1. Introduction
In recent decades, the aim of minimizing environmental damages, and the disposal and use of waste has gained prominence in the spirit of sustainable development. One of the most involved industries is building material production, with a significant amount of research already conducted regarding the production of bricks, roof tiles, concrete, and mortar from different types of waste [
1].
From the beginning of using the Bayer process in aluminum production, a severe and in practice still unsolved problem has arisen: the treatment of the produced red mud [
2]. This non-soluble waste poses a serious environmental problem, with 1–1.5 metric t of red mud produced for every metric ton of aluminum-oxide [
3,
4]. Currently, the most prominent and almost only way of disposal is depositing, which is still a cause of concern, due to the real and potential events of catastrophe, such as the leaking of the reserve, or the collapse of the holding dam.
One possible field of use for treating red mud is in the building industry, more specifically in brick production. The possibilities for using it in manufacturing technologies that are using sintered red mud by itself have been a subject of research since the 1980s [
5,
6,
7,
8]. Currently, the most effective field of use is as a mixing material in brick and roof tile production, where it can be mixed in large amounts with clay. Currently, there are two existing approaches in the industry, depending on whether the final product is fired or not. Traditional bricks are sintered at 1000 °C to reach the desired properties (strength, porosity, vapor permeability, color, etc.). For the production of non-fired products, an inorganic binding agent (such as cement) is used. Between 1972 and 2008, 14 patents were filed in the field of using red mud in the building ceramics industry, 3 for burned and 11 for non-burned products [
9].
The already existing heterogeneity of natural clay grants the possibility of mixing in large amounts of red mud. The high iron, sodium, calcium, magnesium, and other melting point-reducing component content of red mud even makes it advantageous to mix in, particularly, if the final product is sintered [
8,
10].
These properties of red mud motivated researchers to conduct experiments on its use as a raw material. These experiments sought a way to use the red mud in itself and fully [
5,
6,
7,
8,
9]. Xu et al. [
9] sintered four differently composed red mud at 1140 °C. Their study showed that all products were suitable for internal and external brickwork. Moya et al. [
5] reported that the optimal sintering temperature for bricks containing red mud should be at least 1200 °C, as this is the temperature where the optimal properties, such as the largest shrinkage and the lowest water absorption, can be achieved.
The work of Kavas [
11] can be considered an in-between solution. While processing borate minerals, a significant amount of boron-containing matter remains as a byproduct. If this matter is mixed with red mud, advantageous building ceramic products can be obtained, because of the plasticizing effect of boron. Samples containing 15 wt% of red mud were fired at 900 °C and reached a bending strength of 45.3 MPa.
Another main solution is mixing red mud and clay together. According to several authors [
12,
13,
14,
15,
16,
17,
18], this mixing step can improve the mechanical-technical properties of the product. Pérez-Villarejo [
14] found that the mass fraction of red mud in clay should be 0.5 maximum for brick production. In this case, the optimal sintering temperature should be 950 °C, with one hour of holding time. The final products had a compressive strength of 52.5 MPa, comparable to bricks made of pure clay. He et al. [
15] tried different mixing ratios, even a raw material with 80 wt% red mud in it. In this case, the samples were fired at 1050 °C for two hours. They found that the compressive strength of the bricks increased with the red mud concentration, but this increasing tendency stopped and reversed above 40 wt%. They explained that the compressive strength was affected by the amount of the glass phase being present. Ribero et al. [
17] mixed 60 wt% red mud into clay, and they found that the maximal linear shrinkage, minimal water absorption, and maximal bending strength of 2.5 MPa was achieved by sintering the brick at 900 °C if 20 wt% of red mud has been added to the clay. Babisk et al. [
16] wrote that the optimal mixing ratio for the clay-red mud system was 50 wt%. The largest measured pressing strength was around 13.1 MPa. The works listed above demonstrate that it is possible to use red mud as an additive if the red mud content is kept around 40–50 wt% and the firing is done at temperatures below 1050 °C [
16].
Scribot et al. [
18] used a modified red mud with reduced pH in their experiments. The production was done using the method developed by the company Alto. The aluminum oxide is extracted in the Bayer process with sodium hydroxide, which is reclaimed by the following method: the product is first washed with water, and then the residue is filtered under pressure. This process allows the production of red mud that contains less sodium hydroxide, which greatly helps in using it as an additive. They found that clay mixtures containing 30 wt% pH-reduced red mud sintered at 1015 °C showed excellent properties, with a maximal compressive strength of 64.9 MPa.
Apart from the article mentioned above, no other credible description has been given of a technology where the strong alkalinity of the used red mud is reduced, and this quasi-pH-neutral additive is mixed with the clay. pH is an important factor since the high pH of red mud makes large-scale production impossible due to the rapid and severe corrosion of the metallic structural equipment. Industrial experience has shown that the maximum usable red mud content is 5 wt%.
Although not directly related to bricks made by adding reduced alkalinity red mud, Inge Rörig-Dalgaard et al. [
19] investigated the effects of different pH on bricks. During the experiments, the bricks were soaked in solutions with pH values of 3.5, 7, 9, 11, and 13 for 340 days. The acidic solutions were sulfuric acid, while the alkaline solutions were sodium hydroxide. In the pH = 13 environment, the concentrations of potassium, aluminum, and silicate ions increased significantly. The weight of the sample decreased by 2 wt%, indicating that the potassium ions originated from the corrosion of the silicate phase. The physical properties did not change significantly compared to the bricks soaked in the neutral environment, except for the increased porosity and reduced bulk density to a lesser extent. Lewin and Charola [
20] conducted similar experiments and found that with the increasing porosity and the increased reactivity of the decomposition products, water, and other soluble pollutants can penetrate the structure more easily, leading to further property degradation.
