Automotive Paint Sludge: A Review of Pretreatments and Recovery Options
Abstract
1. Introduction
- employment of physically/chemically treated PS in the production of primers and sealants.
- Utilization of PS for the production of building materials as supplementary components of cement concrete, mortar, or bituminous mixtures.
- Extraction of valuable organic and inorganic products by thermal processes (pyrolysis, gasification).
- Biological or chemical stabilization before disposal.
- Other solutions.
2. PS Characterization
| Parameter | Gautam et al., 2010 [9] | Januri et al., 2015 [13] | Tian et al., 2015 [14] | Dalmazzo et al., 2017 WB [17] | Dalmazzo et al., 2017 C [17] | Salihoglu et al., 2018 [15] | Yenikaya et al., 2018 [16] | Gadhekar et al., 2019 [10] | Abu Bakar et al., 2022 [11] | Yeganeh et al., 2022 [12] |
|---|---|---|---|---|---|---|---|---|---|---|
| Water content (%) | 29.9 | 2.4 | NA | 50.9 | 60.8 | 63.4 | 63.4 | 54.5 | 29.9 | 70.65 |
| Organic matter or VS (%, d.b.) | 75.2 | 75.9 | NA | 72.4 | 94.5 | 72.6 | NA | 75.66 | 75.2 | NA |
| Ash (%, d.b.) | 22.7 | 2.9 | NA | 27.6 | 5.5 | NA | NA | 24.83 | 22.7 | NA |
| Fixed carbon (%, d.b.) | 2.1 | 3.2 | NA | NA | NA | NA | NA | NA | 2.1 | NA |
| Heating value (kcal/kg) | 4330 | 22.6 (MJ/kg) | NA | 4280 | 4820 | 7682 (kJ/kg) | NA | 5705 | NA | NA |
| Loss on ignition (%) | 0.2 | NA | NA | NA | NA | NA | 71.0 | NA | NA | NA |
| Fe (%) | 6.2 (Fe2O3) | NA | 0.16 | 0.268 | 0.08 | NA | NA | 0.025 (mg/L?) | 6.6 | 0.43 (Fe2O3) |
| Al (%) | 18.0 (Al2O3) | NA | 2.26 | 2.42 | 1.02 | NA | NA | NA | 19.1 | 18.0 (Al2O3) |
| Ti (%) | 53.0 (TiO2) | NA | NA | 6.81 | 0.0044 | NA | NA | NA | 56.2 | 54.2 (TiO2) |
| Si (%) | 6.8 (SiO2) | NA | NA | NA | NA | NA | NA | NA | 7.2 | 2.15 (SiO2) |
| Ba (%) | 8.5 (BaO) | NA | 6210 (mg/kg) | 6.57 | 41.7 | NA | 6.4 mg/kg (L) | NA | 9.0 | NA |
| P (%) | 0.4 (P2O5) | NA | 0.19 (g/kg) | NA | NA | NA | NA | NA | 0.4 | NA |
| Na (%) | 0.2 (Na2O) | NA | NA | NA | NA | NA | NA | NA | 0.2 | NA |
| K (%) | 0.2 (K2O) | NA | 0.34 (g/kg) | NA | NA | NA | NA | NA | 0.2 | NA |
| Ca (%) | 0.4 (CaO) | NA | 1.28 (g/kg) | NA | NA | NA | NA | NA | 0.4 | 2.30 (CaO) |
| Mg (%) | 0.4 (MgO) | NA | 2.02 (g/kg) | NA | NA | NA | NA | NA | 0.4 | 0.66 (MgO) |
| Cl (%) | 0.14 | NA | NA | NA | NA | NA | 440 mg/kg (L) | NA | 0.14 | NA |
| Co (mg/kg) | NA | NA | 4.80 | 144 | 0.847 | NA | NA | NA | NA | NA |
| Cr (mg/kg) | NA | NA | 38.1 | 228 | 9.17 | 152.2 | NA | ND | NA | 106 |
| Cu (mg/kg) | NA | NA | 47.5 | 171 | 7.07 | NA | <1 mg/kg (L) | ND | NA | NA |
| Ni (mg/kg) | NA | NA | 42.4 | 18.3 | 5.21 | 10.7 | <0.5 mg/kg (L) | 0.19 mg/kg | ND | 16.2 |
| Pb (mg/kg) | NA | NA | < 0.003 | 17.0 | 9.85 | 10.5 | NA | ND | NA | 15.2 |
| Zn (mg/kg) | NA | NA | 28.1 | 172 | 186 | NA | <1 mg/kg (L) | 0.12 mg/kg | ND | NA |
| pH | NA | NA | NA | NA | NA | 9.4 | 9.44 | 7.6 | NA | NA |
3. Pretreatment of PS: Dewatering and Drying
4. Recovery of PS in the Paint or Sealant Industry
- It reduced the final volume of the waste and made its disposal easier and more economical.
