1. Introduction
The world is facing a growing waste crisis that threatens the environment, public health, and the climate. Currently, over 2 billion metric tons of waste are generated globally each year, with projections indicating an increase of 70% to nearly 4 billion tons by 2050 if current practices continue [
1]. A significant portion of this waste is mismanaged, with 38% being openly dumped or burned, releasing harmful pollutants into the air, soil, and water [
2]. Various types of waste, including municipal solid waste, industrial waste, agricultural waste, hazardous waste, construction and demolition debris, electronic waste, and medical waste, require careful disposal to minimize their environmental impact and promote sustainability [
3]. These waste types encompass food waste, plastics, paper, hazardous materials, and construction debris, all of which can lead to contamination and spread disease if not managed properly. Urgent action is needed to modernize waste collection, increase recycling and composting, and transition to a circular economy that minimizes waste and pollution. Governments, businesses, and citizens all play crucial roles in tackling this global crisis before it escalates further [
4].
Agro-industrial wastes, including residues from agricultural and food processing industries, pose a global environmental challenge. These wastes are often left untreated and underutilized, leading to increased greenhouse gas emissions and the unnecessary use of fossil fuels [
5]. The improper disposal of these wastes also causes pollution and can harm human and animal health. Industries like juice, coffee, and cereal production generate large amounts of waste, including fiber sources, wheat straw residues, and rice straws [
6].
Despite their potential uses, these wastes are frequently treated as worthless rather than as valuable resources [
7]. These wastes mainly consist of cellulose, hemicellulose, and lignin, which can be converted into a variety of value-added products. Hazardous agro-industrial wastes, such as phytosanitary products, pose immediate and long-term problems if not managed correctly. Therefore, it is crucial to find sustainable ways to utilize these wastes and convert them into useful products to reduce environmental impact and create economic opportunities [
8].
Agro-industrial waste is increasingly recognized as a valuable resource for various applications, particularly due to its rich content of bioactive compounds, carbohydrates, and proteins. This waste, which includes byproducts from food processing and agricultural activities, can be transformed into valuable products such as biofuels, enzymes, and biodegradable materials [
6]. However, a significant challenge in utilizing agro-industrial waste lies in its lignin content [
8]. Lignin, a complex and recalcitrant polymer, complicates the breakdown of biomass into fermentable sugars, which are essential in the production of bio-based products. The presence of lignin can hinder microbial fermentation processes, making it difficult to efficiently convert these wastes into desired bioactive compounds or energy sources [
9]. Therefore, while agro-industrial waste holds great potential as a sustainable resource, overcoming the barriers posed by lignin is crucial for maximizing its utilization and achieving effective waste valorization.
Hydrothermal processing has emerged as a promising solution for managing the vast amounts of agro-industrial waste generated worldwide. This thermochemical conversion method utilizes high temperatures and pressures to transform biomass into valuable products such as biofuels, biochar, and other bio-based chemicals [
10]. The efficiency of hydrothermal processes is significantly influenced by the composition of the biomass, particularly the content of hemicellulose and lignin [
11]. Hemicellulose, which is more amenable to hydrolysis, can be converted into fermentable sugars that serve as precursors for biofuel production. In contrast, the lignin, as mentioned above, is a complex and rigid polymer that poses challenges during hydrothermal reactions. Its presence can hinder the breakdown of biomass, leading to lower yields of desired products and complicating the overall conversion process [
12]. Therefore, optimizing the hydrothermal treatment conditions to effectively manage the lignin content while maximizing hemicellulose utilization is crucial for enhancing the viability of agro-industrial waste as a sustainable resource for bioenergy and bioproducts [
13].
The hydrothermal conversion of lignocellulosic biomass can generate a range of valuable platform chemicals that serve as essential building blocks for biofuels, biochemicals, and biomaterials. Notable platform chemicals produced through this process include furans, such as 5-hydroxymethylfurfural (HMF) and furfural, which are derived from the dehydration of hexoses and pentoses, respectively [
14]. Additionally, organic acids like acetic acid, formic acid, and levulinic acid can be produced from the breakdown of cellulose and hemicellulose, with applications spanning use in food or pharmaceuticals, and alongside other platform chemicals. The use of catalysts in hydrothermal conversion can significantly influence the yield and selectivity of these platform chemicals [
15]. The hydrothermal carbonization (HTC) process, a specific type of hydrothermal conversion, not only facilitates the production of these chemicals but also converts biomass into a carbon-rich solid known as hydrochar, which can be used as a soil amendment or in energy applications [
16].
Non-traditional cellulose-containing raw materials, such as peapods and coffee cherries, represent an underutilized but promising category for valorization through hydrothermobaric methods. These materials typically have a cellulose content ranging from 20 to 28%, which is lower than traditional sources like wood or agricultural residues but sufficient for conversion into valuable compounds. Their unique composition, which often includes a higher proportion of non-cellulosic components such as proteins, fats, and bioactive compounds, provides additional opportunities for the production of diverse value-added products. Utilizing these non-traditional materials not only diversifies the feedstock base but also contributes to waste reduction in industries like agriculture and food processing. Exploring the use of hydrothermobaric processing for these substrates can unlock their potential sustainable applications while addressing the challenges posed by their distinct composition.
The use of catalysts in hydrothermal conversion can significantly influence the yield and selectivity of these platform chemicals, enhancing the overall efficiency of the process. Homogeneous acid catalysts like sulfuric acid (H
2SO
4) and heterogeneous catalysts like zeolites can promote the depolymerization and upgrading of biomass components [
17]. For example, the addition of a H
2SO
4 catalyst in the hydrothermal liquefaction of peapod waste increased its selectivity to phenol, furfural, and HMF. Optimizing the catalyst type, loading, and reaction conditions is crucial for maximizing the production of desired platform chemicals from biomass [
18].
The article proposes to determine the impact of catalysts on the generation of platform chemicals from peapod waste using hydrothermal carbonization. This investigation has proven the effectiveness of both organic and inorganic acid catalysts. By carefully choosing the right catalyst and optimizing the hydrothermal process, this significant agricultural waste stream can be used to improve the yield, efficiency, and specificity of the desired platform chemicals. This research aspires to contribute to the expanding field of biomass conversion through a methodical investigation of hydrothermal carbonization parameters and catalyst selection. It encourages the creation of environmentally friendly technologies that can reduce energy use and the environmental effects while converting agricultural waste into high-value products.
3. Results and Discussion
3.1. Preliminary Characterization of Biomass
Table 1 presents a comparative analysis of the chemical composition of the coffee cherries and peapods utilized in this research. The moisture level is a key aspect that may affect how the materials are processed and stored, as well as their quality. In the first stage, the biomass of coffee cherries had a moisture content of 80.79%, which is comparable to the peapod moisture content of 80.77%. This implies that a comparable drying or dehydration process may be necessary for both biomass types prior to additional processing. After the biomass was dried, the moisture content of each sample was measured again. The moisture percentage in the BHP (Biomass for Hydrothermal Processes) for both biomass types was lower compared to the initial measurements, indicating that the drying process was successful. A moisture content of 10.94% was obtained for coffee cherries and 7.77% for peapods.
The percentage of inorganic mineral stuff in the materials is represented by the ash content. When compared to peapods (4.22%), coffee cherries have a higher ash concentration (7.79%). This implies that coffee cherries might contain more minerals, which could be important for some uses, including the production of biofuels or as a source of nutrients. Alternatively, this concentration could represent inorganic matter that poses a risk to the reactor and cannot be recovered.
Fixed carbon, a residual carbon after thermal processing, is a key component of biomass, indicating its energy content. Higher levels correlate with greater energy density, making them suitable for combustion and gasification applications [
25]. Coffee cherries may be more reactive during thermal processing—such as combustion or pyrolysis—due to their higher volatile matter concentration (79.91%) compared to peapods (74.18%). In contrast, peapods exhibit a significantly higher fixed carbon content (13.0%) than coffee cherries (1.36%). This difference suggests that peapods possess a higher energy density, making them better suited for certain energy-related applications.
The percentages of cellulose, hemicellulose, and lignin in a material might affect its suitability for a specific use, such as producing biofuels, bioplastics, or other products with added value. Coffee cherries contain more lignin (13.7%) and cellulose (27.6%) than peapods (20.2% cellulose and 5.0% lignin). On the other hand, hemicellulose content in peapods is higher (17.4%) than in coffee cherries (12.5%). The prospective applications of each material may be impacted by these variations in the composition of their structural components. It is important to note that lignin can inhibit hydrothermal processes, lowering output yield [
26].
3.2. Overall Yields
The yields and catalysts tested are presented in
Table 2.
