Abstract
Mangrove ecosystems are some of the most productive on our planet but have declined globally by 30–50%. Many species rely on mangrove habitats; thus, their conversion to aquaculture farms has led to noticeable losses in commodities such as wild fish stocks. This study aimed to assess the influence of aquaculture and restoration projects on the ecosystem’s ability to provide resources. We collected data on mangrove vegetation (i.e., biomass, richness, and abundance), soil nutrients (i.e., organic carbon, aluminium, and nitrogen), crab abundance, and fishing pressure at six sites. We set up 15 plots at each site and collected data between May and July 2023. Via generalised linear mixed models, we found that the abundance and richness of crabs was significantly higher in aquaculture plots than in non-aquaculture plots. Aquaculture plots had higher topsoil aluminium, higher topsoil and subsoil nitrogen, and lower topsoil carbon than non-aquaculture sites. Restored sites had less nitrogen in the topsoil than unmanaged sites. The biomass did not change between aquaculture, restored, and unmanaged plots. We found a negative correlation between crab abundance and richness and mangrove diameter at breast height (DBH), suggesting that the species of crabs present preferred areas with propagules for feeding (e.g., Grapsidae crabs). The content of nitrogen in the subsoil was positively correlated with mangrove richness, diversity, and height, suggesting the importance of nitrogen availability for mangrove growth. The content of aluminium in the subsoil was negatively correlated with the content of organic carbon in both the topsoil and subsoil, suggesting the detrimental effect of aluminium on the carbon cycle. Fishing practices were observed at all sites during the data collection period. Despite the lack of significant impact on most vegetation parameters and the limited differences between managed and unmanaged sites, key variables such as soil aluminium, carbon, and nitrogen contents and crab assemblages exhibited high variability, highlighting the complex interactions within mangrove ecosystems.
Keywords:
biomass; carbon; aluminium; nitrogen; crab; fishing pressure; restoration; soil; management 1. Introduction
Mangroves are highly specialised plant species that provide significant ecological and economic value [1]. By definition, mangroves are subtropical-to-tropical trees or shrubs adapted to the extreme conditions posed by intertidal regions of brackish-to-coastal saline waters [2]. Here, mangroves rely on highly specialised traits to deal with high salinity, anoxic soil, exposure, and tidal fluxes that can be very large [2,3,4]. Because of the traits required to occupy these zones, mangroves have an exceedingly high capacity for carbon capture and storage, through both biomass and soil [5,6,7]. Estimates suggest that they exceed all other aquatic systems and tropical forests (estimates of 5×) and, thus, have a major role to play in managing global C [8,9]. Likewise, their specialised roots stabilise the shoreline by trapping sediment, which reduces coastal erosion and buffers against storm surges [10,11,12]. Mangroves intrinsically have high biodiversity value given their resilience to high salinity, with only a few other species of plant able to occupy the areas where they thrive [13]. They support a rich diversity of terrestrial and marine life that relies on mangroves for feeding, spawning, or sheltering during some phase of their life cycle, with many highly specialised to the habitat [1,2,14,15]. Their presence also enhances the diversity and biomass of communities in adjacent coral reefs and sea grasses [16]. Their socio-economic benefits are diverse, extending from sequestering pollutants to providing timber products, medicinal products, and food, with 80% of fisheries (e.g., crabs, prawns, oysters, and many fish) being linked to mangroves [17,18,19].
Despite the importance of mangroves, they are continuing to be lost at an alarming rate, for a variety of reasons [20,21]. Globally, it has been estimated that between 30 and 50% of mangrove habitats have disappeared in half a century [9,20]. In some parts of the world, up to 40% of mangrove species are threatened with extinction [22], as are the other organisms that rely on mangroves (e.g., [23]). The loss of mangroves has alarming ramifications for carbon emissions [24]. Stressors on mangrove survival include human-induced climate change from increased coastal storm intensity and sea levels [21], while more direct human impacts include overexploitation of fisheries and deforestation for urban and agricultural development or aquaculture conversions [25,26].
Deforestation of mangroves for agriculture and aquaculture is particularly noticeable in South-East Asia, which is a mangrove hotspot [13]; for instance, Indonesia alone hosts 22% of the world’s mangroves [27]. Across South-East Asia, approximately 30% of mangroves have been lost to aquaculture [28]. Some of these losses come from the planting of palm oil and rice paddies, requiring felling of the mangroves, and this is predicted to increase [29], but by far the greatest loss comes from conversion to aquacultural ponds. It is estimated that up to 85% of former mangrove areas in Borneo and Indonesia have been converted to aquaculture ponds [30]. Aquaculture conversion involves creating walls of mangroves aligned with a constructed pond, or a pond with an isolated stand (i.e., assemblage of mangrove trees) central to the pond [31]. It also requires wild mangrove seeds to ensure the stands, which is ironically made difficult by the loss of wild populations of mangroves. Irrespective of the strategy used for aquaculture, the stands of mangroves ultimately become fragmented and lose much of their ecosystem service value beyond cultivating a select group of commercially consumable species. This is concerning, given that it has been estimated that an intact mangrove can generate 70% higher output from goods and services than aquaculture ponds [32]. But simply ceasing aquaculture is unfeasible, as it represents job opportunities, food security, and substantial profit, especially within countries with low socio-economic development [26,28]. Therefore, there is a need to find more sustainable practices and to incentivise the protection and restoration of mangroves to ensure that mangrove populations are maintained and the essential ecosystem services are restored.
There is no question that there is a global need to preserve, manage, and restore mangrove populations, but there are barriers to this aim. For instance, local communities still need to benefit from mangroves to incentivise their restoration [33], which needs to be managed in a sustainable way [25,34]. One of the many challenges here includes tracking the fluxes in goods and services relative to the mangroves’ health [25,35,36,37,38]. Indeed, it is difficult to measure the success of restoration if success is not well defined. Indonesian mangrove ecosystems have been shown to host high numbers of marine and terrestrial species that are at risk of extinction, with active management of mangrove ecosystems suggested as an effective solution [39]. The aim of this study, then, was to gain some initial insights into the influence of (1) aquaculture and (2) restoration projects on mangrove sites in Indonesia. Targeted features were attributes reputed to be useful for detecting shifts in mangrove health:
- -
- Mangroves’ above- and belowground biomass: A lack of biodiversity is typically linked with a loss of biomass [40]. Likewise, tree characteristics such as age and density have been found to directly correlate with biomass [41]. Evaluating the level of biomass that a forest contains is vital to ensure that the correct forestry management practices are implemented [42].
- -
- Mangrove density: The density of the mangroves affects their ability to protect the coast from storm or wave damage [11]. Although mangroves attenuate waves’ action [12], the more dispersed they are, the less of an impact they have. Density can also potentially impact wildlife relying on the mudflats, e.g., crabs.
- -
- Soil quality: Soil composition can provide an indication of mangrove ecosystems’ health [36]. Mangroves regulate carbon found within coastal soils through CO2 sequestration and biomass accumulation [43,44]. They can also nullify pollutants and denitrify waterways that are secured within the soils [45]. The presence of aluminium, however, can hinder propagule growth and is often associated with aquaculture [46].
- -
- Crab assemblages: Crabs are intertwined with the growth and development of mangroves [37]. Ferreira et al. [47] suggested that monitoring the abundance of crabs could be used to measure the success of mangrove restoration. Crabs facilitate litter decomposition through leaf processing, burying of leaves and mixing of soil and decomposing bacteria through excavations [15,48,49,50]. Concurrently, they may also influence the community density and structure through direct consumption of the propagules. Furthermore, crabs are also a main food source for local communities.
- -
- Fishing pressure: Pressure from fishing activities can directly impact mangrove health. Local communities rely on resources from mangroves, but they can be overexploited through intense use.
2. Materials and Methods
2.1. Site Selection and Descriptions
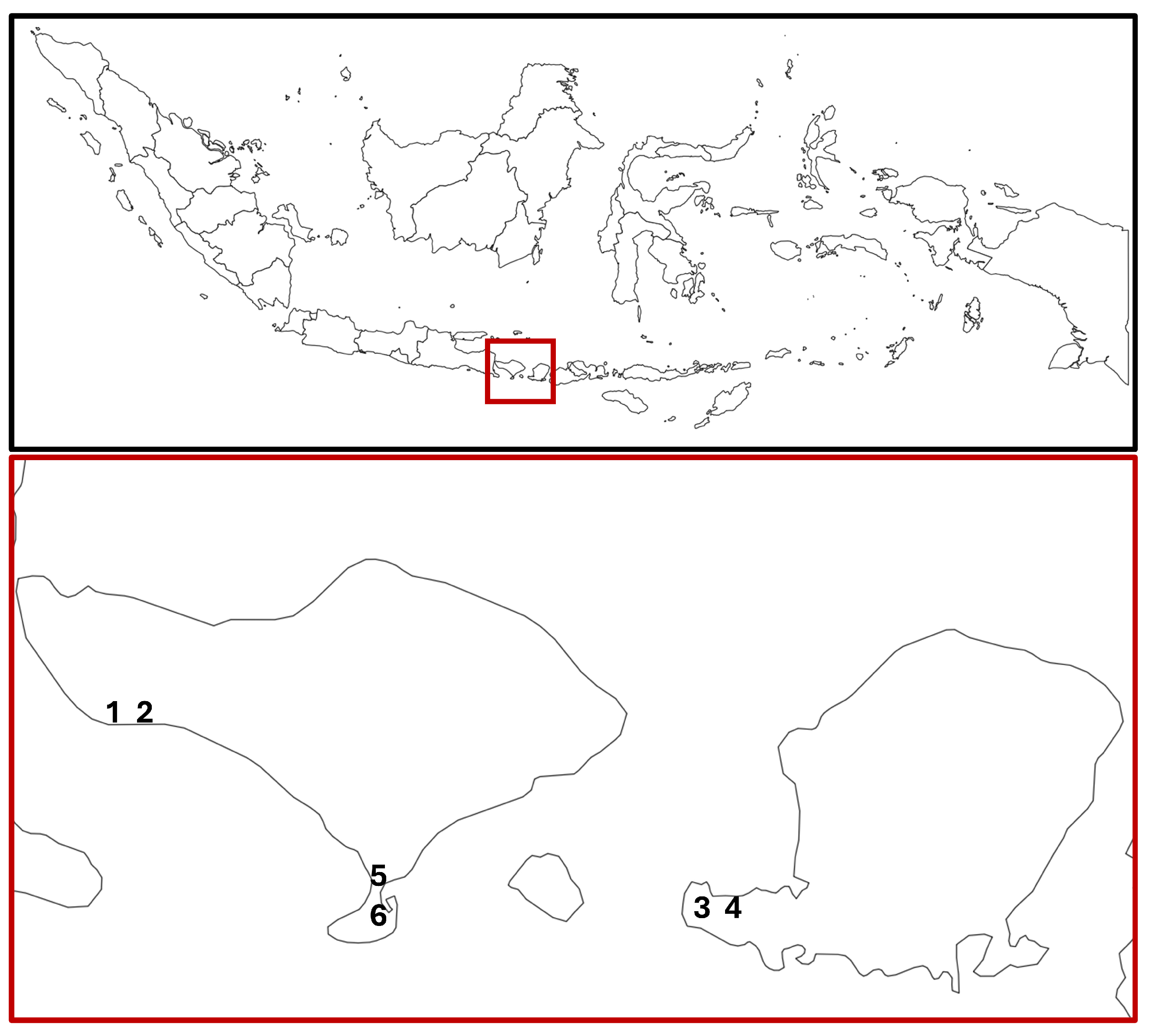
The Bali Nusa Tenggara region (including Bali, Lombok, Nusa Tenggara, Sumba, and Timor) hosts ~35,000 ha of mangroves and a similar mangrove diversity to other regions of Indonesia [51]. Six sites, located on the islands of Bali and Lombok in Indonesia, were investigated in this study (Figure 1). The sites were categorised as unmanaged or managed prior to surveying (Table 1). Designations were made based on local knowledge, historical evidence, and the current use of the land. All sites varied in their perceived mangrove ecosystem health and management. The sites were matched as best as possible, so managed and unmanaged sites were in close proximity. One of the couplets (Sites 1–2 in Budeng) was selected to include a site currently under aquaculture operations. The area near Budeng includes around 125 ha of mangrove sites on abandoned aquaculture ponds, 35 ha of which has been restored and is still under management [52,53]. All transects were labelled as originating from either an unmanaged or managed site, irrespective of whether there was within-site variation in management.

