1. Introduction
Energy produced from fossil fuels has detrimental social, political, and environmental effects. Moreover, burning fossil fuel has increased carbon dioxide (CO
2) levels globally and accelerated climate change. These implications have attracted more attention to renewable energy source development [
1]. over the decreasing supply of fossil fuels and the increasing emissions of greenhouse gases (GHGs) are conducive to a growing emphasis on utilizing biomass energy as an alternative fuel, particularly from forestry and agricultural wastes, due to its low cost and availability [
2]. Biomass resources have the ability to be transformed to solid, liquid, and gaseous fuels. When compared with fossil fuels, biomass energy emits less CO
2 and has lower content of nitrogen and sulfur [
3]. Biomass derives either directly or indirectly from sun energy. The emitted CO
2, a byproduct of biomass combustion, can be reabsorbed via photosynthesis through the growth of green plants. Consequently, biomass is a source of renewable energy that can potentially satisfy the needs of sustainable development [
4]. Cotton stalks (CSs), a byproduct of the cultivation of cotton, is becoming an important agricultural waste via its potential uses throughout many industries. Annually, huge amounts of CSs are generated worldwide—about 90.3 to 129 million tons—but this material is commonly neglected and categorized as waste [
5]. However, CSs have high percentages of lignin, hemicellulose, and cellulose contents, which make it a good candidate for bioenergy production via thermo-chemical conversion methods. Therefore, exploiting CS biomass for bioenergy generation could contribute significantly to emission reduction, as well as providing further income for cotton farmers.
Biomass as a solid fuel has attracted researchers’ interest due to its potential to aid in reducing dependency on fossil fuels as well as address climate change issues [
6]. Biomass energy has gained great attention due to its low CO
2 emissions, high content of hydrogen, and widespread distribution. By 2050, energy derived from biomass will account for almost half of the energy consumption in the world; of this, developed countries consume approximately 80%. Furthermore, biomass resources have been used as fuels for long periods throughout history and, recently, it has been found that biomass can be utilized with different thermal methods (mixed combustion, pure combustion, pyrolysis, and gasification) for generating energy along with other value-added outputs [
7].
The pyrolysis process is considered the first stage in all thermal processes, and plays an important role in the thermo-chemical conversion of biomass [
7]. It refers to the thermal degradation of biomass in an oxygen-free atmosphere. In terms of operations, the pyrolysis method can be categorized into three different stages: flash, fast, and slow pyrolysis [
8]. Many studies have been conducted on CS pyrolysis using different reactors and conditions. For instance, the impact of CS particle size and reaction temperature on the fast pyrolysis technique was investigated in a bubbling fluidized-bed reactor. The findings determined the highest bio-oil output to be at a temperature of 490 °C and a 1 mm feedstock particle size [
9]. Another study examined the microwave-assisted pyrolysis of CSs under various parameters (reaction temperature, microwave power, and retention time). These conditions were optimized to obtain maximum bio-oil production (32.47%) [
10]. Valorized colored cotton residue was investigated using a micro-pyrolyzer. The results demonstrated significant energetic ability and depicted a presence of oxygenated substances, light organic acids, and phenols in pyrolysis products, implying its use as an alternative energy source and bioenergy generator [
11]. On the other hand, investigating CSs via slow pyrolysis is still not well established. Therefore, studying CSs through slow pyrolysis, adopting different conditions for bio-oil production, could contribute significantly to the utilization of these residues and produce clean energy as well.
The pyrolysis of solid fuels involves two major steps: primary and secondary pyrolysis. The primary stage takes place at a lower temperature, where solid fuel deteriorates into tar, char, lighter gases, and ash. Secondary pyrolysis is a process in which primary pyrolysis products, particularly tar, undergoes additional chemical reactions at a higher temperature and longer retention time [
12]. Cellulose, hemicellulose, and lignin of biomass degrade through the primary reaction, yielding primary as well as intermediate byproducts. The intermediate products undergo a secondary reaction which depends on the reaction parameters and feedstock characteristics [
13]. Consequently, understanding the biomass’s thermal decomposition is critical for determining the optimal temperature of biomass conversion for combustion or pyrolysis. Kinetic studies of thermal and chemical processes can help to predict the reaction’s performance and energy [
14]. Furthermore, estimating the pyrolysis kinetics is crucial for predicting biomass decomposition behavior through the pyrolysis process, which provides the basis of designing the reactors. The thermal decomposition of biomass involves several steps, making it a complex process. However, the International Confederation for Thermal Analysis and Calorimetry (ICTAC) strongly recommends the use of iso-conversional (IC) approaches to determine the kinetic triplet of thermal degradation, which includes activation energy [
15]. The IC, also known as the model-free method, is the most extensively used method for studying biomass pyrolysis kinetics. Generally, there are two kinds of model-free approaches: differential and integral. The differential iso-conversional technique’s reliance on the instantaneous rate values makes it vulnerable to trial noise, resulting in numerical instability. Using the integral method, particularly in TGA experiments, can effectively avoid this matter [
16]. The Kissinger–Akahira–Sunose (KAS) and Flynn–Wall–Ozawa (FWO) approaches are common integral non-isothermal iso-conversion methods [
17]. In the present study, the FWO and KAS models were employed to determine the activation energy (E
a) of CSs using TGA data. However, more attention is given to the pyrolysis technique due to several benefits. It can convert biomass materials to biochar, gases, and bio-oil. Each of these products can be utilized for various purposes. The gases are capable of being used to generate heat and electricity. Bio-oil could serve as a boiler fuel or a raw material for chemicals. Biochar can be gasified to produce syngas, or utilized as an adsorbent, biofuel, or amendment for soil [
18]. However, in this research, a kinetic analysis was first carried out to investigate CS decomposition behavior. The effects of reaction conditions (including biomass characteristics) on pyrolysis products were then explored, while many other investigations focusing on other conditions including heating rate, reaction temperature, and carrier gas flow rate, not including feedstock particle size, were performed.
