Comparison of Growth, Yield, and Carbon Dioxide Emission After Cultivation of Five Edible Mushrooms
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyses of the Physicochemical Properties and Nutrient Content of Mushroom Substrate Before and After Cultivation
2.2. Substrate Preparation and Mushroom Cultivation
2.3. Study of Growth and Yield in Five Mushroom Species
2.4. Carbon Dioxide Emission of Five Mushroom Species
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analyses of the Physicochemical Properties and Nutrient Content of Mushroom Substrate Before and After Cultivation
3.2. Study of Growth and Yield in Five Mushroom Species
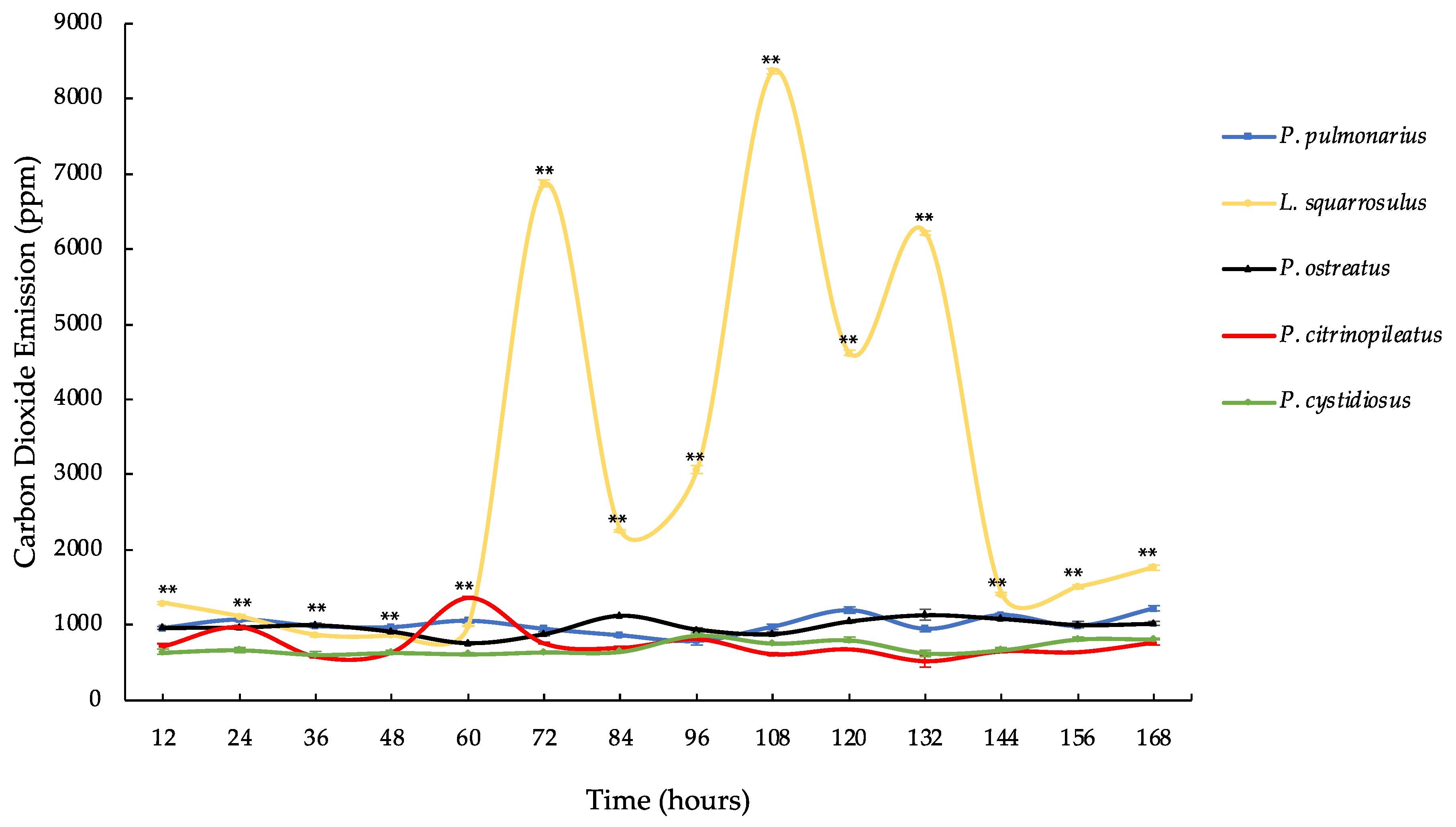
3.3. Carbon Dioxide Emission of Five Mushroom Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kongsomboonwet, S. Medicinal mushroom and health. Food J. 2011, 41, 361–366. [Google Scholar]
- Wattanasiritham, S.L. Medicinal mushroom polysaccharides. Food J. 2020, 50, 33–41. [Google Scholar]
- The Collection of Production and Marketing Data for Economic Mushrooms and Edible Wild Mushrooms in Thailand. Available online: https://waa.inter.nstda.or.th/stks/pub/2021/20210817-economic-mushroom-market.pdf (accessed on 10 October 2024).
