Enhancing the Growth, Bioactive Compounds, and Antioxidant Activity of Kangkong (Ipomoea aquatica Forssk.) Microgreens Using Dielectric Barrier Discharge Plasma
Abstract
1. Introduction
2. Materials and Methods
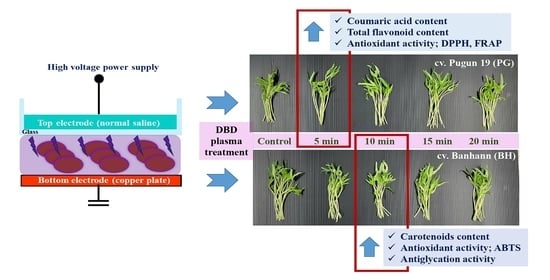
2.1. Setup for an Experiment, Initial Seed Sample, and Plasma Treatment
2.2. Growth of Microgreens
2.3. Chlorophyll and Carotenoids Contents
2.4. Crude Extraction
2.5. Total Ascorbic Acid Content
2.6. Total Phenolic Content
2.7. Total Flavonoid Content
2.8. Phenolic Profiling by HPLC
2.9. Antioxidant Activity
2.9.1. DPPH Assay
2.9.2. FRAP Assay
2.9.3. ABTS Assay
2.10. Antiglycation Activity
2.11. Experimental Design and Statistical Analysis
3. Results
3.1. Growth of Microgreens
3.2. Chlorophyll and Carotenoids Contents
3.3. Bioactive Compounds
3.3.1. Total Ascorbic Acid Content
3.3.2. Total Phenolic Content
3.3.3. Total Flavonoid Content
3.3.4. Phenolic Profile
3.4. Antioxidant Activity
3.5. Antiglycation Activity
3.6. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolonen, H.; Reinikainen, J.; Zhou, Z.; Härkänen, T.; Männistö, S.; Jousilahti, P.; Paalanen, L.; Lundqvist, A.; Laatikainen, T. Development of non-communicable disease risk factors in Finland: Projections up to 2040. Scand. J. Public Health 2023, 51, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Akinyede, A.I.; Fehintola, A.B.; Oluwajuyitan, T.D. Antioxidant activity and blood glucose reduction potential of malabar chestnut in streptozotocin induced diabetic rats. J. Agric. Food Res. 2022, 8, 100299. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and microgreens-novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; DePascale, S. Sprouts, microgreens and edible flowers as novel functional foods. Agronomy 2021, 11, 2568. [Google Scholar] [CrossRef]
- Manvar, M.N.; Desai, T.R. Phytochemical and pharmacological profile of Ipomoea aquatica. Indian J. Med. Sci. 2013, 67, 49–60. [Google Scholar] [CrossRef]
- Sajak, A.A.B.; Abas, F.; Ismail, A.; Khatib, A. Effect of different drying treatments and solvent ratios on phytochemical constituents of Ipomoea aquatica and correlation with α-glucosidase inhibitory activity. Int. J. Food Prop. 2016, 19, 2817–2831. [Google Scholar] [CrossRef]
- Datta, S.; Sinha, B.K.; Bhattacharjee, S.; Seal, T. Nutritional composition, mineral content, antioxidant activity and quantitative estimation of water soluble vitamins and phenolics by RP-HPLC in some lesser used wild edible plants. Heliyon 2019, 5, e01431. [Google Scholar] [CrossRef] [PubMed]
- Sigala Aguilar, N.A.; González Fuentes, J.A.; Valdez Aguilar, L.A.; López Pérez, M.G.; Medrano Macias, J.; Benavides-Mendoza, A.; González Morales, S. Effect of elicitors and biostimulants on the content of bioactive compounds in raspberry fruits. Hort. Sci. 2023, 50, 101–111. [Google Scholar] [CrossRef]