3.3. Hydrothermal Valorization Without Catalysts
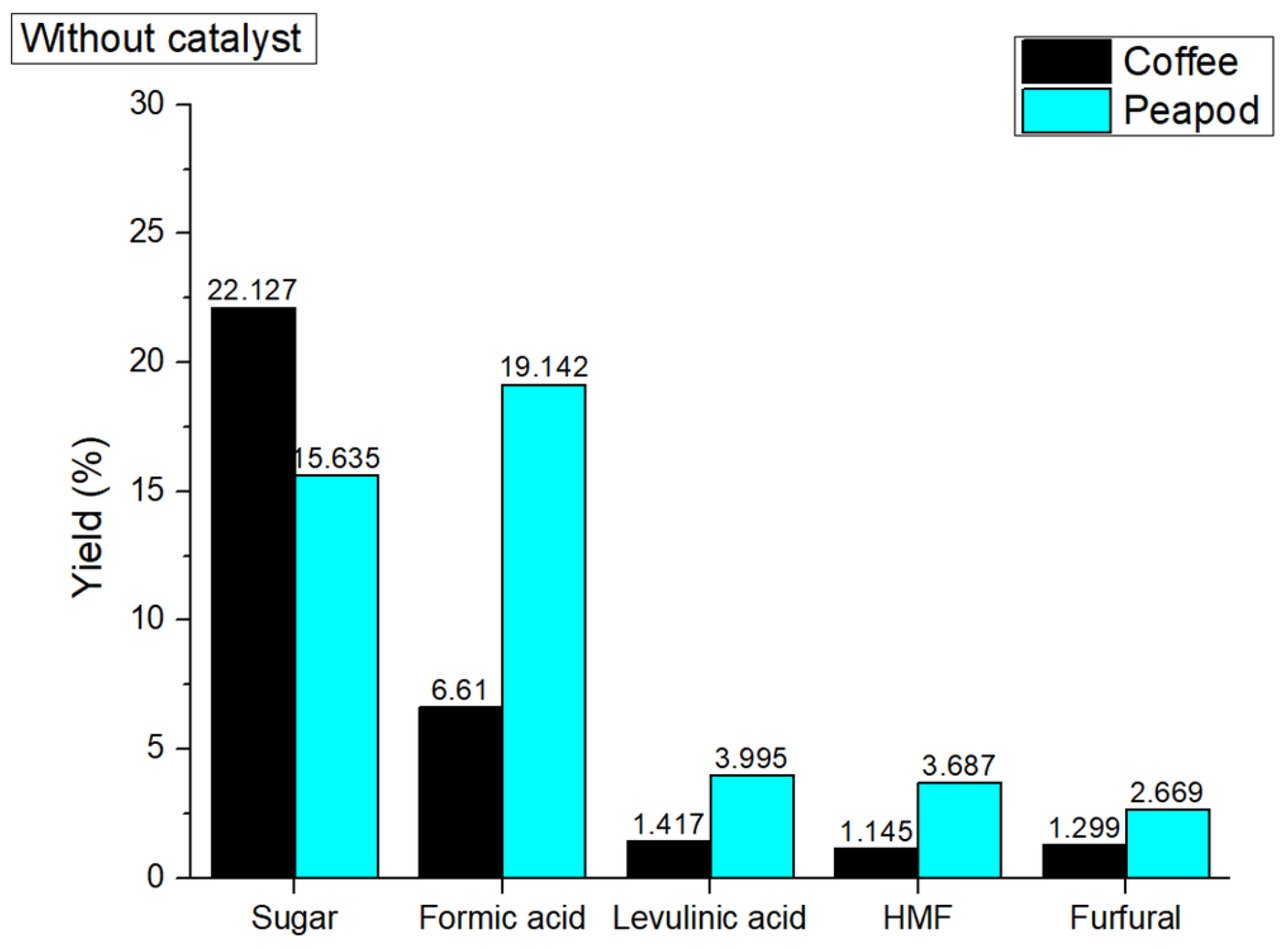
In order to obtain a base idea of how the reactions take place without a catalyst, an initial graph (
Figure 1) is presented. Each individual platform chemical’s yield is shown depending on the initial biomass from which it was produced (blue for peapod and black for coffee). The differing yields of platform chemicals (PC) from coffee cherries (32.598 wt%) and peapods (45.128 wt%) during hydrothermal valorization at 180 °C can be attributed to their distinct chemical compositions [
27]. Coffee cherries have a higher volatile matter content (79.91%) and more cellulose (27.6%), which can enhance sugar production, but they also contain more lignin (13.7%), which may impede breakdown. In contrast, peapods possess a higher fixed carbon content (13.0%) and greater hemicellulose content (17.4%), while having a lower lignin content (5.0%), allowing them to decompose more efficiently and yield more platform chemicals. The balance of these components, along with the specific processing conditions, ultimately determines the effectiveness of each biomass in producing valuable chemicals during hydrothermal treatment.
The varying yields of platform chemicals from coffee cherries and peapods during hydrothermal valorization at 180 °C can be attributed to the distinct chemical compositions and structural properties of each biomass.
For coffee cherries, the production of sugars (22.127 wt%), formic acid (6.61 wt%), levulinic acid (1.417 wt%), HMF (1.417 wt%), and furfural (1.299 wt%) is primarily driven by the breakdown of cellulose and hemicellulose under hydrothermal conditions. The high cellulose content (27.6%) in coffee cherries provides a significant source of glucose, which can be further converted into various platform chemicals through dehydration and fragmentation reactions [
28]. The presence of hemicellulose (12.5%) also contributes to the formation of sugars and furan derivatives like HMF and furfural. The acidic environment created during hydrothermal treatment facilitates the hydrolysis of glycosidic bonds, releasing monosaccharides that can undergo subsequent reactions. Formic acid and levulinic acid are produced through the dehydration and fragmentation of sugars, with formic acid being a primary product and levulinic acid forming through the further degradation of HMF [
29].
In contrast, peapods show a different distribution of platform chemicals, comprising sugars (15.635 wt%), formic acid (19.142 wt%), levulinic acid (3.995 wt%), HMF (3.687 wt%), and furfural (2.669 wt%). The higher hemicellulose content (17.4%) in peapods compared to coffee cherries contributes to the formation of pentoses, which can be dehydrated to produce furfural. The lower lignin content (5.0%) in peapods relative to coffee cherries (13.7%) facilitates the breakdown of the lignocellulosic structure, allowing for their more efficient conversion into platform chemicals. Additionally, the presence of catalytic minerals in peapods, such as potassium and sodium, may promote certain reactions and influence the distribution of platform chemicals.
3.4. Hydrotermal Valorization with Sulfuric Acid as a Catalyst
Sulfuric acid is effectively used in hydrothermal valorization processes due to its strong acidic properties, which facilitate the hydrolysis of lignocellulosic biomass components such as cellulose and hemicellulose [
30]. At elevated temperatures, typically around 180 °C, sulfuric acid catalyzes the breakdown of complex carbohydrates into simpler sugars, enhancing the yield of valuable platform chemicals like levulinic acid and furfural [
31]. The mechanism involves the protonation of glycosidic bonds in polysaccharides, making them more susceptible to hydrolysis. This process not only increases the efficiency of biomass conversion but also helps in the subsequent dehydration reactions that produce organic acids and furan derivatives [
32]. Additionally, using sulfuric acid in a dilute form minimizes equipment corrosion while maintaining effective catalytic activity, making it a practical choice for biomass valorization in sustainable biorefineries [
33].
The almost 20 wt% increase in platform chemical yields from both coffee cherries and peapods during hydrothermal valorization with sulfuric acid compared to non-catalyzed reactions can be attributed to the catalytic action of sulfuric acid, which enhances the hydrolysis and decomposition of complex biomass components [
34]. In a non-catalyzed reaction, the breakdown of cellulose and hemicellulose into fermentable sugars and other chemicals occurs at a slower rate and with reduced efficiency due to the stability of the glycosidic bonds in these polysaccharides [
35]. However, the presence of sulfuric acid significantly accelerates this process by protonating the glycosidic bonds, making them more susceptible to hydrolysis. This results in a more efficient release of monomeric sugars, which can be further converted into valuable platform chemicals such as HMF, furfural, and organic acids [
36].
Peapods yield more platform chemicals compared (62.936 wt%) to coffee cherries (51.236 wt%), primarily due to their favorable chemical composition (
Figure 2), particularly their higher hemicellulose content (17.4% vs. 12.5%) and lower lignin content (5.0% vs. 13.7%). Hemicellulose is more easily hydrolyzed into pentoses, which can be converted into furfural—a valuable platform chemical—under acidic conditions. The lower lignin content in peapods allows for the more efficient breakdown of the lignocellulosic structure, enhancing the overall conversion efficiency. Additionally, the catalytic effect of sulfuric acid not only increases the yield of sugars but also promotes the formation of organic acids like formic acid and levulinic acid, further contributing to the higher overall yield of platform chemicals from peapods compared to coffee cherries.
The yields of platform chemicals from coffee cherries (sugars: 40.328 wt%; formic acid: 5.405 wt%; levulinic acid: 0.636 wt%; HMF: 2.666 wt%; furfural: 2.201 wt%) and peapods (sugars: 56.193 wt%; formic acid: 3.179 wt%; levulinic acid: 1.085 wt%; HMF: 2.479 wt%; furfural: 0 wt%) during hydrothermal valorization at 180 °C with sulfuric acid as a catalyst show significant increases compared to non-catalyzed reactions for both coffee (sugars: 22.127 wt%; formic acid: 6.61 wt%; levulinic acid: 1.417 wt%; HMF: 1.417 wt%; furfural: 1.299 wt%) and peapods (sugars: 15.635 wt%; formic acid: 19.142 wt%; levulinic acid: 3.995 wt%; HMF: 3.687 wt%; furfural: 2.669 wt%).
The differences in yield between coffee cherries and peapods can be explained by their distinct chemical compositions. Peapods, with a higher hemicellulose content (17.4% vs. 12.5% in coffee cherries), produce more sugars during hydrolysis, contributing to the higher overall yield of platform chemicals. The presence of sulfuric acid allows for the efficient conversion of hemicellulose into pentoses, which can be further transformed into valuable chemicals. Conversely, coffee cherries, while yielding more sugars, have a higher lignin content (13.7% vs. 5.0% in peapods), which can inhibit the hydrolysis process and limit the overall yield of platform chemicals. Furthermore, the absence of furfural in peapods indicates that the conditions favored the conversion of sugar into products other than furan derivatives, which may be due to the different structural characteristics of the biomass and the specific catalytic pathways promoted by sulfuric acid.