- Mushroom and Mushroom Production. Available online: http://wb.yru.ac.th/xmlui/handle/yru/6381 (accessed on 10 October 2024).
- Thavivongse, S.; Buppachat, M. Grey Oyster Mushroom for Food Security Versus CO2 Emission. J. Environ. Res. Dev. 2013, 7, 1363–1368. [Google Scholar]
- Meilleur, M.-A.; Bastien, D.; Monfet, D. Modeling Mushrooms’ Carbon Dioxide Emission and Heat Exchange Rates for Synergistic Cultivation with Leafy Greens. Sustainability 2023, 15, 6740. [Google Scholar] [CrossRef]
- Pavlík, M.; Fleischer, P.; Fleischer, P., Jr.; Pavlík, M., Jr.; Šuleková, M. Evaluation of the Carbon Dioxide Production by Fungi under Different Growing Conditions. Curr. Microbiol. 2020, 77, 2374–2384. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Zhang, L.; Yang, X.; Li, Q.; Zhang, C.; Guo, L.; Yu, H.; Yu, H. Responses of the Mushroom Pleurotus ostreatus under Different CO2 Concentration by Comparative Proteomic Analyses. J. Fungi. 2022, 8, 652. [Google Scholar] [CrossRef]
- Zi-Piao, Y.E. A Review on Modeling of Responses of Photosynthesis to Light and CO2. Chin. J. Plant Ecol. 2010, 34, 727–740. [Google Scholar] [CrossRef]
- Jung, D.-H.; Son, J.-E. CO2 Utilization Strategy for Sustainable Cultivation of Mushrooms and Lettuces. Sustainability 2021, 13, 5434. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, L.; Yu, H.; Song, C.; Zhang, M.; Tan, Q.; Shang, X. CO2 Release from Lentinula edodes Strains under Different Cultivation Modes. Acta Edulis Fungi 2020, 36, 37–44. [Google Scholar] [CrossRef]
- Horwitz, W.; International, A. Official Methods of Analysis of AOAC International, 17th ed.; Current Through Revision; AOAC International: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Imtiaj, A.; Jayasinghe, C.; Lee, G.W.; Shim, M.J.; Rho, H.S.; Lee, H.S.; Hur, H.; Lee, W.M.; Lee, U.Y.; Lee, T.S. Vegetative Growth of Four Strains of Hericium erinaceus Collected from Different Habitats. Mycobiology 2008, 36, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Giménez, A.; Pardo-González, J.E. Evaluation of Casing Materials made from Spent Mushroom Substrateand Coconut Fibre Pith for Use in Production of Agaricus bisporus (Lange) Imbach. Span. J. Agric. Res. 2008, 6, 683–690. [Google Scholar] [CrossRef]
- Dissasa, G. Cultivation of Different Oyster Mushroom (Pleurotus species) on Coffee Waste and Determination of Their Relative Biological Efficiency and Pectinase Enzyme Production, Ethiopia. Int. J. Microbiol. 2022, 2022, 5219939. [Google Scholar] [CrossRef] [PubMed]
- Seephueak, P.; Preecha, C.; Seephueak, W. Effect of Nutrient in Palm Oil Sludge on Mycelium Growth of Auricularia polytricha (Mont.) Sacc. Khon Kaen Agric. J. 2016, 44 (Suppl. S1), 219–224. [Google Scholar]
- Tesfaw, A.; Tadesse, A.; Kiros, G. Optimization of Oyster (Pleurotus ostreatus) Mushroom Cultivation Using Locally Available Substrates and Materials in Debre Berhan, Ethiopia. J. App. Biol. 2015, 3, 015–020. [Google Scholar] [CrossRef]
- Han, J.; Sun, R.; Huang, C.; Xie, H.; Gao, X.; Yao, Q.; Yang, P.; Li, J.; Gong, Z. Effects of Different Carbon and Nitrogen Ratios on Yield, Nutritional Value, and Amino Acid Contents of Flammulina velutipes. Life 2024, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Juan, J.; Xiao, T.; Zhang, J.; Chen, H.; Song, X.; Chen, M.; Huang, J. The Physical Structure of Compost and C and N utilization During composting and Mushroom Growth in Agaricus bisporus Cultivation with Rice, Wheat, and Reed-Based Composts. Appl. Microbiol. Biotechnol. 2021, 105, 3811–3823. [Google Scholar] [CrossRef] [PubMed]