Table 1.
Description and designation of the study areas in Bali and Lombok, Indonesia.
Table 1.
Description and designation of the study areas in Bali and Lombok, Indonesia.
| Site | Locality | Designation | Use |
|---|---|---|---|
| Site 1 | Budeng (Bali Barat) | Unmanaged | Current aquaculture site that has been unmanaged for more than 10 years, with local communities extracting resources (shrimp and fish). |
| Site 2 | Budeng (Bali Barat) | Managed | Current aquaculture site that has been managed and restored over the last 20 years and is still used as an aquaculture farm for mangrove mud crabs, shrimp, and oysters. |
| Site 3 | Batu Putih (Lombok Barat) | Managed | Under restoration since 2021 by the NGO Sustainable Oceanic Research, Conservation, and Education initiative (SORCE). This site of around 10 ha has been subjected to deforestation of the mangrove habitat to create a road through the landward area of the mangrove forest [54]. |
| Site 4 | Batu Putih (Lombok Barat) | Unmanaged | Site selected for future restoration by SORCE but that was unmanaged at the time of the surveys [54]. This site of around 13 ha has seen more natural recruitment in these areas than Site 3, with many young Ceriops species in the areas that could be classed as mudflats. |
| Site 5 | Tahura Ngurah Rai (Kuta, Bali) | Managed | A tourist attraction with wooden walkways built within the mangrove forest; however, this has been closed off to the public for several years and needs permission to be entered. There is evidence that restoration was conducted recently and is ongoing. |
| Site 6 | Tahura Ngurah Rai (Kuta, Bali) | Unmanaged | Used by local fishermen to moor boats and fish along the mudflats. This site is unmanaged and has been historically deforested for economic development. Sites 5 and 6 are part of the same mangrove ecosystem of around 1100 ha [55]. |
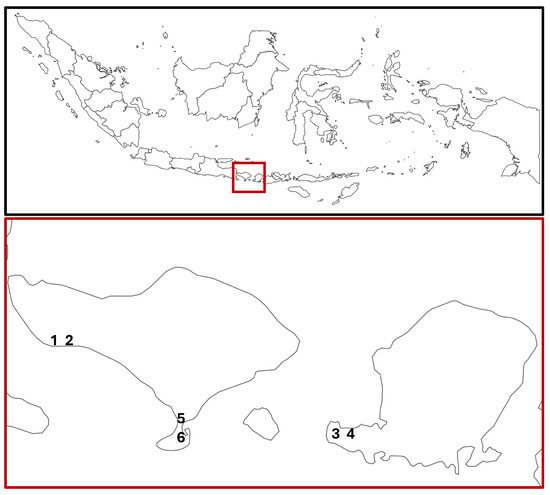
Figure 1.
Location of the study sites in Bali and Lombok, Indonesia. Sites 1 (unmanaged) and 2 (managed) are aquaculture mangrove sites in Budeng, Bali Barat. Sites 3 (managed) and 4 (unmanaged) are costal mangrove sites in Batu Putih, Lombok Barat. Sites 5 (managed) and 6 (unmanaged) are costal mangrove sites in Tahura Ngurah Rai, Kuta. Baseline map taken from [56].
Figure 1.
Location of the study sites in Bali and Lombok, Indonesia. Sites 1 (unmanaged) and 2 (managed) are aquaculture mangrove sites in Budeng, Bali Barat. Sites 3 (managed) and 4 (unmanaged) are costal mangrove sites in Batu Putih, Lombok Barat. Sites 5 (managed) and 6 (unmanaged) are costal mangrove sites in Tahura Ngurah Rai, Kuta. Baseline map taken from [56].
2.2. Survey Design
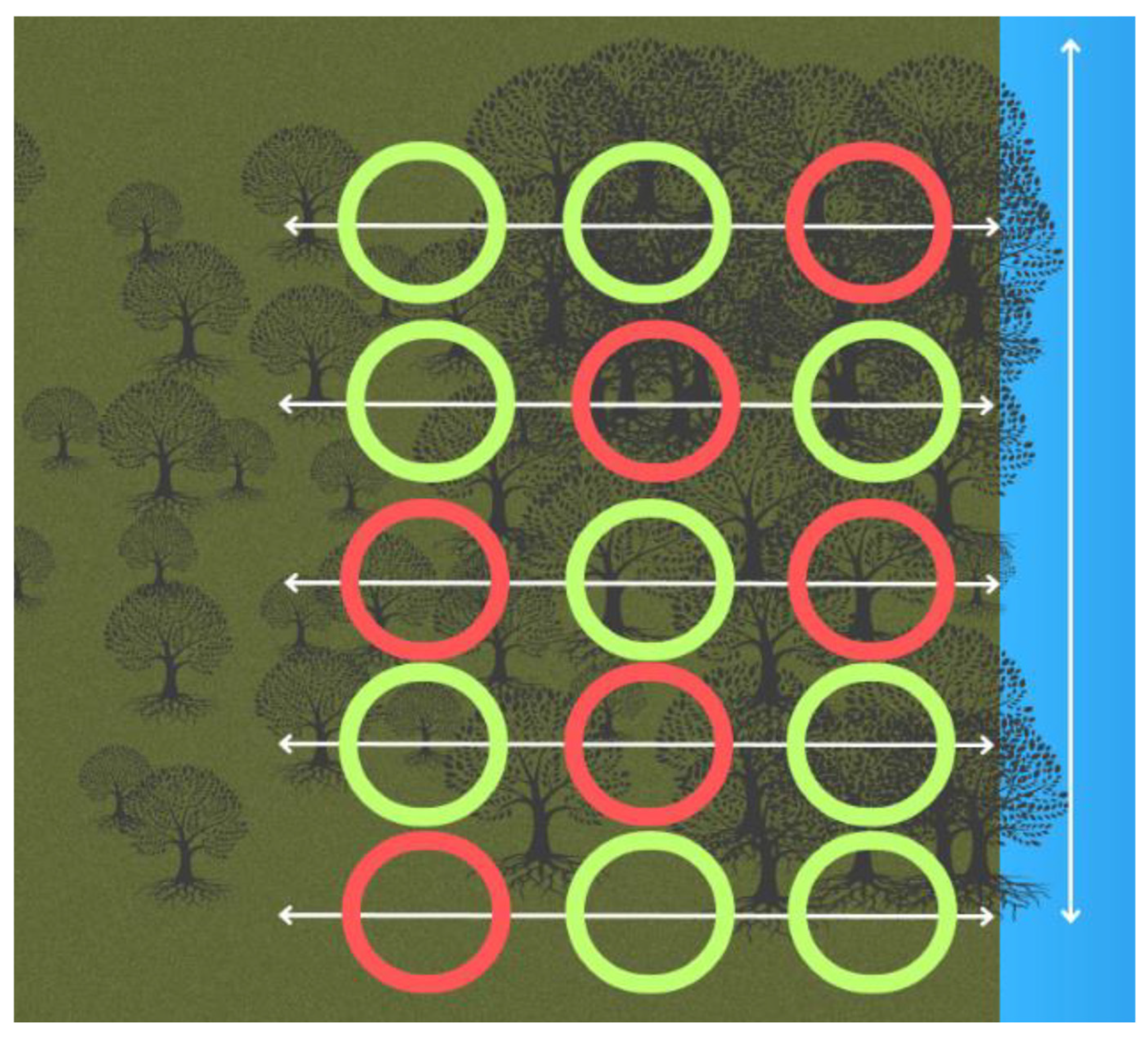
At each site, five transects were placed 60 m apart. On each transect, three 5 m2 plots were measured using a reel marked out at every 5 m (Figure 2) [37,47]. This was for ease of use in the field to ensure that the corners of the plots were the same throughout the surveys. The plots started at 5 m, 42.5 m, and 90 m, with a 37.5 m gap in between the plots [37]. Mangroves were classed as trees if they had leaves, due to the premise that propagules that were less than two months old had not produced leaves and, therefore, may not have established themselves.
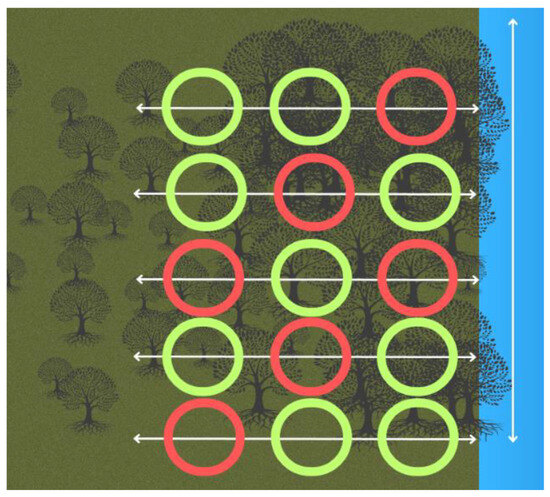
Figure 2.
Layout of the transects and plots to conduct the surveys at each mangrove site in Bali and Lombok, Indonesia. The red plots show where the crab surveys were conducted and soil samples were taken. Vegetation data were collected from all plots.
2.3. Mangrove Vegetation
Every mangrove tree in each plot was identified by species, and then their diameter at breast height (DBH) was measured using a measuring tape. AGB and BGB were calculated for all of the species present in the study sites. Estimates were calculated using either species-specific equations (when available) or a common allometric equation specific for mangroves (Table 2). This common allometric equation required wood density data, which were sourced from the Tree Functional Attributes and Ecological Database ([57,58]; Table 2). We further calculated mangrove richness via the Shannon index and abundance (as the total number of individuals per plot or site) (Table S1). In addition, we differentiated between different growth stages: saplings (DBH < 5 cm), small trees (5 cm ≤ DBH < 10 cm), medium trees (10 cm ≤ DBH ≤ 20 cm), and large trees (DBH > 20 cm) (based on [59]).

Table 2.
List of mangrove species found across the study sites, along with their respective species-specific AGB and BGB equations and the sources of the equations. A common allometric equation was used for all other species. All equations required diameter at breast height (D), and the general equation required wood density (ρ).
2.4. Soil
Soil samples were taken from six plots per site (Figure 2). Cores were taken from the centre of the plots of topsoil (0–30 cm) and subsoil (deeper than 30 cm). If the centre was occupied by a mangrove or crab burrow, the sample was taken immediately adjacent. The organic carbon, nitrogen, and aluminium levels were analysed at Universitas Udayana. We calculated the organic carbon (%) via the Walkley and Black method and UV–vis spectrophotometry, nitrogen (%) via the Kjeldahl method and titration, and available aluminium (mg/kg) via spark atomic emission spectrometry [68] (Table S2).
2.5. Crabs
A 1 m2 quadrat was marked out in six plots per site (Figure 2). This was carried out in an area along the transect where the entire plot was visible. This was to ensure that no crabs were missed and that mangrove roots were not obstructing the view of the plot [47]. All members of the team moved away from the area, and a 3 min adjustment period was given. This was decided because the crabs were previously observed to come out of hiding after a few minutes after any stimulus was removed. The crab species and abundance were observed within the quadrat for 10 min to avoid double counting of any individuals [69]. The member of the team conducting this observation sat 1 m away from the plot and remained motionless for the 10 min period. Furthermore, where the team member sat was chosen to prevent any visual stimulation, such as shadows, from influencing the crabs’ behaviours or choice to emerge from their burrows. We used morphospecies and identified the genus when possible (Table S2).