In this study, sulfuric acid (H
2SO
4) is shown to play a crucial role in biomass hydrolysis during hydrothermal valorization. It acts as a catalyst, breaking down the complex structures of lignocellulosic biomass into simpler, fermentable sugars. The mechanism involves the protonation of glycosidic bonds in cellulose and hemicellulose, weakening these bonds and facilitating their cleavage. This process, known as acid-catalyzed hydrolysis, results in the formation of monomeric sugars such as glucose and xylose. Sulfuric acid is particularly effective at lower concentrations (0.9 wt%) and can achieve high conversion rates, with studies showing 100% hemicellulose and 97.3% cellulose conversion to xylose and glucose, respectively [
37]. The acid also helps to disrupt the lignin structure, making cellulose more accessible for hydrolysis.
3.5. Hydrotermal Valorization with Hydrogen Chloride as a Catalyst
Hydrochloric acid (HCl) can be used as a catalyst in hydrothermal valorization processes to enhance the conversion of biomass into valuable products [
38]. When added to feedwater, HCl facilitates the hydrolysis of lignocellulosic structures in the biomass, promoting the formation of platform chemicals like sugars, furans, and organic acids. In the case of peapod waste and coffee cherry biomass, HCl-assisted hydrothermal carbonization (HTC) can selectively produce target platform molecules by optimizing the reaction conditions. The acid catalyst helps to break down the complex carbohydrates and lignin in the biomass into simpler compounds that can be more easily converted into biofuels, biochemicals, and other value-added products [
39].
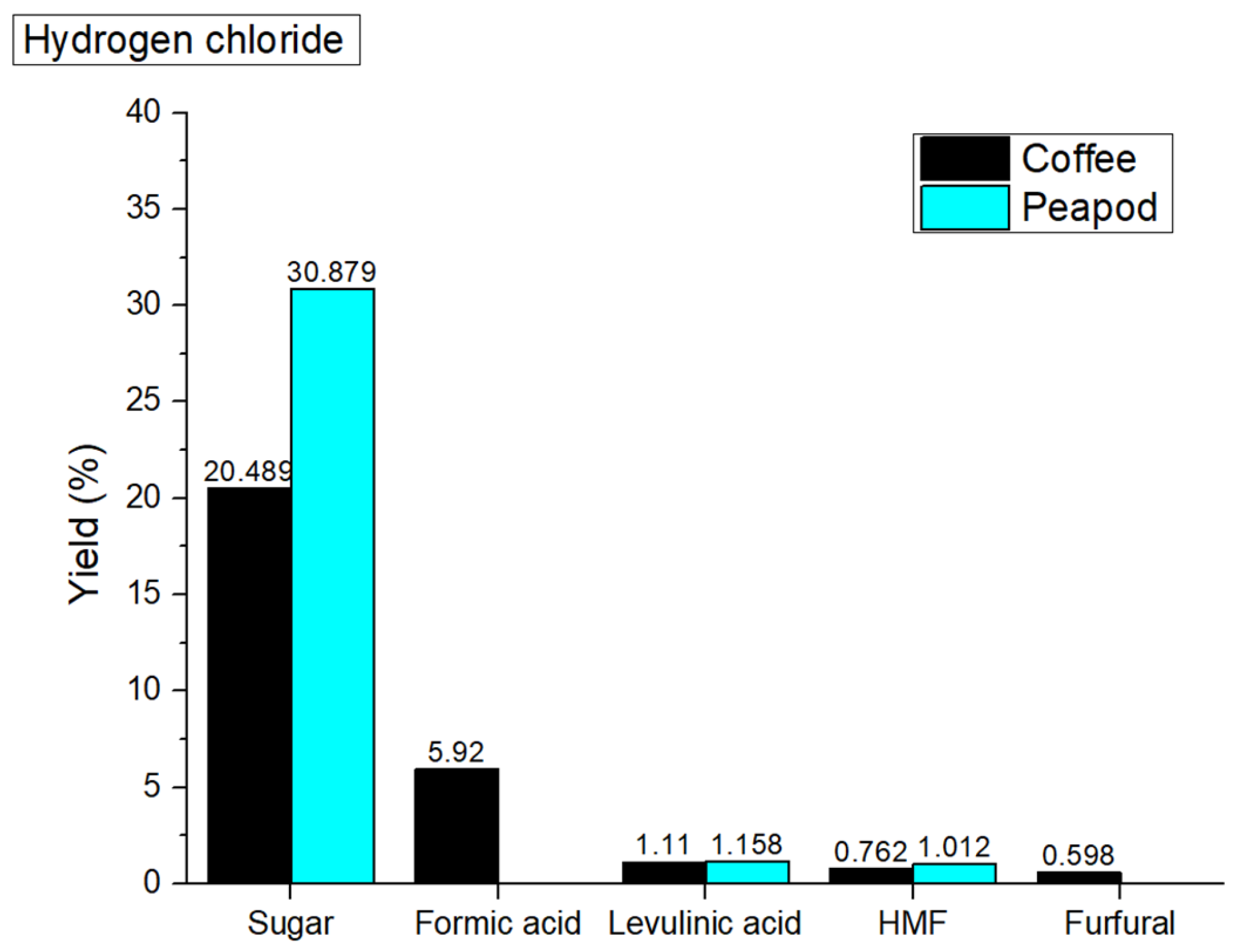
Figure 3 shows the yields of platform chemicals obtained from peapods and coffee cherry waste. The observed decrease in total yield from the hydrothermal valorization of coffee cherries and peapods when catalyzed with hydrochloric acid (HCl) can be attributed to the mechanisms of acid-catalyzed hydrolysis and the subsequent degradation of biomass components. While this can enhance the solubilization of sugars and other platform chemicals, it may also result in the loss of some biomass as gasses or other byproducts, thus reducing the overall yield. Specifically, the yields of coffee cherries and peapods dropped to 28.879 wt% and 33.049 wt% (
Figure 3), respectively, from their non-catalyzed yields of 32.598 wt% and 45.128 wt%.
The hydrothermal valorization of peapods and coffee cherries catalyzed by hydrochloric acid (HCl) yields distinct profiles of platform chemicals, reflecting the differing compositions of these biomass types. For peapods, the process results in a notably high sugar yield of 30.879 wt%, indicating the effective hydrolysis of cellulose and hemicellulose under acidic conditions. The absence of formic acid and furfural suggests that the reaction conditions may not favor the conversion of pentoses into furfural, potentially due to the specific structural characteristics of the peapod biomass. In contrast, coffee cherries yield lower sugars (20.489 wt%) but produce significant amounts of formic acid (5.92%) and other degradation products like levulinic acid (1.11%) and HMF (0.762 wt%). This variation highlights the influence of biomass composition on the hydrothermal valorization process.
The differences in yields can be attributed to the higher lignin content in coffee cherries, which makes them more resistant to hydrolysis compared to the more cellulose-rich peapods. The presence of lignin can hinder the accessibility of HCl to the polysaccharides, leading to lower sugar yields and a shift towards the formation of organic acids and furans as byproducts. This suggests that optimizing reaction conditions, such as temperature and pressure, is crucial for maximizing the production of the desired platform chemicals from each biomass type.
Hydrochloric acid (HCl) functions similarly to sulfuric acid in biomass hydrolysis, but with some distinct characteristics. HCl is a strong acid that can effectively penetrate the biomass structure, catalyzing the hydrolysis of cellulose and hemicellulose. Its smaller molecular size compared to sulfuric acid allows for better diffusion into the biomass particles. The mechanism involves the protonation of oxygen atoms in the glycosidic bonds, leading to their cleavage and the formation of sugar monomers. HCl is often used in its concentrated form for biomass hydrolysis, offering high sugar yields. However, it can be more corrosive than sulfuric acid, which may impact equipment and downstream processing.
3.6. Hydrothermal Valorization with Nitric Acid as a Catalyst
Nitric acid (HNO
3) is a strong oxidizing agent that plays a crucial role in various chemical reactions, including the oxidation of organic compounds. Its effectiveness in hydrolysis reactions is lower than that of other acids like sulfuric acid or hydrochloric acid [
40].
A thorough review of the literature reveals that dilute nitric acid has been effectively used for the treatment of lignocellulosic biomass, offering unique advantages in the hydrolysis process. Nitric acid exhibits higher efficiency for hemicellulose removal compared to other acids, like hydrochloric or sulfuric acid, and the process is generally faster. Studies have shown that under optimal conditions, nitric acid pretreatment can yield significant amounts of fermentable sugars while minimizing the production of inhibitory compounds. The peculiarity of nitric acid’s effect on lignocellulose polymers lies in its ability to effectively break down hemicellulose while leaving cellulose more accessible for subsequent enzymatic hydrolysis. This two-step process, where dilute nitric acid first targets hemicellulose, and then cellulose, under more severe conditions, allows for a more controlled and efficient breakdown of the lignocellulosic structure. Furthermore, nitric acid pretreatment has been successfully applied to various feedstocks, including corn stover and Jerusalem artichoke stalks, demonstrating its versatility in lignocellulose processing. It is worth noting that none of these processes included hydrothermal valorization [
41,
42].
In hydrothermal processes, nitric acid serves as a catalyst that enhances the efficiency of reactions involving biomass and organic materials [
43]. This catalytic behavior is crucial for optimizing reaction conditions and achieving higher efficiencies in biomass conversion processes.
In
Figure 4, the results of hydrothermal reactions using nitric acid as a catalyst are presented for two different biomass feedstocks: peapod and coffee cherry. When comparing the results for peapod biomass with the experiment conducted without a catalyst, it can be observed that the sugar yield was higher when using the catalyst (6.815 wt%). Additionally, the yields of HMF (4.383 wt%) and furfural also increased with the use of nitric acid. However, the formic acid yield was lower (10.029 wt%) compared to the no-catalyst experiment, and levulinic acid was not produced under these conditions. In contrast, the use of coffee cherry biomass resulted in different trends; the yields of the other platform chemicals, besides the sugar yield, were lower than the results obtained without a catalyst.