- Asan, H.S. Elicitors enhanced the production of bioactive compounds in shoot cultures of Hypericum amblysepalum. Bot. Serbica 2023, 47, 271–277. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Thomas, M.; Angers, P.; Arul, J. Hydrogen peroxide can enhance the synthesis of bioactive compounds in harvested broccoli florets. Front. Sustain. Food Syst. 2022, 6, 812123. [Google Scholar] [CrossRef]
- Ji, S.H.; Choi, K.H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.K.; Choi, E.H.; et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Abarghuei, F.M.; Etemadi, M.; Ramezanian, A.; Esehaghbeygi, A.; Alizargar, J. An application of cold atmospheric plasma to enhance physiological and biochemical traits of basil. Plants 2021, 10, 2088. [Google Scholar] [CrossRef] [PubMed]
- Rithichai, P.; Jirakiattikul, Y.; Singhawiboon, M.; Poolyarat, N. Enhancement of seed quality and bioactive compound accumulation in sunflower sprouts by dielectric barrier discharge plasma treatment. ScienceAsia 2021, 47, 441–448. [Google Scholar] [CrossRef]
- Saengha, W.; Karirat, T.; Buranrat, B.; Matra, K.; Deeseenthum, S.; Katisart, T.; Luang-In, V. Cold plasma treatment on mustard green seeds and its effect on growth, isothiocyanates, antioxidant activity and anticancer activity of microgreens. Int. J. Agric. Biol. 2021, 25, 667–676. [Google Scholar] [CrossRef]
- Burducea, I.; Burducea, C.; Mereuta, P.-E.; Sirbu, S.-R.; Iancu, D.A.; Istrati, M.-B.; Straticiuc, M.; Lungoci, C.; Stoleru, V.; Teliban, G.-C.; et al. Helium atmospheric pressure plasma jet effects on two cultivars of Triticum aestivum L. Foods 2023, 12, 208. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Pradhan, S.P.; Pandey, B.P.; Shrestha, B.; Fronczak, M.; Kierzkowska-Pawlak, H.; Subedi, D.P. Growth enhancement of radish seed induced by low-temperature argon plasma. Plasma Chem. Plasma Process. 2023, 43, 111–137. [Google Scholar] [CrossRef]
- Nicoletto, C.; Falcioni, V.; Locatelli, S.; Sambo, P. Non-thermal plasma and soilless nutrient solution application: Effects on nutrient film technique lettuce cultivation. Horticulturae 2023, 9, 208. [Google Scholar] [CrossRef]
- Attri, P.; Koga, K.; Okumura, T.; Shiratani, M. Impact of atmospheric pressure plasma treated seeds on germination, morphology, gene expression and biochemical responses. Jpn. J. Appl. Phys. 2021, 60, 040502. [Google Scholar] [CrossRef]
- Lim, J.S.; Kim, D.; Ki, S.; Mumtaz, S.; Shaik, A.M.; Han, I.; Hong, Y.J.; Park, G.; Choi, E.H. Characteristics of a rollable dielectric barrier discharge plasma and its effects on spinach-seed germination. Int. J. Mol. Sci. 2023, 24, 4638. [Google Scholar] [CrossRef]
- Bilea, F.; Garcia-Vaquero, M.; Magureanu, M.; Mihaila, I.; Mildažienė, V.; Mozetič, M.; Pawłat, J.; Primc, G.; Puač, N.; Robert, E.; et al. Non-thermal plasma as environmentally-friendly technology for agriculture: A review and roadmap, CRC. Crit. Rev. Plant Sci. 2024, 43, 428–486. [Google Scholar] [CrossRef]