2.6. Fishing Pressure
Fishing activity was recorded ad hoc at each site during the survey period, ranging from 7 hr 29 min to 20 hr 20 min. We recorded any boats engaged in netting, line fishing, or returning to the mangrove site. The various fishing pressures observed were categorised as described in [19]. Fishing pressure, even just from local fishing, can have a large impact on the fish species richness, their size, and the abundance with which they are found within the mangroves [70]. In addition to this, if the fishing methods are destructive or continuous, then the impact and damage caused will be expected to increase over time [70,71].
2.7. Data Analysis
We tested whether the variables indicating the resource availability and health of mangrove ecosystems were different between considering management and the presence of aquaculture. In particular, we considered vegetation parameters (AGB, BGB, species richness), soil parameters (aluminium, organic carbon, nitrogen), and crab assemblages (richness, abundance, diversity) as dependent variables. We considered the combined effect of habitat management (managed vs. unmanaged) and aquaculture (presence vs. absence of aquaculture) as a fixed factor. We used the nested term (1|Region/Site) as A random effect to account for the spatial closeness of the plots. We used the function “glmmTMB” to run generalised linear mixed models and tested the family distributions offered by the corresponding package [72]. We then checked the model residuals plotted via the package “DHARMa” to decide on the family fit [73]. We additionally checked for multicollinearity of the predictors via the “check_collinearity” function from the package “performance” and detected low correlations (Variance Inflation Factor (VIF) < 4) [74]. To check for potential links between the variables of investigation, we ran Pearson correlations via the “rcorr” function and plotted them via the “corrplot” function [75]. For this, we considered only the 36 plots from which we collected all of the data. We used p = 0.05 as the threshold for significance and p = 0.1 as the threshold to define trends towards significance.
3. Results
3.1. Mangrove Vegetation
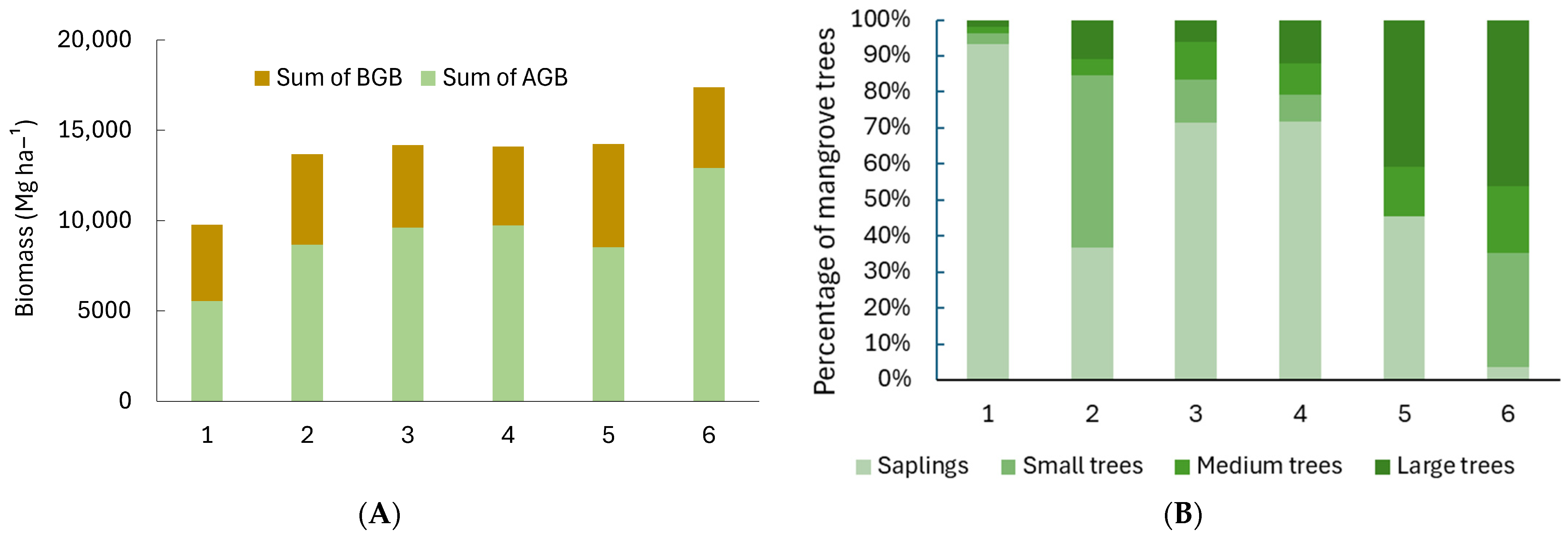
Between the six sites, we found 13 species of mangroves, with Site 1 having the highest richness. Sites 5 and 6 had the highest proportion of large trees, while the other sites, especially Site 1, were dominated by tree saplings (Table 3; Figure 3). Most of the tree saplings in Site 1 (98.2%) were from Avicennia marina.

Table 3.
Vegetation parameters by site and in total, considering the six sites, in mangrove ecosystems in Bali and Lombok, Indonesia.
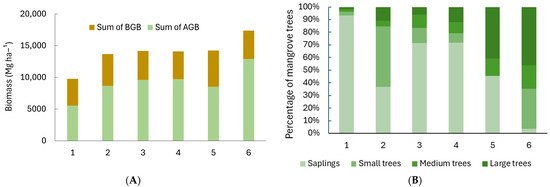
Figure 3.
(A) Total biomass (ABG: aboveground biomass; BGB: belowground biomass) and (B) percentages of mangrove trees at different growth stages (saplings (DBH < 5 cm), small trees (5 cm ≤ DBH < 10 cm), medium trees (10 cm ≤ DBH ≤ 20 cm), and large trees (DBH > 20 cm)) at six sites in Bali and Lombok, Indonesia.
3.2. Soil
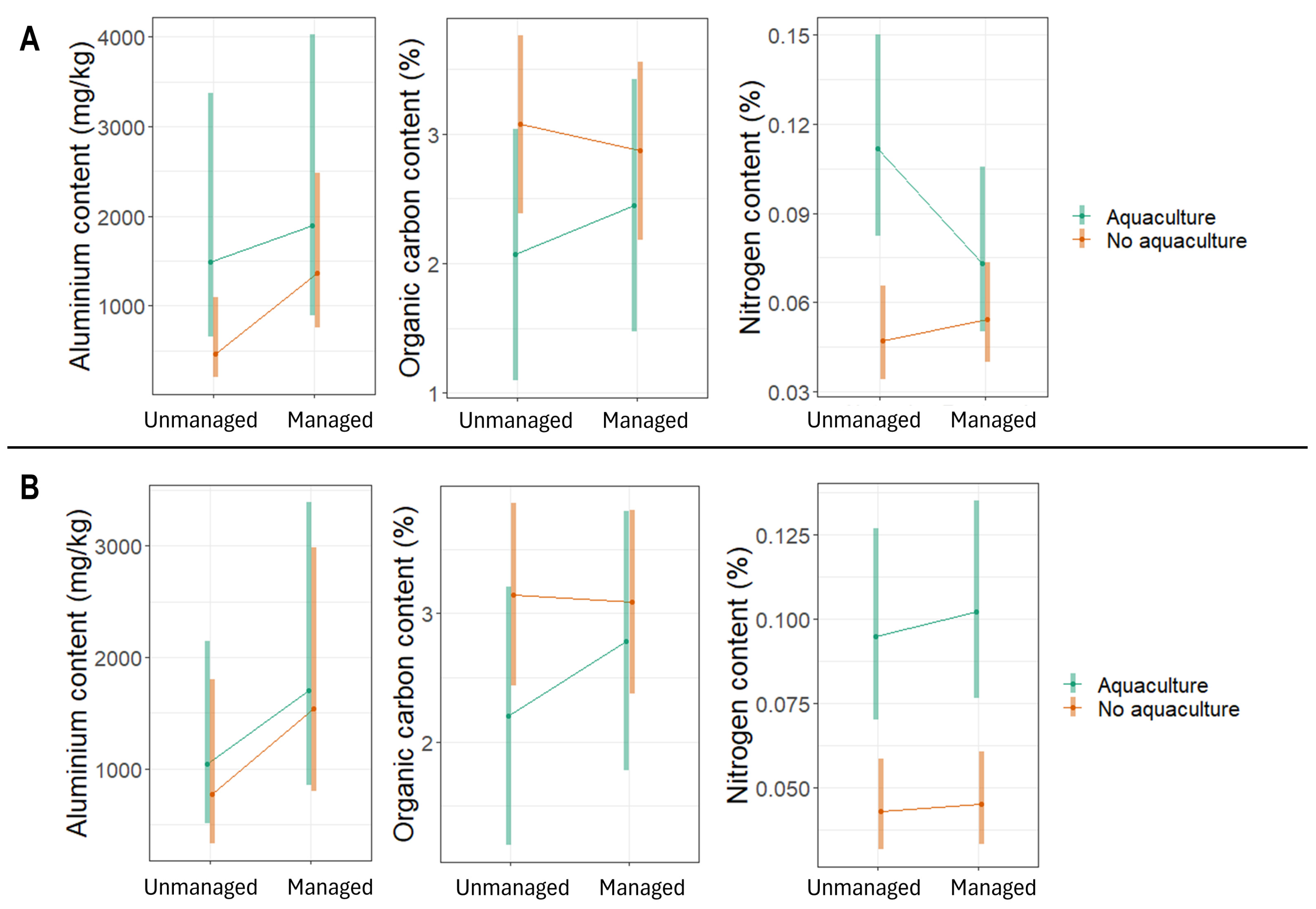
Aquaculture plots had higher topsoil Al, higher topsoil and subsoil N, and lower topsoil C than non-aquaculture sites. Restored sites had less N than unmanaged sites (Figure 4). In particular, Al in topsoil tended to be higher in restored aquaculture plots than in unmanaged non-aquaculture plots. The unmanaged aquaculture plots had higher nitrogen contents in the topsoil than non-aquaculture sites (both unmanaged and restored). The aquaculture plots (both unmanaged and restored) had higher nitrogen contents in the subsoil than non-aquaculture sites (both unmanaged and restored).
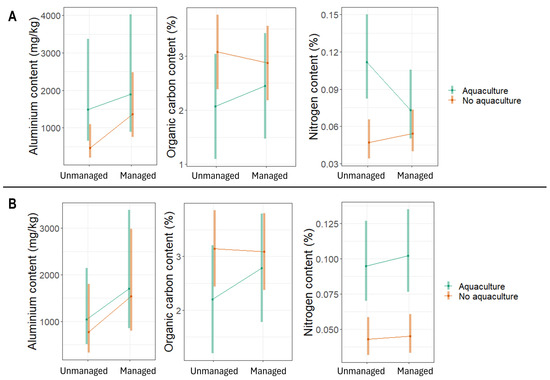
Figure 4.
Contents of aluminium (mg/kg), organic carbon (%), and nitrogen (%) in (A) topsoil and (B) subsoil samples from mangrove ecosystems in Bali and Lombok, Indonesia. Values are estimated marginal means and 95% confidence intervals.
3.3. Crabs
We found 18 species of crabs overall among the six sites, with Site 1 hosting the highest richness and abundance, Site 2 hosting the highest diversity, Site 4 hosting the lowest richness and diversity, and Site 6 hosting the lowest abundance (Table 4).

Table 4.
Crab assemblages in the six sites in Bali and Lombok, Indonesia.