3.7. Hydrothermal Valorization with Phosphoric Acid as a Catalyst
Phosphoric acid (H
3PO
4) is a versatile chemical that functions both as an acid and a catalyst in various chemical reactions. It is particularly effective in the polymerization of olefins and in alkylation reactions, such as the conversion of benzene to cumene using propylene [
44]. Phosphoric acid catalysts have demonstrated significant promise in these environments due to their ability to maintain their stability and activity under harsh conditions [
45]. In hydrothermal processes, they can catalyze the conversion of biomass into fermentable sugars, which can subsequently be transformed into biofuels or other valuable chemicals [
46]. For this reason, the decision was made to use phosphoric acid as a catalyst and to analyze its effect on the yields being studied.
The yields of the platform chemicals with peapod and coffee biomass, obtained using phosphoric acid as a catalyst, are shown in
Figure 5. In general, it was observed that the yields obtained from peapod biomass were higher compared to those obtained from coffee biomass. When comparing the yields from coffee biomass to the experiment in which no catalyst was used, no significant difference was observed. Therefore, it can be concluded that the percentage of lignin affects the catalysis with phosphoric acid, as the yields of the reaction were not significantly increased.
On the other hand, the experiment involving pea biomass revealed a different trend. The sugar yield (24.097 wt%) was higher than that obtained without a catalyst and exceeded the yield achieved with coffee under the same conditions. In this setup, the reaction was more selective for sugar production, as it decreased the formation of formic acid and resulted in a better yield of HMF (6.346 wt%) and furfural (2.558 wt%). In conclusion, catalysis with phosphoric acid was effective for hydrothermal treatment using peapod biomass but did not lead to good results when using the coffee biomass.
3.8. Hydrothermal Valorization with Chloroacetic Acid as a Catalyst
Chloroacetic acid (CAA) can effectively catalyze hydrothermal reactions due to its acidic properties, which increase the concentration of H+ ions in high-temperature and high-pressure aqueous environments. This acid catalysis enhances the breakdown and rearrangement of chemical bonds in refractory pollutants, facilitating their conversion into more biodegradable forms [
47]. Overall, CAA serves as a crucial catalyst in the optimization of various hydrothermal processes [
47].
As seen in
Figure 6, the overall yields of platform chemicals from coffee cherries (31.865 wt%) and peapods (39.557 wt%) following hydrothermal valorization catalyzed by chloroacetic acid (CAA) are lower than the non-catalyzed yields (coffee cherries: 32.598 wt%; peapods: 45.128 wt%) for several reasons. While CAA enhances the concentration of H+ ions and promotes certain reactions, it can also lead to the degradation of valuable compounds into lower-molecular-weight products, reducing the yield of the desired platform chemicals. Additionally, the presence of CAA may alter reaction pathways and selectivity, favoring side reactions that consume target products rather than producing them. Furthermore, the optimal conditions for maximizing yields may not align with those that favor CAA catalysis, and the varying compositions of the biomass feedstocks can influence how effectively the catalyst interacts with the materials.
In the hydrothermal valorization process at 180 °C with chloroacetic acid, the yields of platform chemicals from coffee cherries and peapods exhibited notable changes compared to non-catalyzed processes. For coffee cherries, the yields of sugar and other products such as formic acid and HMF slightly improved, but the overall yield of formic acid was lower than in the non-catalyzed process. In contrast, the yields from peapods showed a significant increase in sugar production (26.749 wt%) but a complete absence of HMF and furfural, which may indicate that the catalyst alters the reaction pathways, enhancing sugar formation while suppressing the formation of these other compounds. This shift suggests that the presence of CAA increases selectivity towards certain products, potentially at the expense of others, leading to the more efficient conversion of carbohydrates to sugars in the case of peapods.
The decrease in the yields of HMF, furfural, and formic acid in the CAA-catalyzed process can be advantageous for subsequent valorization steps, particularly those involving microorganisms.
3.9. Hydrothermal Valorization with Phthalic Acid as a Catalyst
Phthalic acid can catalyze the hydrothermal valorization of biomass by acting as a proton donor, which enhances the hydrolysis and depolymerization of complex biomass structures under high-temperature and high-pressure conditions. The mechanism involves the formation of an acidic environment that facilitates the cleavage of glycosidic bonds in polysaccharides, leading to the release of fermentable sugars [
48]. Additionally, phthalic acid can promote the formation of valuable platform chemicals such as levulinic acid and furfural through dehydration and rearrangement reactions. By providing a source of protons, phthalic acid accelerates the conversion of biomass components into simpler molecules, thereby improving the overall yield and selectivity of the desired products [
49].
Figure 7 shows the yields of platform chemicals obtained via phthalic acid catalysis. In hydrothermal valorization catalyzed by phthalic acid, the overall yields of platform chemicals from coffee cherries (27.583 wt%) and peapods (49.714 wt%) are lower compared to the non-catalyzed yields (coffee cherries: 32.598 wt%; peapods: 45.128 wt%) due to their distinct reaction mechanisms and biomass compositions. The presence of phthalic acid enhances the acidity of the reaction environment, which can lead to increased hydrolysis rates; however, it may also promote the degradation of valuable products into lower-molecular-weight compounds or byproducts that are not quantified as platform chemicals. In the case of coffee cherries, which have a higher lignin content, the catalyst may drive reactions that lead to the formation of more complex byproducts rather than the desired sugars and acids. Conversely, peapods, with their higher cellulose and hemicellulose content, may benefit more from the catalytic action, resulting in a relatively higher sugar yield.
In the hydrothermal valorization process at 180 °C with phthalic acid, the yields of platform chemicals from coffee cherries (sugar: 19.582 wt%; formic acid: 4.218 wt%; levulinic acid: 0.942 wt%; HMF: 1.593 wt%; furfural: 1.248 wt%) and peapods (sugar: 45.529 wt%; formic acid: 3.125 wt%; levulinic acid: 1.06 wt; HMF: 0 wt%, furfural: 0 wt%) are affected by the catalytic environment and the inherent composition of the biomass. Peapods show a significant improvement in sugar yield (from 15.635% to 45.529 wt%), suggesting that the catalyst effectively enhances the conversion of polysaccharides into sugars, achieving one of the highest yields in this study. Its mechanism of hydrothermal valorization involves the donation of protons to catalyze the breakdown of cellulose and hemicellulose. The presence of two carboxyl groups in phthalic acid allows for a more controlled release of protons during the hydrolysis process. This can lead to a more gradual breakdown of biomass components, potentially reducing the formation of degradation products that can inhibit subsequent fermentation processes. While phthalic acid may not be as strong as sulfuric or hydrochloric acid, its milder nature can be advantageous in certain applications where a less aggressive hydrolysis is desired.
The difference in performance compared to coffee can be attributed to the distinct structural compositions of the biomass; coffee cherries, with a higher lignin content, may experience more complex reactions leading to the formation of byproducts, while the cellulose-rich peapods benefit from the catalytic action.
The selectivity and efficiency of the hydrothermal valorization process are influenced by the catalytic effects of phthalic acid, which alters the reaction pathways and product distribution. The decrease in the yields of HMF, furfural, and formic acid in the catalyzed process can be beneficial for subsequent valorization steps, particularly those involving microorganisms, as high concentrations of these compounds can inhibit microbial growth and fermentation efficiency.
3.10. Hydrothermal Valorization with Adipic Acid as a Catalyst
Adipic acid can function as a catalyst in hydrothermal valorization processes through facilitating the conversion of biomass into valuable platform chemicals using its acidic properties [
50]. The mechanism involves the protonation of biomass components, which enhances the cleavage of glycosidic bonds in polysaccharides, leading to the release of fermentable sugars. As an acid catalyst, adipic acid promotes hydrolysis and dehydration reactions, aiding in the transformation of sugars into other valuable products, such as levulinic acid and furfural [
51]. Additionally, the presence of adipic acid can help stabilize intermediates during the reaction, thus improving the selectivity towards desired products while minimizing the formation of unwanted byproducts. The catalytic action of adipic acid not only enhances the efficiency of biomass conversion but also aligns with green chemistry principles by utilizing a renewable feedstock and reducing reliance on harsher chemical catalysts [
52].