This study aimed to investigate new possibilities in the use of CO2-treated red mud. It is important to reduce the pH of the raw materials as the production equipment is prone to corrosion. Moreover, the carbonates formed during the alkaline deoxidation process can positively impact the porosity of small solid bricks. The method presented here is a viable alternative to using CO2 as a red mud semi-refiner, and could potentially utilize the large quantities of red mud waste available to capture the CO2 emitted to the atmosphere. In terms of utilization, one of the most important parameters for small-sized solid bricks is compressive strength. Therefore, the mechanical properties of the test specimens prepared in the study were compared with the strength values of commercially available products in Hungary.
3. Results and Discussion
3.1. Moisture Content
The moisture content values for each specimen are summarized in
Figure 3. The water requirement for processing samples containing untreated and CO
2-treated red mud in varying amounts changes in a similar trend. In both cases, as the amount of red mud added to the clay increased, the amount of water required for processing decreased. This fact is advantageous for possible industrial use, since during the drying step following shaping, a smaller amount of water needs to be removed from the mass, which can save energy.
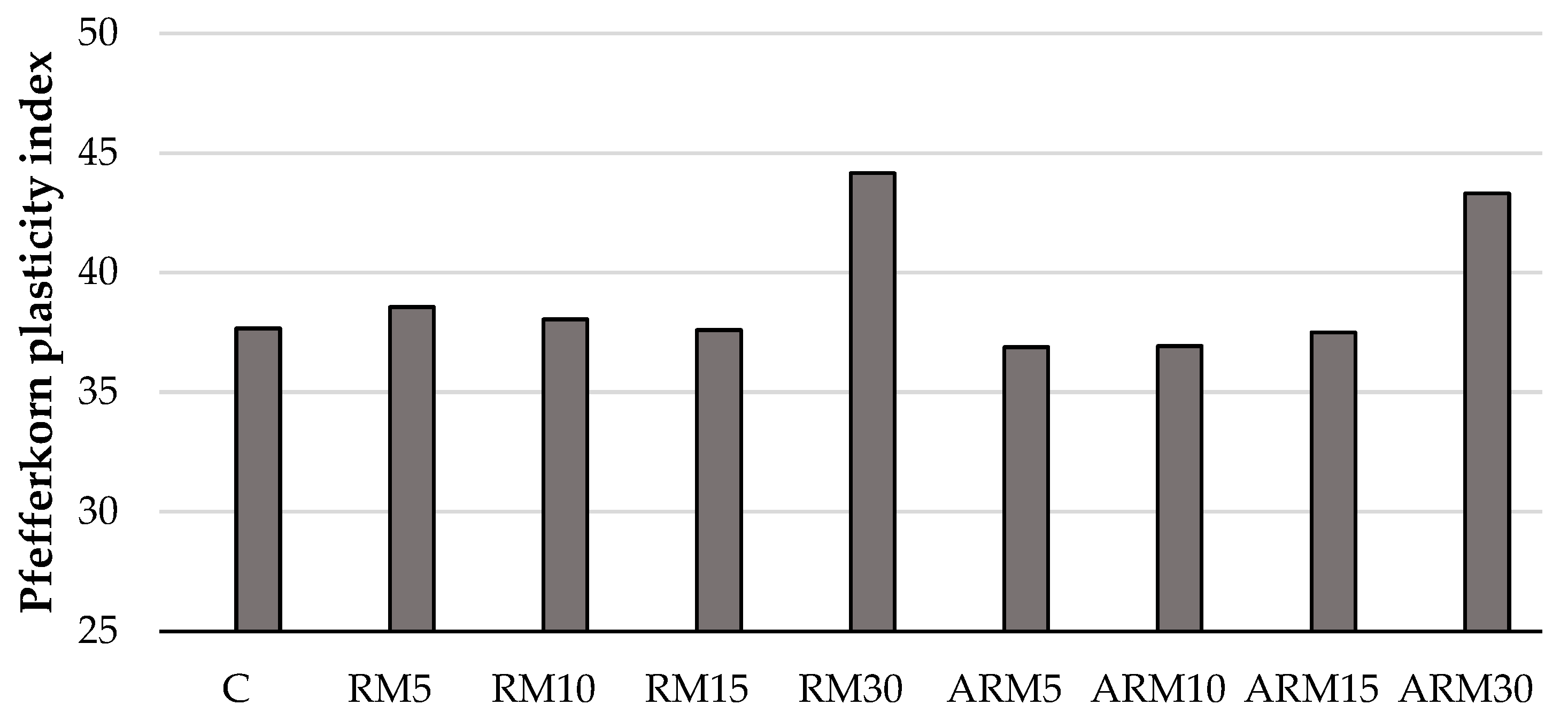
3.2. Pfefferkorn-Plasiticity Index
The results obtained from the determination of plasticity (
Figure 4) showed that the Pfefferkorn plasticity number hardly changed at lower amounts of different red muds, and its values were practically the same. However, at 30 wt% amounts of untreated and reduced alkalinity red mud, its value increased slightly, indicating that adding red mud in such amounts increases the plasticity of the ceramic wet mass. Considering these facts, adding red mud is advantageous from a manufacturing technological aspect, as plasticity can be increased even further, thus making it possible to produce more complex, hollow products.