- The power produced can be handled easier than wet sludge.
- It reduced the hazardousness of the waste because liquid hydrocarbons were evaporated and toxic metals were bound into the cured resin product.
5. Recovery in Building Materials
5.1. Recovery in Portland Cement Concrete
- Mixing PS with NaOH so as to chemically stabilize the free aluminum contained in the sludge.
- Heating the PS-NaOH admixture to a temperature of approx. 60 °C for 10 min, so as to facilitate hydrogen generation from the reaction between free aluminum and NaOH.
- Mixing the PS-NaOH admixture with quicklime (CaO), so as to absorb the residual water within the PS.
- Agitating the obtained admixture so as to minimize any localized heterogeneity.
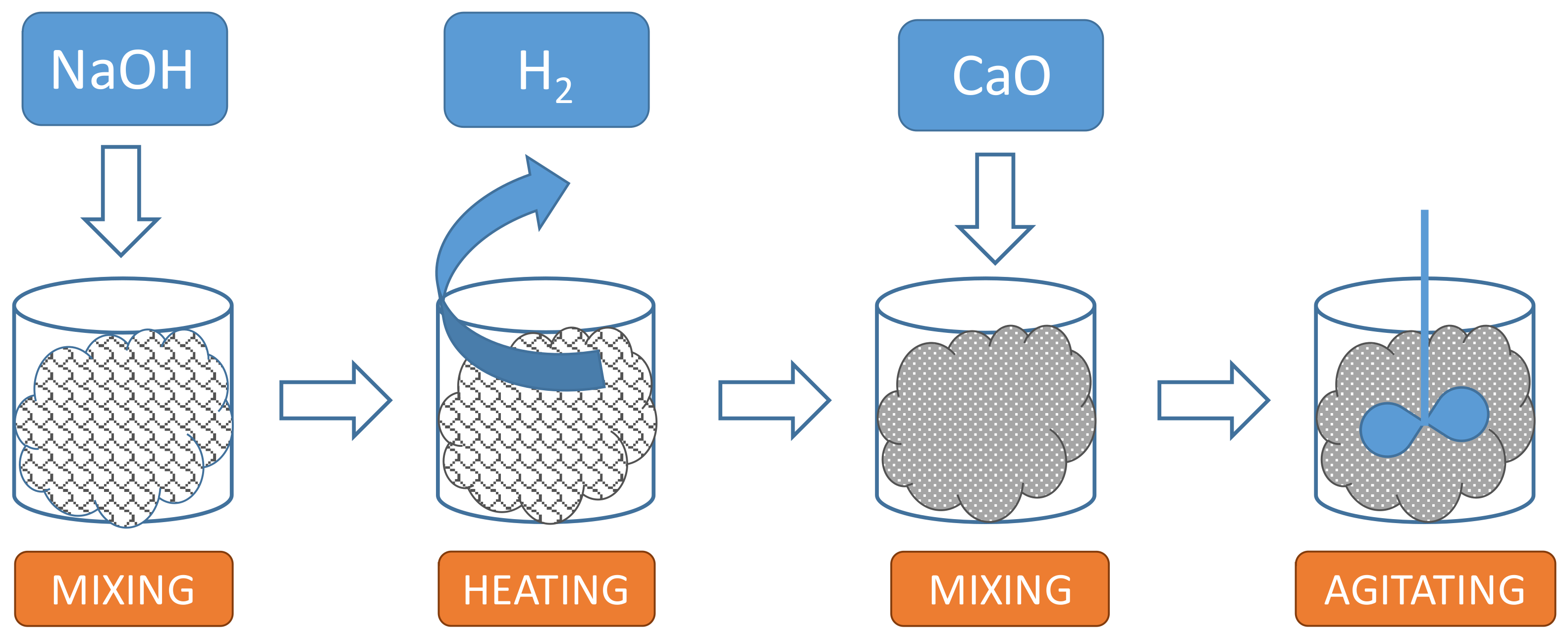
- It does not alter the mechanical properties of the solid lime/paint product.