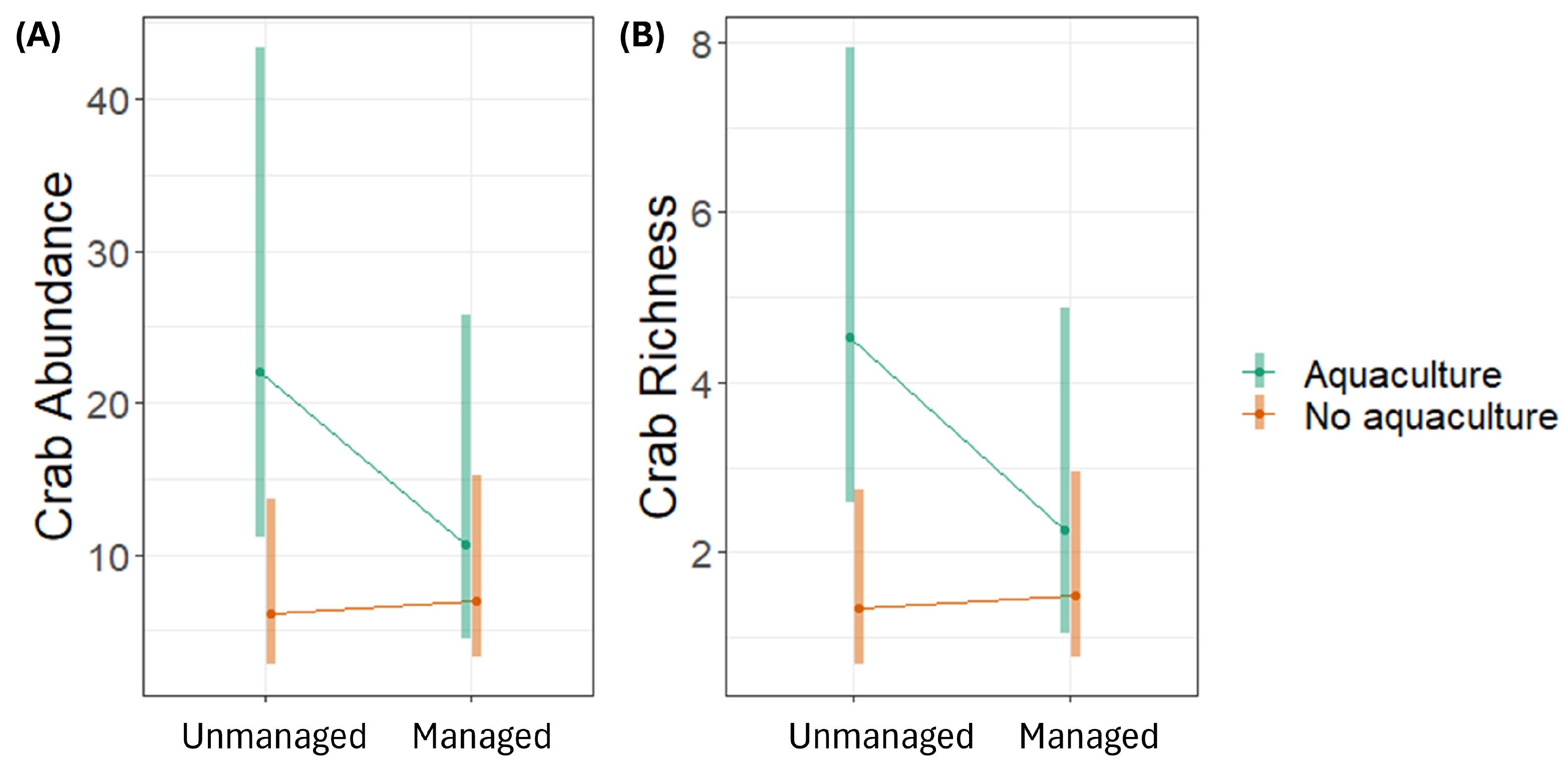
The abundance and richness of crabs was significantly higher in aquaculture sites than in non-aquaculture sites; in particular, the unmanaged aquaculture sites had higher abundance and richness than non-aquaculture sites (either unmanaged or restored) (Figure 5).
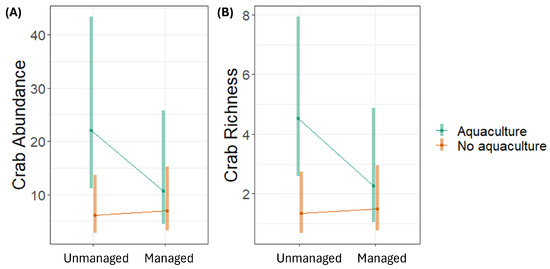
Figure 5.
Estimated marginal means and 95% confidence intervals of crabs’ (A) abundance and (B) richness in 5 m2 plots in mangrove ecosystems in Bali and Lombok, Indonesia, based on generalised linear mixed models.
3.4. Correlations between Variables
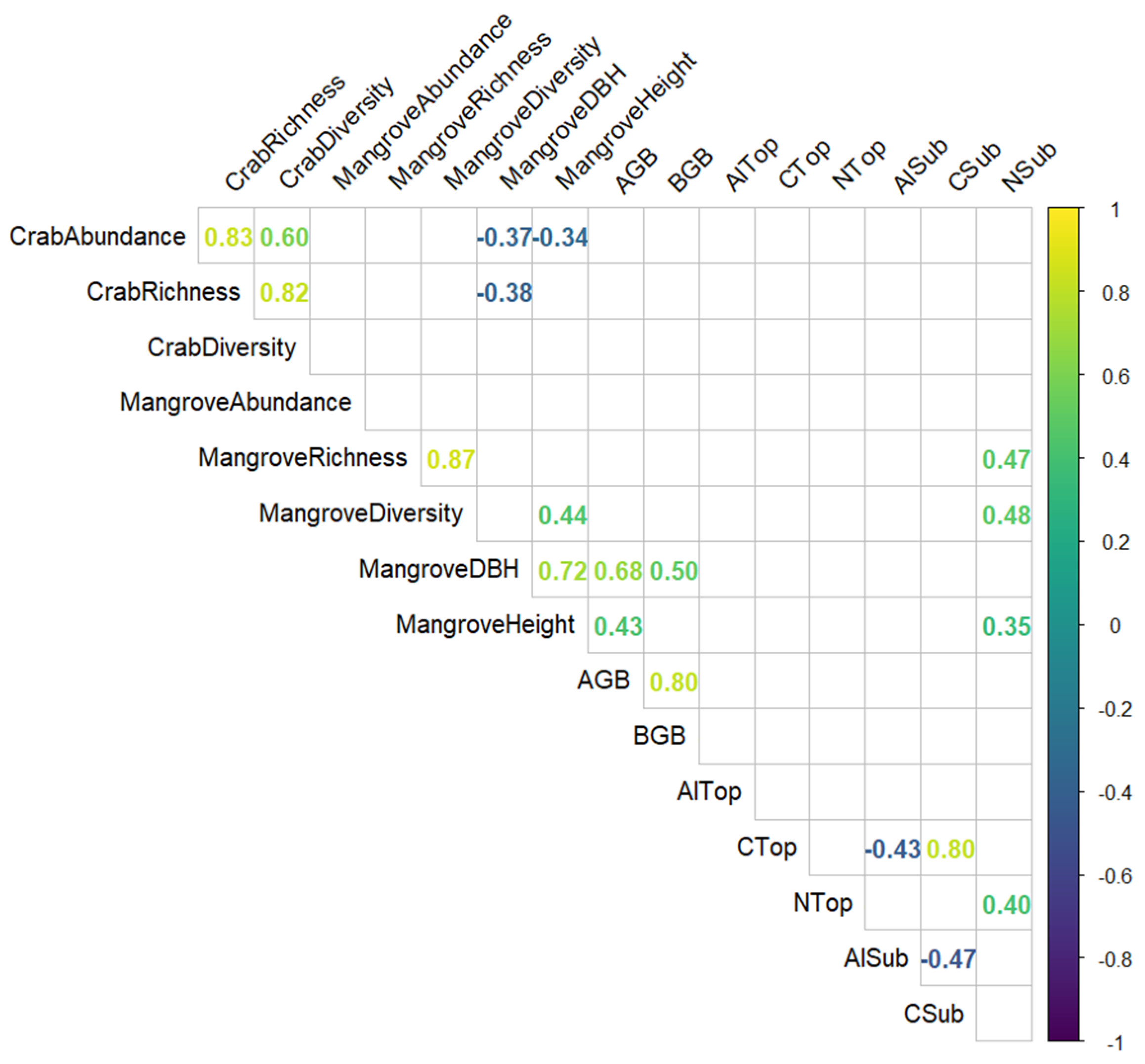
Apart from the correlations that were expected between richness, abundance, and diversity, we found the following correlations that are worth highlighting (Figure 6): The mean DBH of mangroves in plots was negatively correlated with crab abundance and richness, and the mean height of mangroves in plots was negatively correlated with crab abundance. The content of nitrogen in the subsoil was positively correlated with mangrove richness, diversity, and height. The content of aluminium in the subsoil was negatively correlated with the content of organic carbon in both topsoil and subsoil.
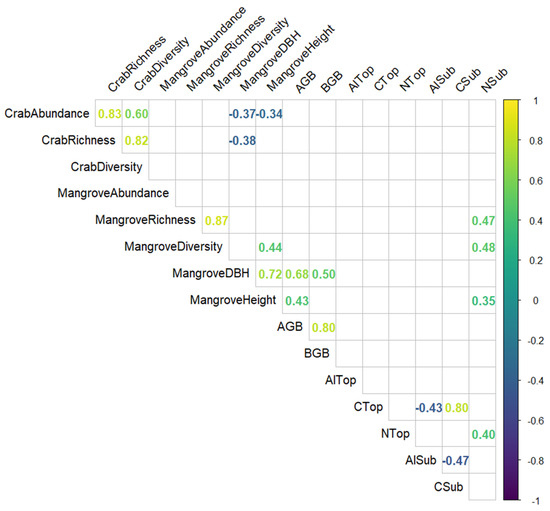
Figure 6.
Correlation matrix of the variables taken into consideration for the investigation of mangrove ecosystems in Bali and Lombok, Indonesia. Only significant (p < 0.05) correlation coefficients are shown.
3.5. Fishing Pressure
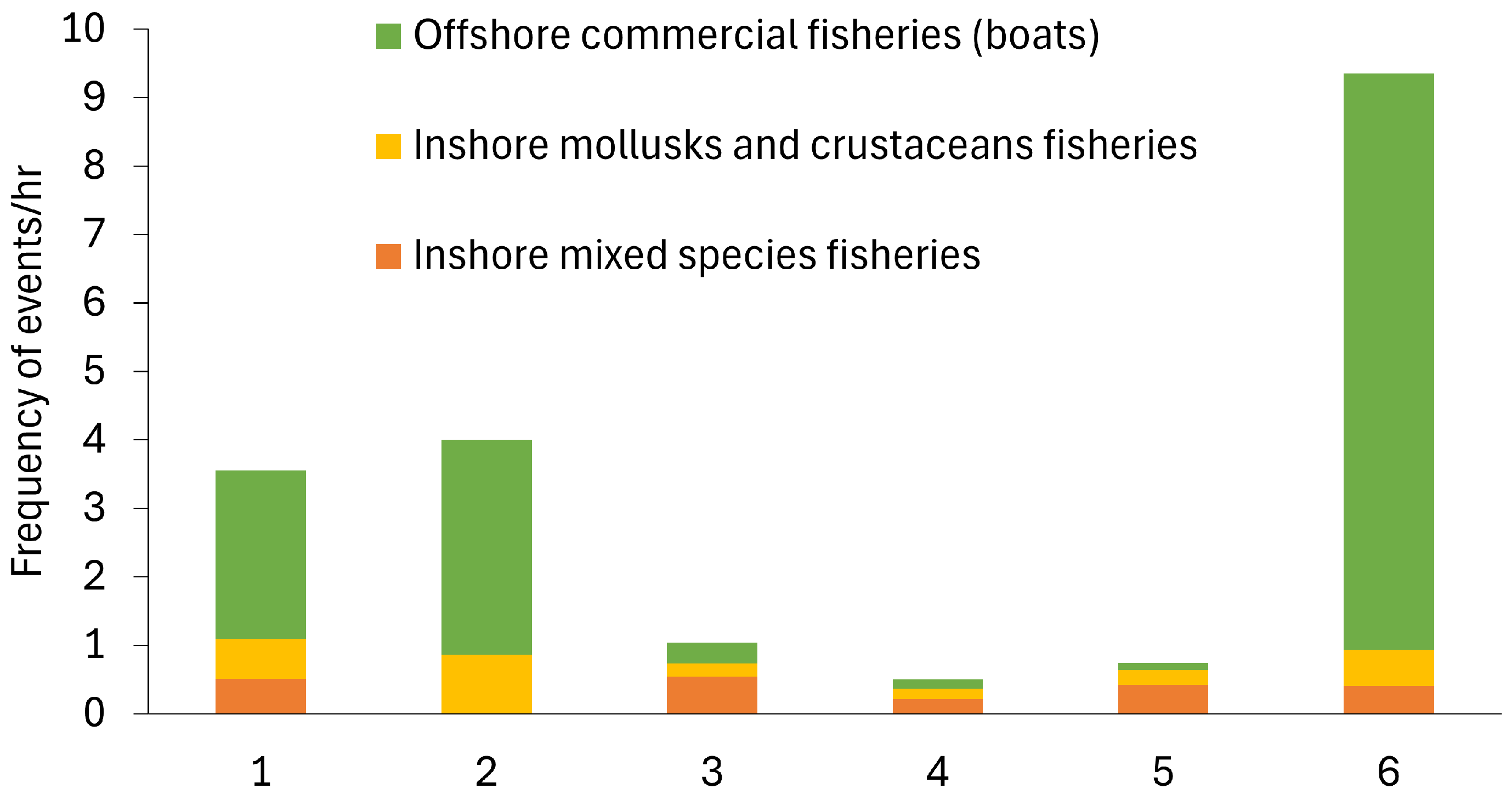
Fishing practices were observed at all sites during the data collection periods (Figure 7). Boats would sell their fish at a fish market near the mangrove sites; thus, they were classed as offshore commercial fisheries. Inshore mixed-species fisheries included people standing onshore or waist-deep in the ocean using a rod-and-line fishing method. Other fishing practices were also observed, including the use of gill nets cast in a circular motion by people standing in hip-deep shallow water, who then reduced the net area to entrap the fish, as well as people casting small gill nets or using the rod-and-line method from a small boat. The inshore mollusc and crustacean fishing included mangrove crab traps, digging for molluscs and crustaceans when the tide was lower, and sifting the water to collect shrimp.