3.3. Drying Sensitivity
Figure 5 shows that all compositions were categorized as “not sensitive to drying”. It can also be observed that increasing the amount of red mud reduces the Macey drying sensitivity numbers, which is advantageous from a technological perspective, as the test specimens are less prone to cracking and have a lower chance of developing microcracks and pores in their internal structure.
Previously, important technological parameters of the raw mixtures were presented. Overall, it can be said that adding red mud has a favorable effect on plasticity and drying sensitivity. Furthermore, in the case of bricks containing red mud, less water is needed for processing, which is important for reducing the energy required for drying while preserving the available stocks of water. There was no significant difference observed in the processing parameters between untreated and reduced alkalinity red mud during the investigation.
In the following sections, characteristic parameters of products sintered at different temperatures (850 °C, 950 °C, and 1050 °C) are presented.
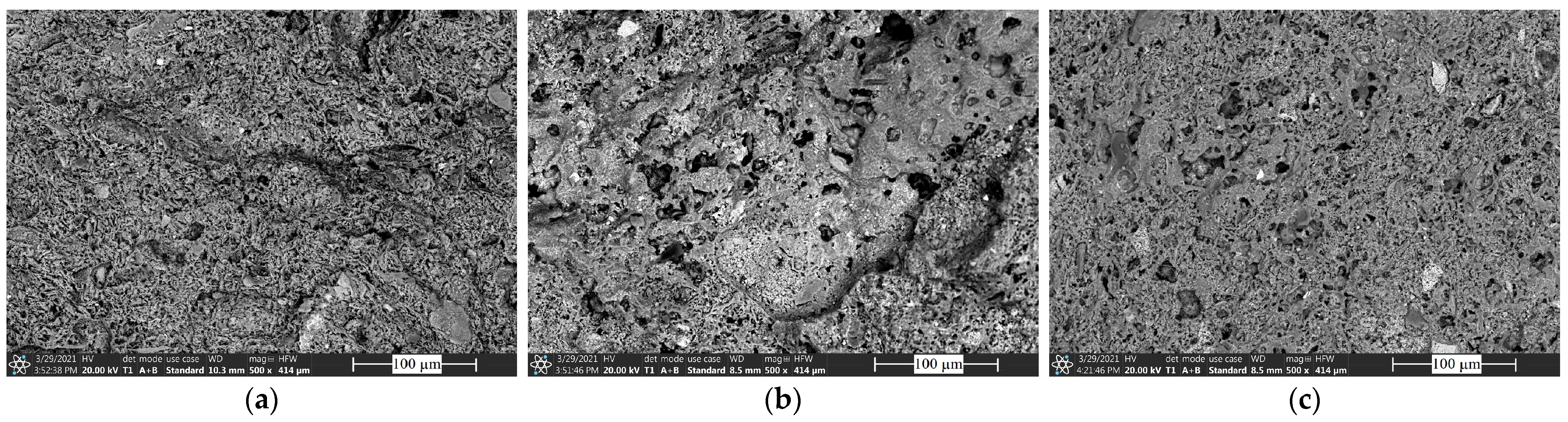
3.4. Morphology and Phase Composition of the Sintered Bricks
Figure 6 shows the microstructure of the clay (C) sintered at a peak temperature of 1050 °C (
Figure 6a), a brick containing 30 wt% untreated red mud (
Figure 6b), and a specimen containing 30 wt% red mud treated with carbon dioxide (
Figure 6c). The structure of the samples labeled RM30 and ARM30 indicates that the process of vitrification began at this firing temperature, resulting in a denser structure of the bricks. The amount of network-modifying oxides (Na
2O, CaO, Fe
2O
3) likely has a significant influence on the vitrification process compared to the raw clay. In the case of the untreated brick, many small pores were observed, while the red mud-containing samples had a denser structure. This phenomenon is expected to significantly affect the mechanical properties.
Table 5 shows the phase composition of the test samples fired at different temperatures. The main crystalline phases present in the samples are: diopside (D; JCPDS: 01-71-1067), feldspathic minerals (Fp: orthoclase; JCPDS: 31-0966, albite, anorthite; JCPDS: 41-1486), quartz (Q; JCPDS: 33-1161), mullite (M; JCPDS: 15-0776), gehlenite (Ge; JCPDS: 35-0755), hematite (H; JCPDS: 33-0664), nepheline (N; JCPDS: 35-0424), tridymite (Tr; JCPDS: 41-1401), muscovite (Mu; JCPDS: 7-0042), and amorphous phase (A). The results showed that as a result of the sintering quartz, gehlenite, and nepheline are likely to enter the molten phase, and at the firing temperature of 1050 °C, the mullite crystalline phase was formed. The firing process significantly increased the amorphous content of the samples, meaning that a glassy phase was formed, as confirmed by SEM images (
Figure 6). When 30 wt% red mud-based additives were added to the clay, a significant amorphous phase was formed at the firing temperature of 950 °C, and this phase content decreased slightly at 1050 °C. This is confirmed by the results of the Rietveld analysis.