- It is of proven efficacy, inexpensive, and readily available.
- It generates sufficient heat upon dissolution into the PS to facilitate the reaction with free aluminum.
- (1)
- Mixing liquid PS with one or more materials used to produce building materials, such as cement mix or concrete mix, or portions thereof.
- (2)
- Allowing the mixture to cure, thereby producing a building material.
5.2. Recovery in Lightweight Construction Mortars
5.3. Recovery in Bituminous Mixture for Paving Applications
6. Recovery through Pyrolysis and Other Thermal Processes
- (i)
- Fuels to be burned.
- (ii)
- Activated char to be further used to capture VOCs from the exhaust air generated in painting operations.
- (iii)
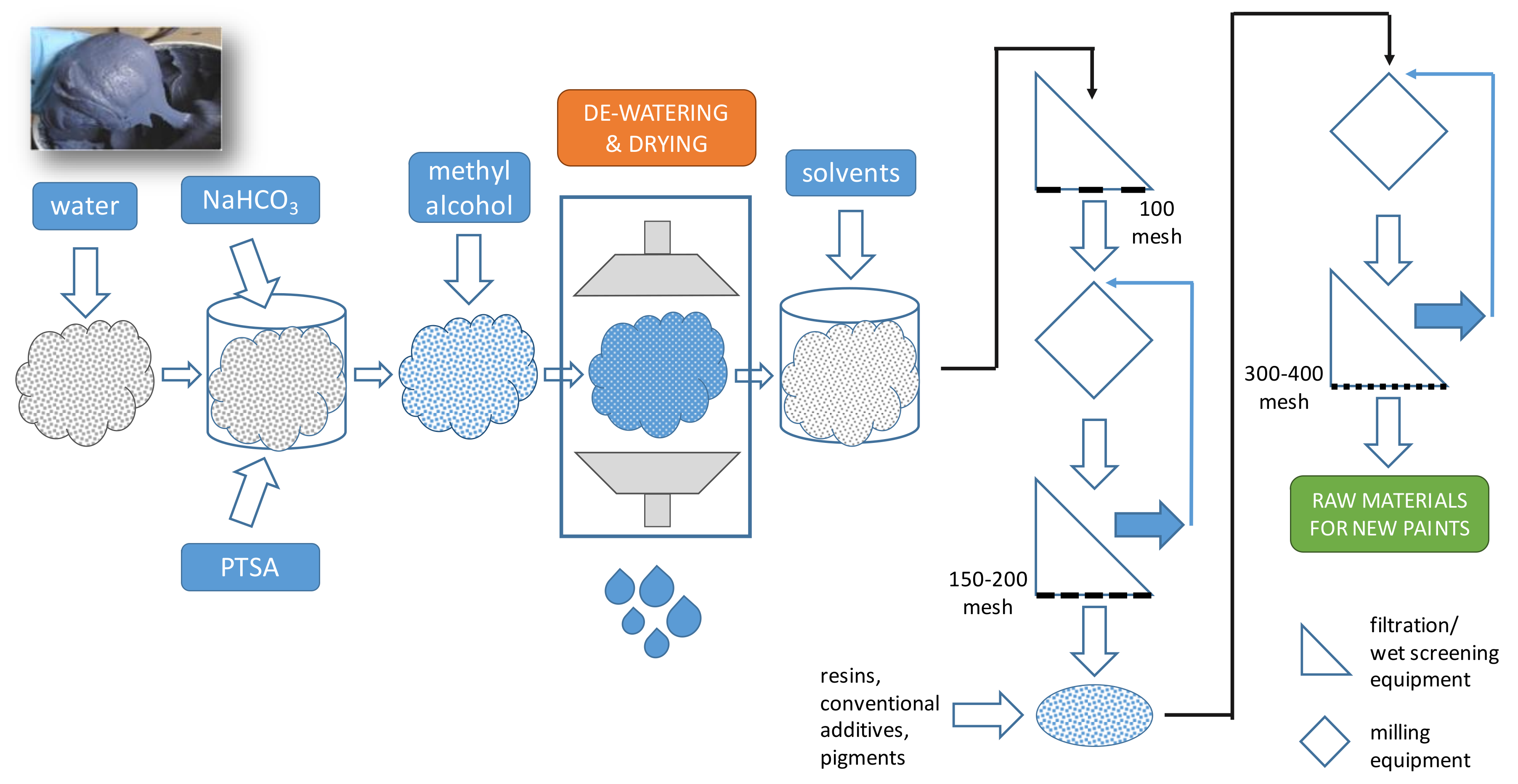
- Inorganic oxides to be recycled into new paints.