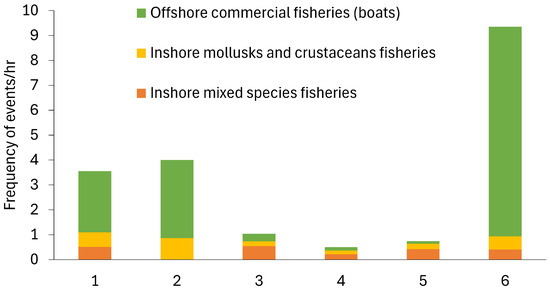
Figure 7.
Fishing pressure events recorded during the observation times in mangrove ecosystems in Bali and Lombok, Indonesia.
4. Discussion
From our investigation, we found that the vegetation parameters were not affected by the presence of aquaculture or restoration management, although we found site-specific variability. Crab richness and abundance were higher in aquaculture sites, and plots with smaller trees were preferred. Soil characteristics (carbon, nitrogen, and aluminium) were highly impacted by the presence of aquaculture and restoration management. In addition, nitrogen in the subsoil was positively correlated with mangrove richness, diversity, and mean height, and aluminium in the subsoil was linked with a decrease in organic carbon in both the subsoil and topsoil. There were no differences between the AGB and BGB throughout the sites that were surveyed. Site 1, with non-restored aquaculture, was the site with the least amount of biomass. This could have been due to the lack of natural recruitment able to take place due to the typographic changes created by the aquaculture’s establishment, therefore limiting the access for seeds or propagules to secure themselves. Most of the propagules at Site 1 were Avicennia marina, a pioneer mangrove species that has relatively low sapling survival rates [76,77]. Therefore, the active restoration of the mangroves with the promotion of a diversity of saplings has been successful in increasing the biomass of the ecosystem. Studies suggest that this allows for increased resources with regard to carbon sequestration and a healthier ecosystem [78].
It has been reported that the higher the biomass of a forest, the more potential for carbon sequestration it contains, and that biomass data can be used to calculate carbon storage [6]. Organisations such as the International Union for Conservation of Nature (IUCN) are driving for blue carbon to be introduced to REDD+ [79], which may be able to provide short-term funding for conservation projects involved in the restoration of mangrove ecosystems. This short-term funding could be provided by governments or private businesses involved in offsetting carbon emissions [79,80]. This provides stakeholders with the motivation to support the agreements that governments have made to reach an equilibrium between human-made emissions and their absorption by 2100 [81]. Even though this may not be applicable on a global scale, for countries such as Indonesia, blue carbon could become a large source of carbon storage to help meet the objectives set by the Paris Agreement [81,82].
The collected soil samples showed that there were increased levels of Al and N compared to those of non-aquaculture sites. This was mostly likely due to human disturbances, such as aquaculture, disrupting the natural levels of N within the ecosystem [21]. This is also supported by other research showing that the levels of N increase in mangrove ecosystems where there is increased human influence [37]. This suggests that mangrove ecosystems that have had aquaculture present have potentially influenced the nitrifying bacteria in the ecosystem, which have the ability to break down and release N [21]. Even though N is essential for growth, the increased levels can create an unbalanced ecosystem. However, areas that have been restored showed decreased levels of N compared to the unmanaged sites. This could be because the activities required for restoration to occur hinder the amount of N within the ecosystem. Furthermore, areas that are undergoing restoration or are exposed to any human disturbances could potentially alter ecosystem resources provided by the mangroves [83]. Whilst the restoration influence the observed N content, the benefits of restoring these ecosystems should outweigh the impacts of increasing the N levels during any restoration activities.
The topsoil of the restored aquaculture site had higher levels of Al than that of the unmanaged site. This could be due to the restored site having more active aquaculture occurring than the unmanaged site, therefore increasing the level of Al observed. Whilst this was true for the aquaculture site, overall the influence of aquaculture showed an increase in the amount of Al, thus suggesting that any aquaculture present increases the amount of Al released into the ecosystem [84]. This can be harmful to the growth of propagules, therefore influencing the amount of growth occurring within the ecosystem, although it has also been stated that mangroves are able to withstand aluminium, and this does not influence their growth as much as the varying environmental factors [85,86]. This is supported by the little variance in the biomass across the sites; therefore, it could be argued that metals found in mangroves are not necessarily influenced by aquaculture but, rather, through the composition of the soil itself [87]. The results gathered from this study support this due to the increased levels of Al observed at the aquaculture sites. However, this could be explained by the positioning of these sites being set further inland compared to the others, as a result of which the soil composition would be different. Even though this is true, the other sites showed similarities in their subsoil Al levels, suggesting that the soil composition differences may not be the sole contributing factor to the increase in Al in the topsoil.
The most frequently observed family of crabs, throughout all ecosystems, was Grapsidae, which typically predate upon propagules as their main source of food [88]. As such, increased propagule availability could also increase the abundance of crabs from this family, given the propensity of these species to feed on the propagules. In particular, smaller propagules have been shown to be at greater risk of predation from crabs [89]. On the other hand, since it can take up to six years for a mangrove to produce propagules [90], areas where there are predominantly younger mangroves are likely to have fewer propagules. For example, mangrove ecosystems that are being restored from a completely degraded state could see fewer Grapsidae species when conducting these surveys at the beginning of the restoration. It should be noted, however, that the average age of individual trees present is not the only factor that could influence propagule abundance. For example, mangrove ecosystems that are degraded could also produce fewer propagules due to a change in the coastal environment [35] and could therefore see fewer crabs. In addition, the diversity of saplings in restored areas has been found to be a key factor for maintaining high diversity of crabs [50].
Sites 3 and 4 are located within the Gita Nada Marine Protected Area (MPA), which has an area of 210 km2 [91]. At both of these sites, rod-and-line fishing methods were observed. When looking at this area on the Marine Conservation Institute [91] database of MAPs, the level of protection for fishing in this area is classed as “Less Protected/Unknown”. According to local knowledge, the area where the mangrove ecosystems that were surveyed are located is classed as a rod-and-line or sustenance-only fishing zone. This could also explain why there was an increase in the number of fish sightings at these sites, as the zone implemented minimises the impact that fishing has on the fish assemblages in the area [92]. Sites 1 and 2 proved to be interesting in terms of their aquaculture observations. The utilisation of the mangroves in this way was seen at both sites; however, Site 1 was found to have more additional fishing pressure. This is most likely due to the protection offered by the local department of forestry at Site 2, where they are conducting the restoration and allowing locals to use the site for aquaculture and, thus, may be restricting fishing access. Additionally, the aquaculture permitted appears to be minimal based on the observations made at the site during this study.
This was not the same for the restored Site 5, where numerous fishing techniques were observed. Given that there appeared to be very little presence of the organisations running the restoration program at that site, it is highly probable that the site is not being effectively monitored, which may have led to an increase in fishing pressure. Additionally, the survey at this site only took a total of ten hours and ten minutes, so this only provides a snapshot of the fishing pressure, suggesting that it could be greater than was observed. On the other hand, the short survey time also means that any monitoring that is in place for this site could have been missed. Additionally, Site 6 appeared to have few-to-no regulations regarding any of the fishing vessels seen or the fishing methods being used. Monitoring fishing pressure is vital to ensure the productivity of the mangrove ecosystems and to ensure that local people are able to benefit from them not only now, but in the future as well [93]. Sites 5 and 6 both support a large fishing industry for the local communities surrounding them [94]. As such, both sites should be continually monitored, with data being collected not only on the health of the mangroves but also regarding the fishing pressure and catch per unit effort. These data could not only provide vital information on the fish diversity and richness of the species that are occupying the mangroves but also provide insight into those of the areas outside of the MPAs or protected ecosystems that are benefitting from a spillover effect [95].
5. Conclusions
In conclusion, we found that presence of aquaculture and management does not necessarily impact the vegetation structure in mangrove ecosystems, but there are more hidden mechanisms that should be considered. First, the aluminium content is high in aquaculture sites, and this also causes a negative flow of organic carbon available. Second, the nitrogen content, which is positively linked with mangrove growth, was higher in aquaculture sites, but this may be due to the presence of nitrifying bacteria in the ecosystem that have the ability to break down and release N. The presence of restoration projects decreased the available nitrogen in the ecosystem, potentially suggesting that the activities required for restoration to occur are hindering the amount of N that is available within the ecosystem. Crab richness and abundance were higher at aquaculture sites and in plots with tree saplings, suggesting that the species of crabs present preferred areas with propagules for feeding. Finally, on a worrying note, we found fishing pressure at all of the sites surveyed during our assessment, and we suggest that the sustainability of fishing should be evaluated. Management is a potential solution to preserve the variety of fauna and flora hosted and the ecosystem services provided by mangrove ecosystems, but our study did not find substantial improvements of vegetation, soil, crabs, or fishing pressure compared to unmanaged sites. We suggest promoting the diversity of mangrove saplings, targeting larger areas for restoration (restoration activities focused on only a small fraction of the sites selected for restoration), and monitoring restoration success (including soil and biodiversity parameters and resource use) over time.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/10.3390/resources13090117/s1: Table S1: Dataset used on mangrove vegetation parameters; Table S2: Dataset used on soil, crabs, and linked mangrove vegetation.
Author Contributions
Conceptualisation, C.H. and M.C.; methodology, C.H. and M.C.; software, C.H. and M.C.; validation, C.H. and M.C.; formal analysis, C.H. and M.C.; investigation, C.H.; resources, C.H., I.N.Y.P., K.M., and M.C.; data curation, C.H.; writing—original draft preparation, C.H., M.W.B., and M.C.; writing—review and editing, J.C., I.N.Y.P., and K.M.; visualisation, C.H. and M.C.; supervision, M.C. and M.W.B.; project administration, M.C. and K.M.; funding acquisition, C.H., J.C., and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Oxford Brookes University’s HLS Developing Potential Research Excellence Award and by the Royal Geographical Society Geographical Fieldwork Grant.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
We thank Indonesia’s National Research and Innovation Agency in Jakarta (BRIN) for authorising this study. We thank the editor and four reviewers for their constructive comments and suggestions for improvement.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Results of the generalised linear mixed models.