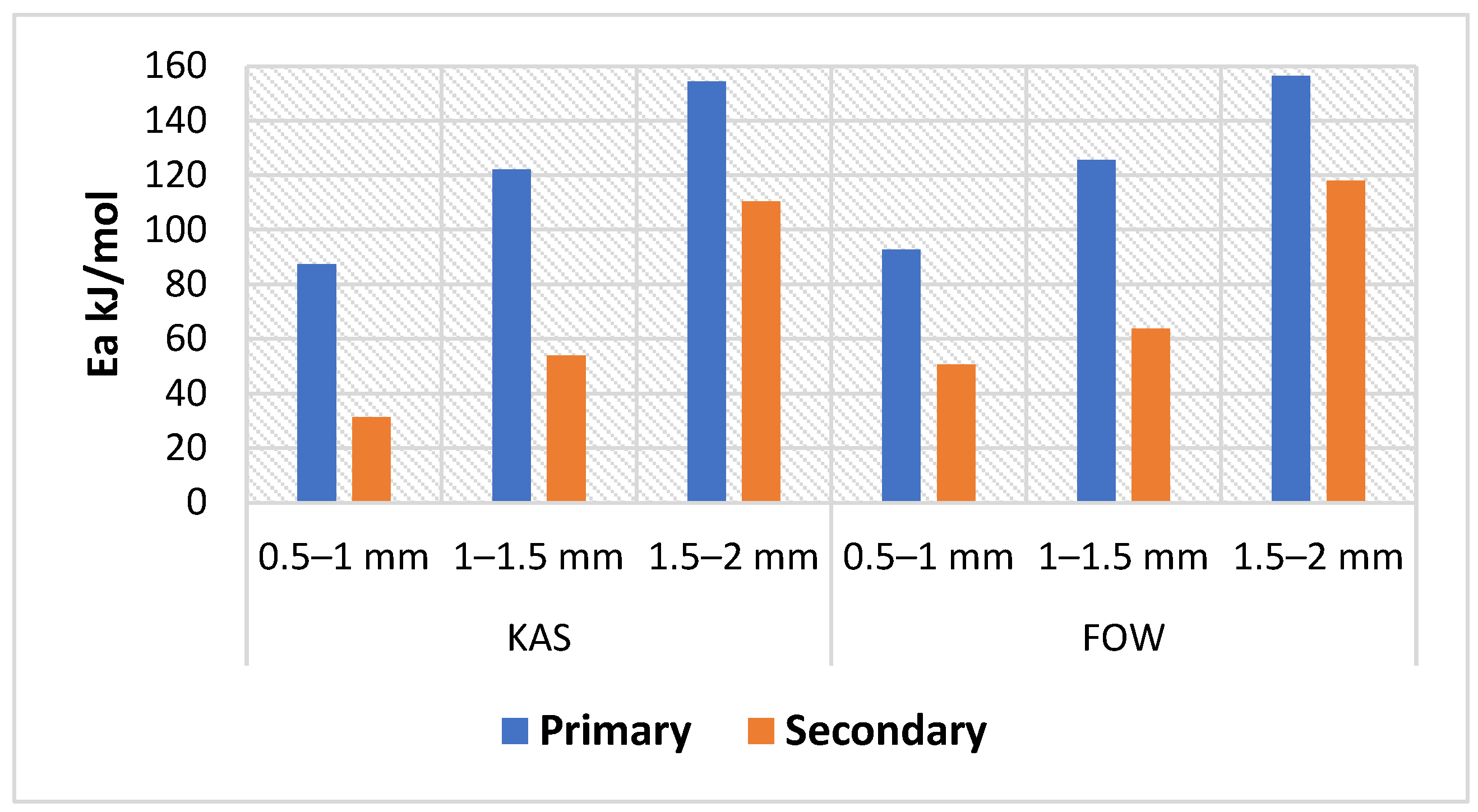
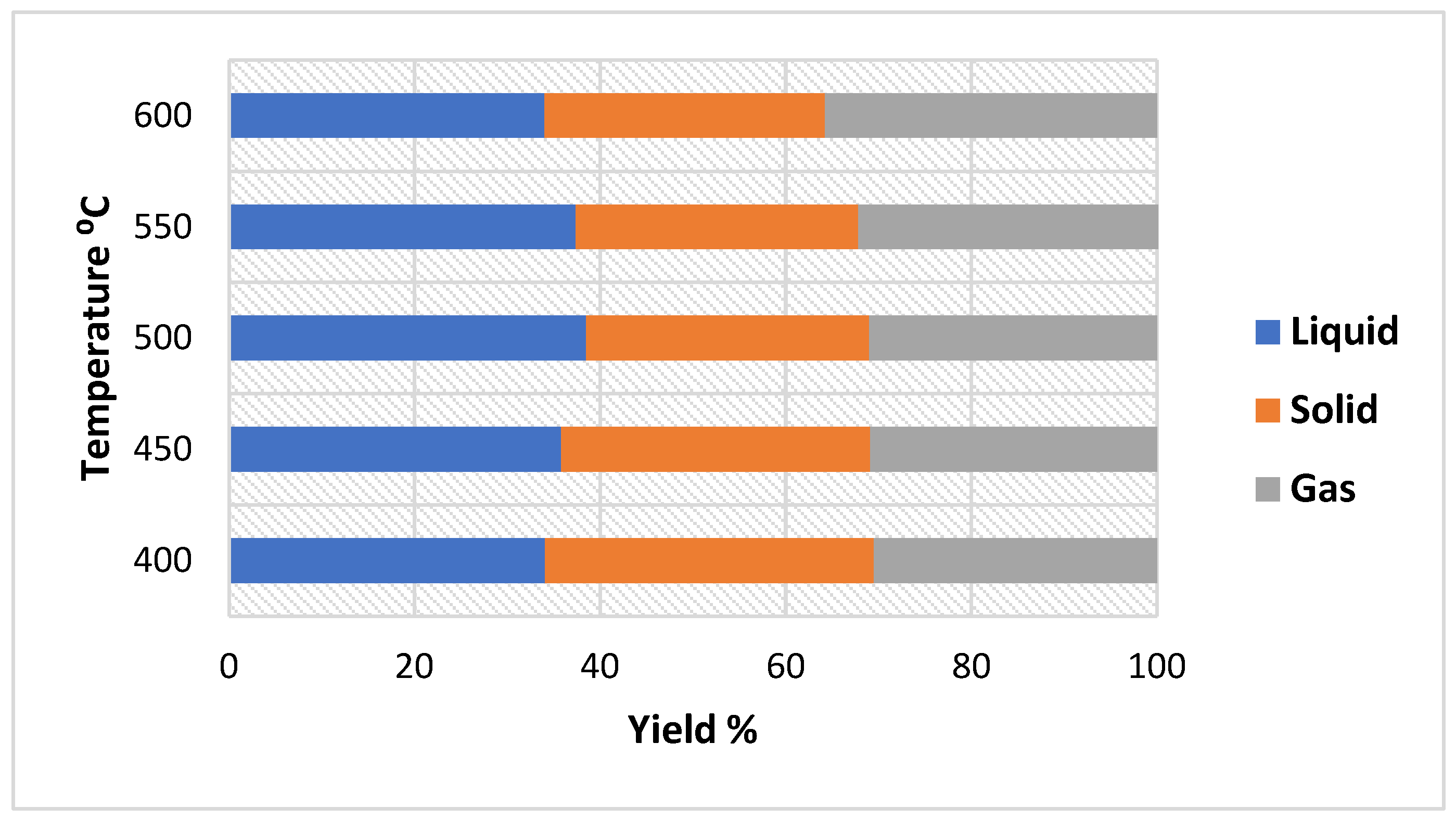
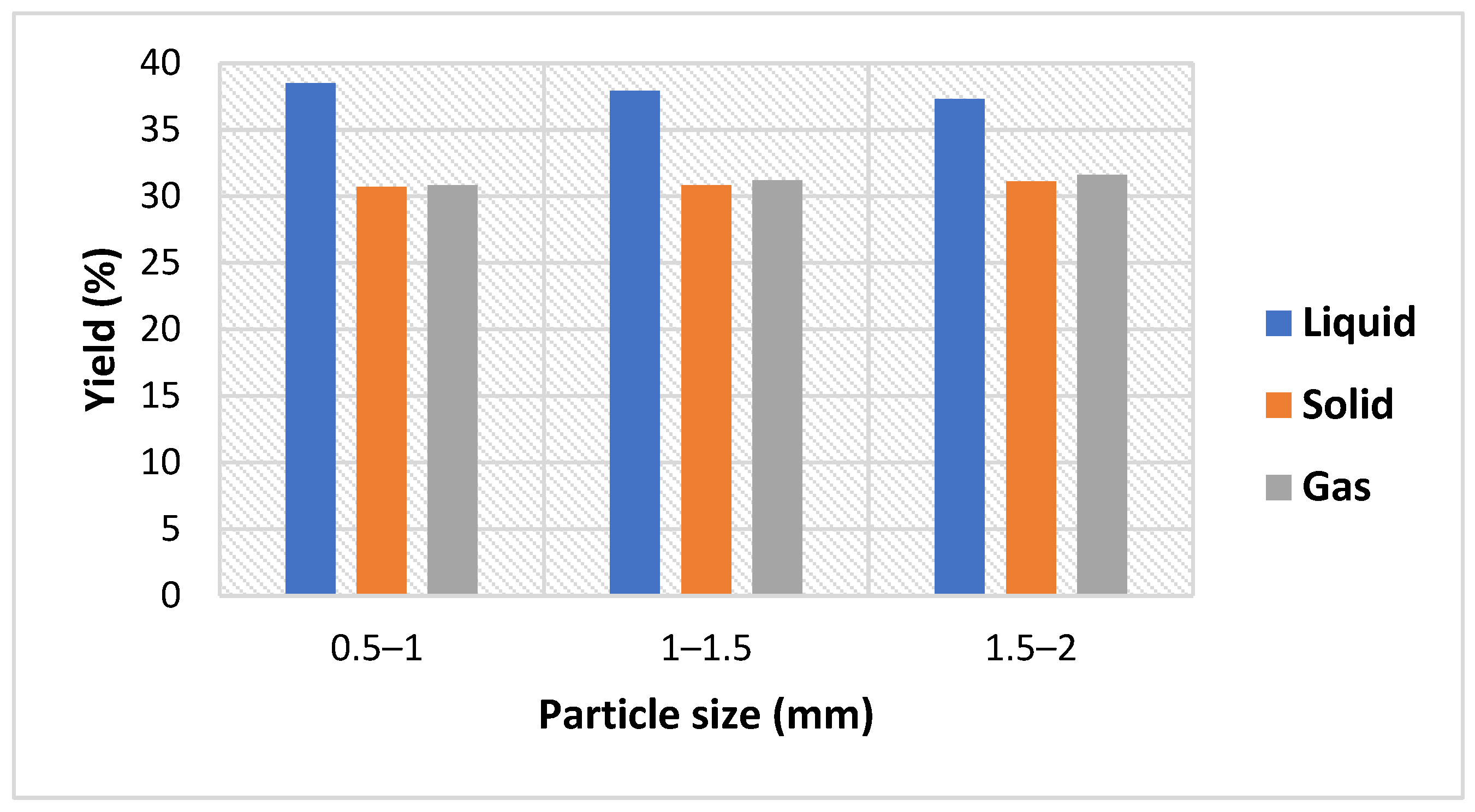
Exploiting biomass wastes as a renewable energy using efficient techniques can provide alternative green fuels which contributes positively to the reduction of GHG emissions in the environment. Reaction parameters such as residence time, temperature, and biomass characteristics have an impact on the yield and properties of the products produced during the pyrolysis process. Therefore, this study aimed to perform kinetic analysis to estimate the activation energy of CSs using thermogravimetric analysis (TGA) data and to estimate the impact of reaction conditions (particle size, temperature, and retention time) on bio-oil yield derived from CS pyrolysis adopting the single factor method, and further characterize the bio-oil and biochar products utilizing analytical methods including gas chromatography–mass spectrometry (GC–MS), TGA, scanning electron microscopy (SEM), X-ray diffraction (XRD) analysis, energy yield, and energy