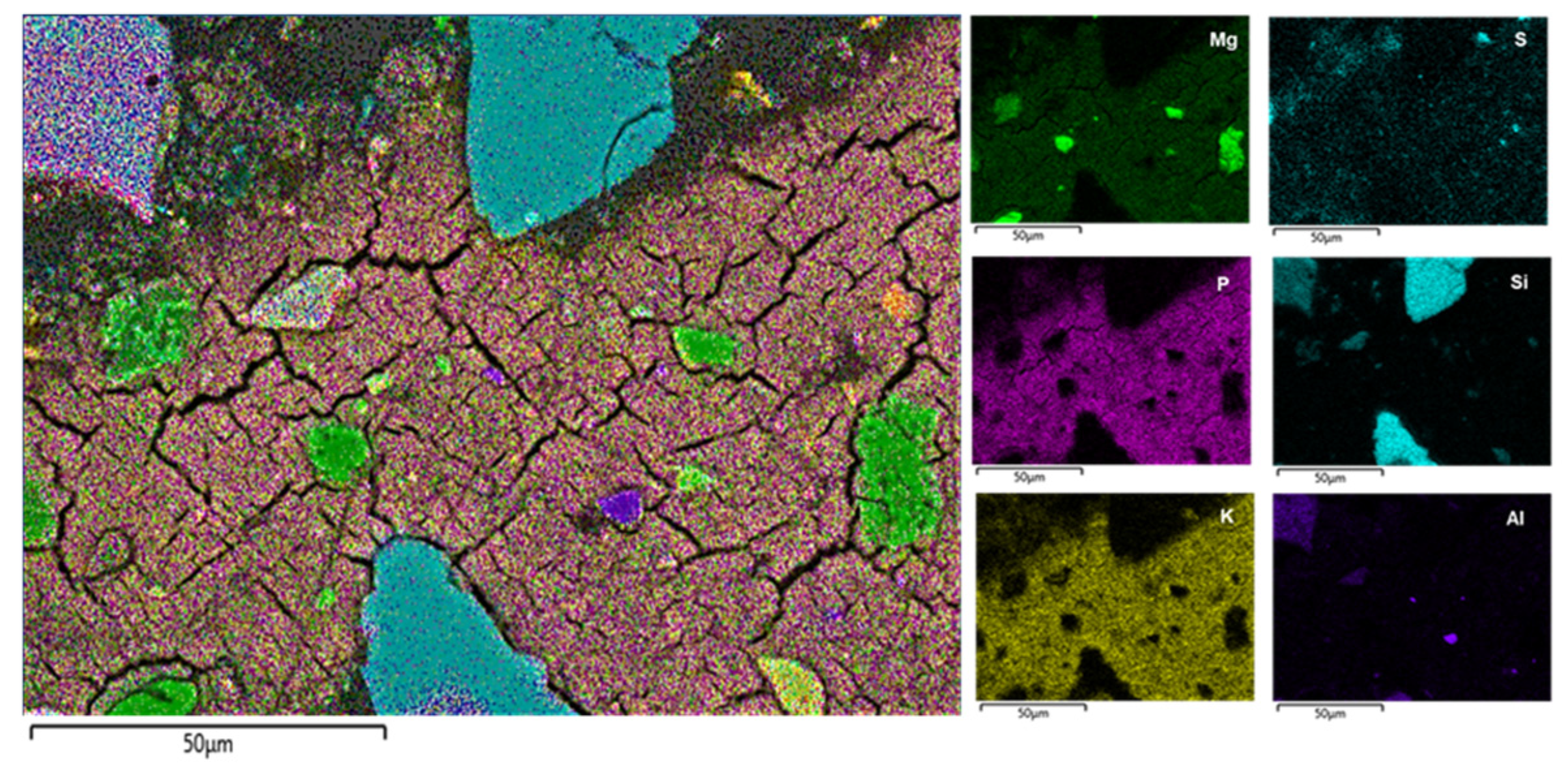
3.9. SEM-EDS
3.10. Dynamic Leaching Test
4. Conclusions
- The utilization of TUN waste from the metallurgical industry as a residual source of MgO allows the production of a binder, named MPC-TUN, which is adequate for developing mortars. MPC-TUN mortars exhibit properties that make them suitable for their utilization as precast material. Consequently, this research marks the beginning of a path toward exploring alternative mortars, which may play an important role in the development of alternative construction materials.
