1. Introduction
In 2008, with the “Raw Materials Initiative”, the European Union (EU) began addressing the future challenges of sustainable raw materials (RMs) supply [
1]. Through this initiative, the EU aims to implement a strategy to ensure access to RMs from international markets under the same conditions as other industrial competitors, set the right framework conditions within the EU to foster a sustainable supply of RMs from EU sources, boost overall resource efficiency, and promote recycling to reduce the EU’s consumption of primary RMs and decrease the relative dependence on imports. Following this initiative, the EU introduced the concept of Critical Raw Materials (CRMs), a list of RMs at high risk of supply shortages with significant economic impact compared with most other RMs [
1]. Since 2011, the EU has published the CRMs list every three years, updated based on changes in supply risks and the economic importance of candidate RMs [
2,
3,
4,
5,
6]. Starting from May 2024, the Critical Raw Materials Act (CRMA) came into force within the European Union, with specific objectives and strategies aimed at reducing the EU’s dependency on CRMs and promoting sustainable and uniform competitive conditions for CRMs value chains within the EU [
7]. The CRMA has also established benchmarks for the CRMs supply chain and for diversifying the EU’s supplies, with at least 10% of the EU’s annual consumption for extraction, at least 40% of the EU’s annual consumption for processing, at least 25% of the EU’s annual consumption for recycling, and no more than 65% of the EU’s annual consumption from a single third country [
7]. In terms of recycling, Article 27 of the CRMA is dedicated to the recovery of CRMs from EW, requiring member states to adopt measures by November 2027 to promote CRMs recovery from EW [
7]. In this context, various authors worldwide are investigating, for example, the potential of using mining tailings as an alternative source for CRMs supply (e.g., [
8,
9,
10,
11,
12,
13]). In Italy, due to its long history of mining, mining tailing storage sites are widespread [
14], and some authors have begun investigating the extraction potential of this waste [
15,
16,
17,
18,
19].
In recent years, in addition to mining tailings, some researchers in Italy have also begun to focus on EW derived from the extraction of silicate-rich rocks for ornamental purposes to investigate their extraction potential in terms of CRMs [
20,
21,
22]. Ornamental rocks such as gneiss and granite can host significant amounts of rare-earth element (REE)-bearing minerals (e.g., monazite and allanite), making them potential resources for the recovery and extraction of REEs. [
20,
21]. In this context, the region of Sardinia (Italy) has large quantities of ornamental granite extractive waste (EW), having formerly been one of the world leaders in granite extraction [
23]. Therefore, investigating the extraction potential of these materials could make an important contribution to the objectives set by the CRMA regarding CRMs recovery from EW.
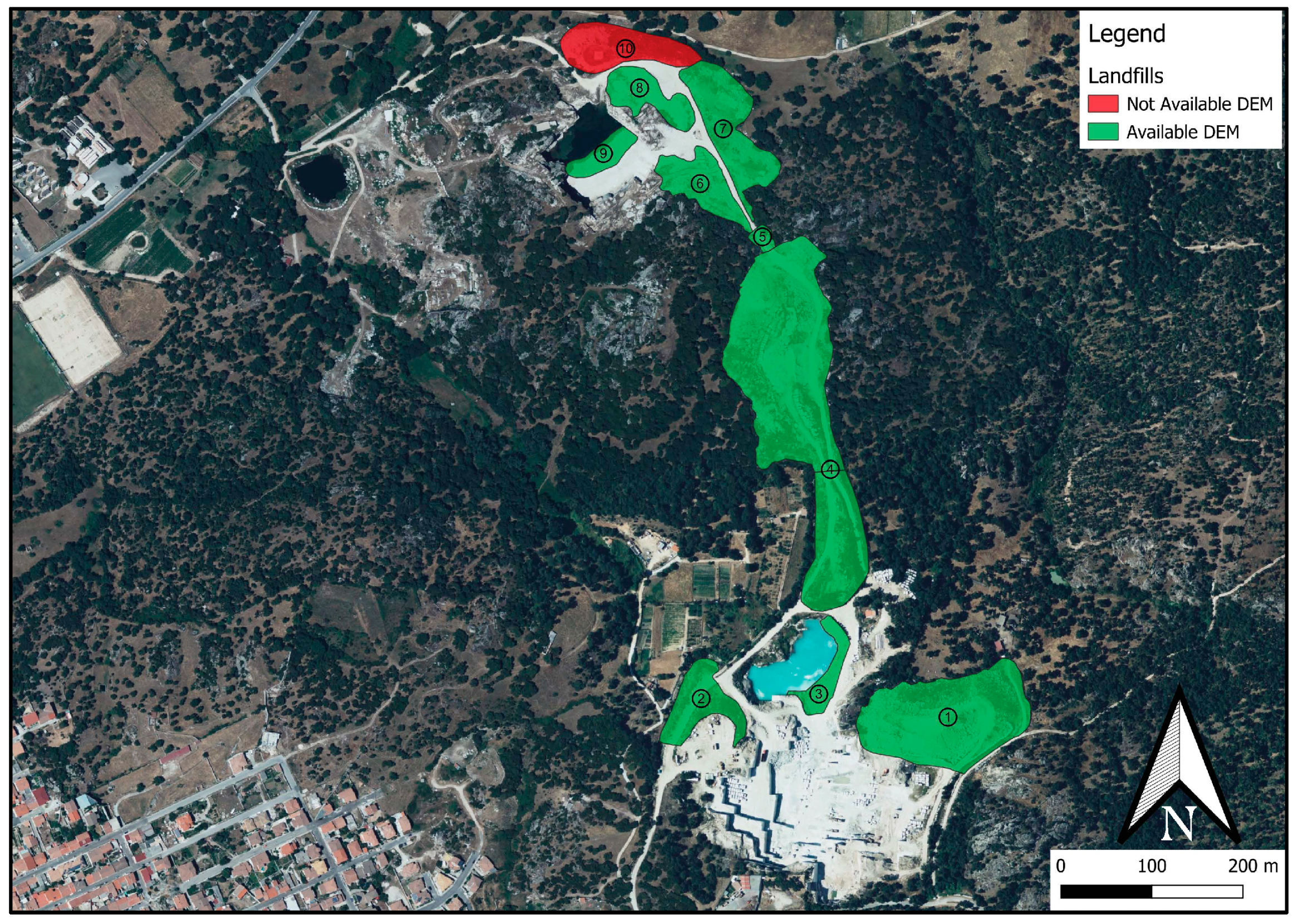
The present research represents a case study of a granite quarry within the municipality of Buddusò, located in one of the most productive areas of the region for granite extraction—the Buddusò-Alà dei Sardi extractive district (northern Sardinia). The rocks of this area belong to the high-K calc-alkaline plutonic association, related to the Corsica–Sardinian Batholith, which formed during the post-collisional phase of the Variscan orogeny [
24,
25,
26]. More specifically, they belong to the so-called Buddusò Pluton, which comprises tonalites, granodiorites, and monzogranites with widespread microgranular mafic enclaves [
25,
27,
28]. These rocks primarily consist of quartz, K feldspar, and plagioclase, with smaller amounts of biotite and hornblende. They are further characterized by accessory minerals including zircon, magnetite, ilmenite, titanite, apatite, rutile, and allanite [
29]. Rocks that constitute the EW from the investigated quarry are classified as monzogranites belonging to the calc-alkaline series, and they are generally slightly metaluminous [
22].