densification. This work integrates both the determination of Ea and investigation of unique reaction conditions (temperature, retention time, and particle size) via TGA and analysis of the pyrolysis process of CS biomass, respectively. According to kinetic Ea values, it has been found that smaller biomass particle sizes are more suited to the pyrolysis process due to increased heat transmission efficiency, which is in conformity with the pyrolysis experiments, where the highest bio-oil yield occurred using smaller sizes of CSs (0.5–1 mm) at a reaction temperature of 500 °C, while the other sizes (1–1.5 and 1.5–2 mm) generated more gases. Therefore, this research provides insightful information about the kinetic and pyrolysis processes of CSs; hence, it can be used as a valuable reference for researchers and stakeholders.
2. Materials and Methods
2.1. Samples Preparation and Characteristics
Cotton stalk samples were collected from Xinjiang Province, China. CS samples were collected in the 2023 season, directly after the cotton harvest, and used in experiments after 45 days. The dried samples were cut into smaller sizes and then crushed to sizes of 0.5–1, 1–1.5, and 1.5–2 mm. The prepared raw materials were placed in an oven at 105 °C for 4 h. After that, they were packed in bags and stored in a dry place to be used later in experiments.
A German Element Analyzer (Model: UNICUBE) was used for the ultimate analysis. The sample was decomposed by catalyst oxidation in an oxygen environment at a high temperature (1800 °C), the non-detected gases such as volatile halogen were removed and different component gases were separated by a special adsorption column, then the corresponding gases were detected by a Thermal Conductivity Detector using nitrogen as a carrier and purge gas. The proximate test was performed in accordance with the current Chinese national standard (GB/T28732-2012). Calorific value was measured using a bomb calorimeter. The cellulose, hemicellulose, and lignin percentages in CS raw materials were measured using the Van Soest Method of Detergent Fiber Analysis [
19].