Table A1.
Results of the generalised linear mixed models.
| Response Variable | Predictor | Estimate | Std. Error | Z Value | p Value |
|---|---|---|---|---|---|
| Crab abundance | Intercept | 3.09 | 0.35 | 8.93 ** | <0.001 |
| No aquaculture vs. aquaculture | −1.30 | 0.49 | −2.59 ** | 0.010 | |
| Managed vs. unmanaged | −0.72 | 0.53 | −1.36 | 0.174 | |
| Interaction effect | 0.85 | 0.73 | 1.16 | 0.246 | |
| Crab richness | Intercept | 1.51 | 0.29 | 5.27 ** | <0.001 |
| No aquaculture vs. aquaculture | −1.21 | 0.45 | −2.69 ** | 0.007 | |
| Managed vs. unmanaged | −0.70 | 0.48 | −1.46 | 0.146 | |
| Interaction effect | 0.80 | 0.68 | 1.18 | 0.239 | |
| Crab diversity | Intercept | −0.58 | 0.56 | −1.04 | 0.297 |
| No aquaculture vs. aquaculture | −1.13 | 0.83 | −1.37 | 0.171 | |
| Managed vs. unmanaged | −0.36 | 0.85 | −0.43 | 0.668 | |
| Interaction effect | −0.01 | 1.26 | −0.01 | 0.994 | |
| Mangrove abundance | Intercept | 4.16 | 2.02 | 2.06 * | 0.039 |
| No aquaculture vs. aquaculture | −1.13 | 2.02 | −0.56 | 0.575 | |
| Managed vs. unmanaged | −0.10 | 0.62 | −0.16 | 0.874 | |
| Interaction effect | −0.26 | 0.80 | −0.33 | 0.744 | |
| Mangrove AGB | Intercept | 5.91 | 0.39 | 15.15 ** | <0.001 |
| No aquaculture vs. aquaculture | 0.71 | 0.47 | 1.53 | 0.126 | |
| Managed vs. unmanaged | 0.45 | 0.54 | 0.83 | 0.409 | |
| Interaction effect | −0.67 | 0.65 | −1.02 | 0.306 | |
| Mangrove BGB | Intercept | 5.64 | 0.36 | 15.48 ** | <0.001 |
| No aquaculture vs. aquaculture | 0.04 | 0.45 | 0.10 | 0.925 | |
| Managed vs. unmanaged | 0.17 | 0.51 | 0.33 | 0.742 | |
| Interaction effect | −0.02 | 0.62 | −0.03 | 0.979 | |
| Mangrove richness | Intercept | 1.18 | 1.22 | 0.94 | 0.335 |
| No aquaculture vs. aquaculture | −0.79 | 1.23 | −0.64 | 0.522 | |
| Managed vs. unmanaged | −0.23 | 0.33 | −0.68 | 0.500 | |
| Interaction effect | 0.09 | 0.41 | 0.23 | 0.822 | |
| Subsoil Al | Intercept | 6.95 | 0.37 | 19.01 ** | <0.001 |
| No aquaculture vs. aquaculture | −0.31 | 0.57 | −0.54 | 0.589 | |
| Managed vs. unmanaged | 0.49 | 0.47 | 1.04 | 0.297 | |
| Interaction effect | 0.21 | 0.60 | 0.34 | 0.731 | |
| Subsoil C | Intercept | 2.20 | 0.49 | 4.48 ** | <0.001 |
| No aquaculture vs. aquaculture | 0.95 | 0.60 | 1.57 | 0.116 | |
| Managed vs. unmanaged | 0.59 | 0.70 | 0.84 | 0.400 | |
| Interaction effect | −0.64 | 0.85 | −0.76 | 0.450 | |
| Subsoil N | Intercept | −2.26 | 0.17 | −13.53 ** | <0.001 |
| No aquaculture vs. aquaculture | −0.85 | 0.23 | −3.64 ** | <0.001 | |
| Managed vs. unmanaged | 0.08 | 0.23 | 0.37 | 0.715 | |
| Interaction effect | −0.04 | 0.32 | −0.12 | 0.902 | |
| Topsoil Al | Intercept | 7.30 | 0.42 | 17.48 ** | <0.001 |
| No aquaculture vs. aquaculture | −1.17 | 0.60 | −1.93 t | 0.054 | |
| Managed vs. unmanaged | 0.24 | 0.57 | 0.42 | 0.672 | |
| Interaction effect | 0.84 | 0.78 | 1.08 | 0.280 | |
| Topsoil C | Intercept | 2.07 | 0.48 | 4.35 ** | <0.001 |
| No aquaculture vs. aquaculture | 1.01 | 0.58 | 1.73 t | 0.084 | |
| Managed vs. unmanaged | 0.38 | 0.67 | 0.56 | 0.573 | |
| Interaction effect | −0.58 | 0.82 | −0.71 | 0.480 | |
| Topsoil N | Intercept | −2.07 | 0.17 | −11.97 ** | <0.001 |
| No aquaculture vs. aquaculture | −0.93 | 0.24 | −3.82 ** | <0.001 | |
| Managed vs. unmanaged | −0.47 | 0.27 | −1.75 t | 0.080 | |
| Interaction effect | 0.61 | 0.36 | 1.72 t | 0.086 |
t p < 0.1; * p < 0.05; ** p < 0.01.

Table A2.
Contrast ratios (means and SE) and corresponding Z values and p values for the interaction effects between no aquaculture (NoA) vs. aquaculture (Aqu) and managed (Man) vs. unmanaged (Unm).
Table A2.
Contrast ratios (means and SE) and corresponding Z values and p values for the interaction effects between no aquaculture (NoA) vs. aquaculture (Aqu) and managed (Man) vs. unmanaged (Unm).
| Variable | AquUnm/ NoAUnm | AquUnm/ AquMan | AquUnm/ NoAMan | NoAUnm/ AquMan | NoAUnm/ NoAMan | AquMan/ NoAMan |
|---|---|---|---|---|---|---|
| Crab abundance | 3.60 ± 1.78 Z = 2.6 *, p = 0.047 | 2.05 ± 1.08 Z = 1.4, p = 0.524 | 3.14 ± 1.53 Z = 2.4 t, p = 0.087 | 0.57 ± 0.31 Z = −1.0, p = 0.737 | 0.87 ± 0.45 Z = −0.3, p = 0.994 | 1.53 ± 0.84 Z = 0.8, p = 0.862 |
| Crab richness | 3.35 ± 1.50 Z = 2.7 *, p = 0.035 | 2.01 ± 0.96 Z = 1.5, p = 0.465 | 3.01 ± 1.33 Z = 2.5 t, p = 0.059 | 0.60 ± 0.31 Z = −1.0, p = 0.758 | 0.90 ± 0.44 Z = −0.2, p = 0.996 | 1.50 ± 0.77 Z = 0.8, p = 0.859 |
| Crab diversity | 3.10 ± 2.56 Z = 1.4, p = 0.520 | 1.44 ± 1.23 Z = 0.4, p = 0.974 | 4.50 ± 4.05 Z = 1.7, p = 0.337 | 0.47 ± 0.41 Z = −0.9, p = 0.823 | 1.45 ± 1.35 Z = 0.4, p = 0.978 | 3.13 ± 2.98 Z = 1.2, p = 0.630 |
| Mangrove abundance | 3.11 ± 6.27 Z = 0.6, p = 0.944 | 1.10 ± 0.69 Z = 0.2, p = 0.999 | 4.45 ± 9.23 Z = 0.7, p = 0.889 | 0.36 ± 0.58 Z = −0.6, p = 0.920 | 1.43 ± 0.71 Z = 0.7, p = 0.888 | 4.03 ± 6.80 Z = 0.8, p = 0.842 |
| Mangrove AGB | 0.49 ± 0.23 Z = −1.5, p = 0.420 | 0.64 ± 0.35 Z = −0.8, p = 0.842 | 0.61 ± 0.29 Z = −1.0, p = 0.722 | 1.31 ± 0.59 Z = 0.6, p = 0.935 | 1.25 ± 0.46 Z = 0.6, p = 0.931 | 0.96 ± 0.44 Z = −0.1, p = 0.999 |
| Mangrove BGB | 0.96 ± 0.43 Z = −0.1, p = 0.999 | 0.85 ± 0.43 Z = −0.3, p = 0.988 | 0.82 ± 0.37 Z = −0.4, p = 0.972 | 0.88 ± 0.39 Z = −0.3, p = 0.992 | 0.85 ± 0.31 Z = −0.4, p = 0.975 | 0.98 ± 0.43 Z = −0.1, p = 1.000 |
| Mangrove richness | 2.20 ± 2.71 Z = 0.6, p = 0.919 | 1.25 ± 0.42 Z = 0.7, p = 0.907 | 2.51 ± 3.15 Z = 0.7, p = 0.884 | 0.57 ± 0.56 Z = −0.6, p = 0.941 | 1.14 ± 0.28 Z = 0.5, p = 0.949 | 2.00 ± 2.03 Z = 0.7, p = 0.902 |
| Subsoil Al | 1.36 ± 0.77 Z = 0.5, p = 0.995 | 0.61 ± 0.29 Z = −1.0, p = 0.880 | 0.68 ± 0.34 Z = −0.8, p = 0.968 | 0.45 ± 0.25 Z = −1.4, p = 0.634 | 0.50 ± 0.19 Z = −1.9, p = 0.326 | 1.11 ± 0.54 Z = 0.2, p = 1.000 |
| Subsoil C | 0.71 ± 0.16 Z = −1.6, p = 0.454 | 0.80 ± 0.24 Z = −0.8, p = 0.741 | 0.73 ± 0.16 Z = −1.5, 0.454 | 1.13 ± 0.23 Z = 0.6, p = 0.742 | 1.02 ± 0.22 Z = 0.1, p = 0.907 | 0.90 ± 0.24 Z = −0.5, p = 0.742 |
| Subsoil N | 2.33 ± 0.54 Z = 3.6 **, p = 0.002 | 0.92 ± 0.21 Z = −0.4, p = 0.983 | 2.23 ± 0.51 Z = 3.47 **, p = 0.003 | 0.40 ± 0.09 Z = −4.1 **, p < 0.001 | 0.96 ± 0.22 Z = −0.2, p = 0.997 | 2.42 ± 0.55 Z = 3.9 **, p < 0.001 |
| Topsoil Al | 3.21 ± 1.94 Z = 1.9, p = 0.281 | 0.79 ± 0.45 Z = −0.4, p = 0.999 | 1.09 ± 0.56 Z = 0.2, p = 1.000 | 0.25 ± 0.14 Z = −2.4 t, p = 0.090 | 0.34 ± 0.18 Z = −2.0, p = 0.226 | 1.34 ± 0.68 Z = 0.66, p = 0.986 |
| Topsoil C | 0.67 ± 0.19 Z = −1.7, p = 0.535 | 0.85 ± 0.20 Z = −0.6, p = 0.673 | 0.72 ± 0.18 Z = −1.4, p = 0.535 | 1.26 ± 0.21 Z = 1.1, p = 0.580 | 1.07 ± 0.14 Z = 0.4, p = 0.673 | 0.85 ± 0.20 Z = −0.7, p = 0.673 |
| Topsoil N | 2.54 ± 0.62 Z = 3.8 **, p < 0.001 | 1.60 ± 0.43 Z = 1.7, p = 0.122 | 2.20 ± 0.52 Z = 3.32 **, p = 0.003 | 0.63 ± 0.17 Z = −1.7, p = 0.122 | 0.87 ± 0.20 Z = −0.6, p = 0.538 | 1.38 ± 0.36 Z = 1.2, p = 0.264 |
t p < 0.1; * p < 0.05; ** p < 0.01.