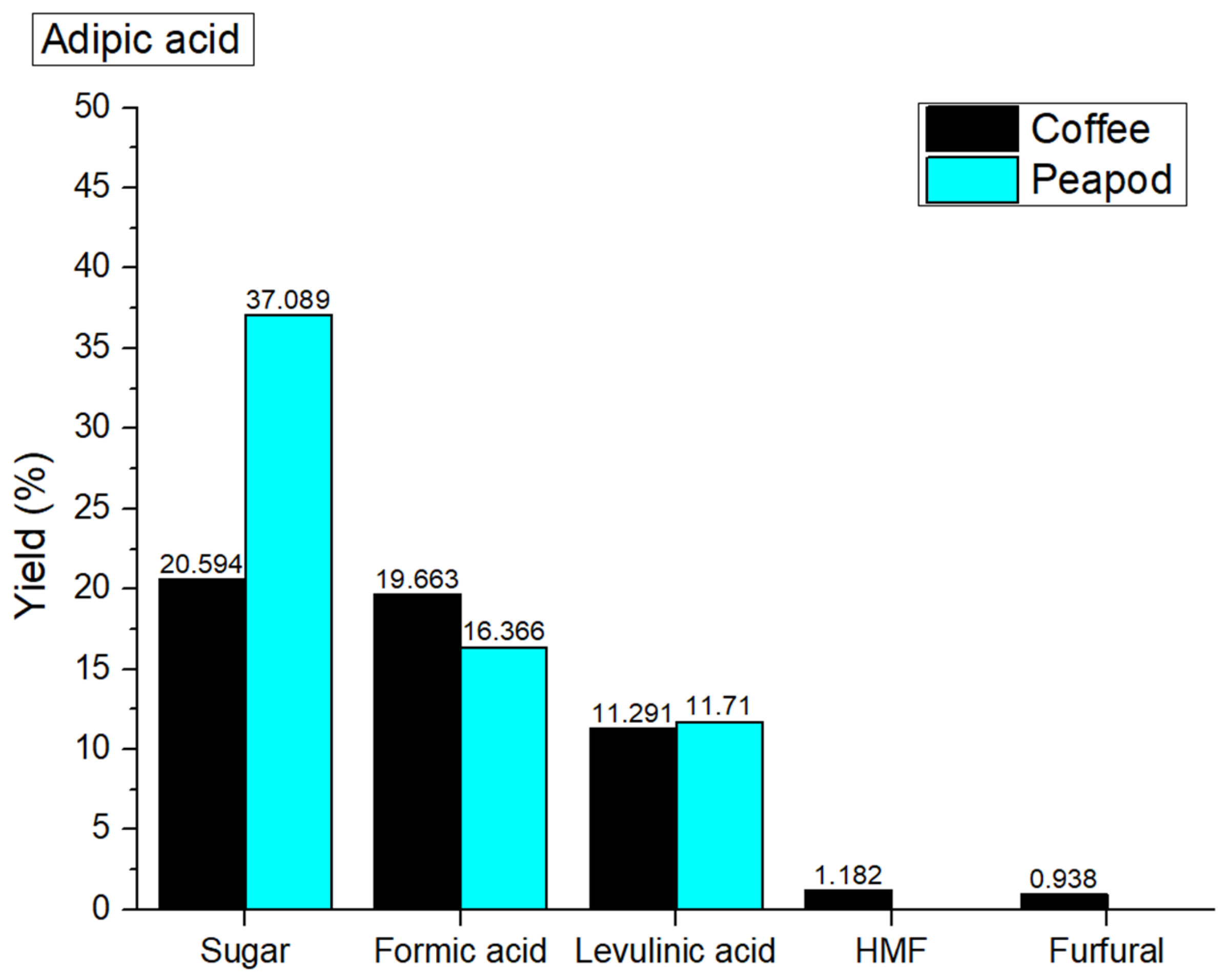
In hydrothermal valorization processes catalyzed with adipic acid, the overall yields of platform chemicals from coffee cherries (53.668%) and peapods (65.165 wt%) significantly surpass the non-catalyzed yields (coffee cherries: 32.598 wt%; peapods: 45.128 wt%) due to the enhanced catalytic activity of adipic acid, which promotes more efficient biomass conversion (
Figure 8). The mechanism involves adipic acid acting as a proton donor, which facilitates the hydrolysis of polysaccharides and the cleavage of glycosidic bonds, leading to an increase in the release of fermentable sugars. The acidic environment created by adipic acid not only accelerates the breakdown of complex carbohydrates but also stabilizes reaction intermediates, allowing for the selective formation of valuable products like levulinic acid and furfural. The differences in biomass composition further influence the outcomes; coffee cherries, which contain more lignin, may experience more complex reactions that can lead to byproduct formation, while the higher cellulose content in peapods allows for the more efficient extraction and conversion of sugar. This catalytic enhancement not only increases the overall yield of platform chemicals but also improves the selectivity towards desired products, making the process more effective for subsequent applications.
In the hydrothermal valorization process at 180 °C with adipic acid, the yields of platform chemicals show significant differences compared to the non-catalyzed process, particularly for coffee cherries and peapods. For coffee cherries, the sugar yield decreased to 20.594% from 22.127 wt%, while formic acid yield increased substantially to 19.663% from 6.61 wt%, and levulinic acid also saw a notable increase to 11.291% from 1.417 wt%. This indicates that while the overall sugar yield is lower, the catalytic action of adipic acid enhances the formation of other valuable acids, suggesting a shift in selectivity towards products that may be more favorable for downstream applications. In contrast, peapods exhibited a significant increase in sugar yield to 37.089% from 15.635%, while formic acid decreased slightly to 16.366% from 19.142 wt%. The increase in sugar yield from peapods indicates that adipic acid effectively catalyzes the breakdown of cellulose, resulting in a higher rate of fermentable sugar production, which is advantageous for subsequent bioconversion processes.
3.11. Hydrothermal Valorization with Acetic Acid as a Catalyst
Acetic acid (CH
3COOH) is a valuable organic compound that is widely utilized as a catalyst in various chemical processes. One of its significant applications is in the N-acylation of amines, where it facilitates the acetylation reaction, often yielding high product concentrations with minimal catalyst loading [
53]. Additionally, acetic acid plays a crucial role in hydrogen generation from sodium borohydride, demonstrating comparable reactivity to stronger acids like hydrochloric acid under specific conditions. These applications underscore acetic acid’s effectiveness and efficiency as a catalyst in organic synthesis [
54].
Acetic acid is a key catalyst in hydrothermal reactions, particularly in biomass conversion processes. It aids in breaking down complex organic materials into simpler compounds, enhancing the yield of valuable products like lactic acid and acetic acid [
55]. Studies show that adding acetic acid during the amylopectin hydrothermal reaction improves conversion efficiency, leading to higher yields of glucose and other metabolites [
56]. Acetic acid can also act synergistically with metal-based catalysts like copper-supported biochar to optimize lignocellulosic biomass conversion. This combination increases acetic acid yield and promotes the formation of other organic acids, showcasing its versatility in hydrothermal processes [
57]. It stabilizes reactive intermediates and enhances sugar solubility, making acetic acid a promising approach for improving biomass valorization’s efficiency and sustainability [
58]. Because of this, experiments were carried out using acetic acid with coffee and pea biomass. The results obtained are shown in
Figure 9.
Total yields of 35.436 wt% were obtained using coffee biomass, while yields reached 50.427 wt% with peapods. Overall, the sugar yield was significantly higher when using peapod biomass with a catalyst (38.423 wt%) compared to without a catalyst (15.635 wt%). This indicates that the presence of a catalyst greatly enhances the extraction of sugars from the peapod biomass, suggesting that optimizing catalytic conditions could lead to even higher yields. In contrast, no significant difference in sugar yields was observed between coffee biomass with a catalyst (20.939 wt%) and coffee biomass without a catalyst (22.127 wt%). This finding implies that coffee biomass has consistent sugar extraction properties, regardless of the catalytic intervention used, which may be due to its inherent composition or structure.
When comparing the production of other platform chemicals, such as levulinic acid, HMF, and furfural, there was no significant difference between using coffee biomass and peapod biomass. This suggests that both types of biomasses can effectively produce these valuable chemicals, although they may differ in their efficiency for sugar production.
In conclusion, the results demonstrated a high selectivity for sugar production from both coffee and peapod biomass. The enhanced yield from peapod biomass when using a catalyst underscores the importance of optimizing catalytic processes for biomass conversion. Furthermore, since the production of carboxylic acids, HMF, and furfural was quite low when using acetic acid as a catalyst, this approach can be considered a viable alternative for producing sugars suitable for fermentation. This could be particularly beneficial in biorefinery processes, where maximizing sugar yield while minimizing unwanted byproducts is crucial for economic viability and efficiency. The findings suggest that focusing on the catalytic mechanisms involved in sugar production could lead to more sustainable and profitable biomass utilization strategies in the future.
3.12. Hydrothermal Valorization with Butyric Acid as a Catalyst
Butyric acid, or butanoic acid, is a straight-chain fatty acid. It is a colorless liquid characterized by a strong, unpleasant odor, often associated with rancid butter, from which it derives its name. While butyric acid is not widely found in nature, its esters, known as butyrates, are common [
59]. This compound plays a significant role in various biological processes, particularly in the mammalian gut, where it serves as a source of energy for colon cells and has implications for gut health and the metabolism. In terms of industrial applications, butyric acid is increasingly recognized for its potential as a catalyst in the production of biofuels and fine chemicals [
60].
Figure 10 shows the lack of potential of butyric acid as a catalyst. In a hydrothermal valorization process at 180 °C catalyzed with butyric acid, the overall yields of platform chemicals from coffee cherries (17.168 wt%) and peapods (36.301 wt%) are significantly lower than the non-catalyzed yields (coffee cherries: 32.598 wt%, peapods: 45.128 wt%). This reduction in yield can be attributed to the specific catalytic properties of butyric acid, which may not effectively promote the hydrolysis and depolymerization of biomass components compared to other acids, like adipic acid. The mechanism of butyric acid as a catalyst involves its ability to donate protons, but its shorter carbon chain may lead to the less effective stabilization of reaction intermediates, resulting in the reduced conversion of polysaccharides into fermentable sugars and other valuable products.
In the hydrothermal valorization process at 180 °C with butyric acid, the yields of platform chemicals from coffee cherries (sugar: 16.793 wt%; formic acid: 0 wt%; levulinic acid: 0 wt%; HMF: 0.37538 wt%; furfural: 0%) and peapods (sugar: 33.691 wt%; formic acid: 2.612 wt%; levulinic acid: 0 wt%; HMF: 0 wt%; furfural: 0 wt%) are significantly lower than the non-catalyzed yields of coffee cherries (sugar: 22.127 wt%; formic acid: 6.61 wt%; levulinic acid: 1.417 wt%; HMF: 1.145 wt%; furfural: 1.299 wt%) and peapods (sugar: 15.635 wt%; formic acid: 19.142 wt%; levulinic acid: 3.995 wt; HMF: 3.687 wt%; furfural: 2.669 wt%). The reduction in yields can be attributed to the weaker catalytic properties of butyric acid compared to other acids, which may not effectively promote the hydrolysis and depolymerization of biomass components. While butyric acid can donate protons to facilitate reactions, its shorter carbon chain may result in the less effective stabilization of reaction intermediates, leading to the reduced conversion of polysaccharides into fermentable sugars and other valuable products. This is particularly evident in coffee cherries, where the significant drop in sugar yield indicates that the catalyst may favor degradation pathways rather than hydrolysis, yielding fewer fermentable sugars.