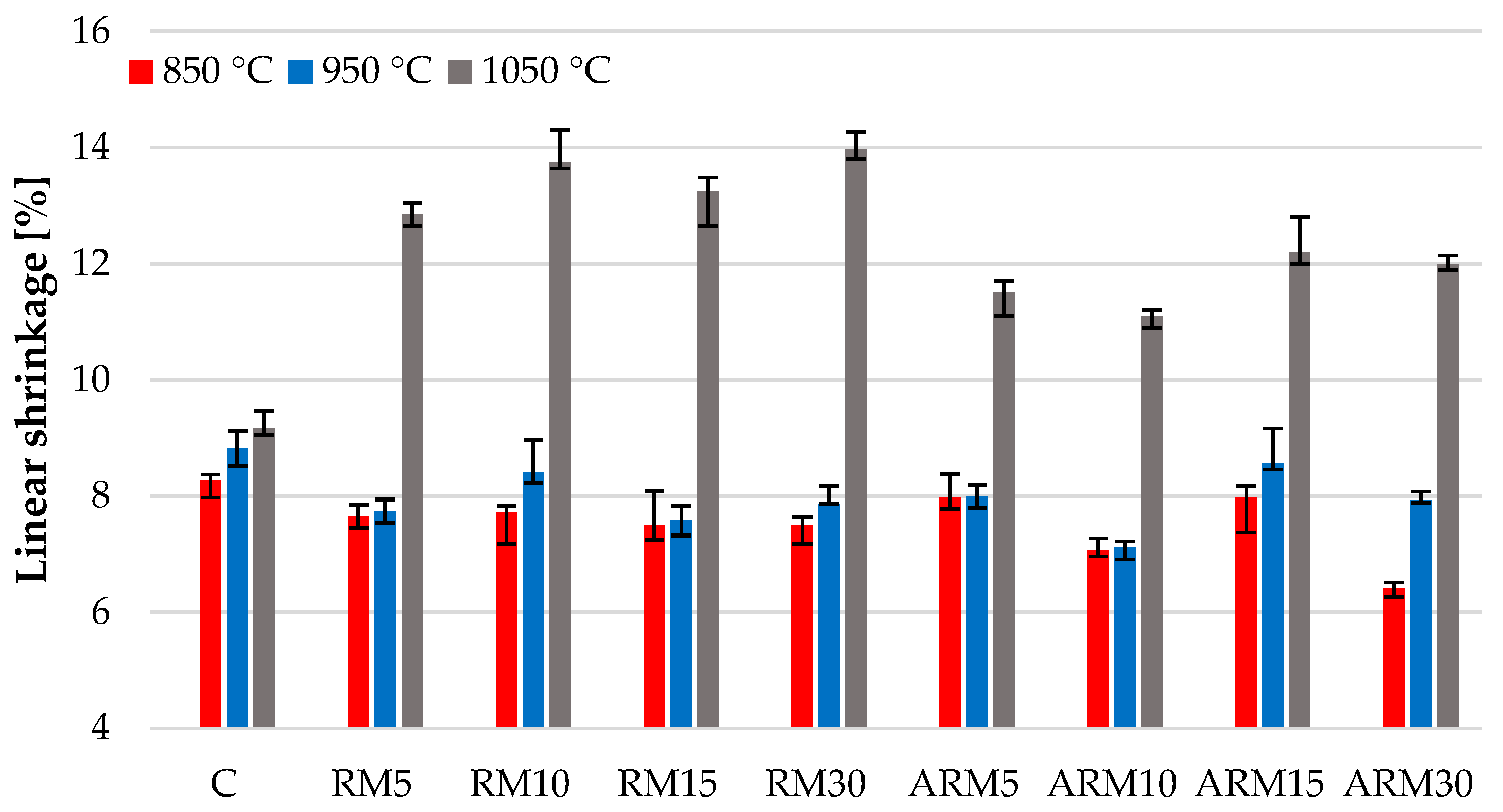
3.5. Linear Shrinkage
In
Figure 7, it is clearly visible that the increase in the content of red mud decreased the shrinkage of the test samples during firing at 850 °C and 950 °C compared to the additive-free clay. This is an advantageous property from a technological aspect, as it reduces the possibility of deformation and cracking. At 1050 °C, significant shrinkage was caused by the presence of large amounts of glassy (amorphous) phase (see
Table 5). The diagram shows that the firing shrinkage was similar for the samples fired at 850 °C and 950 °C. For the samples fired at 1050 °C, the absolute value of shrinkage was greater, indicating densification, which is expected to result in greater compressive strength.
3.6. Porosity
The changes in the apparent porosity (
Figure 8) can be divided into two parts. The data obtained at 850 and 950 °C indicate a property change opposite to the data acquired at 1050 °C. During firing at 850 °C and 950 °C, an increase in porosity was observed, which can be attributed to the release of CO
2 from the decomposition of carbonate minerals (dolomite, calcite, and thermonatrite). However, firing at the highest temperature led to the shrinkage of the specimens, induced by the increasingly developed molten phase. This is indicated by the increase in the amorphous fraction obtained from the quantitative evaluation of XRD data (
Table 5), the evolution of firing shrinkage, and the morphology observed in the SEM images (
Figure 6). The molten phase contracts the pores, partly filling them, explaining the increase in shrinkage, the decrease in porosity, and the subsequent changes in strength.
This fact can lead to two different directions for practical use, depending on the application: the production of a product with high strength or the manufacture of a building element with high porosity, providing good thermal insulation properties. Since achieving sufficient strength can be accomplished by using organic additives that burn out, creating porosity, it is more important to achieve the necessary strength than to create a large in-situ porosity. Based on this, sintering at 1050 °C seems to be the optimal choice, even with 30 wt% red mud content.