- Hati, S.; Patel, M.; Yadav, D. Food bioprocessing by non-thermal plasma technology. Curr. Opin. Food Sci. 2018, 19, 85–91. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, A.; Heroux, P.; Sand, W.; Sun, Z.; Zhan, J.; Wang, C.; Hao, S.; Li, Z.; Guo, Y.; et al. Effect of dielectric barrier discharge cold plasma on pea seed growth. J. Agric. Food Chem. 2019, 67, 10813–10822. [Google Scholar] [CrossRef]
- Guragain, R.P.; Baniya, H.B.; Dhungana, S.; Chhetri, G.K.; Sedhai, B.; Basnet, N.; Shakya, A.; Pandey, B.P.; Pradhan, S.P.; Joshi, U.M.; et al. Effect of plasma treatment on the seed germination and seedling growth of radish (Raphanus sativus). Plasma Sci. Technol. 2022, 24, 015502. [Google Scholar] [CrossRef]
- Ongrak, P.; Poolyarat, N.; Suksaengpanomrung, S.; Saidarasamoot, K.; Jirakiattikul, Y.; Rithichai, P. Germination, physicochemical properties, and antioxidant enzyme activities in kangkong (Ipomoea aquatica Forssk.) seeds as affected by dielectric barrier discharge plasma. Horticulturae 2023, 9, 1269. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Ranaweera, K.K.D.S. Antioxidative properties of 34 green leafy vegetables. J. Funct. Foods 2016, 26, 176–186. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, R. Determinations of total carotenoids and chlorophyll a and b of leaf extract in different solvents. Biochem. Soc. Trans. 1983, 11, 519–592. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, Z.; Cheng, K.; Shan, F.; Ren, G.; Chen, F.; Wang, M. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008, 106, 475–481. [Google Scholar] [CrossRef]
- Kammapana, L.; Mulalin, S.; Tangteerawattana, S. Effects of exogenous methyl jasmonate treatment with polyethylene bag storage on decay reduction and enhanced total ascorbic acid, total phenolic, and antioxidant activities in ‘Kamphaeng Saen 42’ mulberry fruit. Trends Sci. 2022, 19, 3069. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S. Phytochemicals and antioxidant activity of different fruit fractions (peel, pulp, aril and seed) of Thai gac (Momordica cochinchinensis Spreng). Food Chem. 2011, 127, 1138–1145. [Google Scholar] [CrossRef]
- Jirakiattikul, Y.; Ruangnoo, S.; Sangmukdee, K.; Chamchusri, K.; Rithichai, P. Enhancement of plumbagin production through elicitation in in vitro-regenerated shoots of Plumbago indica L. Plants 2024, 13, 1450. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Liu, R.; Lu, Z.; Marcone, M.F.; Tsao, R. Amber, red and blue LEDs modulate phenolic contents and antioxidant activities in eight Cruciferous microgreens. J. Food Bioact. 2020, 11, 95–109. [Google Scholar] [CrossRef]
- Puccinelli, M.; Maggini, R.; Angelini, L.G.; Santin, M.; Landi, M.; Tavarini, S.; Castagna, A.; Incrocci, L. Can light spectrum composition increase growth and nutritional quality of Linum usitatissimum L. sprouts and microgreens? Horticulturae 2022, 8, 98. [Google Scholar] [CrossRef]
- Rahbar, S.; Natarajan, R.; Yerneni, K.; Scott, S.; Gonzales, N.; Nadler, J.L. Evidence that pioglitazone, metformin and pentoxifylline are inhibitors of glycation. Clin. Chim. Acta 2000, 301, 65–77. [Google Scholar] [CrossRef]