- There is a correlation between the progression of curing days and the increase in mechanical strengths, with the 0.55 formulation yielding the best mechanical performance at 28 days among the tested compositions. The consistency results further support this, as the 0.55 formulation achieves an optimal balance between adequate fluidity and adequate strength development. Moreover, this formulation meets the compressive strength requirements to be classified as M-10 masonry mortars, making it suitable for building applications.
- The characterization of MPC-TUN mortars, including FTIR-ATR, XRD, TG, ICC, and SEM-EDS, was essential for a comprehensive analysis. These techniques provided key insights, confirming K-struvite as the main phase and identifying secondary phases derived from TUN.
- The development of mortars using TUN waste demonstrates the potential for future MPC applications, promoting the principles of the circular economy, sustainability, and implementing proper waste management practices. By incorporating solid waste materials as substitutes for pure DBM, these strategies address the high energy consumption and CO2 emissions typically associated with MPC production. The implementation of waste recovery strategies not only promotes the efficient management of natural resources but also highlights a paradigm shift towards sustainable practices, contributing to the preservation of environmental ecosystems and the mitigation of climate change.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Z.; Zhu, X.; Wang, J.; Mu, M.; Wang, Y. Comparison of CO2 Emissions from OPC and Recycled Cement Production. Constr. Build. Mater. 2019, 211, 965–973. [Google Scholar] [CrossRef]
- Worrell, E.; Price, L.; Martin, N.; Hendriks, C.; Media, L.O. Carbon Dioxide Emissions from the Global Cement Industry. Annu. Rev. Energy Environ. 2001, 26, 303–329. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 Emission Strategies to Achieve Net Zero Target in Cement Sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Mañosa, J.; Calderón, A.; Salgado-Pizarro, R.; Maldonado-Alameda, A.; Chimenos, J.M. Research Evolution of Limestone Calcined Clay Cement (LC3), a Promising Low-Carbon Binder—A Comprehensive Overview. Heliyon 2024, 10, e25117. [Google Scholar] [CrossRef]
- Bernard, E.; Nguyen, H.; Kawashima, S.; Lothenbach, B.; Manzano, H.; Provis, J.; Scott, A.; Unluer, C.; Winnefeld, F.; Kinnunen, P. MgO-based Cements—Current Status and Opportunities. RILEM Tech. Lett. 2023, 8, 65–78. [Google Scholar] [CrossRef]
- Yang, M.; Chen, L.; Lai, J.; Osman, A.I.; Farghali, M.; Rooney, D.W.; Yap, P.-S. Advancing Environmental Sustainability in Construction through Innovative Low-Carbon, High-Performance Cement-Based Composites: A Review. Mater. Today Sustain. 2024, 26, 100712. [Google Scholar] [CrossRef]
- Huete-Hernández, S.; Maldonado-Alameda, A.; Giro-Paloma, J.; Chimenos, J.M.; Formosa, J. Fabrication of Sustainable Magnesium Phosphate Cement Micromortar Using Design of Experiments Statistical Modelling: Valorization of Ceramic-Stone-Porcelain Containing Waste as Filler. Ceram. Int. 2021, 47, 10905–10917. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Furszyfer Del Rio, D.D.; Foley, A.M.; Bazilian, M.D.; Kim, J.; Uratani, J.M. Decarbonizing the Cement and Concrete Industry: A Systematic Review of Socio-Technical Systems, Technological Innovations, and Policy Options. Renew. Sustain. Energy Rev. 2023, 180, 113291. [Google Scholar] [CrossRef]