It is worth noting that red mud content exceeding 10 wt% represents a significant amount in terms of material volume, and there was a significant difference in the volume of clay and red mud added as an additive.
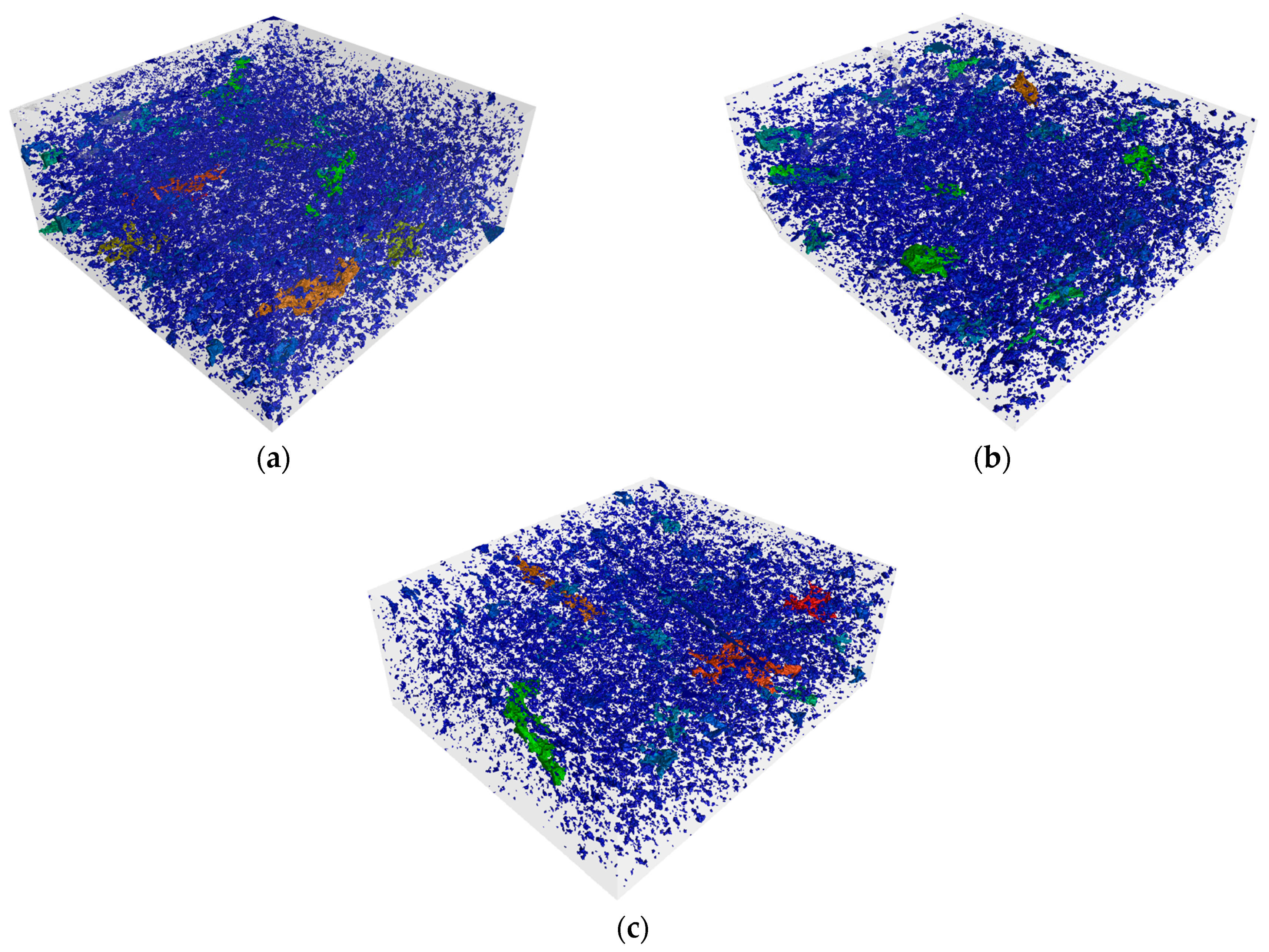
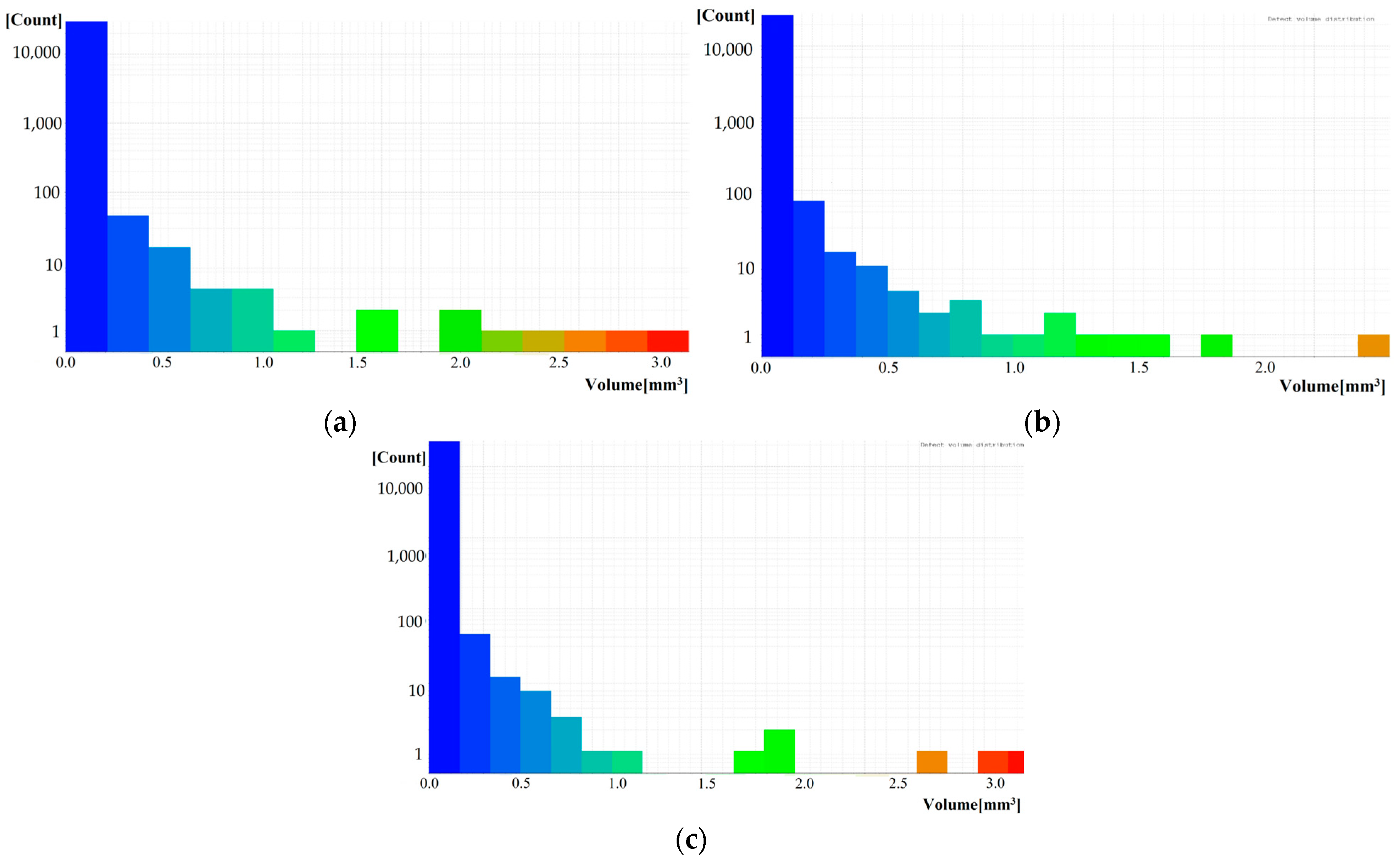
The results obtained from the CT scan are well correlated with the previous findings. The following
Figure 9 and
Figure 10 shows the development of closed porosity in samples fired at different temperatures, using the ARM30 sample. The highest amount of closed pores is observed in the sample fired at 850 °C (
Figure 10a), which decreased slightly in the sample fired at 950 °C (
Figure 10b). At 1050 °C, the larger pores tended to merge, resulting in a more compact pore structure (
Figure 10c). Although the apparent porosity value decreased significantly, numerous small, closed pores were still present in the test samples, as observed in the scanning electron microscopy images (
Figure 6).
During the closed porosity analysis carried out with X-ray tomography, blocks of 50 mm × 50 mm × 30 mm were cut using VG Studio 3.4 software. According to the closed porosity analysis performed on the blocks, the closed porosity of bricks fired at 850 °C was 2.04 vol%, while for the sintered brick at 950 °C, it was 1.77 vol%. In the case of the heat-treated brick at 1050 °C with a 30 wt% of alkalinity red mud content, the measured porosity was 1.66 vol%. For RM30 samples, the closed porosity values were 3.43 vol%, 3.05 vol%, and 2.13 vol% during sintering at 850 °C, 950 °C, and 1050 °C, respectively. As observed in the open porosity measurement, the proportion of closed pores decreased with higher sintering temperatures, which is consistent with the melting processes occurring during heat treatment.