- Wu, C.H.; Yen, G.C. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. J. Agric. Food Chem. 2005, 53, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Yu, N.N.; Zheng, W.; Zhang, L.N.; Liu, Y.; Yu, J.B.; Zhang, Y.Q.; Park, G.; Sun, H.N.; Kwon, T. Effect of non-thermal plasma (NTP) on common sunflower (Helianthus annus L.) seed growth via upregulation of antioxidant activity and energy metabolism-related gene expression. Plant Growth Regul. 2021, 95, 271–281. [Google Scholar] [CrossRef]
- Billah, M.; Sajib, S.A.; Roy, N.C.; Rashid, M.M.; Reza, M.A.; Hasan, M.M.; Talukder, M.R. Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo L.) seed germination and growth. Arch. Biochem. Biophys. 2020, 681, 108253. [Google Scholar] [CrossRef] [PubMed]
- Amnuaysin, N.; Korakotchakorn, H.; Chittapun, S.; Poolyarat, N. Seed germination and seedling growth of rice in response to atmospheric air dielectric-barrier discharge plasma. Songklanakarin J. Sci. Technol. 2018, 40, 819–823. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef] [PubMed]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of plasma-seed treatments as a potential seed processing technology. Front. Phys. 2021, 9, 617345. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Ahmad, A.; Sripong, K.; Uthairatanakij, A.; Photchanachai, S.; Pankasemsuk, T.; Jitareerat, P. Decontamination of seed borne disease in pepper (Capsicum annuum L.) seed and the enhancement of seed quality by the emulated plasma technology. Sci. Hortic. 2022, 291, 110568. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Josna, K.; Pandiselvam, R.; Kothakota, A.; Gavahian, M.; Khaneghah, A.M. Impacts of cold plasma treatment on physicochemical, functional, bioactive, textural, and sensory attributes of food: A comprehensive review. Food Chem. 2022, 368, 130809. [Google Scholar] [CrossRef]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Liao, X.; Cullen, P.J.; Liu, D.; Xiang, Q.; Wang, J.; Chen, S.; Ye, X.; Ding, T. Effects of nonthermal plasma technology on functional food components. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1379–1394. [Google Scholar] [CrossRef]
- Du, Y.; Mi, S.; Wang, H.; Yuan, S.; Yang, F.; Yu, H.; Xie, Y.; Guo, Y.; Cheng, Y.; Yao, W. Intervention mechanisms of cold plasma pretreatment on the quality, antioxidants and reactive oxygen metabolism of fresh wolfberries during storage. Food Chem. 2024, 431, 137106. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Pateiro, M.; Lorenzo, J.M. Influence of plasma treatment on the polyphenols of food products—A review. Foods 2020, 9, 929. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2020, 63, 180–209. [Google Scholar] [CrossRef]