References
- Biswas, P.L.; Biswas, S.R. Mangrove Forests: Ecology, Management, and Threats. In Life on Land, Encyclopedia of the UN Sustainable Development Goals; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–14. [Google Scholar]
- Kathiresan, K.; Bingham, B.L. Biology of Mangroves and Mangrove Ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Krauss, K.W.; Ball, M.C. On the halophytic nature of mangroves. Trees-Struct. Funct. 2013, 27, 7–11. [Google Scholar] [CrossRef]
- Naidoo, T.; Rajkaran, A. Sershen Impacts of plastic debris on biota and implications for human health: A South African perspective. S. Afr. J. Sci. 2020, 116, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Suratman, M.N. Carbon Sequestration Potential of Mangroves in Southeast Asia. In Managing Forest Ecosystems: The Challenge of Climate Change; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Kauffman, J.C.H.; Thomas, G.C.; Kathleen, A.D.; Daniel, C.; Donato, D. Ecosystem carbon stocks of micronesian mangrove forests. Wetlands 2011, 31, 343–352. [Google Scholar] [CrossRef]
- Ferraz, A.; Saatchi, S.S.; Xu, L.; Hagen, S.C.; Chave, J.; Yu, Y.; Meyer, V.; Garcia, M.; Silva, C.A.; Roswintiart, O.; et al. Carbon storage potential in degraded forests of Kalimantan, Indonesia. Environ. Res. Lett. 2018, 13, 095001. [Google Scholar] [CrossRef]
- Donato, D.C.; Kauffman, J.B.; Murdiyarso, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Chatting, M.; Al-Maslamani, I.; Walton, M.; Skov, M.W.; Kennedy, H.; Husrevoglu, Y.S.; Vay, L.L. Future mangrove carbon storage under climate change and deforestation. Front. Mar. Sci. 2022, 9, 781876. [Google Scholar] [CrossRef]
- Sánchez-Núñez, D.A.; Bernal, G.; Pineda, J.E.M. The relative role of mangroves on wave erosion mitigation and sediment properties. Estuaries Coasts 2019, 42, 2124–2138. [Google Scholar] [CrossRef]
- Kamil, E.A.; Takaijudin, H.; Hashim, A.M. Mangroves As Coastal Bio-Shield: A Review of Mangroves Performance in Wave Attenuation. Civ. Eng. J. 2021, 7, 1964–1981. [Google Scholar] [CrossRef]
- Lee, W.K.; Tay, S.H.X.; Ooi, S.K.; Friess, D.A. Potential short wave attenuation function of disturbed mangroves. Estuar. Coast. Shelf Sci. 2021, 248, 106747. [Google Scholar] [CrossRef]
- Giesen, W.; Wulffraat, S.; Zieren, M.; Scholten, L. Mangrove Guidebook for Southeast Asia; UN Food and Agricultural Organization: Phnom Penh, Cambodia, 2007. [Google Scholar]
- Faridah-Hanum, I.; Kudus, K.A.; Saari, N.S. Plant Diversity and Biomass of Marudu Bay Mangroves in Malaysia. Pakistan J. Bot. 2012, 44, 151–156. [Google Scholar]
- Twilley, R.R.; Snedaker, S.C.; Yaez-Arancibia, A.; Medina, E. Biodiversity and ecosystem processes in tropical estuaries: Perspectives of mangrove ecosystems. In Scope-Scientific Committee on Problems of the Environment International Council of Scientific Unions; John and Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Mumby, P.J.; Edwards, A.J.; Rias-Gonzalez, J.E.; Lindeman, K.C.; Blackwell, P.G.; Gall, A.; Gorczynska, M.I.; Harborne, A.R.; Pescod, C.L.; Renken, H.; et al. Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 2004, 427, 533–536. [Google Scholar] [CrossRef]
- Satyanarayana, B.; Quispe-Zuniga, M.R.; Hugé, J.; Sulong, I.; Mohd-Lokman, H.; Dahdouh-Guebas, F. Mangroves Fueling Livelihoods: A Socio-Economic Stakeholder Analysis of the Charcoal and Pole Production Systems in the World’s Longest Managed Mangrove Forest. Front. Ecol. Evol. 2021, 9, 621721. [Google Scholar] [CrossRef]
- Aziz, A.A.; Dargusch, P.; Phinn, S.; Ward, A. Using REDD+ to balance timber production with conservation objectives in a mangrove forest in Malaysia. Ecol. Econ. 2015, 120, 108–116. [Google Scholar] [CrossRef]
- Hutchison, J.; Spalding, M.; zu Ermgassen, P. The Role of Mangroves in Fisheries Enhancement; The Nature Conservancy: Arlington County, VA, USA; Wetlands International: Wageningen, The Netherlands, 2014; pp. 1–54. [Google Scholar]
- Alongi, D.M. Present State and Future of the World’s Mangrove Forests. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Alongi, D.M. Climate Change and Mangroves. In Mangroves: Biodiversity, Livelihoods and Conservation; Springer Nature: Singapore, 2022; pp. 175–198. [Google Scholar]
- Polidoro, B.A.; Carpenter, K.E.; Collins, L.; Duke, N.C.; Ellison, A.M.; Ellison, J.C.; Farnsworth, E.J.; Fernando, E.S.; Kathiresan, K.; Koedam, N.E.; et al. The Loss of Species: Mangrove Extinction Risk and Geographic Areas of Global Concern. PLoS ONE 2010, 5, e10095. [Google Scholar] [CrossRef]
- Luther, D.A.; Greenberg, R. Mangroves: A Global Perspective on the Evolution and Conservation of Their Terrestrial Vertebrates. BioScience 2009, 59, 602–612. [Google Scholar] [CrossRef]
- Adame, M.F.; Connolly, R.M.; Turschwell, M.P.; Lovelock, C.E.; Fatoyinbo, T.; Lagomasino, D.; Goldberg, L.A.; Holdorf, J.; Friess, D.A.; Sasmito, S.D.; et al. Future carbon emissions from global mangrove forest loss. Glob. Chang. Biol. 2021, 27, 2856–2866. [Google Scholar] [CrossRef]
- Arifanti, V.B.; Sidik, F.; Mulyanto, B.; Susilowati, A.; Wahyuni, T.; Subarno; Yulianti; Yuniarti, N.; Aminah, A.; Suita, E.; et al. Challenges and Strategies for Sustainable Mangrove Management in Indonesia: A Review. Forests 2022, 13, 695. [Google Scholar] [CrossRef]
- Luom, T.T.; Phong, N.T.; Smithers, S.; Van Tai, T. Protected mangrove forests and aquaculture development for livelihoods. Ocean Coast. Manag. 2021, 205, 105553. [Google Scholar] [CrossRef]
- Miteva, D.A.; Murray, B.C.; Pattanayak, S.K. Do protected areas reduce blue carbon emissions? A quasi-experimental evaluation of mangroves in Indonesia. Ecol. Econ. 2015, 119, 127–135. [Google Scholar] [CrossRef]
- Tengku Hashim, T.M.Z.; Engku Ariff, E.A.R.; Suratman, M.N. Aquaculture in Mangroves. In Mangroves: Ecology, Biodiversity and Management; Springer: Singapore, 2021; pp. 419–438. [Google Scholar]
- Richards, D.R.; Friess, D.A. Rates and drivers of mangrove deforestation in Southeast Asia, 2000–2012. Proc. Natl. Acad. Sci. USA 2016, 113, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Valiela, I.; Bowen, J.L.; York, J.K. Mangrove Forests: One of the World’s Threatened Major Tropical Environments. Bioscience 2001, 51, 807–815. [Google Scholar] [CrossRef]
- McSherry, M.; Davis, R.P.; Andradi-Brown, D.A.; Ahmadia, G.N.; Van Kempen, M.; Brian, S.W. Integrated mangrove aquaculture: The sustainable choice for mangroves and aquaculture? Front. For. Glob. Chang. 2023, 6, 1094306. [Google Scholar] [CrossRef]
- Balmford, A.; Bruner, A.; Cooper, P.; Costanza, R.; Farber, S.; Green, R.E.; Jenkins, M.; Jefferiss, P.; Jessamy, V.; Madden, J.; et al. Economic Reasons for Conserving Wild Nature. Science 2002, 297, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Aheto, D.W.; Kankam, S.; Okyere, I.; Mensah, E.; Osman, A.; Jonah, F.E.; Mensah, J.C. Community-Based Mangrove Forest Management: Implications for Local Livelihoods and Coastal Resource Conservation along the Volta Estuary Catchment Area of Ghana. Ocean. Coast Manag. 2016, 127, 43–54. [Google Scholar] [CrossRef]
- Mesta, P.; Setturu, B.; Chandran, M.D.S.; Ramachandra, T.V. Inventorying, Mapping and Monitoring of Mangroves towards Sustainable Management of West Coast, India. Geophys. Remote Sens. 2014, 3, 3–9. [Google Scholar]
- Field, C.D. Rehabilitation of mangrove ecosystems, a review. Mar. Pollut. Bull. 1998, 37, 383–392. [Google Scholar] [CrossRef]
- Ibrahim, F.H.; Yusoff, F.M.; Fitrianto, A.; Nuruddin, A.A.; Gandaseca, S.; Samdin, Z.; Kamarudin, N.; Nurhidayu, S.; Kassim, M.R.; Hakeem, K.R.; et al. How to develop a comprehensive Mangrove Quality Index? MethodsX 2019, 6, 1591–1599. [Google Scholar] [CrossRef]
- Faridah-Hanum, I.; Yusoff, F.M.; Fitrianto, A.; Ainuddin, N.A.; Gandaseca, S.; Zaiton, S.; Norizah, K.; Nurhidayu, S.; Roslan, M.K.; Hakeem, K.R.; et al. Development of a comprehensive mangrove quality index (MQI) in Matang Mangrove; Assessing mangrove ecosystem health. Ecol. Indic. 2019, 102, 103–117. [Google Scholar] [CrossRef]
- Biswas, S.R.; Mallik, A.U.; Choudhury, J.K.; Nishat, A. A unified framework for the restored of Southeast Asian mangroves—Bridging ecology, society and economics. Wetl. Ecol. Manag. 2009, 17, 365–383. [Google Scholar] [CrossRef]
- Rahman; Lokollo, F.F.; Manuputty, G.D.; Hukubun, R.D.; Krisye; Maryono; Wawo, M.; Wardiatno, Y. A review on the biodiversity and conservation of mangrove ecosystems in Indonesia. Biodivers Conserv. 2024, 33, 875–903. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nat. Cell Biol. 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Zaman, M.R.; Rahman, M.S.; Ahmed, S.; Zuidema, P.A. What drives carbon stocks in a mangrove forest? The role of stand structure, species diversity and functional traits. Estuar. Coast. Shelf Sci. 2023, 295, 108556. [Google Scholar]
- Duncanson, L.; Armston, J.; Disney, M.; Avitabile, V.; Barbier, N.; Calders, K.; Carter, S.; Chave, J.; Herold, M.; Crowther, T.W.; et al. The importance of consistent global forest aboveground biomass product validation. Surv. Geophys. 2019, 40, 979–999. [Google Scholar] [CrossRef]
- Mcleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.E.; Schlesinger, W.H.; Silliman, B.R. A Blueprint for Blue Carbon: Toward an Improved Understanding of the Role of Vegetated Coastal Habitats in Sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef]
- Inoue, T. Carbon sequestration in Mangroves. In Blue Carbon in Shallow Coastal Ecosystems. Carbon Dynamics, Policy, and Implementation; Springer: Singapore, 2019; pp. 73–99. [Google Scholar]
- Valiela, I.; Cole, M.L. Comparative evidence that saltmarshes and mangroves may protect sea grass meadow from land-derived nitrogen loads. Ecosystems 2002, 5, 92–102. [Google Scholar] [CrossRef]
- Ma, L.; Yang, S. Growth and physiological response of Kandelia obovata and Bruguiera sexangula seedlings to aluminum stress. Environ. Sci. Pollut. Res. 2022, 29, 43251–43266. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ganade, G.; de Attayde, J.L. Restoration versus natural regeneration in a neotropical mangrove: Effects on plant biomass and crab communities. Ocean Coast. Manag. 2015, 110, 38–45. [Google Scholar] [CrossRef]
- Aschenbroich, A.; Michaud, E.; Stieglitz, T.; Fromard, F.; Gardel, A.; Tavares, M.; Thouzeau, G. Brachyuran crab community structure and associated sediment reworking activities in pioneer and young mangroves of French Guiana, South America. Estuar. Coast. Shelf Sci. 2016, 182, 60–71. [Google Scholar] [CrossRef]
- Kristensen, E.; Penha-Lopes, G.; Delefosse, M.; Valdemarsen, T.; Quintana, C.O.; Banta, G.T. What is bioturbation? The need for a precise definition for fauna in aquatic sciences. Mar. Ecol. Prog. Ser. 2012, 446, 285–302. [Google Scholar] [CrossRef]
- Agusto, L.E.; Sara, F.; Jimenez, P.J.; Quadros, A.; Cannicci, S. Structural characteristics of crab burrows in Hong Kong mangrove forests and their role in ecosystem engineering. Estuar. Coast. Shelf Sci. 2020, 248, 106973. [Google Scholar] [CrossRef]
- Sidharta, B.R. Peta mangrove nasional 2021. Kedaulatan Rakyat, 21 October 2021. [Google Scholar] [CrossRef]
- Proisy, C.; Viennois, G.; Sidik, F.; Andayani, A.; Enright, J.A.; Guitet, S.; Gusmawati, N.; Lemonnier, H.; Muthusankar, G.; Olagoke, A.; et al. Monitoring mangrove forests after aquaculture abandonment using time series of very high spatial resolution satellite images: A case study from the Perancak estuary, Bali, Indonesia. Mar. Pollut. Bull. 2018, 131, 61–71. [Google Scholar] [CrossRef]
- Seary, R.; Spencer, T.; Bithell, M.; McOwen, C.; Ota, Y. Defining mangrove-fisheries: A typology from the Perancak Estuary, Bali, Indonesia. PLoS ONE 2021, 16, e0249173. [Google Scholar] [CrossRef] [PubMed]
- Rigby, I.; Siangian, R.A.S. SORCE bi-monthly report for TreeApp. Unpublished report. 2023. [Google Scholar]
- Nurhaliza, A.P.; Damayanti, A.; Dimyati, M. Monitoring area and health changes of mangrove forest using multitemporal landsat imagery in Taman Hutan Raya Ngurah Rai, Bali Province. IOP Conf. Ser. Earth Environ. Sci. 2021, 673, 012050. [Google Scholar] [CrossRef]
- Free Indonesia GIS Map Files. Available online: https://simplemaps.com/gis/country/id (accessed on 15 May 2024).