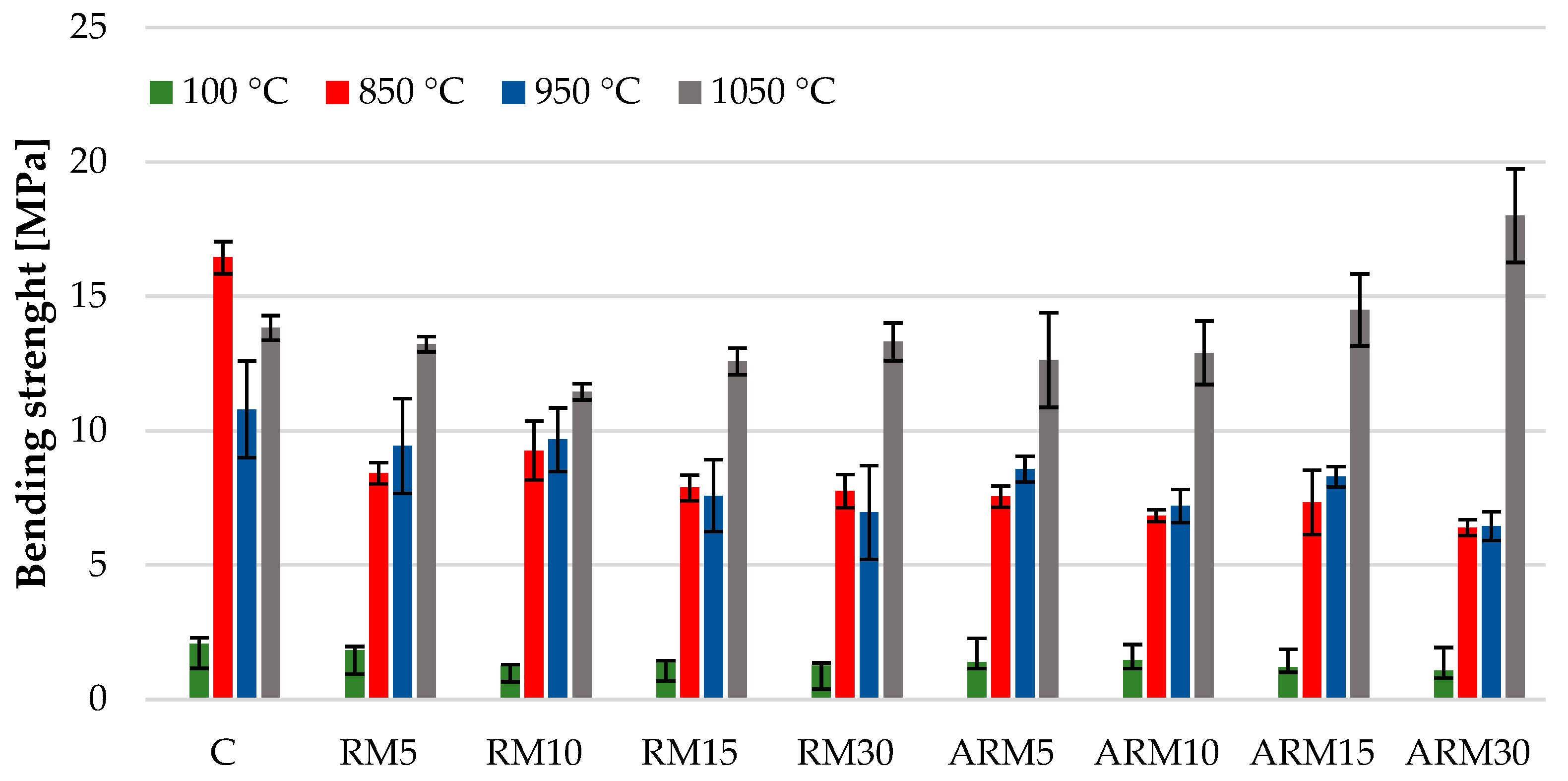
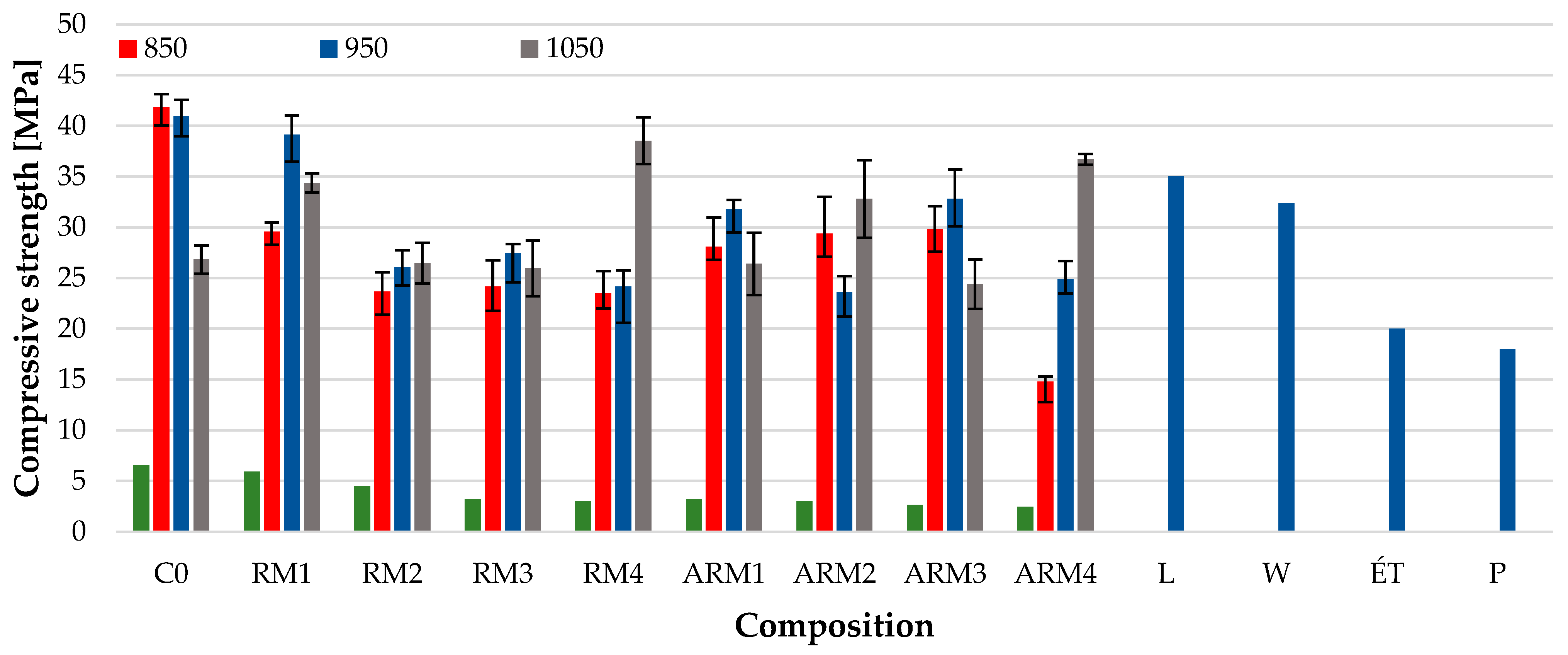
3.7. Flexural and Compressive Strenght
Both flexural and compressive strength tests (
Figure 11 and
Figure 12) showed similar trends, with the difference being that the strength-increasing effect of the compact structure formed at 1050 °C was more pronounced in the flexural strength.