In contrast, peapods achieved a relatively better performance, with a sugar yield of 33.691 wt%, which is an improvement compared to the non-catalyzed yield of 15.635 wt%. However, the overall yield remained low compared to other catalysts, indicating that while butyric acid can enhance sugar production from peapods, it does not achieve the same efficiency as stronger acids. The presence of formic acid in peapods is also much lower than that in the non-catalyzed process, suggesting that butyric acid shifts selectivity towards sugar production but at the expense of other valuable products, like formic acid and HMF. The complete absence of levulinic acid and furfural in both biomass types further highlights the limitations of butyric acid as a catalyst in promoting a diverse range of platform chemicals. Overall, while butyric acid can facilitate some conversion of biomass, its effectiveness is limited, leading to lower overall yields of platform chemicals compared to non-catalyzed processes.
3.13. Hydrothermal Valorization with Anthranilic Acid as a Catalyst
Anthranilic acid, also known as 2-aminobenzoic acid, can be used as a catalyst in hydrothermal valorization processes to enhance the conversion of biomass into valuable platform chemicals. As a weak acid, anthranilic acid can donate protons to facilitate the hydrolysis and depolymerization of biomass components, particularly polysaccharides, under high-temperature and high-pressure conditions [
61].
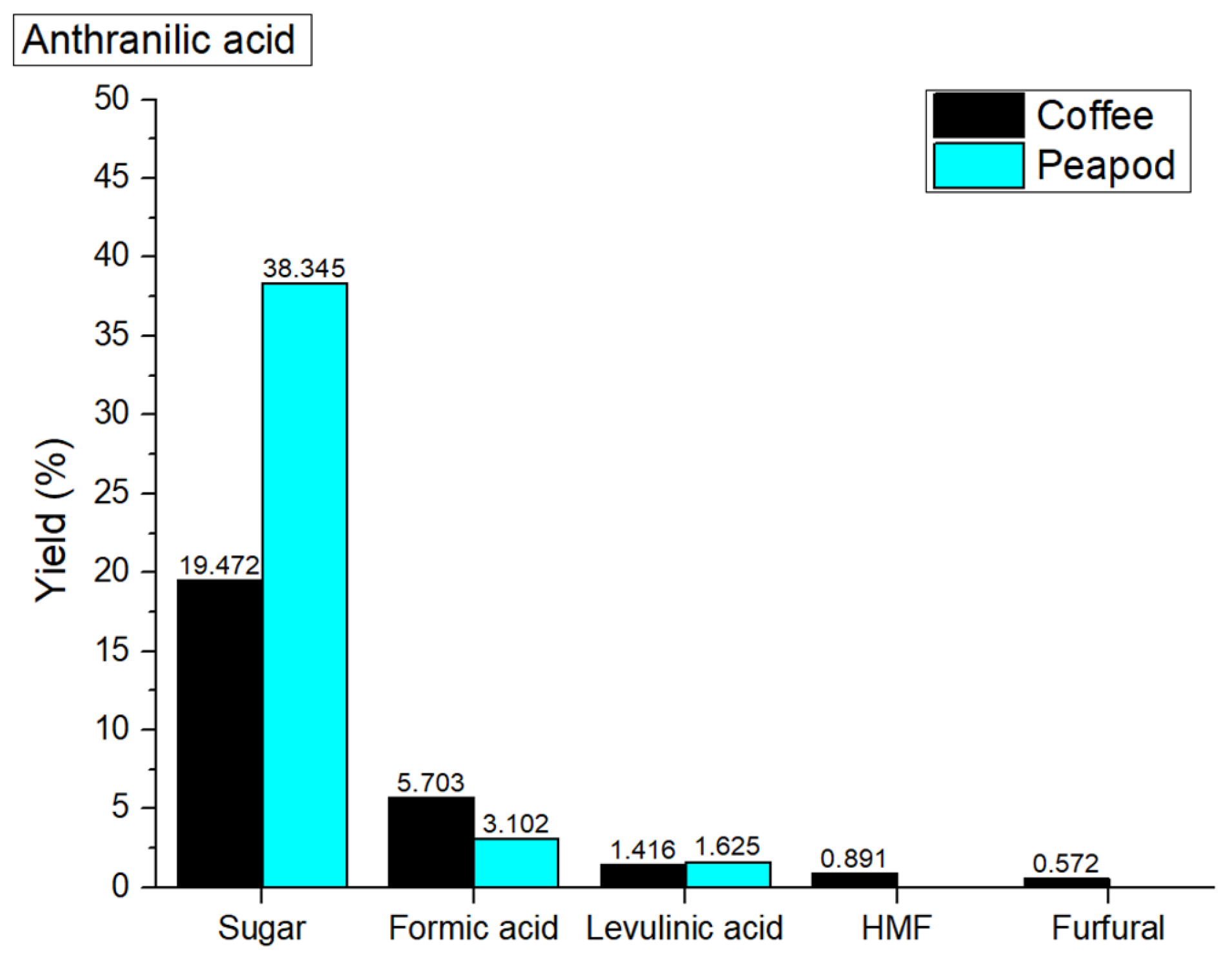
Figure 11 shows that in hydrothermal valorization processes catalyzed with anthranilic acid, the overall yields of platform chemicals from coffee cherries (28.054 wt%) and peapods (43.072 wt%) are lower compared to the non-catalyzed yields (coffee cherries: 32.598 wt%; peapods: 45.128 wt%). This reduction in yield may occur due to the specific catalytic properties of anthranilic acid, which, while capable of donating protons to facilitate hydrolysis, may not be as effective in promoting the breakdown of complex biomass structures as other stronger acids. The mechanism involves anthranilic acid’s ability to stabilize certain intermediates, but its effectiveness is limited by its molecular structure, which may not provide optimal conditions for the cleavage of glycosidic bonds in polysaccharides. Additionally, the inherent differences in biomass composition play a significant role; coffee cherries, which have a higher lignin content, may undergo more complex reactions that lead to byproduct formation rather than the desired platform chemicals. Conversely, while peapods have a higher cellulose content, the weaker catalytic action of anthranilic acid may limit the overall efficiency of sugar extraction, resulting in lower yields of platform chemicals compared to the non-catalyzed process.
In the hydrothermal valorization process at 180 °C with anthranilic acid, the yields of platform chemicals from coffee cherries (sugar: 19.472 wt%; formic acid: 5.703 wt%; levulinic acid: 1.416 wt%; HMF: 0.891 wt%; furfural: 0.572 wt%) and peapods (sugar: 38.345 wt%; formic acid: 3.102 wt%; levulinic acid: 1.625 wt%; HMF: 0 wt%; furfural: 0 wt%) are lower than the non-catalyzed yields of coffee cherries (sugar: 22.127 wt%; formic acid: 6.61 wt%; levulinic acid: 1.417 wt%; HMF: 1.145 wt%; furfural: 1.299 wt%) and peapods (sugar: 15.635 wt%; formic acid: 19.142 wt%; levulinic acid: 3.995 wt%; HMF: 3.687%; furfural: 2.669 wt%). The decrease in sugar yield for coffee cherries indicates that anthranilic acid may not effectively facilitate the hydrolysis of polysaccharides, leading to lower overall sugar production. Although formic acid yield remains relatively stable, the lack of HMF and furfural production suggests that the catalyst may promote pathways that favor sugar retention at the expense of other valuable products [
62]. In contrast, peapods show a significant increase in sugar yield, which indicates that anthranilic acid can enhance the breakdown of cellulose; however, the overall yield of other platform chemicals remains low.
3.14. Hydrothermal Valorization with Propanoic Acid as a Catalyst
Propanoic acid, also known as propionic acid, can be used as a catalyst in hydrothermal valorization processes to enhance the conversion of biomass into valuable platform chemicals. As a weak acid, propanoic acid can donate protons to facilitate the hydrolysis and depolymerization of biomass components, particularly polysaccharides, under high-temperature and high-pressure conditions. The presence of the carboxyl group in the molecule can promote specific reactions and stabilize reaction intermediates, leading to improved selectivity towards the desired product [
63].