2.2. Experimental Design and Procedures
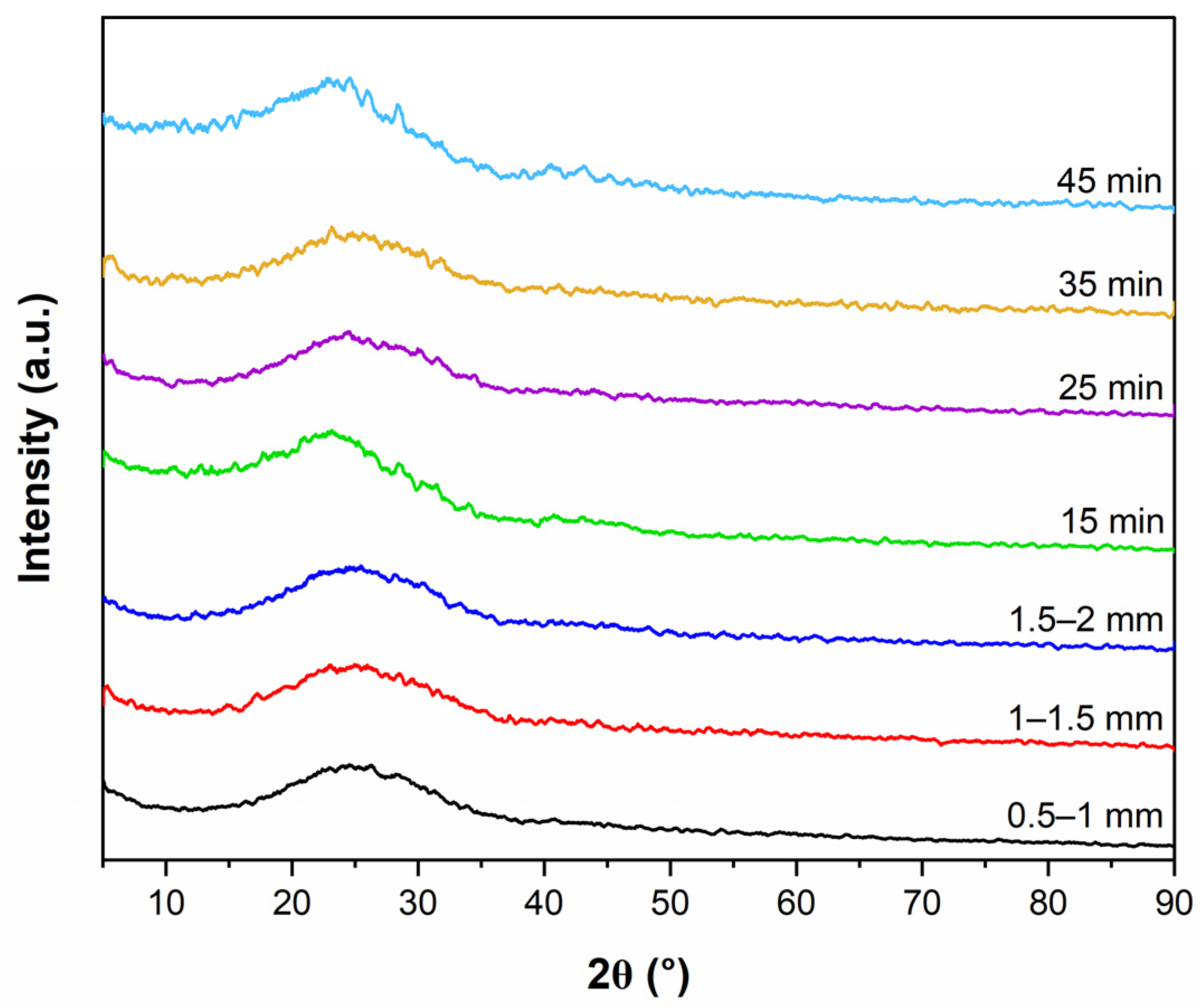
Pyrolysis products were investigated adopting various operation conditions, including temperature (°C), particle size (mm), and retention time (min). The single factor method was used to conduct the experiments and to understand the effect of factors on the liquid yield. The single factor research approach involves changing a single factor within a specific range while keeping all other variables constant. The adopted operation conditions of CS slow pyrolysis were: temperature (400, 450, 500, 550, and 600 °C); particle size (0.5–1, 1–1.5, and 1.5–2 mm); and retention time (15, 25, 35, and 45 min).
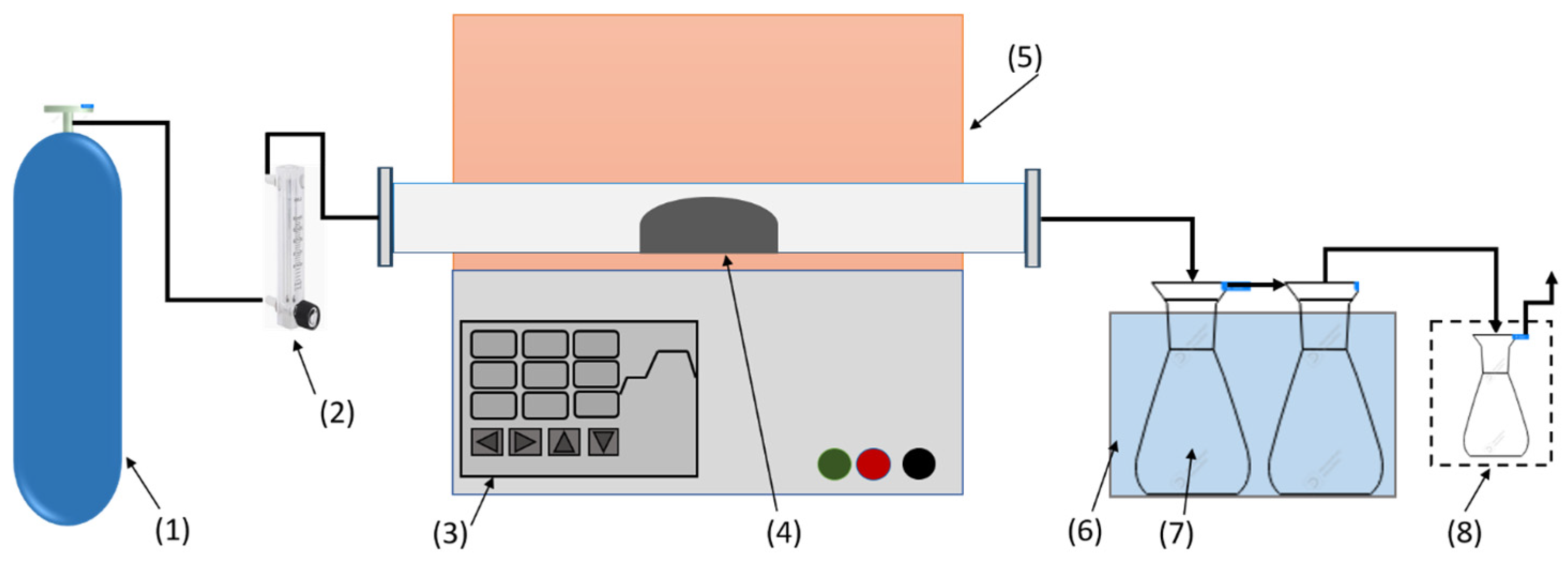
The slow pyrolysis experiments were conducted in a fixed-bed reactor (tube furnace) Hefei Kejing Material Technology Co., Ltd., (OTF-1200X, Kejing, Hefei, China). As demonstrated in
Figure 1, the system contains a gas supply, gas flowmeter, quartz tube, liquid yield collector, and flue gas purifier (a water filter was used to trap gases which include heavy hydrocarbons). The sample of CS (30 g) was placed directly in the center of the tube (1000 mm length, internal diameter 54 mm, length of temperature zone 150 mm). The tube was purged with high purity Ar gas (99.99%) at a flow rate of 200 mL/min. To start the experiment, the temperature of the furnace was programed from 20 °C to increase gradually to the selected temperatures of 400, 450, 500, 550, and 600 °C, with 10 °C/min as the rate of heat increase. Liquid products were collected by condensation using two glass bottles (500 mL size) placed inside iced water. To ensure accurate results, each experiment was conducted in three replicates.
2.3. Yield Calculations
The liquid yield was determined by weighing the bottles before and after the experiment, then it was computed as a percentage of the sample’s initial weight. Biochar yield was obtained by the difference between the final solid product and the sample weight. For the gas yield, it was calculated by the difference between 100% and the sum of solid and liquid yields. Equations (1) and (2) show the calculation of liquid and solid yields [
20,
21].
The energy densification ratio and energy yield of CS biochar were determined using Equations (3) and (4) [
22].
2.4. Thermogravimetric Tests
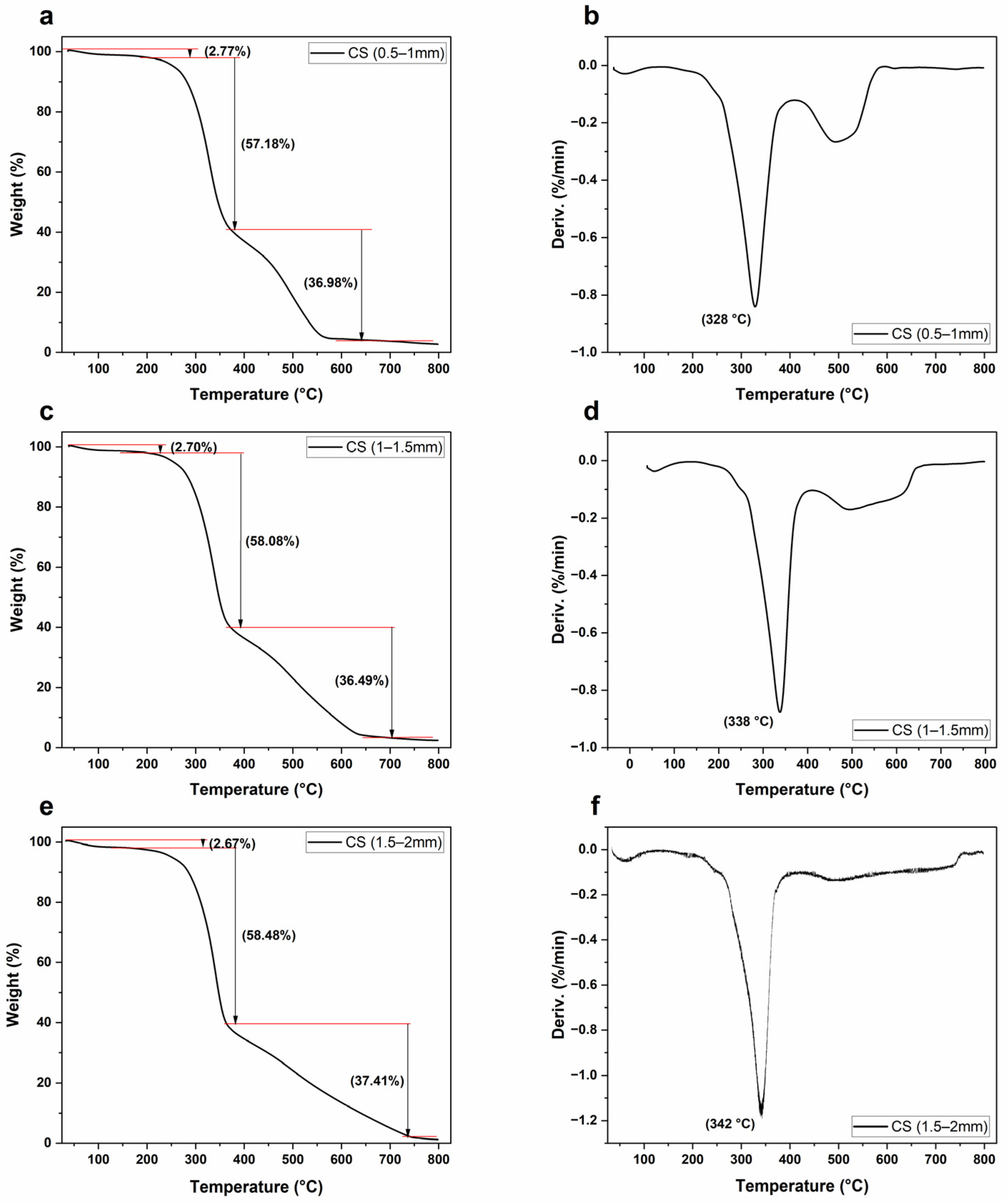
Thermogravimetric tests of CS samples were performed using a TG analyzer (TGA 5500, TA Instruments, United State of America (USA)). Approximately 6.5 mg of CS sample was placed in a heat-resistant platinum pot. The reactor’s temperature was adjusted to gradually increase from 30 °C to 800 °C at a rate of 10 °C/min. The furnace was purged with helium gas at a flow rate of 25 mL/min. The TRIOS program was used to analyze the recorded data.
Pyrolysis progress (conversion degree devolatilization) α (−) was calculated using sample weight loss as a function of temperature:
where M
0, M
t, and M
f are the initial, actual, and final weights of the sample, respectively.
2.4.1. Non-Isothermal Model-Free Iso-Conversion Method
The activation energy of the pyrolysis process was determined by employing IC approaches. These methods assume the same conversion degree (α) of a given value of Ea, regardless of heating rate, and that the E
a fluctuates as the progress of process. Every chemical process is progressive, reflecting various aspects of the mechanism across the whole reaction route [
23]. To conduct kinetic analysis using IC methods, numerous TG measurements at various heating rates are required. The values of E
a are calculated using data from multiple kinetic curves rather than, as with a single fitting approach, at varied heating rates for a constant α. The E
a value can be obtained at any stage of the process, allowing for a thorough evaluation of the kinetics and reaction mechanism, and its degree of complexity as it advances [
24]. The IC study of kinetics provides for exact, near-actual activation energy estimates, limiting issues caused by the impacts of mass transfer and energy at varying heating rates. This analysis also helps to avoid problems caused by the imprecise evaluation of the reaction models.
The model-free approach employed in this study served as the foundation for the IC methods. The IC techniques of FWO and KAS were applied to determine various kinetic parameters that occur via the pyrolysis of CS. The ICTAC has recommended that FWO and KAS are precise procedures for kinetic parameter calculations. Differential or integral approaches are the two types of IC methods. Two integral approaches that are frequently used are FWO and KAS.