Previous studies on this quarry have already highlighted the potential of granite EW as a source of RMs for the ceramics and glass industries [
22]. The current study was conducted to assess the efficiency of the application of gravity and magnetic separation methods on granite EW in order to concentrate CRMs. The work first involves estimating the quantity of granite EW stored within the site and conducting SEM-EDS measurements and microanalysis on the granite EW to identify any CRMs-bearing minerals. Finally, laboratory tests using gravity and magnetic separation processes are performed on the granite EW to evaluate the possibility of obtaining different CRM-rich fractions.
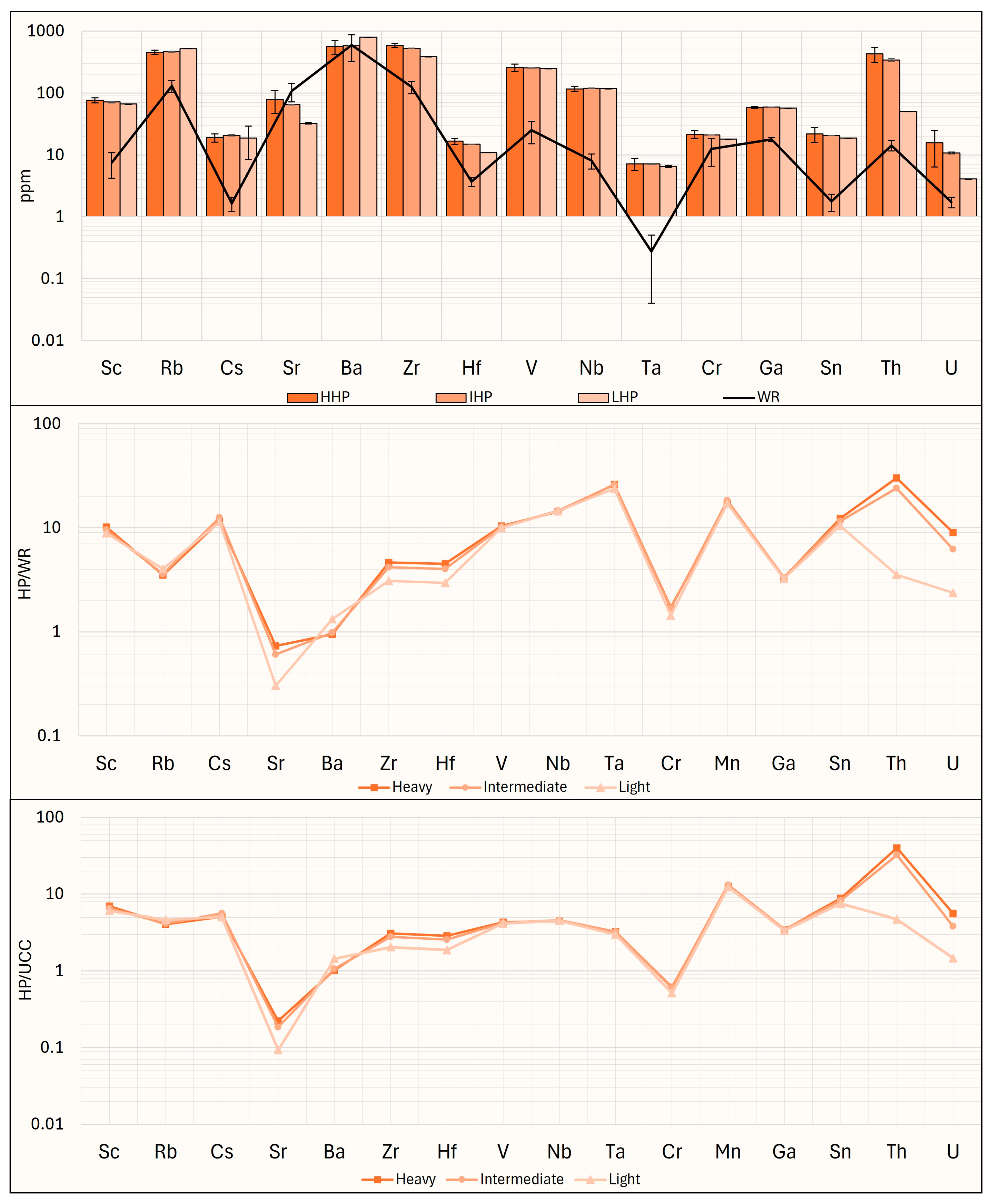
4. Potential CRMs Recovery from the Granite EW
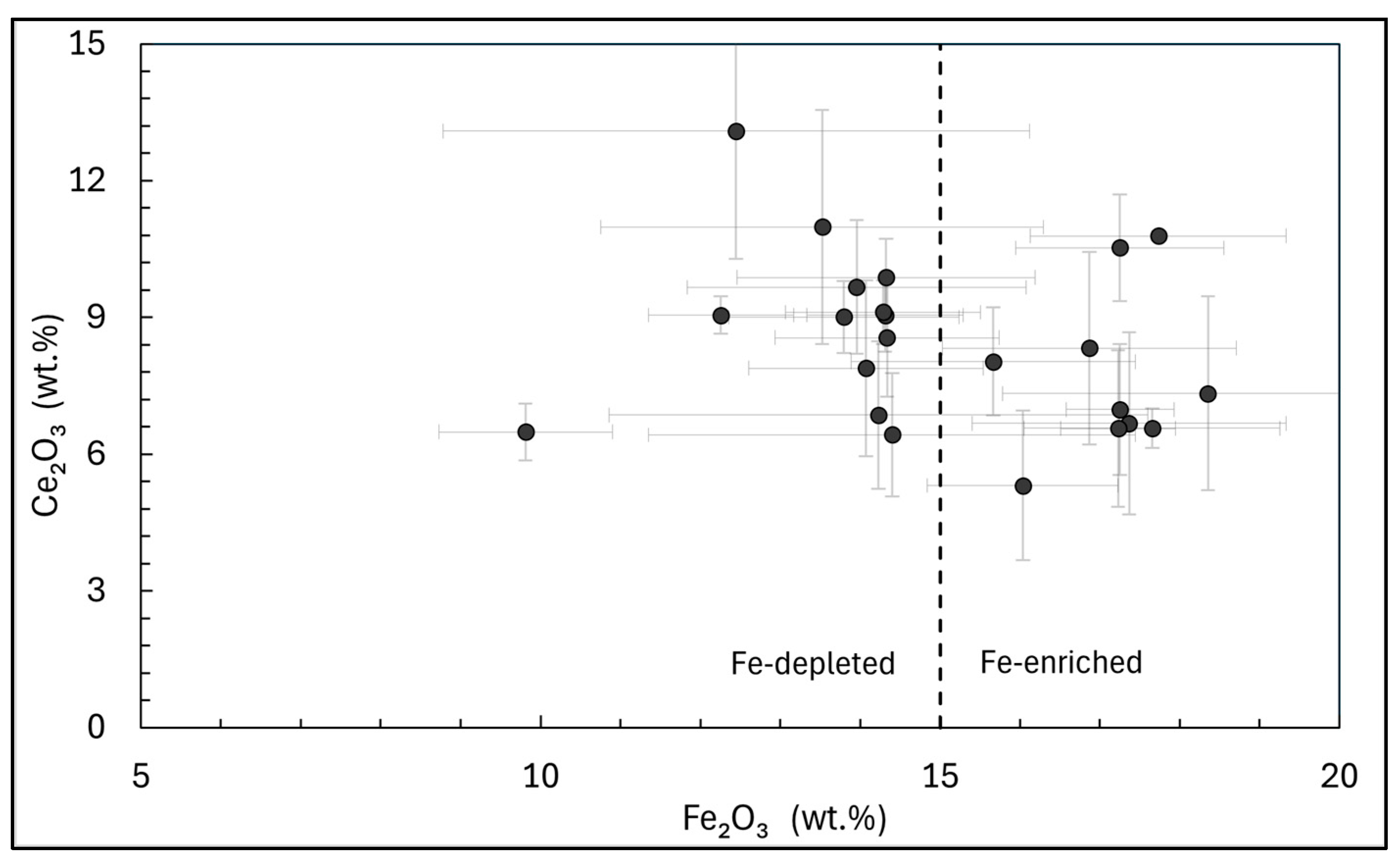
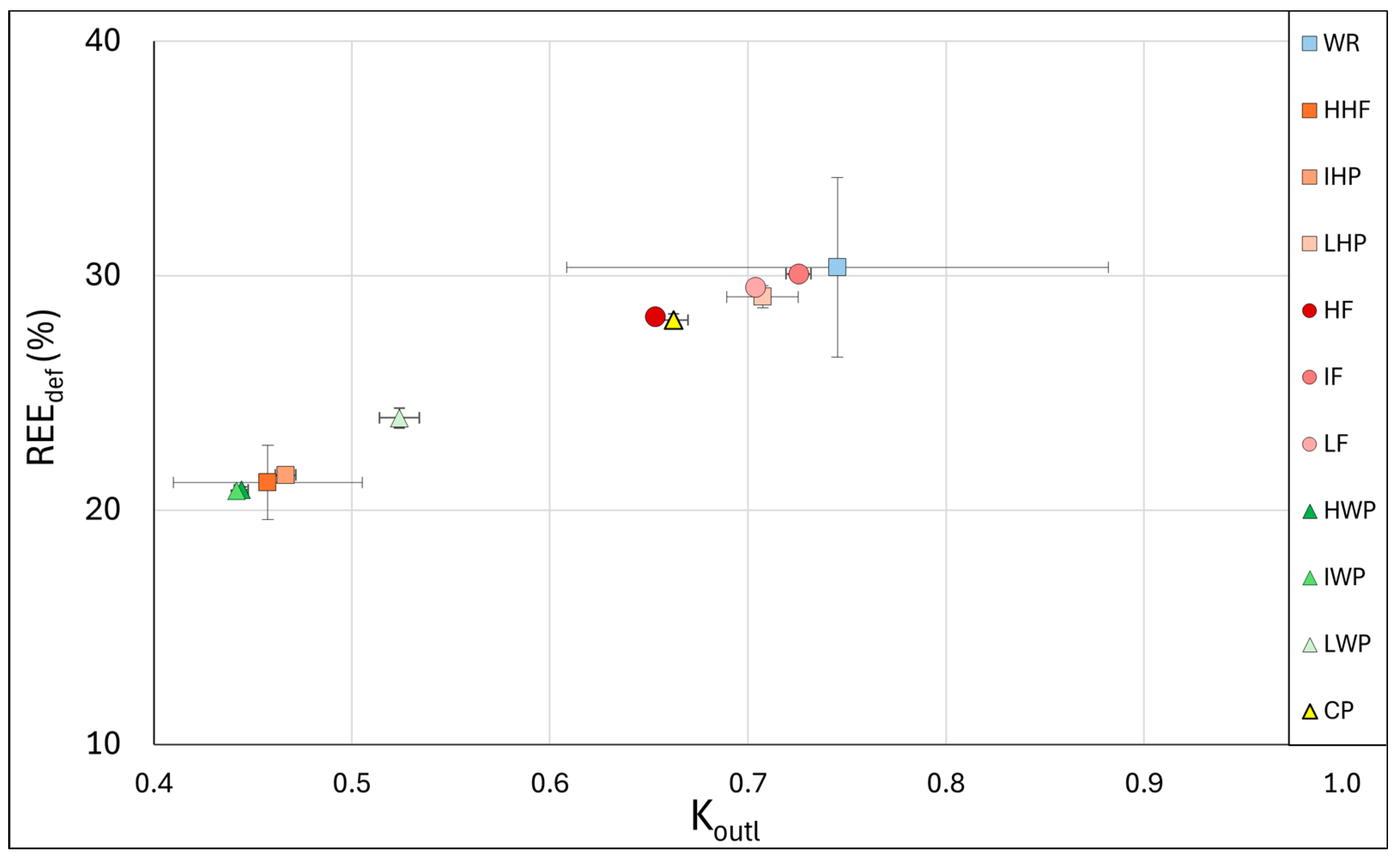
To perform a preliminary evaluation of the materials studied, the outlook coefficient (K
outl) proposed by Seredin (2010) [
58] is calculated. This coefficient is based on the concept that a deposit is more attractive if it has a higher content of critical REEs (Nd, Tb, Dy, Y, Eu, Er) relative to non-critical and excess REEs (La, Pr, Sm, Gd, Ce, Ho, Tm, Yb, Lu). The formula for calculating K
outl is as follows:
Generally, EW materials with a K
outl above 0.7 and a critical REEs content (REE
def) exceeding 30% of the total can be considered to have high industrial recovery potential [
58,
59]. In this study (
Figure 15), the only material with both K
outl and REE
def values above these thresholds is WR. Meanwhile, the LHP, the IF, and the LF have K
outl values above 0.7 but REE
def below 30%. All other fractions have values below these thresholds. Although the outlook coefficients of the investigated fractions are relatively low, it is important to note that all fractions have a fairly high content of REEs. Given that the generally accepted minimum industrial grade for the recovery of REEs is 300 ppm [
21], the fractions obtained through this process could still be promising for potential REEs recovery. It should also be noted that some of the obtained fractions, such as the highly and weakly paramagnetic ones, are characterized by Sc concentrations exceeding 60 ppm, the minimum threshold at which magmatic rocks can be considered as Sc deposits [
60]. Additionally, some fractions, such as the HF, the IF, the LF, the HWP, and the IWP, include Ga concentrations exceeding 30 ppm, representing the minimum industrial requirement [
61], while the highly paramagnetic fractions contain Ga concentrations above 50 ppm, which is the average Ga content in bauxites and zinc deposits from which this metal is extracted in different international localities [
62].
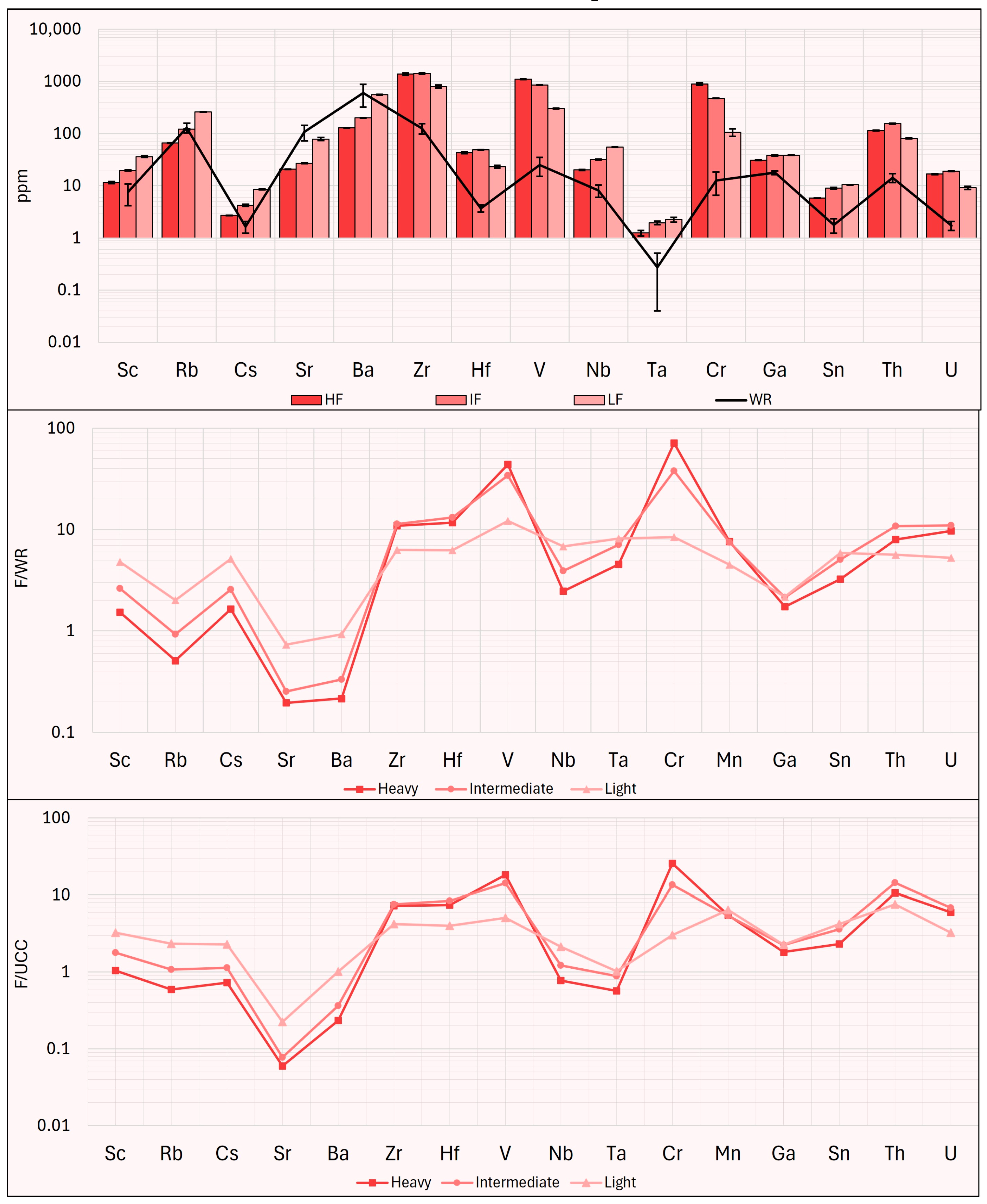
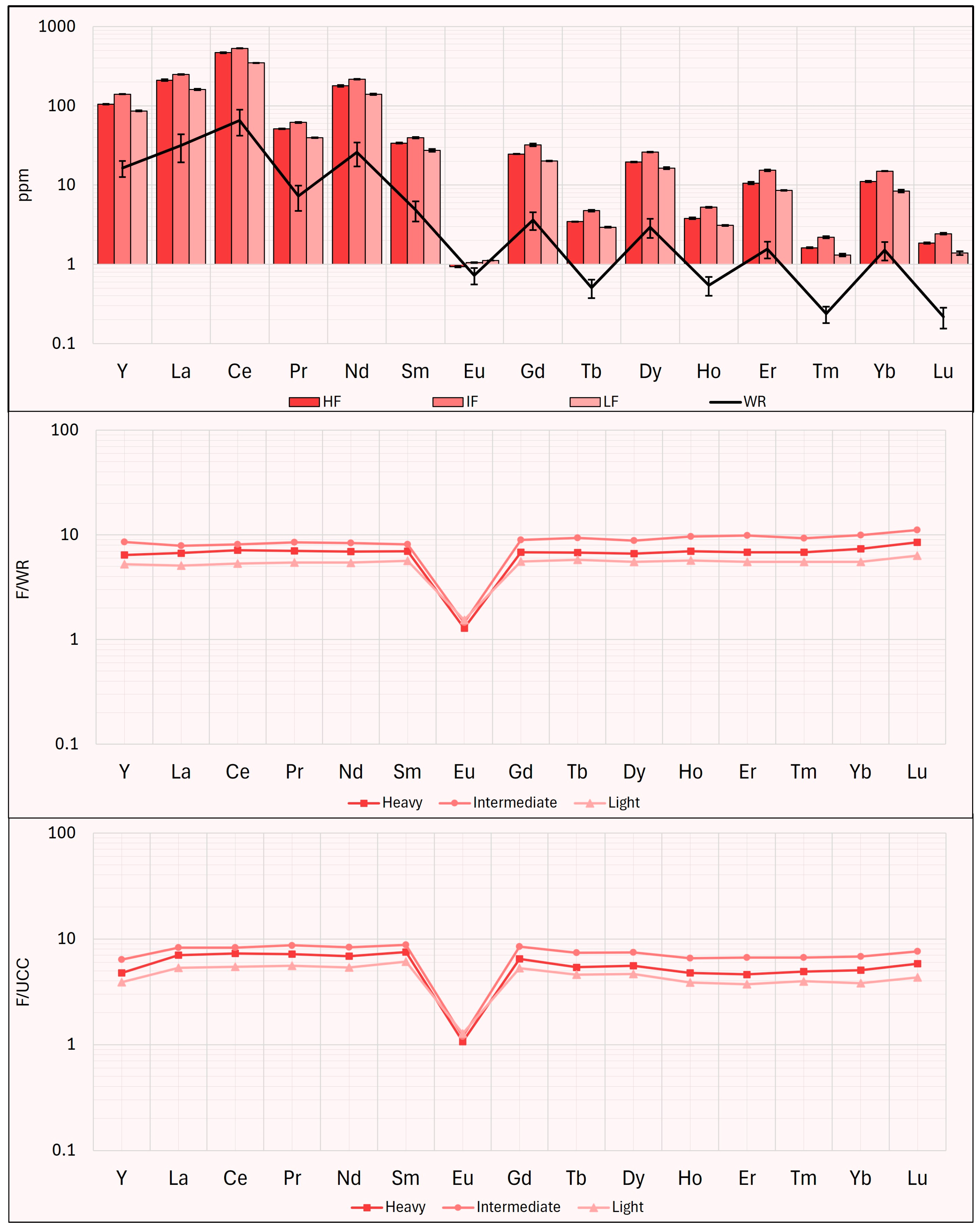
Taking into consideration the method proposed by Zhao et al. [
21], the potential market value of the CRMs content in the products obtained from this study is calculated. In the work of Zhao et al., this calculation is applied solely to REEs [
21], while in this study, it is extended to include other CRMs for which concentration data were available. This calculation assumes that the potential market value of each sample is the total market price (P
total) of the selected CRMs recovered from the disposal of 1000 tons of EW. The calculation equation is as follows:
where K
CRM is the recovery rate of each REEs in the waste; following [
21], for the convenience of calculation, the recovery rate was assumed to be 100%. C
w is the concentration of single CRM in the examined samples (ppm), ∑K
crm c
w is the recoverable amount of CRMs per kg of EW; P
CRM is the average market price (EUR/kg) in the form of metal (except Gd, Ho, Er, V, Nb in oxide form) during the six months before 20 October 2024. CRMs prices are reported in
Table A1—
Appendix A.
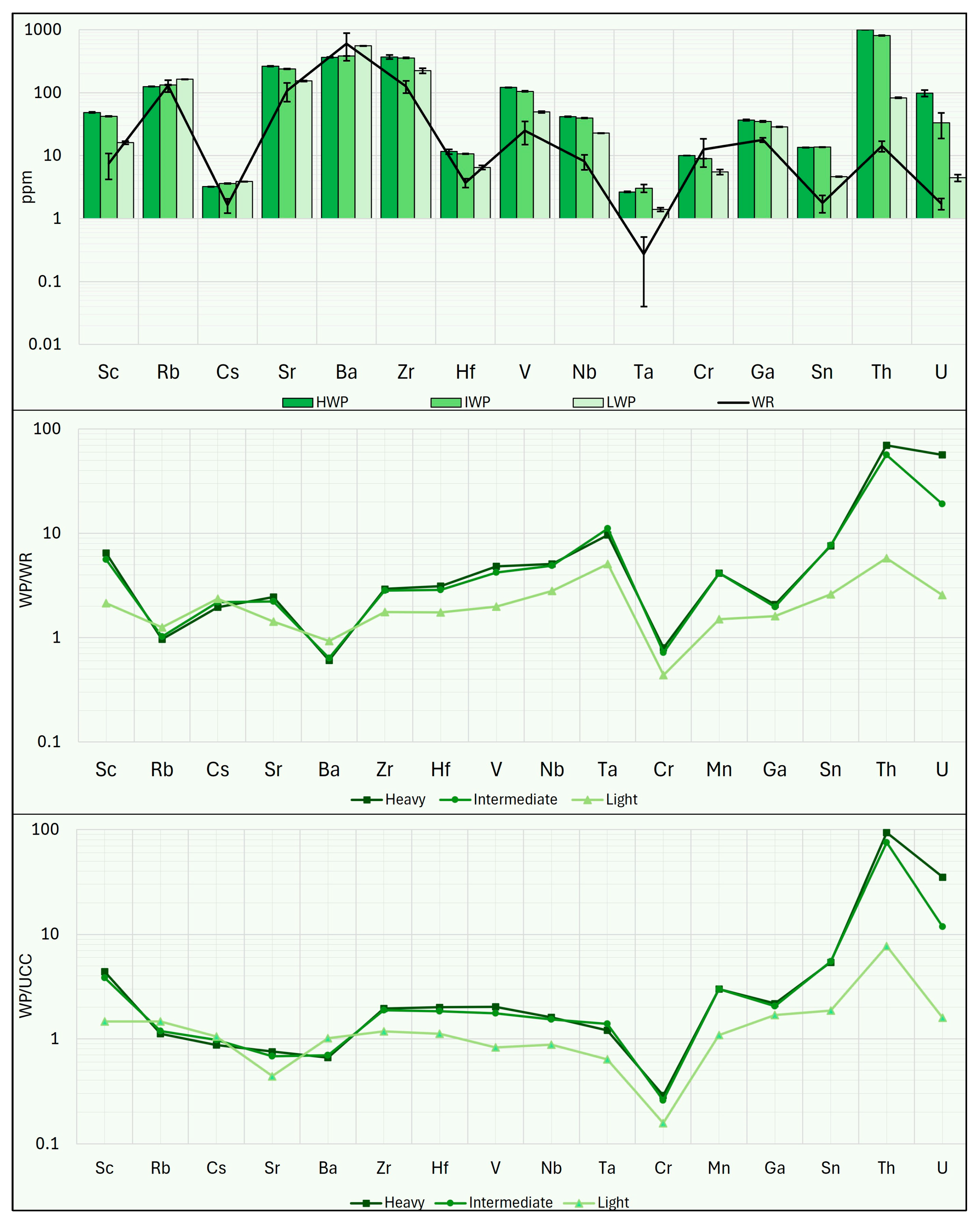
As reported in
Figure 16, the results of this calculation show that the HWP, the IWP, the HHP, and the IHP fractions have high market value (EUR >30 × 10
4); lower values are found in the LHP fraction (approximately EUR 23 × 10
4), while the IF and the LF fractions have market values exceeding EUR 10 × 10
4. All other fractions have market values below EUR 10 × 10
4, with the CP displaying the lowest market value (approximately EUR 4 × 10
4).
Indeed, all these values refer to fractions that were processed in order to concentrate allanite from the granite EW. Referring to the work by Vaccaro et al. [
22], it is found that the entire diamagnetic fraction obtained through this process holds significant potential for use in the ceramics and glass industries. Thus, if the granite EW is processed exclusively to obtain the quartz and feldspar fractions (excluding gravity separation and various magnetic separation steps), it could be reasonable to forego these concentration processes and instead perform single separation. In this scenario, reversing the purpose of this study, the magnetic material would become the byproduct of this process. Assuming this, the theoretical concentration of CRMs contained in the magnetic material—obtained without the various separation steps—is calculated using the following formula:
where [CRM]
V is the virtual concentration of the element considered (ppm), [CRM]
i is the concentration of the element in fraction i, and X
i is the percentage abundance of fraction i. The percentage yields of the fractions obtained through the processing of granite waste are presented in
Table A2 in
Appendix A; the theoretical composition is reported in
Table A3 in
Appendix A.
Based on this theoretical composition, the market value of the magnetic fraction was then calculated using Equation (2). The results of this calculation indicate that this process could produce a magnetic fraction with a CRMs content of 2155 kg per 1000 tons, with a relatively high market value amounting to EUR 21 × 104.
Based on the estimated quantities of granite waste and the percentage yields obtained through the processing, the total stock quantity of each fraction is estimated. This calculation is performed using two scenarios: the most favourable scenario is calculated using the maximum yield values obtained for each fraction and a pile porosity of 5%; the least favourable scenario is calculated using the minimum yield values for each fraction and a pile porosity of 30%. Based on this, the total market value (MV
total) for each fraction is then calculated using the following formula [
21]:
where
is the total stock quantity of material with fraction I, and
is the market value of CRMs within fraction i.