- Priatama, R.A.; Pervitasari, A.N.; Park, S.; Park, S.J.; Lee, Y.K. Current advancements in the molecular mechanism of plasma treatment for seed germination and plant growth. Int. J. Mol. Sci. 2022, 23, 4609. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Yin, Y.; Wang, J.; Wang, Z.; Ding, H.; Ma, R.; Jiao, Z. Research on the physio-biochemical mechanism of non-thermal plasma-regulated seed germination and early seedling development in Arabidopsis. Front. Plant Sci. 2019, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Iranbakhsh, A.; Ardebili, Z.O.; Ardebili, N.O.; Ghoranneviss, M.; Safari, N. Cold plasma relieved toxicity signs of nano zinc oxide in Capsicum annuum cayenne via modifying growth, differentiation, and physiology. Acta Physiol. Plant. 2018, 40, 154. [Google Scholar] [CrossRef]
- Sultan, S.M.E.; Yousef, A.F.; Ali, W.M.; Mohamed, A.A.A.; Shalaby, A.M.E.; Teiba, I.I.; Hassan, A.M.; Younes, N.A.; Kotb, E.F. Cold atmospheric plasma enhances morphological and biochemical attributes of tomato seedlings. BMC Plant Biol. 2024, 24, 420. [Google Scholar] [CrossRef] [PubMed]
- Luang-In, V.; Saengha, W.; Karirat, T.; Buranrat, B.; Matra, K.; Deeseenthum, S.; Katisart, T. Effect of cold plasma and elicitors on bioactive contents, antioxidant activity and cytotoxicity of Thai rat-tailed radish microgreens. J. Sci. Food Agric. 2020, 101, 1685–1698. [Google Scholar] [CrossRef]
- Park, H.; Puligundla, P.; Mok, C. Cold plasma decontamination of brown rice grains: Impact on biochemical and sensory qualities of their corresponding seedlings and aqueous tea infusions. LWT—Food Sci. Technol. 2020, 131, 109508. [Google Scholar] [CrossRef]
- El-Sawi, N.; Gad, M.H.; Al-Seeni, M.N.; Younes, S.; El-Ghadban, E.l.M.; Ali, S.S. Evaluation of antidiabetic activity of Ipomoea aquatica fractions in streptozotocin induced diabetic in male rat mode. Sohag J. Sci. 2017, 2, 9–17. [Google Scholar] [CrossRef]
- Matough, F.A.; Budin, S.B.; Hamid, Z.A.; Alwahaibi, N.; Mohamed, J. The role of oxidative stress and antioxidants in diabetic complications. Sultan Qaboos Univ. Med. J. 2012, 12, 5–18. [Google Scholar] [CrossRef]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subrattry, A.H. Antioxidant and anti-glycation activities correlates with phenolic composition of tropical medicinal herbs. Asian Pac. J. Trop. Med. 2013, 6, 561–569. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, S.; Almatroudi, A.; Khan, A.A.; Alsahil, M.A.; Almatroodi, S.A.; Rahmani, A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021, 48, 787–805. [Google Scholar] [CrossRef]
- Guenaou, I.; Nait, I.I.; Errami, A.; Lahlou, F.A.; Hmimid, F.; Bourhim, N. Bioactive compounds from Ephedra fragilis: Extraction optimization, chemical characterization, antioxidant and antiglycation activities. Molecules 2021, 26, 5998. [Google Scholar] [CrossRef] [PubMed]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold plasma in food processing: Design, mechanisms, and application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Shabbir, A.; Hassan, S.A.; Hanif, H.; Rauf, R.; Muntaha, S.T.; Jubbar, M.; Aadil, R.M. Applications of cold plasma technique to enhance the safety and quality of different food products. Meas. Foods 2024, 15, 100183. [Google Scholar] [CrossRef]