Figure 12 showing the change in flexural strength clearly indicates that the flexural strength of the clay samples with varying amounts of red mud content decreased by nearly half during firing at 850 °C and the bending strength of the samples fired at 950 °C also continuously decreased as the red mud content increased. The numerical values of the two samples fired at the lower temperatures were very similar, and considering the error margins in most cases, the values were practically identical. The highest strength values were measured in the case of bricks, which were sintered at 1050 °C. This fact can be explained by the strength-increasing effect of the amorphous/glassy phase that forms. However, it should be noted that according to X-ray diffraction analysis, the bricks fired at 950 °C (
Table 5) contained the most amorphous phase, but these samples also showed the highest porosity values (
Figure 8). The high degree of porosity is presumably due to the high carbonate content of the raw material (calcite, dolomite, and thermonatrite), whose decomposition has a pore-forming effect.
These results correlate well with the changes in other physical and/or chemical characteristics previously presented, such as porosity, shrinkage, morphology, and composition.
In the case of compressive strength, the dominant strength-increasing effect of firing at 1050 °C is less clear, but even in this case, most of the samples had higher strength values. It is worth mentioning the reference strength value as well: the compressive strength of classic small-sized (250 mm × 120 mm × 65 mm) solid bricks rarely exceeds a value of 35 MPa (according to the datasheet for Leier Ltd. (L), Devecser, Hungary). For small-sized solid bricks distributed by Wienerberger Ltd. (W) in Hungary (Budapest, Hungary), the standard compressive strength value is 32.4 MPa. Among other Hungarian brick factories, the manufacturer’s datasheet for small-sized solid wall bricks distributed by Északmagyar Téglaipari Ltd. (ÉT) (Serényfalva, Hungary) indicates a compressive strength value of 20 MPa, while Pápateszéri Téglagyár Ltd. (P) (Pápateszér, Hungary) specifies a strength value of 18 MPa.
For the bricks containing red mud, as presented in the study, the products fired at 950 °C and 1050 °C exceeded the strength requirements of the manufacturers described above. In this regard, the small-sized solid bricks produced by us may be used for outdoor masonry or decorative applications. In addition to manufacturing bricks with an appropriate strength, the addition of red mud is suitable for the production of ceramics with a deep red color for aesthetic purposes.
Therefore, it is clear that in cases where high alkalinity was neutralized in the samples, the remaining Fe3+ and Na+ ions were still capable of exerting their melt-forming effect, which is clearly evident at higher firing temperatures. Based on our research, it can be said that all of the red mud that undergoes the abovementioned treatment can be used in roof tile and brick production technologies. The reduced alkalinity method allows for significantly greater use of red mud (up to 30 wt%, instead of 5 wt%), as the relevant properties do not deteriorate and corrosive effects on steel structural materials are eliminated.
4. Conclusions
In this study, we presented the applicability of red mud treated with carbon dioxide in the production of building materials. The raw materials used in this study are readily available in significant quantities, with the Kolontár tailing pond and the Devecser brick factory located within 5 km of each other.
In summary, the following conclusions can be drawn from the results obtained during the research:
Red mud can be advantageously used as an additive in the production of bricks since it significantly reduces the water demand of the manufacturing process, thereby reducing the energy required for drying;
The reduced alkalinity red mud is particularly advantageous as it has a lower corrosive effect on the steel structures used in the manufacturing process, as well as being suitable for the production of more complex shapes based on the results of the plasticity tests and is less prone to cracking during drying;
The porosity and strength of the sintered products can be fine-tuned based on the chosen additives and firing temperature according to the intended application;
The compressive strength of the bricks produced, which contain red mud, can exceed the compressive strength of small-sized solid bricks available in the market, depending on the quantity of the additive used;
The CO2 treatment of the red mud also has great potential for developing a new inexpensive CO2 capture method.
The material presented in this study is excellent for the production of small solid bricks and can also be used for roofing tiles if the product is fired to the proper density.
As each gram of red mud used reduces the consumption of the primary raw material, clay, the production costs of bricks decrease. This, in turn, leads to a decrease in mining and landscape destruction. Moreover, the utilization of red mud does not pose any foreseeable problems; for instance, it reduces storage costs. Therefore, the use of red mud is financially viable both directly and indirectly.
Taking into account factors such as the extraction and transportation costs of red mud, as well as other energy-intensive technological processes, a direct price reduction of 10–15% can be achieved with a 30% dosage. While this may not appear substantial, considering the massive quantity of bricks produced, the resulting savings can be significant. According to our estimates, around 30 million small-sized bricks can be manufactured in Hungary. If we calculate the price per piece at EUR 1, it amounts to approximately EUR 30 million. Even with a 10% price reduction taken into consideration, the savings would still be around EUR 3 million.