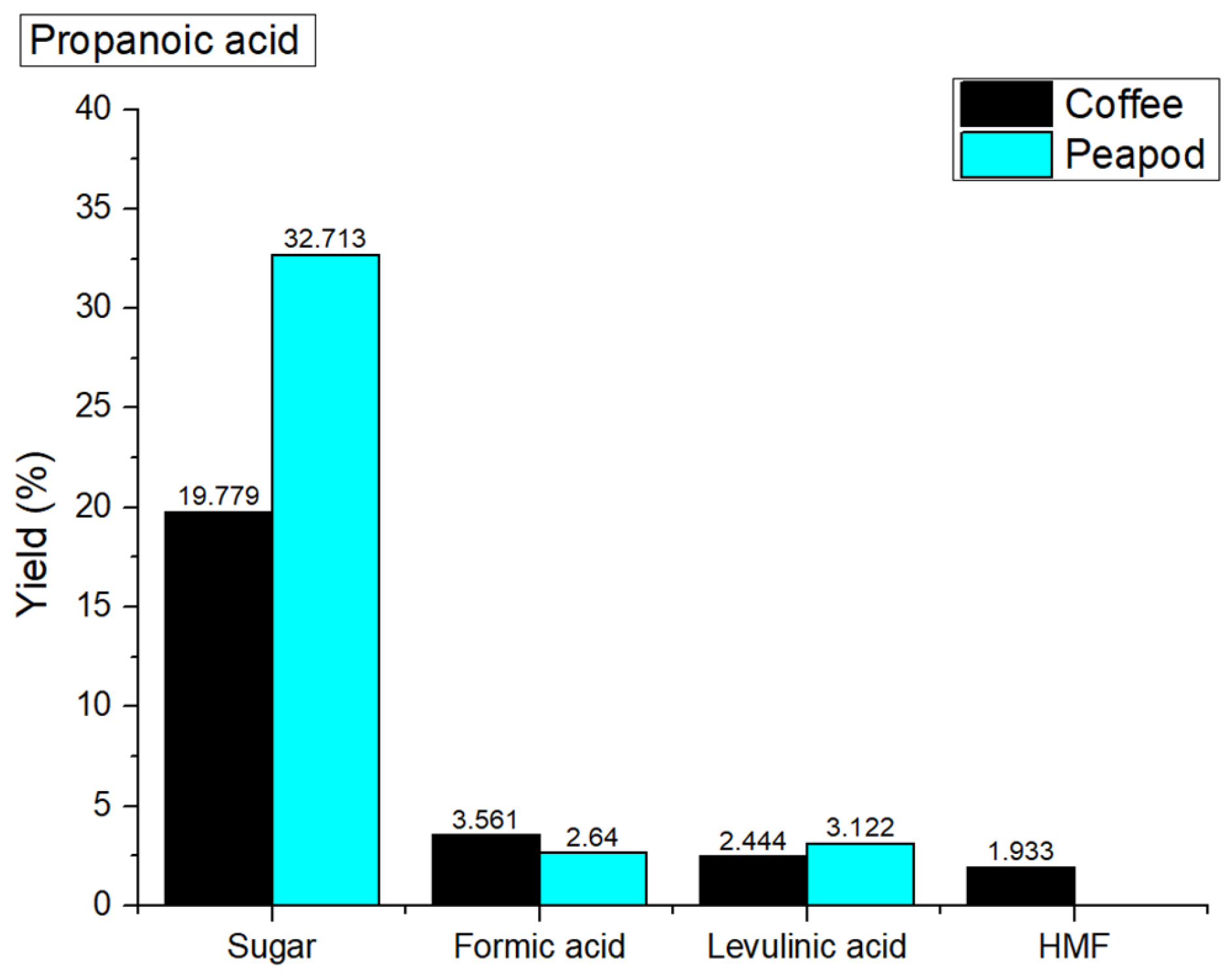
Figure 12 shows the yields obtained with propanoic acid. In hydrothermal valorization processes catalyzed with propanoic acid, the overall yields of platform chemicals from coffee cherries (27.717 wt%) and peapods (38.475 wt%) are notably lower compared to non-catalyzed yields (coffee cherries: 32.598 wt%; peapods: 45.128 wt%). This decline in yield can be attributed to the relatively weak catalytic properties of propanoic acid, which may not effectively facilitate the hydrolysis and depolymerization of biomass components as stronger acids can. While propanoic acid can donate protons to promote certain reactions, its shorter carbon chain may lead to the less effective stabilization of reaction intermediates, resulting in lower conversion rates of polysaccharides into fermentable sugars and other valuable products. Consequently, the use of propanoic acid does not enhance the efficiency of biomass conversion, leading to poorer overall yields compared to non-catalyzed processes.
In the hydrothermal valorization process at 180 °C with propanoic acid, the yields of platform chemicals from coffee cherries (sugar: 19.779 wt%; formic acid: 3.561 wt%; levulinic acid: 2.444 wt%; HMF: 1.933 wt%; furfural: 0 wt%) and peapods (sugar: 32.713 wt%; formic acid: 2.64 wt%; levulinic acid: 3.122 wt%; HMF: 0 wt%; furfural: 0 wt%) are different compared to the non-catalyzed yields of coffee cherries (sugar: 22.127 wt%; formic acid: 6.61 wt%; levulinic acid: 1.417 wt%; HMF: 1.145 wt%; furfural: 1.299 wt%) and peapods (sugar: 15.635 wt%; formic acid: 19.142 wt%; levulinic acid: 3.995 wt; HMF: 3.687 wt%; furfural: 2.669 wt%). The decrease in sugar yield for coffee cherries indicates that propanoic acid may not effectively facilitate the hydrolysis of polysaccharides, leading to lower overall sugar production. The catalyst also appears to suppress the formation of furfural, as no furfural is detected in either biomass type. In contrast, peapods show a significant increase in sugar yield, which indicates that propanoic acid can enhance the breakdown of cellulose. However, the overall yield of other platform chemicals remains low.
3.15. Hydrothermal Valorization with 4-Aminobenzoic Acid as a Catalyst
4-aminobenzoic acid (PABA) can serve as an effective catalyst in hydrothermal valorization processes due to its ability to facilitate the breakdown of biomass into valuable platform chemicals. Acting as a weak acid, PABA donates protons that promote the hydrolysis and depolymerization of complex polysaccharides present in biomass under high-temperature and high-pressure conditions [
64]. The presence of the amino group in PABA not only enhances the acidity of the reaction medium but also stabilizes the reaction intermediates, allowing for the more efficient conversion of carbohydrates into fermentable sugars and organic acids [
65].
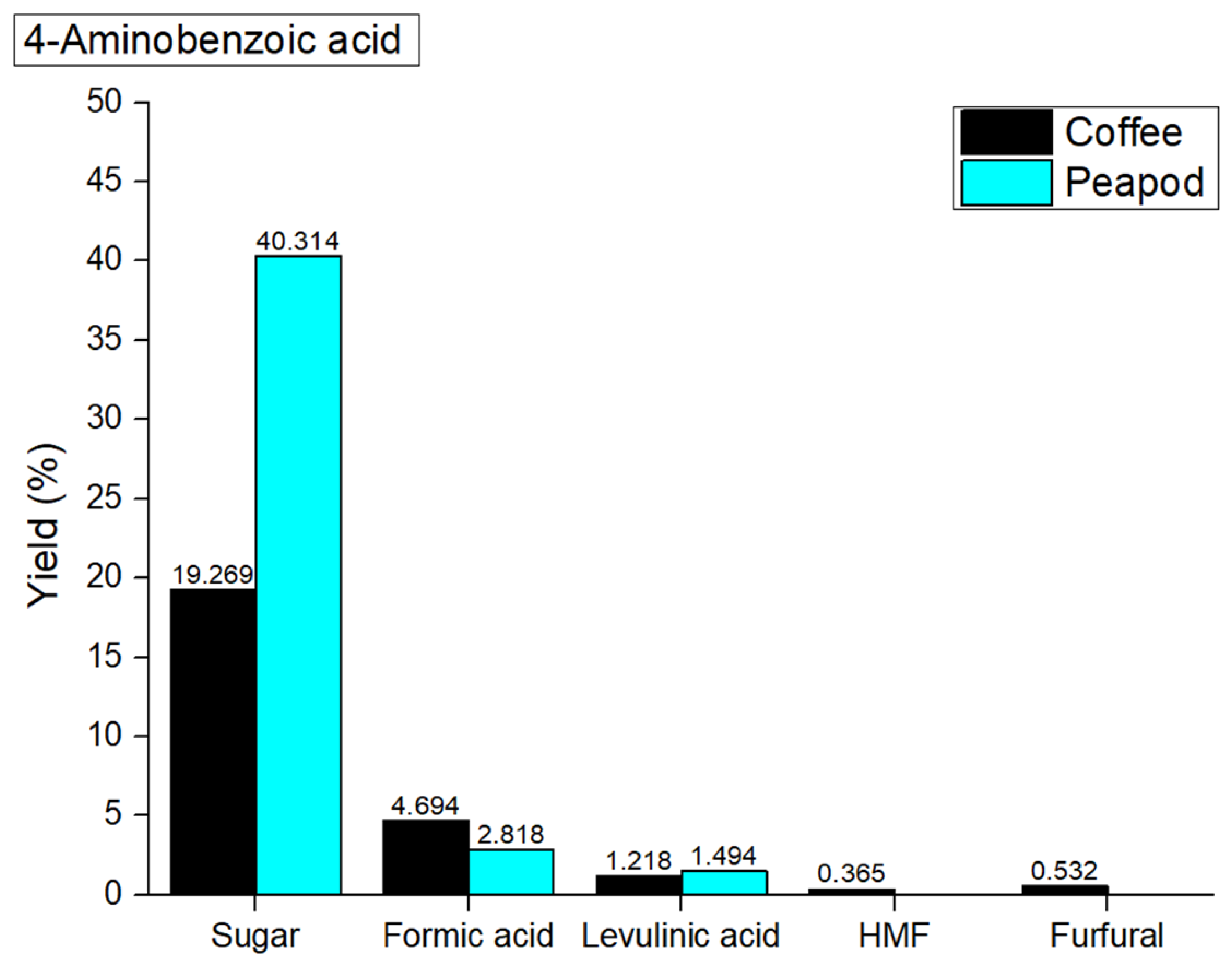
Figure 13 showed the results obtained. In hydrothermal valorization processes catalyzed with 4-aminobenzoic acid, the overall yields of platform chemicals from coffee cherries (26.078 wt%) and peapods (44.626 wt%) are lower compared to the non-catalyzed yields (coffee cherries: 32.598 wt%; peapods: 45.128 wt%). This decline in yield can be attributed to the limited catalytic effectiveness of 4-aminobenzoic acid, which may not sufficiently enhance the hydrolysis and depolymerization of biomass components. While it can donate protons to facilitate reactions, its molecular structure may not allow for the optimal stabilization of reaction intermediates, resulting in the reduced conversion of polysaccharides into fermentable sugars and other valuable products.