| Cultivar (A) | DBD Plasma Treatment (min; B) | SL (cm) | FW (kg/m2) | DW (g/m2) |
|---|---|---|---|---|
| PG | 12.37 ± 0.53 b 1 | 2.99 ± 0.59 b 2 | 169.45 ± 15.18 a 1 | |
| BH | 12.81 ± 0.41 a | 3.25 ± 0.27 a | 160.74 ± 6.81 b | |
| Untreated | 12.04 ± 0.54 c | 2.97 ± 0.25 | 160.23 ± 5.40 ab | |
| 5 | 13.00 ± 0.18 a | 3.11 ± 0.96 | 167.78 ± 13.86 a | |
| 10 | 13.02 ± 0.37 a | 3.25 ± 0.09 | 165.96 ± 13.06 ab | |
| 15 | 12.37 ± 0.32 b | 3.23 ± 0.94 | 165.49 ± 9.36 ab | |
| 20 | 12.27 ± 0.11 bc | 3.15 ± 0.37 | 155.21 ± 5.00 b | |
| PG | Untreated | 11.62 ± 0.18 f | 2.89 ± 0.23 | 161.06 ± 7.31 b |
| 5 | 13.12 ± 0.13 ab | 3.18 ± 3.28 | 177.10 ± 6.17 ab | |
| 10 | 12.66 ± 0.11 b–e | 3.30 ± 3.38 | 192.03 ± 2.22 a | |
| 15 | 12.15 ± 0.23 ef | 3.19 ± 3.29 | 174.01 ± 5.45 ab | |
| 20 | 12.32 ± 0.11 c–e | 2.99 ± 0.08 | 151.85 ± 3.44 b | |
| BH | Untreated | 12.68 ±0.13 b–e | 3.03 ± 0.25 | 159.94 ± 1.72 b |
| 5 | 12.83 ± 0.04 bc | 3.07 ± 0.16 | 155.22 ± 9.90 b | |
| 10 | 13.38 ± 0.04 a | 3.25 ± 0.02 | 168.00 ± 1.10 b | |
| 15 | 12.69 ± 0.04 b–d | 3.26 ± 0.22 | 163.14 ± 5.61 b | |
| 20 | 12.21 ± 0.06 de | 3.33 ± 0.08 | 162.63 ± 0.82 b | |
| A | ** | * | ** | |
| B | ** | ns | ** | |
| A × B | ** | ns | * |
| Cultivar (A) | DBD Plasma Treatment (min; B) | ChA | ChB | TCh | CAR |
|---|---|---|---|---|---|
| (µg/g Dry Weight) | |||||
| PG | 19.93 ± 0.98 | 31.98 ± 3.53 | 51.70 ± 2.63 | 27.13 ± 1.54 | |
| BH | 19.55 ± 0.91 | 32.48 ± 4.07 | 52.03 ± 3.18 | 26.96 ± 2.15 | |
| untreated | 20.76 ± 0.36 a 1 | 27.32 ± 2.36 b | 48.04 ± 2.00 b | 24.41 ± 1.35 b | |
| 5 | 19.85 ± 0.94 ab | 31.47 ± 3.80 ab | 51.32 ± 2.85 ab | 27.13 ± 1.53 ab | |
| 10 | 19.25 ± 1.19 b | 35.59 ± 2.56 a | 54.38 ± 1.91 a | 29.57 ± 1.37 a | |
| 15 | 19.30 ± 0.76 ab | 33.67 ± 2.97 a | 52.96 ± 2.22 a | 27.57 ± 1.60 a | |
| 20 | 19.53 ± 0.36 ab | 32.93 ± 1.62 a | 52.47 ± 1.28 a | 27.34 ± 0.84 a | |
| A | ns | ns | ns | ns | |
| B | * | ** | ** | * | |
| A × B | ns | ns | ns | ns | |
| Cultivar (A) | DBD Plasma Treatment (min; B) | AsA (mg/g Dry Extract) | TPC (mg GAE/g Dry Extract) | TFC (mg QE/g Dry Extract) |
|---|---|---|---|---|
| PG | 285.67 ± 13.33 b 1 | 22.71 ± 2.69 a 2 | 238.40 ± 22.00 a 1 | |
| BH | 312.33 ± 11.33 a | 21.04 ± 2.45 b | 202.40 ± 30.00 b | |
| Untreated | 274.33 ± 15.50 b | 18.36 ± 0.84 c | 181.40 ± 32.00 b | |
| 5 | 304.34 ± 15.99 a | 25.90 ± 1.16 a | 211.40 ± 64.50 ab | |
| 10 | 303.34 ± 31.50 a | 21.99 ± 1.05 b | 219.40 ± 33.50 ab | |
| 15 | 304.00 ± 39.33 ab | 21.31 ± 1.27 b | 249.40 ± 24.00 a | |
| 20 | 299.34 ± 22.00 ab | 21.82 ± 1.70 b | 220.40 ± 39.00 ab | |
| PG | Untreated | 270.33 ± 15.33 b | 18.48 ± 1.16 | 208.40 ± 32.00 ab |
| 5 | 297.67 ± 5.34 ab | 26.41 ± 1.48 | 242.00 ± 14.00 ab | |
| 10 | 289.00 ± 3.33 ab | 22.88 ± 0.18 | 238.40 ± 12.00 ab | |