- Kinnunen, P.; Ismailov, A.; Solismaa, S.; Sreenivasan, H.; Räisänen, M.L.; Levänen, E.; Illikainen, M. Recycling Mine Tailings in Chemically Bonded Ceramics—A Review. J. Clean. Prod. 2018, 174, 634–649. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, Y.; Xing, J.; Li, W.; Huang, X. Progresses of Green Magnesium Phosphate Cement: Production, Energy Consumption, Substitution and Recycling of Magnesia. J. Build. Eng. 2025, 99, 111563. [Google Scholar] [CrossRef]
- Xu, C.; Han, J.; Yang, Y. A Review on Magnesium Potassium Phosphate Cement: Characterization Methods. J. Build. Eng. 2024, 82, 108284. [Google Scholar] [CrossRef]
- Maldonado-Alameda, A.; Alfocea-Roig, A.; Huete-Hernández, S.; Giro-Paloma, J.; Chimenos, J.M.; Formosa, J. Magnesium Phosphate Cement Incorporating Sheep Wool Fibre for Thermal Insulation Applications. J. Build. Eng. 2023, 76, 107043. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, B.; Xing, F.; Han, N.; Li, Z. Cementing Mechanism of Potassium Phosphate Based Magnesium Phosphate Cement. Ceram. Int. 2012, 38, 6281–6288. [Google Scholar] [CrossRef]
- Le Rouzic, M.; Chaussadent, T.; Platret, G.; Stefan, L. Mechanisms of K-Struvite Formation in Magnesium Phosphate Cements. Cem. Concr. Res. 2017, 91, 117–122. [Google Scholar] [CrossRef]
- Pang, B.; Liu, J.; Wang, B.; Liu, R.; Yang, Y. Enhancement of Magnesium Phosphate Cement Solidification of Pb2+ by K-Struvite Whisker in Lead-Contaminated Solution. J. Clean. Prod. 2021, 320, 128848. [Google Scholar] [CrossRef]
- He, Z.; Jiang, Y.; Shi, J.; Qin, J.; Liu, D.; Yalçınkaya, Ç.; He, Y. Effect of Silica Fume on the Performance of High-Early-Strength UHPC Prepared with Magnesium Ammonium Phosphate Cement. Case Stud. Constr. Mater. 2024, 20, e03351. [Google Scholar] [CrossRef]
- Wagh, A.S.; Jeong, S.Y. Chemically Bonded Phosphate Ceramics: I, A Dissolution Model of Formation. J. Am. Ceram. Soc. 2003, 86, 1838–1844. [Google Scholar] [CrossRef]
- Schroeder, P.; Anggraeni, K.; Weber, U. The Relevance of Circular Economy Practices to the Sustainable Development Goals. J. Ind. Ecol. 2019, 23, 77–95. [Google Scholar] [CrossRef]
- Qiu, H.; Yu, J.; Chen, H.; Kuang, D.; He, R. Investigation on a Sustainable Magnesium Phosphate Cement (MPC) with Recycled Waste MPC Powders. J. Environ. Chem. Eng. 2024, 12, 114160. [Google Scholar] [CrossRef]
- Fahad, M.B.; Abdulkarem, A.M.; Hamed, T.H. A Review on Wastes as Sustainable Construction Materials. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012014. [Google Scholar] [CrossRef]
- Seifert, S.; Dittrich, S.; Bach, J. Recovery of Raw Materials from Ceramic Waste Materials for the Refractory Industry. Processes 2021, 9, 228. [Google Scholar] [CrossRef]
- Horckmans, L.; Nielsen, P.; Dierckx, P.; Ducastel, A. Recycling of Refractory Bricks Used in Basic Steelmaking: A Review. Resour. Conserv. Recycl. 2019, 140, 297–304. [Google Scholar] [CrossRef]
- Lopez, G.; Farfan, J.; Breyer, C. Trends in the Global Steel Industry: Evolutionary Projections and Defossilisation Pathways Through Power-to-Steel. J. Clean. Prod. 2022, 375, 134182. [Google Scholar] [CrossRef]
- Muñoz, I.; Soto, A.; Maza, D.; Bayón, F. Life Cycle Assessment of Refractory Waste Management in a Spanish Steel Works. Waste Manag. 2020, 111, 1–9. [Google Scholar] [CrossRef]
- Ding, C.; Lei, H.; Zhang, H.; Xu, M.; Zhao, Y.; Li, Q. New Insight into Relationship between Casting Speed and Inclusion Removal in the Tundish. J. Mater. Res. Technol. 2023, 23, 5400–5412. [Google Scholar] [CrossRef]