- Hultberg, M.; Oskarsson, C.; Bergstrand, K.-J.; Asp, H. Benefits and Drawbacks of Combined Plant and Mushroom Production in Substrate Based on Biogas Digestate and Peat. Environ. Technol. Innov. 2022, 28, 102740. [Google Scholar] [CrossRef]
- Ejigu, N.; Sitotaw, B.; Girmay, S.; Assaye, H. Evaluation of Oyster Mushroom (Pleurotus ostreatus) Production Using Water Hyacinth (Eichhornia crassipes) Biomass Supplemented with Agricultural Wastes. Int. J. Food Sci. 2022, 2022, 9289043. [Google Scholar] [CrossRef] [PubMed]
- Kitaya, Y.; Tani, A.; Kiyota, M.; Aiga, I. Plant Growth and Gas Balance in A Plant and Mushroom Cultivation System. Adv. Space Res. 1994, 14, 281–284. [Google Scholar] [CrossRef] [PubMed]

| Mushroom Species | Nutrient Content in Substrates | Physical—Chemical Content in Substrates | C/N Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | P (%) | K (%) | pH | EC (dS/m) | OC (%) | OM (%) | |||
| Before | 0.46 ± 0.07 | 0.0034 ± 0.00 | 0.21 ± 0.01 | 7.77 ± 0.10 | 2.113 ± 0.12 | 34.86 ± 0.33 | 60.10 ± 0.56 | 78.44 ± 2.95 | |
| After | P. pulmonarius | 0.29 ± 0.03 d 1/ | 0.0012 ± 0.00 | 0.22 ± 0.01 | 4.96 ± 0.04 c | 1.959 ± 0.40 c | 33.62 ± 0.82 a | 57.97 ± 1.42 a | 118.22 ± 0.87 a |
| L. squarrosulus | 0.40 ± 0.03 c | 0.0011 ± 0.00 | 0.29 ± 0.01 | 4.78 ± 0.04 d | 2.116 ± 0.25 b | 35.24 ± 1.26 a | 60.77 ± 2.17 a | 88.50 ± 2.24 b | |
| P. ostreatus | 0.46 ± 0.04 b | 0.0012 ± 0.00 | 0.30 ± 0.02 | 5.05 ± 0.13 c | 2.469 ± 0.18 a | 35.69 ± 1.03 a | 61.53 ± 1.78 a | 77.26 ± 1.43 c | |
| P. citrinopileatus | 0.48 ± 0.02 b | 0.0012 ± 0.00 | 0.21 ± 0.03 | 5.20 ± 0.10 b | 1.765 ± 0.20 d | 31.44 ± 2.39 b | 54.21 ± 4.12 b | 65.93 ± 1.77 d | |
| P. cystidiosus | 0.91 ± 0.06 a | 0.0011 ± 0.00 | 0.25 ± 0.01 | 5.39 ± 0.17 a | 2.004 ± 0.09 c | 31.40 ± 1.42 b | 54.14 ± 2.45 b | 34.66 ± 0.55 e | |
| F-test | ** | ns | ** | ** | ** | ** | ** | ** | |
| C.V. (%) | 7.42 | 4.15 | 3.05 | 1.96 | 2.63 | 4.44 | 4.44 | 1.95 | |
| Mushroom Species | Number of Caps | Diameter of Cap (cm) | Fresh Weight (g) | Dry Weight (g) | Biological Efficiency (%) |
|---|---|---|---|---|---|
| P. pulmonarius | 10.23 ± 0.43 c 1/ | 4.88 ± 0.18 a | 51.53 ± 0.11 d | 9.65 ± 0.30 b | 19.89 ± 0.82 b |
| L. squarrosulus | 3.67 ± 0.48 d | 2.28 ± 0.81 e | 24.90 ± 0.81 e | 3.80 ± 0.46 d | 9.90 ± 0.87 d |
| P. ostreatus | 27.00 ± 0.59 b | 3.84 ± 0.12 c | 65.71 ± 0.82 a | 11.18 ± 0.77 a | 28.22 ± 0.90 a |
| P. citrinopileatus | 60.17 ± 0.79 a | 2.71 ± 0.30 d | 53.37 ± 0.93 c | 7.00 ± 0.57 c | 19.41 ± 0.60 b |
| P. cystidiosus | 3.92 ± 0.28 d | 4.30 ± 0.10 b | 55.95 ± 0.82 b | 7.11 ± 0.49 c | 17.80 ± 0.34 c |
| F-test | ** | ** | ** | ** | ** |
| C.V. (%) | 2.53 | 8.81 | 1.52 | 7.00 | 4.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chutimanukul, P.; Phetkaew, P.; Sukdee, S.; Thepsilvisut, O.; Ehara, H. Comparison of Growth, Yield, and Carbon Dioxide Emission After Cultivation of Five Edible Mushrooms. Resources 2025, 14, 55. https://doi.org/10.3390/resources14040055
Chutimanukul P, Phetkaew P, Sukdee S, Thepsilvisut O, Ehara H. Comparison of Growth, Yield, and Carbon Dioxide Emission After Cultivation of Five Edible Mushrooms. Resources. 2025; 14(4):55. https://doi.org/10.3390/resources14040055
Chicago/Turabian StyleChutimanukul, Preuk, Pawarisa Phetkaew, Siripong Sukdee, Ornprapa Thepsilvisut, and Hiroshi Ehara. 2025. "Comparison of Growth, Yield, and Carbon Dioxide Emission After Cultivation of Five Edible Mushrooms" Resources 14, no. 4: 55. https://doi.org/10.3390/resources14040055
APA StyleChutimanukul, P., Phetkaew, P., Sukdee, S., Thepsilvisut, O., & Ehara, H. (2025). Comparison of Growth, Yield, and Carbon Dioxide Emission After Cultivation of Five Edible Mushrooms. Resources, 14(4), 55. https://doi.org/10.3390/resources14040055