Calculations of E
a were conducted for the α range from 0.1 to 0.9, and four heating rate constants (β = 10, 15, 20, and 25 °C/min). This work concerns pyrolysis at a heating rate of 10 °C/min. However, measurements at other heating rates were used to calculate Ea, and these measurements showed trends similar to those at 10 °C/min. For both formulas, R represents the universal constant of gas (8.314 J/mol·K) [
24].
2.4.2. Flynn–Wall–Ozawa (FWO) Method
The E
a is often determined using the FWO method, which is dependent upon TGA data conducted at heating constant rates. Following that, a linear function with a slope corresponding to the E
a is calculated by combining the natural logarithm of the degradation fraction and the inverse of the absolute temperature. Doyle’s approximations algorithm [
25] was then applied to obtain the subsequent Equation (6):
where β is the heating rate (°C/min), g(α) is a constant at a known conversion value, 1/T is the linear relationship with a given conversion value at different rates, A is the frequency or pre-exponential factor, E
a is the activation energy of the reaction, R is the universal gas constant (8.314 J/mol·K), and T is the absolute temperature. The apparent E
a can be determined via a plot of log(β) versus 1/T at a specific conversion for different heating rates.
2.4.3. Kissinger–Akahira–Sunose (KAS) Method
The Arrhenius formulation serves as the foundation for the KAS, which is a differential method. This approach leads to the conclusion that the heating rate logarithm over the square of absolute temperature versus the reciprocal of absolute temperature is a linear function for a given conversion degree (to understand it another way, the slope and activation energy are proportional). By using the Murray–White approximation [
26] and the Coats–Redfern method [
27], we obtained the KAS model, which is expressed by the Equation (7):
Ea can be calculated using Equation (6) from the slope (−Ea/RT) on a plot of log (β/T2) versus 1/T at a particular conversion for various heating rates.