| 15 | 279.00 ± 14.00 ab | 22.30 ± 0.48 | 236.40 ± 28.00 ab | |
| 20 | 285.67 ± 6.00 ab | 23.46 ± 0.25 | 242.40 ± 18.00 ab | |
| BH | Untreated | 274.33 ± 14.00 ab | 18.24 ± 0.14 | 178.40 ± 0.00 b |
| 5 | 312.33 ± 1.33 ab | 25.39 ± 0.00 | 180.40 ± 4.00 b | |
| 10 | 319.67 ± 2.00 a | 21.09 ± 0.76 | 206.40 ± 8.00 ab | |
| 15 | 318.33 ± 3.33 ab | 20.32 ± 1.02 | 256.40 ± 6.00 a | |
| 20 | 307.37 ± 0.67 ab | 20.17 ± 0.54 | 208.40 ± 6.00 ab | |
| A | ** | ** | ** | |
| B | ** | ** | ** | |
| A × B | ** | ns | ** |
| Cultivar (A) | DBD Plasma Treatment (min; B) | CHL | COU | CAF | FER | RUT |
|---|---|---|---|---|---|---|
| (mg/g Dry Extract) | ||||||
| PG | 0.44 ± 0.06 | 0.19 ± 0.04 a | 0.14 ± 0.03 b | 0.79 ± 0.19 b | 0.25 ± 0.03 | |
| BH | 0.48 ± 0.05 | 0.16 ± 0.03 b | 0.17 ± 0.04 a | 1.09 ± 0.30 a | 0.24 ± 0.03 | |
| Untreated | 0.38 ± 0.05 b 1 | 0.11 ± 0.01 b | 0.11 ± 0.01 b | 0.57 ± 0.11 b | 0.24 ± 0.02 | |
| 5 | 0.48 ± 0.02 a | 0.19 ± 0.02 a | 0.16 ± 0.01 a | 1.04 ± 0.38 a | 0.28 ± 0.03 | |
| 10 | 0.46 ± 0.02 a | 0.18 ± 0.02 a | 0.18 ± 0.02 a | 1.14 ± 0.10 a | 0.24 ± 0.02 | |
| 15 | 0.46 ± 0.04 a | 0.19 ± 0.01 a | 0.16 ± 0.04 a | 1.04 ± 0.11 a | 0.25 ± 0.03 | |
| 20 | 0.52 ± 0.02 a | 0.20 ± 0.04 a | 0.16 ± 0.04 a | 0.97 ± 0.19 a | 0.23 ± 0.01 | |
| PG | Untreated | 0.35 ± 0.00 | 0.11 ± 0.00 | 0.10 ± 0.01 c | 0.46 ± 0.01 e | 0.22 ± 0.01 |
| 5 | 0.47 ± 0.06 | 0.20 ± 0.02 | 0.16 ± 0.01 a–c | 0.74 ± 0.01 de | 0.29 ± 0.03 | |
| 10 | 0.46 ± 0.00 | 0.20 ± 0.01 | 0.18 ± 0.03 ab | 1.08 ± 0. 06 bc | 0.25 ± 0.01 | |
| 15 | 0.44 ± 0.01 | 0.20 ± 0.01 | 0.13 ± 0.02 bc | 0.93 ± 0.01 b–d | 0.27 ± 0.02 | |
| 20 | 0.51 ± 0.01 | 0.23 ± 0.02 | 0.13 ± 0.00 bc | 0.78 ± 0. 01 cd | 0.24 ± 0.02 | |
| BH | Untreated | 0.43 ± 0.04 | 0.10 ± 0.01 | 0.11 ± 0.01 c | 0.65 ± 0.09 de | 0.25 ± 0.02 |
| 5 | 0.49 ± 0.04 | 0.18 ± 0.02 | 0.15 ± 0.01 a–c | 1.48 ± 0.17 a | 0.28 ±0.01 | |
| 10 | 0.47 ± 0.03 | 0.16 ± 0.00 | 0.18 ± 0.00 ab | 1.20 ± 0.10 ab | 0.23 ± 0.02 | |
| 15 | 0.50 ± 0.04 | 0.18 ± 0.01 | 0.19 ± 0.03 ab | 1.15 ± 0.02 b | 0.23 ± 0.02 | |
| 20 | 0.52 ± 0.02 | 0.16 ± 0.01 | 0.21 ± 0.01 a | 1.16 ± 0.02 b | 0.23 ± 0.01 | |
| A | ns | ** | ** | ** | ns | |
| B | ** | ** | ** | ** | ns | |
| A × B | ns | ns | ** | ** | ns | |
| Cultivar (A) | DBD Plasma Treatment (min; B) | DPPH (% Inhibition) | ABTS (% Inhibition) | FRAP (µmol AsAE/ mg Dry Extract) | AG (% Inhibition) |
|---|---|---|---|---|---|
| PG | 56.06 ± 6.38 a 1 | 35.60 ± 2.38 1 | 23.43 ± 1.62 a 1 | 58.49 ± 3.66 2 | |
| BH | 43.98 ± 6.76 b | 36.73 ± 5.56 | 21.51 ± 1.74 b | 60.70 ± 4.24 | |
| Untreated | 40.71 ± 5.80 c | 30.16 ± 2.27 c | 19.76 ± 1.15 b | 52.12 ± 6.35 b | |
| 5 | 50.65 ± 8.46 ab | 35.10 ± 1.34 b | 22.38 ± 1.70 a | 59.53 ± 2.56 ab | |
| 10 | 47.19 ± 8.09 b | 28.78 ± 4.53 a | 23.71 ± 1.29 a | 57.44 ± 3.29 ab | |
| 15 | 53.91 ± 2.30 a | 38.59 ± 1.86 a | 23.23 ± 1.51 a | 61.33 ± 1.03 a | |
| 20 | 55.64 ± 9.61 a | 37.96 ± 1.34 ab | 22.98 ± 1.26 a | 61.67 ± 1.70 a | |