7. Recovery and Treatment of PS through Biological Processes
8. Other Processes
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Streitberger, H.J.; Dössel, K.F. Automotive Paints and Coatings; Wiley-VCH Verlag GmbH & Co KgaA: Weinheim, Germany, 2008. [Google Scholar]
- Toda, K.; Salazar, A.; Saito, K. Automotive Painting Technology: A Monozukuri-Hitozukuri Perspective; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- McCarty, J.P.; St. Louis, D.M. Processing Paint Sludge to Produce a Combustible Fuel Product. U.S. Patent 2008/0216392, 11 September 2008. [Google Scholar]
- Salihoglu, G.; Salihoglu, N.K. A review on paint sludge from automotive industries: Generation, characteristics and management. J. Environ. Manag. 2016, 169, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, B.; Zanetti, M.C. Reuse and recycling of automotive paint sludge: A brief overview. In Proceedings of the CRETE 2010, International Conference on Hazardous Waste Management, Chania, Greece, 5–8 October 2010. [Google Scholar]
- Viguri, J.; Onandía, R.; Roberto Arce, R.; Angel Irabien, A. Alkyd paint waste characterization and distillation. Chem. Eng. Commun. 2005, 192, 1490–1504. [Google Scholar] [CrossRef]
- Gerace, M.J.; Gamboa, S.C.; Landaburu, Y.S. Method of Making Sludge Powder and Sealant from Paint Sludge and Sludge Powder and Sealant Composition Produced Thereby. U.S. Patent 5,254,263, 19 October 1993. [Google Scholar]
- Available online: https://ec.europa.eu/eurostat/web/products-datasets/-/road_eqs_carhab (accessed on 3 March 2023).
- Gautam, S.P.; Bundela, P.S.; Murumkar, M. Paint sludge waste co-processing at the ACC Wadi Cement Works in Karnataka, India. WIT Trans. Ecol. Environ. 2010, 140, 57–66. [Google Scholar] [CrossRef]
- Gadhekar, N.V.; Khedikar, I.P.; Thergaonkar, V.P. Characterization of paint sludge from an automobile industry. In Proceedings of the International Conference on Innovation & Research in Engineering, Science & Technology, ICIREST-19, Nagpur, India, 22–23 March 2019; pp. 3–79. Available online: http://iosrjen.org/Papers/Conf.ICIREST-2019/Volume-4/13.%2077-79.pdf# (accessed on 10 January 2023).
- Abu Bakar, N.F.; Muhd Sidek, M.N.; Suharman, S.M.; Abd Rahman, N. Characterization and evaluation of dried automotive paint sludge as cement-based composite. Mater. Today Proc. 2022, 63, S301–S305. [Google Scholar] [CrossRef]
- Yeganeh, B.; Khatamgooya, A. The feasibility study of stabilizing the automotive paint sludge by recycling, to produce green concrete bloks, considering environmental and mechanical factors. J. Mater. Cycles Waste Manag. 2022, 25, 931–943. [Google Scholar] [CrossRef]
- Januri, Z.; