Impact of Hop Residue Reuse on the Chemical and Sensory Properties of Craft Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Craft Beer Brewing
2.3. Repelletizing of Dry-Hop Residues
2.4. Chemical Characterization
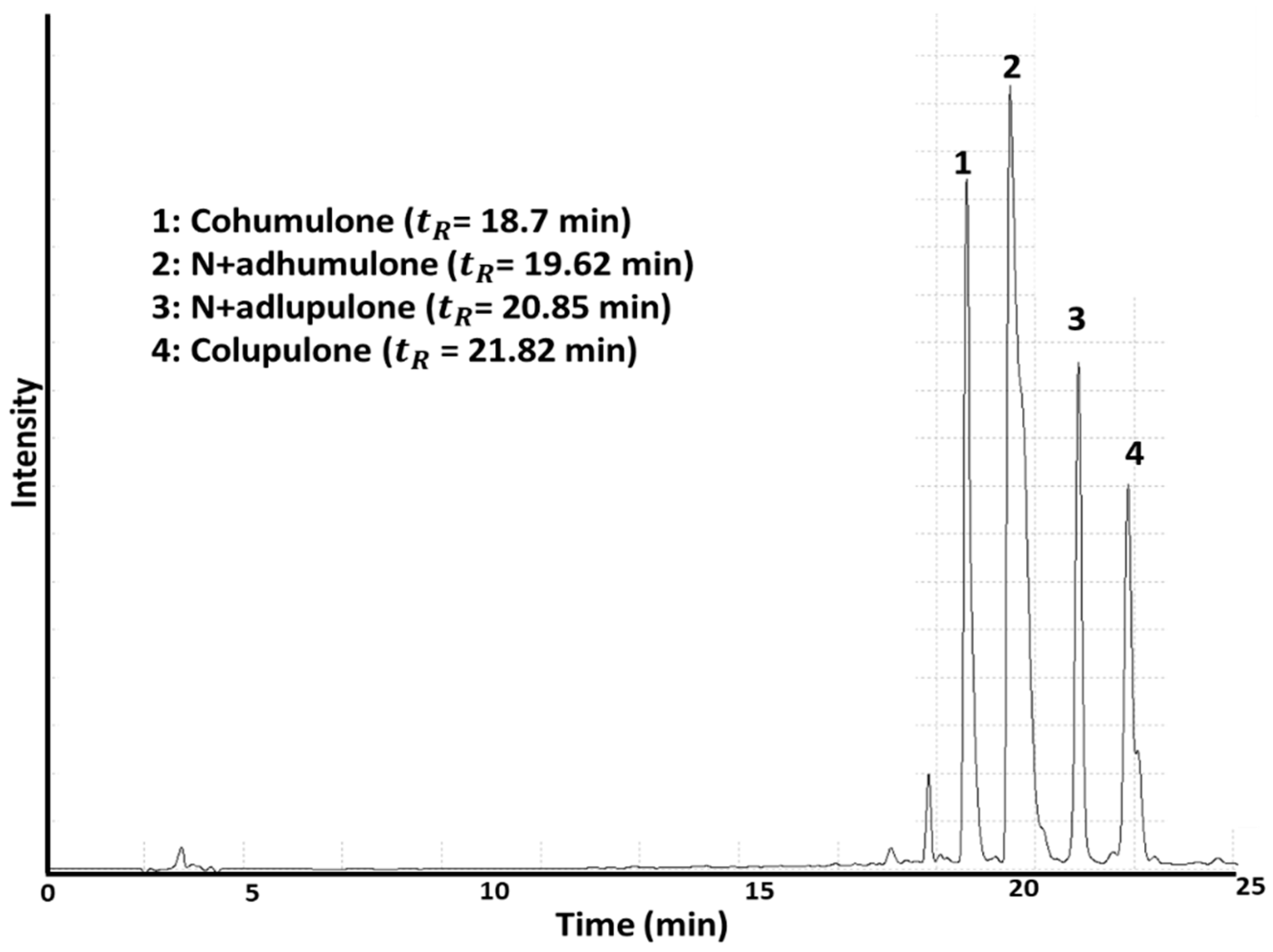
2.5. Determination of α- and β-Acids by HPLC
2.6. Determination of Total Phenolic Compounds
2.7. Antioxidant Capacity
2.8. Determination of International Bitterness Units (IBUs)
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Hop Residues
3.2. Total Phenolic Compounds and Antioxidant Capacity
3.3. IBU Values in Craft Beers and Content of α- and β-Total Acids in Hop Residues
3.4. Sensory Attributes Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marrucci, L.; Daddi, T.; Iraldo, F. Identifying the most sustainable beer packaging through a Life Cycle Assessment. Sci. Total Environ. 2024, 948, 174941. [Google Scholar] [CrossRef]
- Mosher, M.; Trantham, K. Brewing Science: A Multidisciplinary Approach; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Murray, D.W.; O’Neill, M.A. Craft beer: Penetrating a niche market. Br. Food J. 2012, 114, 899–909. [Google Scholar] [CrossRef]
- Salanță, L.C.; Fărcaş, A.C.; Borșa, A.; Pop, C.R. Current strategies for the management of valuable compounds from hops waste for a circular economy. Food Chem. X 2023, 19, 100876. [Google Scholar] [CrossRef]
- Baiano, A. Craft beer: An overview. Compr. Rev. Food Sci. Food Saf. 2020, 20, 1829–1856. [Google Scholar] [CrossRef] [PubMed]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and characteristics of craft beer production processes. Food Biosci. 2021, 45, 101495. [Google Scholar] [CrossRef]
- Huige, N. Brewery by-products and effluents. In Handbook of Brewing, 2nd ed.; Priest, F.G., Stewart, G.G., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 656–707. [Google Scholar]
- Kerby, C.; Vriesekoop, F. An Overview of the Utilisation of Brewery By-Products as Generated by British Craft Breweries. Beverages 2017, 3, 24. [Google Scholar] [CrossRef]
- Dos Santos Mathias, T.R.; De Mello Pedro, P.M.; Eliana, F.C.S. Solid wastes in brewing process: A review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Duarte, L.M.; Aredes, R.S.; Amorim, T.L.; De Carvalho Marques, F.F.; De Oliveira, M.A.L. Determination of α- and β-acids in hops by liquid chromatography or electromigration techniques: A critical review. Food Chem. 2022, 397, 133671. [Google Scholar] [CrossRef] [PubMed]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus-a story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Cvijetić, I.; Bigović, M.; Ristivojević, P.; Vitorović-Todorović, M.; Zloh, M.; Milojković-Opsenica, D. DFT study of the radical scavenging activity of isoxanthohumol, humulones (α-acids), and iso-α-acids from beer. Struct. Chem. 2021, 32, 2051–2059. [Google Scholar] [CrossRef]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial Properties of Spent Hops Extracts, Flavonoids Isolated Therefrom, and Their Derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef]
- Anderson, H.E.; Santos, I.C.; Hildenbrand, Z.L.; Schug, K.A. A review of the analytical methods used for beer ingredient and finished product analysis and quality control. Anal. Chim. Acta 2019, 1085, 1–20. [Google Scholar] [CrossRef]
- Rettberg, N.; Biendl, M.; Garbe, L. Hop Aroma and Hoppy Beer Flavor: Chemical Backgrounds and Analytical Tools—A Review. J. Am. Soc. Brew. Chem. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- Titus, B.M.; Lerno, L.A.; Beaver, J.W.; Byrnes, N.K.; Heymann, H.; Oberholster, A. Impact of Dry Hopping on Beer Flavor Stability. Foods 2021, 10, 1264. [Google Scholar] [CrossRef]
- De Keukeleire, D. Fundamentals of beer and hop chemistry. Quim. Nova 2000, 23, 108–112. [Google Scholar] [CrossRef]
- De Oliveira Gomes, F.; Guimarães, B.P.; Ceola, D.; Ghesti, G.F. Advances in dry hopping for industrial brewing: A review. Food Sci. Technol. 2021, 42, ctaAR60620. [Google Scholar] [CrossRef]
- AOAC. Official Method 931.04 Moisture in Cacao Products Gravimetric Method; AOAC: Rockville, MD, USA, 1931. [Google Scholar]
- AOAC. Method 923.03, Ash of Flour, Official Methods of Analysis, 16th ed.; Adobe® Software® and E-DOC/CJS; AOAC: Rockville, MD, USA, 1997. [Google Scholar]
- AOAC. Official Methods of Analysis. Dumas Method (990.03), 15th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative Study on Total Lipid Determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and Modified Bligh & Dyer Extraction Methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2006, 101, 492–501. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- ASBC. Method of Analysis. American Society of Brewing Chemists; Beer Bitterness, Beer-23A; The Society: St. Paul, MN, USA, 2011. [Google Scholar]
- Roberts, T.R.; Wilson, R.J.H. Hops. In Handbook of Brewing, 2nd ed.; Priest, F.J., Stewart, G.G., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2006; pp. 177–280. [Google Scholar]
- Stevens, R. The Chemistry of Hop Constituents. Chem. Rev. 1967, 67, 19–71. [Google Scholar] [CrossRef]
- Verzele, M. 100 years of hop chemistry and its relevance to brewing. J. Inst. Brew. 1986, 92, 32–48. [Google Scholar] [CrossRef]
- Palamand, S.R.; Aldenhoff, J.M. Bitter tasting compounds of beer. Chemistry and taste properties of some hop resin compounds. J. Agric. Food Chem. 1973, 21, 535–543. [Google Scholar] [CrossRef]
- Hough, J.S.; Briggs, D.E.; Stevens, R.; Young, T.W. Malting and Brewing Science Volume 2: Hopped Wort and Beer, 2nd ed.; Chapman Hall: New York, NY, USA, 1982. [Google Scholar]
- Verzele, M.; De Keukeleire, D. Chemistry and Analysis of Hop and Beer Bitter Acids; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Terpinc, P.; Čeh, B.; Ulrih, N.P.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Collin, S.; Jerkovic, V.; Bröhan, M.; Callemien, D. Natural Products. In Polyphenols and Beer Quality; Ramawat, K., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Petrón, M.; Andrés, A.; Esteban, G.; Timón, M. Study of antioxidant activity and phenolic compounds of extracts obtained from different craft beer by-products. J. Cereal Sci. 2021, 98, 103162. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Del Carmen Esapadas Aldana, G.; Cruz, A.I.C.; Segura-Campos, M.R. Antioxidant Activity of Polyphenols Extracted from Hop Used in Craft Beer; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–310. [Google Scholar] [CrossRef]
- Ambra, R.; Pastore, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef]
- Oladokun, O.; Tarrega, A.; James, S.; Smart, K.; Hort, J.; Cook, D. The impact of hop bitter acid and polyphenol profiles on the perceived bitterness of beer. Food Chem. 2016, 205, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Sharrett, Z.; Perri, M.J.; Lares, M. Quantification of α-Acids in Fresh Hops by Reverse-Phase High-Performance Liquid Chromatography. ACS Omega 2019, 4, 3565–3570. [Google Scholar] [CrossRef]
- Bruner, J.; Marcus, A.; Fox, G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation 2021, 7, 66. [Google Scholar] [CrossRef]
- Castro, R.; Díaz, A.B.; Durán-Guerrero, E.; Lasanta, C. Influence of different fermentation conditions on the analytical and sensory properties of craft beers: Hopping, fermentation temperature and yeast strain. J. Food Compos. Anal. 2021, 106, 104278. [Google Scholar] [CrossRef]
- Castorena-García, J.H.; Juárez-Pérez, V.; Cano-Hernández, M.; Santiago-Santiago, V.; López-Mejía, O.A. Caracterización Físico-química de Cerveza Artesanal don Adjunto de Maíz Azul y Derivados de Caña de Azúcar. Concienc. Tecnol. 2020, 60, 94465715001. Available online: https://www.redalyc.org/articulo.oa?id=94465715001 (accessed on 17 October 2024).
- Jaskula-Goiris, B.; Aerts, G.; De Cooman, L. Hop α-acids isomerisation and utilisation: An experimental review. Cerevisia 2010, 35, 57–70. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, N.; Tang, J. Synthesis, characterization, crystal structure, and antioxidant activity of hexahydro-β-acids. J. Mol. Struct. 2018, 1175, 721–727. [Google Scholar] [CrossRef]
- Formato, A.; Gallo, M.; Ianniello, D.; Montesano, D.; Naviglio, D. Supercritical fluid extraction of α- and β-acids from hops compared to cyclically pressurized solid–liquid extraction. J. Supercrit. Fluids 2013, 84, 113–120. [Google Scholar] [CrossRef]
- Simpson, W.; Smith, A. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 1992, 72, 327–334. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. Antifungal activities of hop bitter resins and related compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar] [CrossRef]
- Bell, K.I.; Tepper, B.J. Short-term vegetable intake by young children classified by 6-n-propylthoiuracil bitter-taste phenotypey. Am. J. Clin. Nutr. 2006, 84, 245–251. [Google Scholar] [CrossRef]
- Dinehart, M.; Hayes, J.; Bartoshuk, L.; Lanier, S.; Duffy, V. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 2005, 87, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.; Hayes, J.; Feeney, E. Understanding taste and texture perception to enhance vegetable acceptance. Proc. Nutr. Soc. 2017, 76, E67. [Google Scholar] [CrossRef]
- Ullrich, N.V.; Touger-Decker, R.; O’Sullivan-Maillet, J.; Tepper, B.J. PROP taster status and self-perceived food adventurousness influence food preferences. J. Am. Diet. Assoc. 2004, 104, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, M.; Bajec, M.; Pickering, G. Orosensory responsiveness and alcohol behaviour. Physiol. Behav. 2017, 177, 91–98. [Google Scholar] [CrossRef]
- Lipchock, S.V.; Spielman, A.I.; Mennella, J.A.; Mansfield, C.J.; Hwang, L.; Douglas, J.E.; Reed, D.R. Caffeine Bitterness is Related to Daily Caffeine Intake and Bitter Receptor mRNA Abundance in Human Taste Tissue. Perception 2017, 46, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Hwang, L.; Zhong, V.W.; An, J.; Gharahkhani, P.; Breslin, P.A.S.; Wright, M.J.; Lawlor, D.A.; Whitfield, J.; MacGregor, S.; et al. Understanding the role of bitter taste perception in coffee, tea and alcohol consumption through Mendelian randomization. Sci. Rep. 2018, 8, 16414. [Google Scholar] [CrossRef]
- Higgins, M.J.; Bakke, A.J.; Hayes, J.E. Personality traits and bitterness perception influence the liking and intake of pale ale style beers. Food Qual. Prefer. 2020, 86, 103994. [Google Scholar] [CrossRef]

| Hop | Moisture | Ashes * | Proteins * | Fat * | Carbohydrates * |
|---|---|---|---|---|---|
| CH | 7.826 ± 0.058 g | 3.564 ± 0.100 c | 20.429 ± 0.252 b | 6.743 ± 0.153 a | 69.624 ± 0.137 cd |
| L1 | 62.961 ± 0.002 a | 2.674 ± 0.066 d | 20.221 ± 0.250 b | 5.592 ± 0.156 abc | 71.412 ± 0.150 ab |
| R1 | 9.801 ± 0.002 e | 1.822 ± 0.002 e | 21.981 ± 0.268 a | 4.781 ± 0.156 bc | 71.534 ± 0.700 a |
| L2 | 60.296 ± 0.002 c | 4.025 ± 0.016 a | 20.354 ± 0.288 b | 6.888 ± 0.161 a | 68.733 ± 0.453 d |
| R2 | 6.918 ± 0.000 h | 4.080 ± 0.009 a | 21.863 ± 0.321 ab | 4.009 ± 1.639 c | 70.420± 0.418 abc |
| L3 | 64.587 ± 0.004 b | 3.800± 0.020 b | 21.180 ± 0.209 b | 6.288 ± 0.158 ab | 69.623 ± 0.497 cd |
| R3 | 8.866 ± 0.002 f | 4.155 ± 0.003 a | 20.324 ± 0.161 b | 6.167 ± 0.210 ab | 69.458 ± 0.388 cd |
| L4 | 59.339 ± 0.001 d | 1.790 ± 0.017 e | 21.069 ± 0.386 a | 6.058 ± 0.213 ab | 70.171 ± 0.683 bc |
| R4 | 7.747 ± 0.000 g | 3.623 ± 0.006 c | 20.289 ± 0.215 ab | 5.381 ± 0.053 abc | 69.927 ± 0.339 cd |
| Hop | Total Phenolic Compounds (mg GAE/100 g) * | Antioxidant Capacity (μmol Trolox/g) * |
|---|---|---|
| CH | 229.333 ± 0.751 a | 496.467 ± 2.060 ab |
| C1 | 216.023 ± 4.240 b | 504.963 ± 17.589 a |
| R1 | 121.583 ± 1.178 c | 286.269 ± 24.125 c |
| C2 | 227.135 ± 1.373 a | 463.669 ± 7.699 a |
| R2 | 123.802 ± 4.685 c | 343.669 ± 19.558 b |
| C3 | 221.288 ± 2.603 ab | 326.449 ± 14.713 bc |
| R3 | 121.940 ± 5.517 c | 148.509 ± 10.090 d |
| C4 | 214.792 ± 4.215 b | 348.835 ± 9.588 b |
| R4 | 118.138 ± 1.319 c | 328.171 ± 10.754 bc |
| Beer | IBU (mg/L) |
|---|---|
| CH | 17.290 ± 4.079 d |
| C1 | 41.197 ± 5.799 a |
| C2 | 40.588 ± 3.727 ab |
| C3 | 27.427 ± 1.106 cd |
| C4 | 29.255 ± 3.499 bc |
| R1 | 28.342 ± 2.921 cd |
| R2 | 18.095 ± 5.031 cd |
| R3 | 23.998 ± 3.935 cd |
| R4 | 26.407 ± 5.212 cd |
| Hop | α-Acids * | β-Acids * | ||
|---|---|---|---|---|
| Cohumulone | N+adhumulone | Colupulone | N+adlupulone | |
| CH | 0.052 ± 0.000 a | 0.001 ± 0.000 i | 0.077 ± 0.002 a | 0.084 ± 0.000 a |
| C1 | 0.033 ± 0.001 a | 0.167 ± 0.001 a | 0.057 ± 0.001 b | 0.067 ± 0.002 b |
| C2 | 0.024 ± 0.001 f | 0.028 ± 0.000 d | 0.019 ± 0.000 f | 0.039 ± 0.001 f |
| C3 | 0.049 ± 0.002 b | 0.062 ± 0.001 b | 0.025 ± 0.001 e | 0.047 ± 0.003 e |
| C4 | 0.027 ± 0.001 e | 0.030 ± 0.002 d | 0.031 ± 0.001 c | 0.062 ± 0.001 c |
| L1 | 0.018 ± 0.001 g | 0.024 ± 0.000 f | 0.025 ± 0.000 e | 0.034 ± 0.001 g |
| L2 | 0.011 ± 0.001 h | 0.029 ± 0.002 de | 0.006 ± 0.000 g | 0.012 ± 0.000 h |
| L3 | 0.030 ± 0.001 d | 0.035 ± 0.001 c | 0.029 ± 0.001 d | 0.031 ± 0.001 g |
| L4 | 0.023 ± 0.001 f | 0.026 ± 0.001 ef | 0.026 ± 0.002 e | 0.055 ± 0.001 d |
| R1 | 0.009 ± 0.001 h | 0.013 ± 0.001 g | 0.001 ± 0.001 h | 0.002 ± 0.001 i |
| R2 | 0.011 ± 0.001 h | 0.011 ± 0.001 g | 0.000 ± 0.000 h | 0.000 ± 0.000 i |
| R3 | 0.023 ± 0.001 f | 0.000 ± 0.000 i | 0.001 ± 0.001 h | 0.002 ± 0.001 i |
| R4 | 0.005 ± 0.001 i | 0.004 ± 0.001 h | 0.000 ± 0.001 h | 0.000 ± 0.000 i |
| Characteristics | Beer | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CH | C1 | C2 | C3 | C4 | R1 | R2 | R3 | R4 | |
| General acceptability | 6.87 ± 1.45 a | 4.26 ± 1.91 d | 4.42 ± 2.03 d | 5.98 ± 1.72 ab | 5.86 ± 1.87 bc | 6.84 ± 1.78 a | 6.55 ± 1.36 ab | 6.49 ± 0.99 ab | 5.02 ± 1.06 cd |
| Aroma | 6.53 ± 1.34 a | 5.26 ± 1.87 b | 4.94 ± 1.88 b | 5.84 ± 1.93 ab | 5.68 ± 1.93 ab | 5.35 ± 2.00 b | 5.55 ± 1.38 b | 5.65 ± 1.21 ab | 5.43 ± 1.18 b |
| Color | 6.76 ± 1.41 a | 5.98 ± 1.14 abcd | 5.39 ± 1.45 d | 6.26 ± 1.67 abc | 6.44 ± 1.30 ab | 6.29 ± 1.97 abc | 5.76 ± 1.55 bcd | 5.79 ± 0.89 bcd | 5.52 ± 1.21 cd |
| Turbidity | 5.45 ± 1.72 abc | 4.97 ± 1.57 bc | 4.96 ± 1.59 bc | 5.78 ± 1.69 ab | 6.01 ± 1.90 a | 5.46 ± 2.38 abc | 5.67 ± 1.67 abc | 5.98 ± 1.41 a | 4.60 ± 1.51 c |
| Carbonation | 5.99 ± 1.65 a | 5.77 ± 1.74 ab | 5.08 ± 1.85 abcd | 5.34 ± 1.90 abcd | 4.89 ± 1.79 bcd | 4.73 ± 1.73 d | 5.69 ± 1.66 abc | 5.64 ± 1.31 abcd | 4.78 ± 1.21 cd |
| Foam | 6.30 ± 1.67 a | 5.70 ± 1.59 ab | 5.11 ± 1.93 b | 5.10 ± 1.77 b | 5.05 ± 1.54 b | 5.15 ± 1.24 b | 5.56 ± 1.60 ab | 5.67 ± 0.99 ab | 5.04 ± 1.00 b |
| Bitterness | 5.56 ± 1.47 bcd | 6.08 ± 2.23 ab | 6.68 ± 1.67 a | 5.62 ± 1.73 bcd | 5.55 ± 1.59 bcd | 5.16 ± 1.40 cd | 5.67 ± 1.29 bcd | 5.92 ± 1.31 abc | 4.98 ± 1.26 d |
| Sweetness | 4.64 ± 1.40 ab | 3.23 ± 1.68d | 3.40 ± 1.67 cd | 4.18 ± 1.74 bc | 3.81 ± 1.67 bcd | 5.10 ± 1.32 a | 4.87 ± 1.38 ab | 5.18 ± 1.40 a | 4.13 ± 1.17 bc |
| Hops flavor | 5.71 ± 1.49 ab | 6.01 ± 1.87 a | 5.46 ± 1.95 ab | 5.56 ± 1.70 ab | 6.15 ± 1.64 a | 4.79 ± 2.01 b | 5.41 ± 1.33 ab | 5.36 ± 1.24 ab | 5.07 ± 1.03 b |
| Malt flavor | 5.41 ± 1.66 bc | 5.86 ± 1.60 ab | 5.31 ± 1.87 bc | 5.63 ± 1.60 abc | 5.07 ± 1.70 bc | 5.17 ± 1.37 bc | 5.91 ± 1.55 a | 5.46 ± 0.89 bc | 4.83 ± 1.06 c |
| Alcohol | 5.02 ± 1.84 ab | 5.04 ± 1.97 ab | 5.10 ± 1.93 ab | 5.33 ± 1.83 ab | 4.94 ± 1.29 ab | 5.63 ± 2.46 a | 5.53 ± 1.73 ab | 5.58 ± 1.49 a | 4.51 ± 1.22 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejia-Llontop, C.I.; Tirado-Rodríguez, C.E.; Acosta-Baca, A.; Aguayo-Flores, M.; Ascate-Pasos, M.; Ayala-Jara, C.; Rodriguez, G.; Villanueva, E.; Aguirre, E. Impact of Hop Residue Reuse on the Chemical and Sensory Properties of Craft Beer. Resources 2025, 14, 2. https://doi.org/10.3390/resources14010002
Mejia-Llontop CI, Tirado-Rodríguez CE, Acosta-Baca A, Aguayo-Flores M, Ascate-Pasos M, Ayala-Jara C, Rodriguez G, Villanueva E, Aguirre E. Impact of Hop Residue Reuse on the Chemical and Sensory Properties of Craft Beer. Resources. 2025; 14(1):2. https://doi.org/10.3390/resources14010002
Chicago/Turabian StyleMejia-Llontop, Cesar I., Carlos E. Tirado-Rodríguez, Alanis Acosta-Baca, Maylee Aguayo-Flores, Manuel Ascate-Pasos, Carmen Ayala-Jara, Gilbert Rodriguez, Eudes Villanueva, and Elza Aguirre. 2025. "Impact of Hop Residue Reuse on the Chemical and Sensory Properties of Craft Beer" Resources 14, no. 1: 2. https://doi.org/10.3390/resources14010002
APA StyleMejia-Llontop, C. I., Tirado-Rodríguez, C. E., Acosta-Baca, A., Aguayo-Flores, M., Ascate-Pasos, M., Ayala-Jara, C., Rodriguez, G., Villanueva, E., & Aguirre, E. (2025). Impact of Hop Residue Reuse on the Chemical and Sensory Properties of Craft Beer. Resources, 14(1), 2. https://doi.org/10.3390/resources14010002