- Tobias, A.; Umali, A.G.A.; Malabrigo, P.; Galang, M.A. Mangrove Forest Inventory and Estimation of Carbon Storage and Sedimentation in Pagbilao. Wealth Account. Valuat. Ecosyst. Serv. 2017, 1–95. Available online: https://www.wavespartnership.org/ (accessed on 30 May 2024).
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sarker, S.K.; Friess, D.A.; Kamruzzaman, M.; Jacobs, M.; Sillanpää, M.; Naabeh, C.S.S.; Pretzsch, H. Mangrove tree growth is size-dependent across a large-scale salinity gradient. For. Ecol. Manag. 2023, 537, 120954. [Google Scholar] [CrossRef]
- Dharmawan, I.W.S.; Siregar, C.A. Karbon tanah dan pendugaan karbon tegakan. J. Penelit. Hutan Dan Konserv. Alam 2008, 5, 317–328. [Google Scholar] [CrossRef]
- Vinh, T.V.; Marchand, C.; Linh, T.V.K.; Vinh, D.D.; Allenbach, M. Allometric models to estimate above-ground biomass and carbon stocks in Rhizophora apiculata tropical managed mangrove forests (Southern Viet Nam). For. Ecol. Manag. 2019, 434, 131–141. [Google Scholar] [CrossRef]
- Clough, B.F.; Scott, K. Allometric relationships for estimating above-ground biomass in six mangrove species. For. Ecol. Manag. 1989, 27, 117–127. [Google Scholar] [CrossRef]
- Fromard, F.; Puig, H.; Mougin, E.; Marty, G.; Betoulle, J.L.; Cadamuro, L. Structure, above-ground biomass and dynamics of mangrove ecosystems: New data from French Guiana. Oecologia 1998, 115, 39–53. [Google Scholar] [CrossRef]
- Hanh, N.T.H.; Tinh, P.H.; Tuan, M.S. Allometry and biomass accounting for mangroves Kandelia obovata Sheue, Liu & Yong and Sonneratia caseolaris (L.) Engler planted in coastal zone of Red River Delta, Vietnam. Int. J. Dev. Res. 2016, 6, 7804–7808. [Google Scholar]
- Komiyama, A.; Poungparn, S.; Kato, S. Common allometric equations for estimating the tree weight of mangroves. J. Trop. Ecol. 2005, 21, 471–477. [Google Scholar] [CrossRef]
- Komiyama, A.; Ong, J.E.; Poungparn, S. Allometry, biomass, and productivity of mangrove forests: A review. Aquat. Bot. 2008, 89, 128–137. [Google Scholar] [CrossRef]
- Gevana, D.; Im, S. Allometric models for Rhizophora stylosa Griff. in dense monoculture plantation in the Philippines. Malays. For. 2016, 79, 39–53. [Google Scholar]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H.; Soltanpour, P.N.; Tabatabai, M.A.; Johnston, C.T.; Sumner, M.E. Methods of Soil Analysis, Part 3–Chemical Methods; Soil Science Society of America Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Stiepani, J.; Gillis, L.G.; Chee, S.Y.; Pfeiffer, M.; Nordhaus, I. Impacts of urbanization on mangrove forests and brachyuran crabs in Penang, Malaysia. Reg. Environ. Chang. 2021, 21, 69. [Google Scholar] [CrossRef]
- Reis-Filho, J.A.; Harvey, E.S.; Giarrizzo, T. Impacts of small-scale fisheries on mangrove fish assemblages. ICES J. Mar. Sci. 2018, 76, 153–164. [Google Scholar] [CrossRef]
- Tahiluddin, A.; Sarri, J. An Overview of Destructive Fishing in the Philippines. Acta. Nat. Sci. 2022, 3, 116–125. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modelling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; R Package Version 020; University of Regensburg: Regensburg, Germany, 2018. [Google Scholar]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- van Bijsterveldt, C.E.J.; Debrot, A.O.; Bouma, T.J.; Maulana, M.B.; Pribadi, R.; Schop, J.; Tonneijck, F.H.; van Wesenbeeck, B.K. To Plant or Not to Plant: When can Planting Facilitate Mangrove Restoration? Front. Environ. Sci. 2022, 9, 690011. [Google Scholar] [CrossRef]
- Tamin, N.M.; Zakaria, R.; Hashim, R.; Yin, Y. Establishment of Avicennia marina mangroves on accreting coastline at Sungai Haji Dorani, Selangor, Malaysia. Estuar. Coast Shelf Sci. 2011, 94, 334–342. [Google Scholar] [CrossRef]
- Zhila, H.; Mahmood, H.; Rozainah, M.Z. Biodiversity and biomass of a natural and degraded mangrove forest of Peninsular Malaysia. Environ. Earth Sci. 2015, 71, 4629–4635. [Google Scholar] [CrossRef]
- Hilmi, N.; Chami, R.; Sutherland, M.D.; Hall-Spencer, J.M.; Lebleu, L.; Benitez, M.B.; Levin, L.A. The role of blue carbon in climate change mitigation and carbon stock conservation. Front. Clim. 2021, 3, 710546. [Google Scholar] [CrossRef]
- Herrera-Silveira, J.A.; Pech-Cardenas, M.A.; Morales-Ojeda, S.M.; Cinco-Castro, S.; Camacho-Rico, A.; Sosa, J.P.C.; Mendoza-Martinez, J.E.; Pech-Poot, E.Y.; Montero, J.; Teutli-Hernandez, C. Blue carbon of Mexico, carbon stocks and fluxes: A systematic review. PeerJ 2020, 8, e8790. [Google Scholar] [CrossRef]
- United Nations. Adoption of the Paris Agreement; I: Proposal by the President (Draft Decision); United Nations: New York, NY, USA, 2015; p. 2. [Google Scholar]
- Taillardat, P.; Friess, D.A.; Lupascu, M. Mangrove blue carbon strategies for climate change mitigation are most effective at the national scale. Biol. Lett. 2018, 14, 20180251. [Google Scholar] [CrossRef]
- Reis, C.R.G.; Nardoto, G.B.; Oliveira, R.S. Global overview on nitrogen dynamics in mangroves and consequences of increasing nitrogen availability for these systems. Plant Soil 2017, 410, 1–19. [Google Scholar] [CrossRef]
- Karstens, S.; Lukas, M.C. Contested aquaculture development in the protected mangrove forests of the Kapuas estuary, West Kalimantan. Geoöko 2014, 35, 78–121. [Google Scholar]
- Reef, R.; Feller, I.C.; Lovelock, C.E. Nutrition of mangroves. Tree Physiol. 2010, 30, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Oxmann, J.F.; Pham, Q.H.; Schwendenmann, L.; Stellman, J.M.; Lara, R.J. Mangrove reforestation in Vietnam: The effect of sediment physicochemical properties on nutrient cycling. Plant Soil 2010, 326, 225–241. [Google Scholar] [CrossRef]
- Tam, N.F.Y.; Yao, M. Normalisation and heavy metal contamination in mangrove sediments. Sci. Total Environ. 2019, 216, 33–39. [Google Scholar] [CrossRef]
- Parman, R.P.; Kamarudin, N.; Ibrahim, F.H.; Nuruddin, A.A.; Omar, H.; Abdul Wahab, Z. Geostatistical Analysis of Mangrove Ecosystem Health: Mapping and Modelling of Sampling Uncertainty Using Kriging. Forests 2022, 13, 1185. [Google Scholar] [CrossRef]
- Clarke, P.J.; Kerrigan, R.A. The effects of seed predators on the recruitment of mangroves. J. Ecol. 2002, 90, 728–736. [Google Scholar] [CrossRef]
- Imbert, D.; Rousteau, A.; Scherrer, P. Ecology of mangrove growth and recovery in the Lesser Antilles: State of knowledge and basis for restoration projects. Restor. Ecol. 2000, 8, 230–236. [Google Scholar] [CrossRef]
- Marine Conservation Institute. Global Marine Fishing Protection, Available at: Global Marine Protection|Marine Protection Atlas 2023. Available online: https://mpatlas.org (accessed on 24 May 2024).
- Grorud-Colvert, K.; Sullivan-Stack, J.; Roberts, C.; Constant, V.; Horta e Costa, B.; Pike, E.P.; Kingston, N.; Laffoley, D.; Sala, E.; Claudet, J.; et al. The MPA Guide: A framework to achieve global goals for the ocean. Science 2021, 373, 1215. [Google Scholar] [CrossRef]
- Russ, G.R.; Alcala, A.C.; Maypa, A.P.; Calumpong, H.P.; White, A.T. Marine reserve benefits local fisheries. Ecol. Appl. 2004, 14, 597–606. [Google Scholar] [CrossRef]
- Gokkon, B. ‘Bali Mangrove Bay Is Now a Conservation Zone, Nixing Reclamation Plan’. Available online: https://news.mongabay.com/2019/10/bali-benoa-bay-mangroves-conservation-reclamation/ (accessed on 6 May 2024).
- Gell, F.R.; Roberts, C.M. Benefits beyond boundaries: The fishery effects of marine reserves. Trends Ecol. Evol. 2003, 18, 448–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).