- Alfocea-Roig, A.; Huete-Hernández, S.; García-Zubiri, X.; Giro-Paloma, J.; Formosa, J. Can Tundish Deskulling Waste Be Used as a Magnesium Oxide Source to Develop Magnesium Phosphate Cement? J. Environ. Chem. Eng. 2023, 11, 110618. [Google Scholar] [CrossRef]
- Zou, Y.; Gu, H.; Huang, A.; Fu, L.; Li, G.; Wang, L.; Chen, D. Simultaneous Enhance of the Thermal Shock Resistance and Slag-Penetration Resistance for Tundish Flow-Control Refractories: The Role of Microporous Magnesia. Mater. Des. 2023, 233, 112245. [Google Scholar] [CrossRef]
- Quintero-Payan, A.C.; Huete-Hernández, S.; Aguilar-Pozo, V.B.; Astals, S.; Chimenos, J.M. Stabilization of Metal and Metalloids from Contaminated Soils Using Magnesia-Based Tundish Deskulling Waste from Continuous Steel Casting. Chemosphere 2024, 348, 140750. [Google Scholar] [CrossRef]
- Muñoz-Ruiz, V.; Cifrian, E.; Alfocea-Roig, A.; Santos, J.; Formosa, J.; Chimenos, J.M.; Andres, A. Ecotoxicity Assessment of Sustainable Magnesium Phosphate Cements (Sust-MPCs) Using Luminescent Bacteria and Sea Urchin Embryo-Larval Development Tests. J. Environ. Chem. Eng. 2024, 12, 113995. [Google Scholar] [CrossRef]
- Spyridakos, A.; Alexakis, D.E.; Vryzidis, I.; Tsotsolas, N.; Varelidis, G.; Kagiaras, E. Waste Classification of Spent Refractory Materials to Achieve Sustainable Development Goals Exploiting Multiple Criteria Decision Aiding Approach. Appl. Sci. 2022, 12, 3016. [Google Scholar] [CrossRef]
- Systematic and Integral Valorization of Refractories Under the “5R” Approach—5RefrACT. 2018. Available online: https://webgate.ec.europa.eu/life/publicWebsite/project/LIFE17-ENV-ES-000228/systematic-and-integral-valorization-of-refractories-under-the-5r-approach (accessed on 1 June 2025).
- Xu, B.; Lothenbach, B.; Leemann, A.; Winnefeld, F. Reaction Mechanism of Magnesium Potassium Phosphate Cement with High Magnesium-to-Phosphate Ratio. Cem. Concr. Res. 2018, 108, 140–151. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Paula, G.R.; Morelli, M.R. Effect of Boric Acid Content on the Properties of Magnesium Phosphate Cement. Constr. Build. Mater. 2019, 214, 557–564. [Google Scholar] [CrossRef]
- Formosa, J.; Chimenos, J.M.; Lacasta, A.M.; Niubó, M. Interaction between Low-Grade Magnesium Oxide and Boric Acid in Chemically Bonded Phosphate Ceramics Formulation. Ceram. Int. 2012, 38, 2483–2493. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef] [PubMed]
- Strydom, C.A.; Van Der Merwe, E.M.; Aphane, M.E. The Effect of Calcining Conditions on the Rehydration of Dead Burnt Magnesium Oxide Using Magnesium Acetate as a Hydrating Agent. J. Therm. Anal. Calorim. 2005, 80, 659–662. [Google Scholar] [CrossRef]
- Alfocea-Roig, A.; Müller, A.; Steubing, B.; Huete-Hernández, S.; Giro-Paloma, J.; Formosa, J. Life Cycle Assessment of the Climate Change Impact of Magnesium Phosphate Cements Formulated with Tundish Deskulling Waste Compared to Conventional Cement. Sustain. Chem. Pharm. 2024, 42, 101802. [Google Scholar] [CrossRef]
- Andrés, A.; Díaz, M.C.; Coz, A.; Abellán, M.J.; Viguri, J.R. Physico-Chemical Characterisation of Bricks All through the Manufacture Process in Relation to Efflorescence Salts. J. Eur. Ceram. Soc. 2009, 29, 1869–1877. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Ma, B.; Rentsch, D.; Lothenbach, B. Influence of Aluminum Sulfate on Properties and Hydration of Magnesium Potassium Phosphate Cements. Cem. Concr. Res. 2022, 156, 106788. [Google Scholar] [CrossRef]
- UNE-EN 1015-9; Methods of Test for Mortar for Masonry—Part 9: Determination of Workable Life and Correction Time of Fresh Mortar. AENOR: Madrid, Spain, 2000.