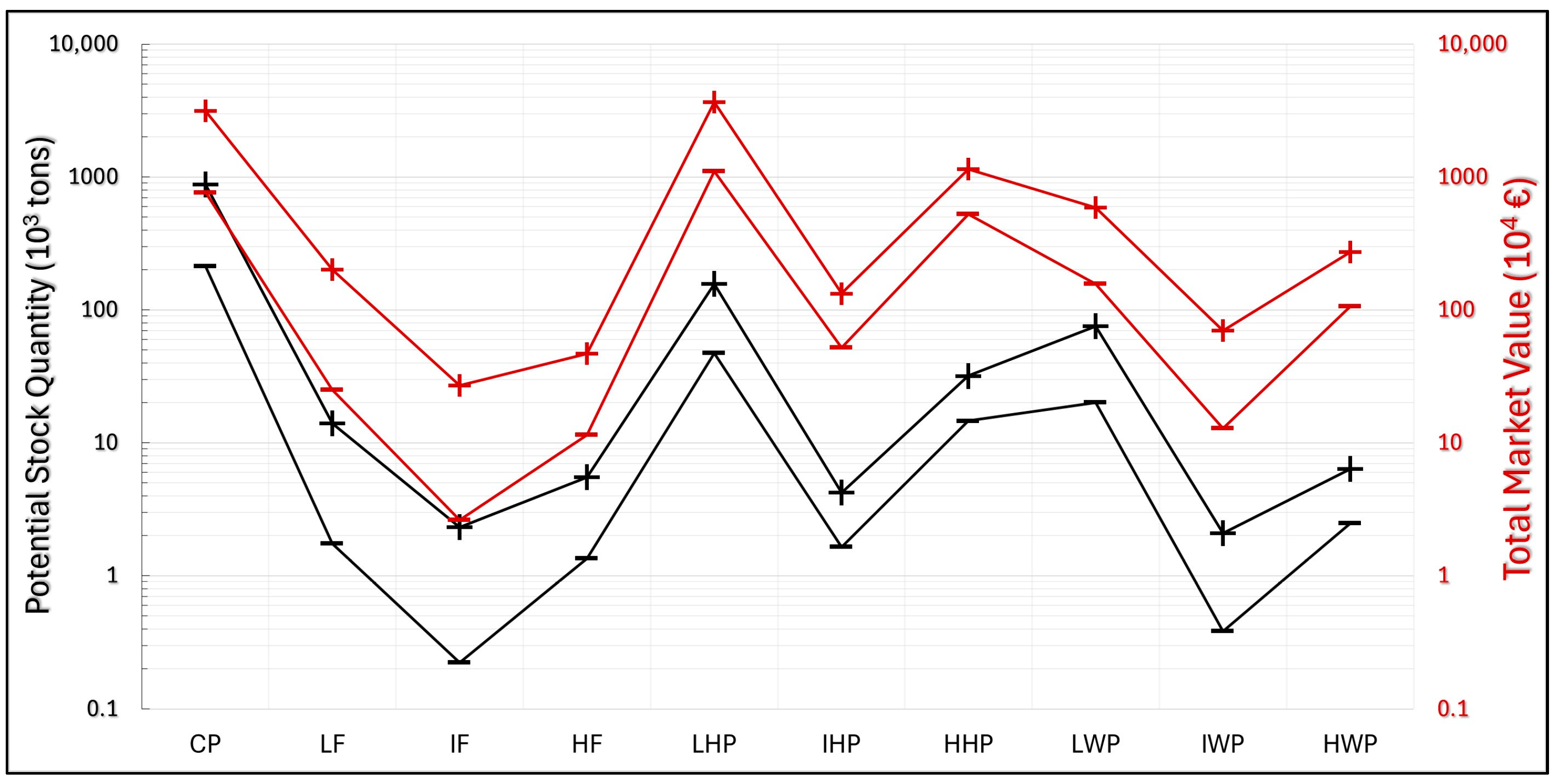
The results of these calculations (
Figure 17) reveal that the CP, representing the most abundant fraction (213–877 × 10
3 tons), holds a very high total market value (EUR 766–3157 × 10
4). Overall, the fraction with the highest MV
total is the LHP, in the range of EUR 1104–3668 × 10
4, and this is also the second most abundant fraction (47–157 × 10
3 tons). The HHP fraction, despite having a lower potential stock quantity (15–32 × 10
3 tons) than the LHP, presents a high MV
total (EUR 527–1148 × 10
4).
Among the weakly paramagnetic fractions, IWP and HWP emerge as those with the highest absolute REEs content, but they are also characterized by lower stock quantities (IWP between 0.4 and 2.1 × 103 tons and HWP between 2 and 6 × 103 tons). Despite these low material quantities, the MVtotal values are relatively high, especially in HWP (EUR 106–272 × 104).
The ferromagnetic fractions all have stock quantities below 20 × 103 tons. The LF fraction, being the most abundant (2–14 × 103 tons), also has the highest MVtotal among them (EUR 25–202 × 104).
In this scenario, under the assumption that the processing of granite waste is limited to a single high-intensity magnetic separation step, bypassing gravity separation and focusing solely on isolating quartz and feldspar from the other fractions, the MVtotal of the resulting hypothetical magnetic fraction is calculated. For this calculation, an average yield value is used for the fraction obtained in the separation tests; thus, the best and worst scenarios refer only to the porosity of the EW piles. The results of the calculations suggest that this virtual fraction could range from 158 to 215 × 103 tons, with an MVtotal varying in the range of EUR 3367–4569 × 104.
Assuming that all the CRMs investigated in this study are recovered from EW granite, the market value could range from a minimum of EUR 2763 × 104 to a maximum of EUR 9312 × 104.
This work does not explore potential steps beyond those previously shown. The recovery of CRMs from granite EW represents a significant challenge. As previously noted, current knowledge on REEs’ beneficiation from allanite is quite limited. It will therefore be essential to conduct focused studies to identify possible pathways for the beneficiation of REEs and CRM recovery, in general, from the obtained materials. Furthermore, the results presented in this study refer to laboratory-scale investigations, whereas data from experiments conducted at a semi-industrial scale will be necessary. In addition to other challenges, the high quantities of U and especially Th in the obtained materials could pose significant obstacles in subsequent beneficiation steps for the recovery of CRMs. Future research will need to address this factor carefully to optimize processing methods and mitigate potential complications associated with elevated Th and U concentrations. On the other hand, in recent years, there has been growing interest in developing thorium-based nuclear energy. Although there are currently no commercial-scale plants, there is potential for this to change in the future [
63,
64].