| PG | Untreated | 45.87 ± 4.30 c–e | 31.95 ± 0.80 cd | 20.57 ± 0.72 | 56.77 ± m ab |
| 5 | 57.45 ± 2.22 ab | 35.07 ± 1.34 bc | 24.02 ± 0.57 | 58.33 ± m ab | |
| 10 | 54.54 ± 1.50 a–c | 35.37 ± 0.57 bc | 24.99 ± 0.19 | 57.27 ± 0.18 ab | |
| 15 | 54.19 ± 3.84 bc | 38.49 ± 1.68 ab | 23.54 ± 0.40 | 61.65 ± m ab | |
| 20 | 64.84 ± 4.74 a | 37.12 ± 0.53 bc | 24.04 ± 0.89 | 60.91 ± 1.81 ab | |
| BH | Untreated | 35.56 ± 3.18 e | 27.46 ± 0.65 d | 18.53 ± 0.23 | 50.59 ± 1.87 b |
| 5 | 40.45 ± 1.12 de | 35.14 ± 1.90 bc | 20.73 ± 0.08 | 60.27 ± 2.51 ab | |
| 10 | 39.83 ±5.64 de | 43.89 ± 3.87 a | 22.44 ± 0.26 | 60.51 ± 3.28 ab | |
| 15 | 53.63 ± 0.95 bc | 38.75 ± 3.39 ab | 22.92 ± 2.05 | 61.33 ± 0.61 ab | |
| 20 | 46.44 ± 0.61 cd | 39.21 ± 1.77 ab | 21.92 ± 0.39 | 62.08 ± 1.16 a | |
| A | ** | ns | ** | ns | |
| B | ** | ** | ** | ** | |
| A x B | ** | ** | ns | ** |
| Variable | PC1 (49.2%) | PC2 (17.8%) | Variable | PC1 (49.2%) | PC2 (17.8%) |
|---|---|---|---|---|---|
| Shoot length | 0.11 | 0.89 | chlorogenic acid | 0.46 | −0.66 |
| Fresh weight | 0.73 | −0.14 | coumaric acid | 0.46 | 0.02 |
| Dry weight | 0.40 | 0.18 | caffeic acid | 0.61 | −0.65 |
| Chlorophyll a | 0.02 | 0.54 | ferulic acid | 0.63 | 0.19 |
| Chlorophyll b | −0.73 | −0.19 | rutin | 0.71 | 0.45 |
| Total chlorophyll | 0.82 | 0.10 | DPPH | −0.06 | −0.45 |
| Carotenoids | 0.83 | 0.09 | ABTS | 0.84 | 0.09 |
| Total ascorbic acid | 0.82 | 0.04 | FRAP | 0.53 | −0.60 |
| Total phenolic content | 0.65 | 0.52 | antiglycation | 0.78 | −0.11 |
| Total flavonoid content | 0.41 | −0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ongrak, P.; Poolyarat, N.; Suksaengpanomrung, S.; Harakotr, B.; Jirakiattikul, Y.; Rithichai, P. Enhancing the Growth, Bioactive Compounds, and Antioxidant Activity of Kangkong (Ipomoea aquatica Forssk.) Microgreens Using Dielectric Barrier Discharge Plasma. Resources 2025, 14, 72. https://doi.org/10.3390/resources14050072
Ongrak P, Poolyarat N, Suksaengpanomrung S, Harakotr B, Jirakiattikul Y, Rithichai P. Enhancing the Growth, Bioactive Compounds, and Antioxidant Activity of Kangkong (Ipomoea aquatica Forssk.) Microgreens Using Dielectric Barrier Discharge Plasma. Resources. 2025; 14(5):72. https://doi.org/10.3390/resources14050072
Chicago/Turabian StyleOngrak, Prapasiri, Nopporn Poolyarat, Suebsak Suksaengpanomrung, Bhornchai Harakotr, Yaowapha Jirakiattikul, and Panumart Rithichai. 2025. "Enhancing the Growth, Bioactive Compounds, and Antioxidant Activity of Kangkong (Ipomoea aquatica Forssk.) Microgreens Using Dielectric Barrier Discharge Plasma" Resources 14, no. 5: 72. https://doi.org/10.3390/resources14050072
APA StyleOngrak, P., Poolyarat, N., Suksaengpanomrung, S., Harakotr, B., Jirakiattikul, Y., & Rithichai, P. (2025). Enhancing the Growth, Bioactive Compounds, and Antioxidant Activity of Kangkong (Ipomoea aquatica Forssk.) Microgreens Using Dielectric Barrier Discharge Plasma. Resources, 14(5), 72. https://doi.org/10.3390/resources14050072