- UNE-EN 1936:2007; Natural Stone Test Methods—Determination of Real Density and Apparent Density, and of Total and Open Porosity. AENOR: Madrid, Spain, 2007.
- UNE-EN 12504-4; Testing Concrete in Structures—Part 4: Determination of Ultrasonic Pulse Velocity. AENOR: Madrid, Spain, 2022.
- Huete-Hernández, S.; Maldonado-Alameda, A.; Alfocea-Roig, A.; Giro-Paloma, J.; Chimenos, J.M.; Formosa, J. Sustainable Magnesium Phosphate Micromortars Formulated with PAVAL® Alumina By-Product as Micro-Aggregate. Bol. Soc. Esp. Ceram. Vidr. 2023, 62, 543–557. [Google Scholar] [CrossRef]
- Rosell, J.R.; Cantalapiedra, I.R. Método Simple Para Determinar El Módulo de Young Dinámico a Partir de Una Excitación Por Impacto, Aplicado a Morteros de Cal y Cemento. Mater. Construcc. 2011, 61, 39–48. [Google Scholar] [CrossRef]
- UNE-EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. AENOR: Madrid, Spain, 2018.
- UNE EN 12457-2; Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size Below 4 mm (Without or With Size Reduction). AENOR: Madrid, Spain, 2003.
- Chau, C.K.; Qiao, F.; Li, Z. Microstructure of Magnesium Potassium Phosphate Cement. Constr. Build. Mater. 2011, 25, 2911–2917. [Google Scholar] [CrossRef]
- UNE-EN 998-1:2018; Specification for Mortar for Masonry—Part 1: Rendering and Plastering Mortar. AENOR: Madrid, Spain, 2018.
- Wang, X.; Meng, L.; Hu, M.; Gao, L.; Lian, B. The Competitive and Selective Adsorption of Heavy Metals by Struvite in the Pb(II)-Cd(II)-Zn(II) Composite System and Its Environmental Significance. Water Res. 2023, 250, 121087. [Google Scholar] [CrossRef]
- Yuanquan, Y.; Guanhua, Z.; Jinbo, G.; Dingwen, Q.; Runqing, L. An Insight into the Thermal Properties of Struvite-K by Rietveld Refinement Method. J. Mater. Res. Technol. 2023, 24, 3683–3690. [Google Scholar] [CrossRef]
- Kabdaşlı, I.; Siciliano, A.; Limonti, C.; Tünay, O. Is K-Struvite Precipitation a Plausible Nutrient Rrecovery Method from Potassium-Containing Wastes? A Review. Sustainability 2022, 14, 11680. [Google Scholar] [CrossRef]
- Le, M.-V.; Duy, T.H.T.; Dang, B.-T.; Luan, V.H.; Huynh, N.-D.-T.; Long, N.Q.; Phuong, L.C.N. Phosphorus Recovery from Fertilizer Industrial Wastewaters Using Bittern: Influence of Wastewater Composition and pH on Struvite Formation. Bioresour. Technol. Rep. 2023, 25, 101752. [Google Scholar] [CrossRef]
- Pimentel Lages, V.; de Campos Vitorino, F.; Bentes Carvalho, R.; da Cunha, A.L.C.; Dweck, J. A New Thermogravimetric Method to Quantify SO2 Absorption Capacity by Limestone. Thermochim. Acta 2018, 667, 140–147. [Google Scholar] [CrossRef]
- Dweck, J.; Melchert, M.B.M.; Viana, M.M.; Cartledge, F.K.; Büchler, P.M. Importance of Quantitative Thermogravimetry on Initial Cement Mass Basis to Evaluate the Hydration of Cement Pastes and Mortars. J. Therm. Anal. Calorim. 2013, 113, 1481–1490. [Google Scholar] [CrossRef]