Despite all these limitations, the obtained results are promising, especially at a time when knowledge of potential alternative sources of CRMs is crucial. Furthermore, with the enforcement of the CRMA, it is likely that EU investments in research in this field will increase, encouraging companies and research institutions to expand their understanding across all aspects investigated in this work.
The present case study focuses on a single quarry, while in the Buddusò area alone, there are numerous granite quarries in similar conditions, containing large quantities of EW. In this context, the creation of a granite waste processing hub in the Buddusò area could hold significant economic potential. Therefore, it would be advisable to extend studies to other quarries in the area, both in terms of volume estimation and material characterization, to gather information that could support the assessment of this opportunity.
5. Conclusions
This study highlights the significant quantities of granite waste in a quarry, with a notable difference between the best-case (2.56 × 106 tons) and worst-case (1.88 × 106 tons) scenarios. However, the results are limited by the heterogeneity of the granite waste and the presence of vegetation, as well as the use of outdated surveys from 2013. Updated studies are needed for more accurate volume estimates.
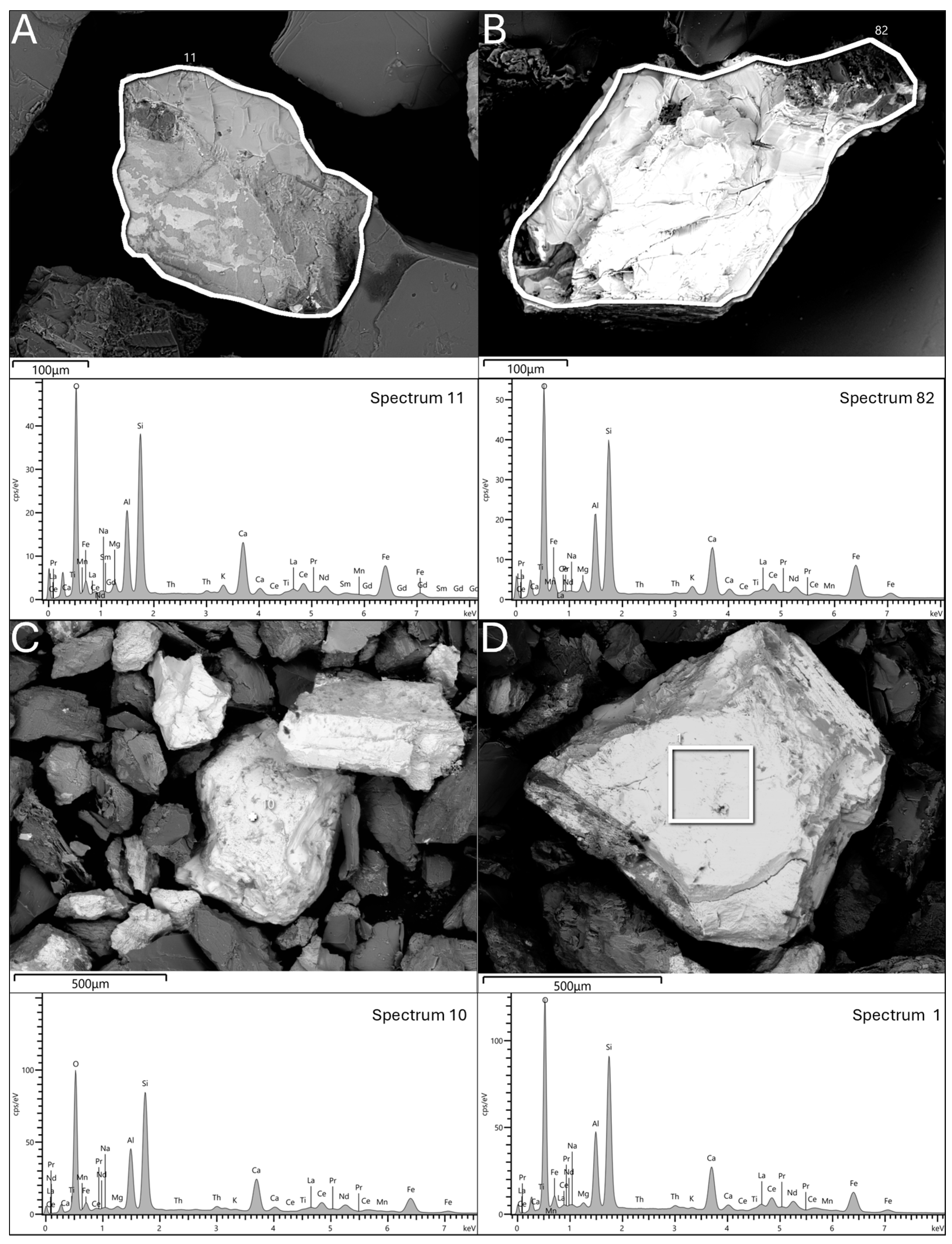
The presence of allanite, an REE-bearing epidote, in the granite EW, is confirmed by the SEM-EDS investigations. Allanite includes high concentrations of LREEs (>16 wt.%) but remains underutilized for REEs extraction due to the historical preference for other minerals. As demand for CRMs rises, research into allanite could become more relevant, supporting the EU’s CRMA objectives.
The gravity and magnetic separation tests demonstrate the potential for enriching fractions with CRMs, especially LREEs, from paramagnetic fractions. However, the maximum particle size of 850 µm should be reduced to improve separation efficiency, as some fractions contained polymineralic grains. Despite low outlook coefficients, the REEs, Sc, and Ga concentrations in these fractions suggest promising recovery potential. If all the CRMs were to be recovered, the market value could range from EUR 2.76 × 104 to EUR 9.31 × 104. The crushing powders, though untested, may also have high market value. Additionally, alternative approaches, such as single magnetic separation for diamagnetic quartz–feldspar concentrates, could yield valuable byproducts.
Although CRMs recovery presents challenges, particularly due to limited knowledge of beneficiation techniques and potential radiogenic elements, it remains a viable goal. Further studies on granite waste in Buddusò and the surrounding areas are needed. Establishing a treatment hub for granite waste could be a key resource for the region and to support the EU’s needs.