In the hydrothermal valorization process that occurs at 180 °C with propanoic acid, the yields of platform chemicals from coffee cherries (sugar: 19.269 wt%; formic acid: 4.694 wt%; levulinic acid: 1.218 wt%; HMF: 0.365 wt%; furfural: 0.532 wt%) and peapods (sugar: 40.314 wt%; formic acid: 2.818 wt%; levulinic acid: 1.494 wt%; HMF: 0 wt%; furfural: 0 wt%) are different compared to the non-catalyzed yields. The decrease in sugar yield for coffee cherries indicates that propanoic acid may not effectively facilitate the hydrolysis of polysaccharides, leading to lower overall sugar production. The catalyst also appears to suppress the formation of HMF and furfural, as the yields of these compounds are significantly reduced in both biomass types. In contrast, peapods show a significant increase in sugar yield, which indicates that propanoic acid can enhance the breakdown of cellulose. However, the overall yield of other platform chemicals remains low.
3.16. Hydrothermal Valorization with Succinic Acid as a Catalyst
Succinic acid, a dicarboxylic acid, serves as a valuable platform chemical with significant potential in various catalytic processes. It is produced through microbial fermentation and is considered a sustainable alternative to petroleum-derived chemicals [
66]. Its versatility allows for it to be transformed into high-value compounds such as 1,4-butanediol, gamma-butyrolactone, and tetrahydrofuran through catalytic hydrogenation [
67]. This process typically employs transition metal catalysts, including ruthenium and cobalt, which have demonstrated effectiveness in facilitating these conversions under specific conditions, such as elevated temperatures and pressures [
68].
Research indicates that succinic acid can be effectively utilized in various catalytic pathways, making it a promising candidate for further exploration in hydrothermal applications [
69]. The integration of succinic acid into hydrothermal treatment processes could lead to more sustainable and efficient methods of biomass conversion, aligning with the broader goals of green chemistry and biorefinery development [
67].
Therefore, experiments were carried out using succinic acid as a catalyst in the hydrothermal treatment of coffee and peapod biomass. The results are shown in
Figure 14.
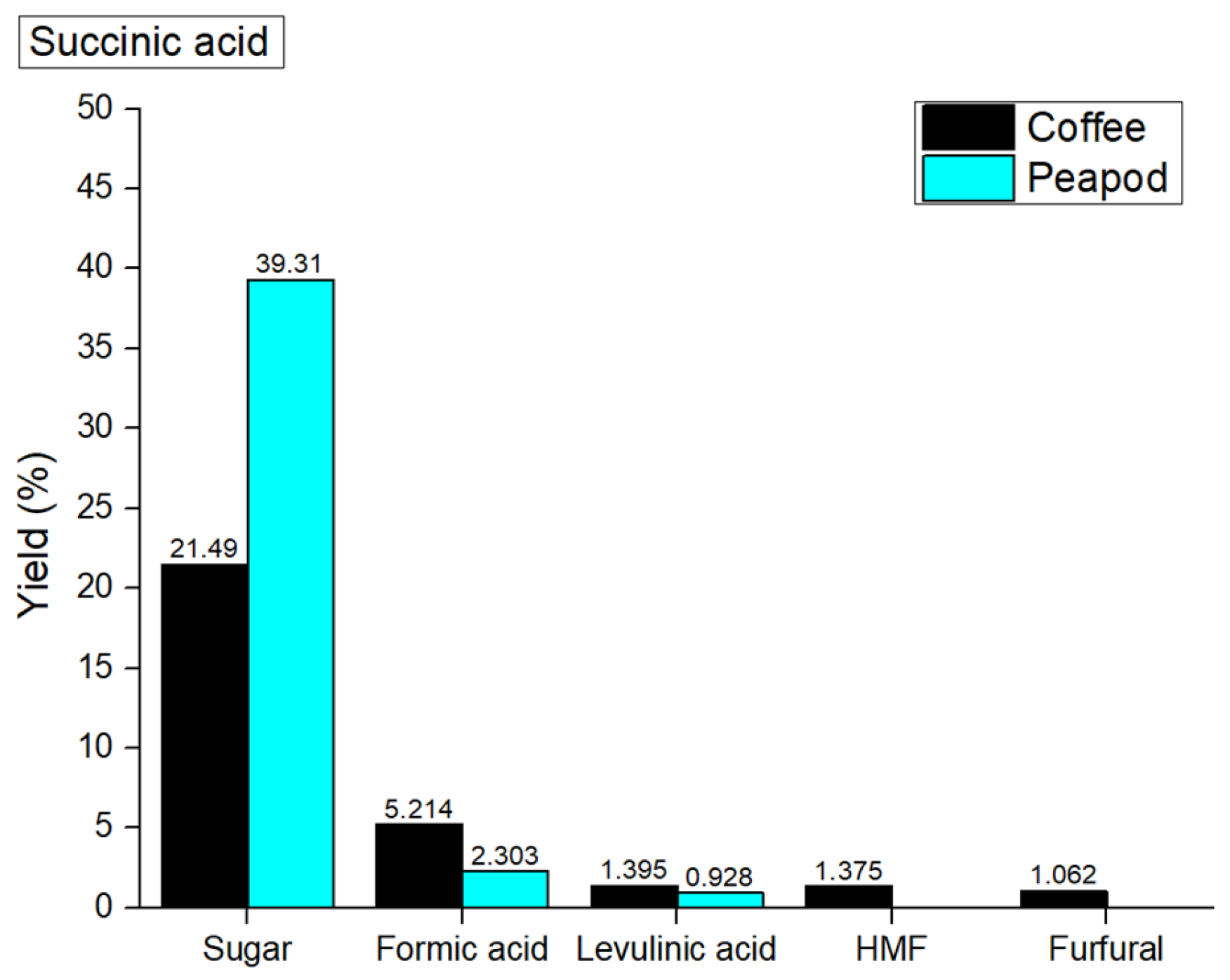
It is important to highlight that the total yield of the reaction was lower when using the catalyst for both types of biomasses. The total yield with coffee and pea biomass was 42.541 wt% and 30.535 wt%, respectively; without the catalyst, the yields were 45.127 wt% and 32.598 wt%. Although the yield was very low, the selectivity was significantly higher when a catalyst was used compared to when it was not. The yields obtained from the peapod biomass were 39.31 wt% sugars, 2.303 wt% formic acid, and 0.928 wt% levulinic acid. Notably, neither HMF (5-hydroxymethylfurfural) nor furfural was produced in this process. In contrast, the yields from coffee biomass were lower than those from peapod biomass, with results of 21.49 wt% sugars, 5.214 wt% formic acid, 1.395 wt% levulinic acid, 1.375 wt% HMF, and 1.062 wt% furfural.
The comparison of yields between pea and coffee biomass highlights the effectiveness of using pea biomass in this catalytic process. The higher sugar yield obtained from the peapod biomass indicates a more efficient conversion of biomass into valuable sugars, which can be further utilized for various applications, including biofuels and bioplastics. The increased selectivity observed with the use of a catalyst suggests that the catalytic process not only enhances conversion efficiency but also minimizes the formation of unwanted byproducts. This is particularly important in biomass conversion, where the goal is to maximize the yield of desired products while reducing the generation of less useful compounds.
In summary, while both biomass sources yield valuable products, pea biomass demonstrates a superior performance in terms of sugar yield and selectivity when a catalyst is employed. This finding suggests that further exploration of the use of pea biomass for industrial applications could be beneficial, especially in the context of sustainable and efficient biomass conversion processes.
5. Conclusions
This comparative analysis of coffee cherries and peapods as biomass feedstocks for hydrothermal valorization reveals differences in their chemical composition and potential for producing platform chemicals. Coffee cherries contain higher levels of cellulose (27.6%) and lignin (13.7%), while peapods exhibit a lower lignin content (5.0%) and higher hemicellulose content (17.4%). These compositional characteristics influence the efficiency of biomass conversion, with peapods yielding a higher percentage of platform chemicals (45.128 wt%) compared to coffee cherries (32.598 wt%) during hydrothermal processing without catalysts. The lower lignin content in peapods facilitates their easier breakdown and conversion into valuable chemicals, highlighting their potential as a more accessible feedstock for biorefinery applications.
The introduction of various acid catalysts during hydrothermal valorization significantly impacts the yields and selectivity of platform chemicals. For instance, sulfuric acid enhances sugar production from both biomass types, yielding 62.936 wt% from peapods and 51.236 wt% from coffee cherries, demonstrating its effectiveness in promoting sugar formation. Conversely, hydrochloric acid resulted in decreased yields, with coffee cherries and peapods producing 28.879 wt% and 33.049 wt%, respectively, while selectively favoring sugar production. Nitric acid also showed promising results, yielding 35.223 wt% from coffee cherries and 40.315 wt% from peapods, indicating its ability to facilitate the conversion of both biomass types into platform chemicals.
Other acids, such as adipic acid and acetic acid, further demonstrated the potential for enhancing overall yields. Adipic acid achieved yields of 53.668 wt% for coffee cherries and 65.165 wt% for peapods, showcasing its ability to increase the production of levulinic acid, alongside other platform chemicals. Acetic acid also yielded significant results, with coffee biomass producing 35.436 wt% and peapods reaching 50.427 wt%, indicating a substantial increase in sugar production. In contrast, butyric acid resulted in lower overall yields (17.168 wt% for coffee cherries and 36.301 wt% for peapods), primarily producing sugars, with the minimal formation of other platform chemicals.