- Lahalle, H.; Patapy, C.; Glid, M.; Renaudin, G.; Cyr, M. Microstructural Evolution/Durability of Magnesium Phosphate Cement Paste over Time in Neutral and Basic Environments. Cem. Concr. Res. 2019, 122, 42–58. [Google Scholar] [CrossRef]
- Dollimore, D.; Lerdkanchanaporn, S. Thermal Analysis. Anal. Chem. 1998, 70, 27–36. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; He, Y. A Novel Preparation of Nano-Sized Hexagonal Mg(OH)2. Procedia Eng. 2015, 102, 388–394. [Google Scholar] [CrossRef]
- Zarzuela, R.; Luna, M.; Carrascosa, L.M.; Yeste, M.P.; Garcia-Lodeiro, I.; Blanco-Varela, M.T.; Cauqui, M.A.; Rodríguez-Izquierdo, J.M.; Mosquera, M.J. Producing C-S-H Gel by Reaction between Silica Oligomers and Portlandite: A Promising Approach to Repair Cementitious Materials. Cem. Concr. Res. 2020, 130, 106008. [Google Scholar] [CrossRef]
- Wagh, A.S. Recent Progress in Chemically Bonded Phosphate Ceramics. ISRN Ceram. 2013, 2013, 983731. [Google Scholar] [CrossRef]
- Qiao, F.; Chau, C.K.; Li, Z. Calorimetric Study of Magnesium Potassium Phosphate Cement. Mater. Struct. Mater. Constr. 2012, 45, 447–456. [Google Scholar] [CrossRef]
- Wang, D.; Yue, Y.; Qian, J. Effect of Carbonation on the Corrosion Behavior of Steel Rebar Embedded in Magnesium Phosphate Cement. Compos. Part B Eng. 2024, 268, 111088. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, Y.; Yang, Y.; Long, W.; Dong, B. Enhancement of Magnesium Phosphate Cement Composites with Sintered Sludge Ash. Dev. Built Environ. 2023, 17, 100313. [Google Scholar] [CrossRef]



| Compounds | MgO | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | P2O5 | Cr2O3 | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 68.70 | 9.43 | 8.67 | 4.68 | 3.56 | 0.15 | 0.11 | 0.07 | 0.02 | 4.61 |
| Techniques | MPC-TUN Mortars | |||
|---|---|---|---|---|
| Fresh state | Slump test | - | - | - |
| Apparent fresh density | - | - | - | |
| Hardened state | Techniques | 2d | 7d | 28d |
| Apparent density | 🗸 | 🗸 | 🗸 | |
| MoE | 🗸 | 🗸 | 🗸 | |
| CS (σc) | 🗸 | 🗸 | 🗸 | |
| FS (σf) | 🗸 | 🗸 | 🗸 | |
| FTIR-ATR | 🗸 | 🗸 | 🗸 | |
| XRD | 🗸 | 🗸 | 🗸 | |
| TG | - | - | 🗸 | |
| Isothermal calorimetry | 🗸 | 🗸 | 🗸 | |
| Leaching tests | - | - | 🗸 | |
| SEM | - | - | 🗸 | |
| Formulation | Base Width (mm) |
|---|---|
| 0.55 | 112.53 |
| 0.60 | 120.88 |
| 0.65 | 126.78 |
| Formulation | Apparent Fresh Density (g·cm−3) |
|---|---|
| 0.55 | 2.044 ± 0.034 |
| 0.60 | 2.042 ± 0.025 |
| 0.65 | 2.054 ± 0.019 |
| Apparent Density (g·cm−3) | |||
|---|---|---|---|
| Formulation | 2d | 7d | 28d |
| 0.55 | 2.064 ± 0.005 | 2.034 ± 0.040 | 1.954 ± 0.030 |
| 0.60 | 2.024 ± 0.003 | 2.030 ± 0.020 | 1.932 ± 0.020 |