2.5. Analysis of Pyrolysis Products
The compositions of tar compounds were determined using GC–MS model 7890A/5975C, Agilent Technologies, Santa Clara, CA, USA. Acetone solution was utilized to swill the condensed bottles to collect tar products. Powder of copper sulfate (anhydrous) was utilized to soak up moisture, followed by filtration via a quartz microporous membrane (0.22 μm). The GC–MS column was DB-5MS (30 m length, 0.25 mm diameter, and film thickness of 0.25 μm). Chromatographic conditions were: 1 μL injection volume, unsplit stream sampling, injector temperature adjusted to 250 °C, flow rate 64.20 mL/min. The temperature of the column was maintained at 60 °C for 5 min before gradually increasing to 270 °C with a rate of heat of 5 °C/min for 10 min. Pure helium gas (He) was employed as the carrier gas. The chromatographic peaks for each target component achieved using the aforementioned chromatographic parameters were compared to the NIST, 2008 (National Institute of Standards and Technology, USA) database of MS. The component yields were obtained as a peak area percentage.
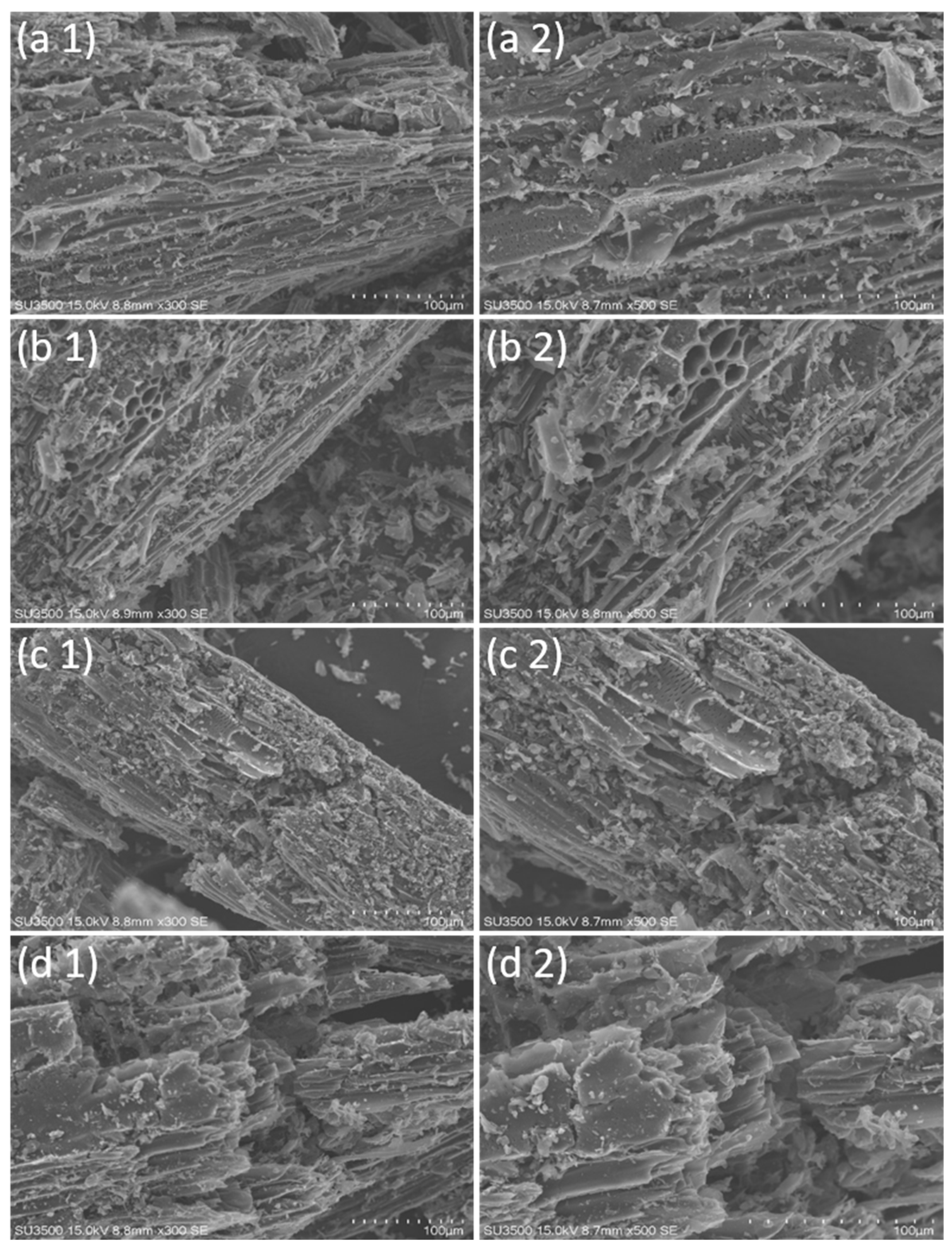
The physical morphology of biochar surface was analyzed using SEM, SU3500, Hitachi, Japan, with parameters of 100 µm resolution and 10.0 kV acceleration. The XRD instrument was used to assess the structure and crystallinity of biochar.
The liquid yield data were assessed utilizing Statistical Package for the Social Sciences (SPSS), and differences among averages were examined via analysis of variance (ANOVA) using LSD (least significant difference) with p-value (p ˂ 0.05).