| 0.65 | 1.985 ± 0.009 | 2.015 ± 0.002 | 1.947 ± 0.010 |
| Band (cm−1) | Assignation |
|---|---|
| 2940–3250 | ν O-H from water |
| 1590–1680 | δ O-H from water |
| 1000–1010 | ν1 P-O4 from K-struvite |
| 575–560 | ν4 P-O4 from K-struvite |
| wt.% | ||||
|---|---|---|---|---|
| Formulation | MgKPO4·6H2O | Mg(OH)2 | CaCO3 | K2SO4 |
| 0.55 | 27.77 | 1.27 | 1.03 | 5.66 |
| 0.60 | 28.72 | 1.05 | 1.01 | 5.55 |
| 0.65 | 28.83 | 1.33 | 0.61 | 7.15 |
| Sample | pH | As | Ba | Cd | Cr | Cu | Hg | Mo | Ni | Pb | Sb | Se | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TUN | 12.54 | <0.01 | 2.20 | <0.02 | 0.02 | 0.05 | <0.01 | 0.29 | 0.03 | <0.05 | 0.01 | 0.49 | <0.10 |
| MPC-TUN (paste) | 11.28 | 0.41 | <0.05 | <0.02 | <0.05 | <0.05 | <0.01 | 1.80 | <0.05 | <0.05 | 0.09 | 0.99 | <0.10 |
| 0.55 | 10.74 | 0.02 | 10.86 | <0.01 | <0.01 | <0.01 | <0.02 | 0.33 | <0.02 | <0.01 | <0.01 | 0.35 | <0.10 |
| 0.60 | 10.63 | 0.01 | 9.18 | <0.01 | <0.01 | <0.01 | <0.02 | 0.40 | <0.02 | <0.01 | <0.01 | 0.42 | <0.10 |
| 0.65 | 10.54 | <0.01 | 9.69 | <0.01 | <0.01 | <0.01 | <0.02 | 0.38 | <0.02 | <0.01 | <0.01 | 0.42 | <0.10 |
| Inert limit (mg/kg) | - | 0.5 | 20 | 0.04 | 0.5 | 2 | 0.01 | 0.5 | 0.4 | 0.5 | 0.06 | 0.1 | 4 |
| Non-hazardous limit (mg/kg) | - | 2 | 100 | 1 | 10 | 50 | 0.2 | 10 | 10 | 10 | 0.7 | 0.5 | 50 |
| Hazardous limit (mg/kg) | - | 25 | 300 | 5 | 70 | 100 | 2 | 30 | 40 | 50 | 5 | 7 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfocea-Roig, A.; Vera-Rivera, D.; Huete-Hernández, S.; Giro-Paloma, J.; Formosa Mitjans, J. Tundish Deskulling Waste as a Source of MgO for Producing Magnesium Phosphate Cement-Based Mortars: Advancing Sustainable Construction Materials. Resources 2025, 14, 107. https://doi.org/10.3390/resources14070107
Alfocea-Roig A, Vera-Rivera D, Huete-Hernández S, Giro-Paloma J, Formosa Mitjans J. Tundish Deskulling Waste as a Source of MgO for Producing Magnesium Phosphate Cement-Based Mortars: Advancing Sustainable Construction Materials. Resources. 2025; 14(7):107. https://doi.org/10.3390/resources14070107
Chicago/Turabian StyleAlfocea-Roig, Anna, David Vera-Rivera, Sergio Huete-Hernández, Jessica Giro-Paloma, and Joan Formosa Mitjans. 2025. "Tundish Deskulling Waste as a Source of MgO for Producing Magnesium Phosphate Cement-Based Mortars: Advancing Sustainable Construction Materials" Resources 14, no. 7: 107. https://doi.org/10.3390/resources14070107
APA StyleAlfocea-Roig, A., Vera-Rivera, D., Huete-Hernández, S., Giro-Paloma, J., & Formosa Mitjans, J. (2025). Tundish Deskulling Waste as a Source of MgO for Producing Magnesium Phosphate Cement-Based Mortars: Advancing Sustainable Construction Materials. Resources, 14(7), 107. https://doi.org/10.3390/resources14070107








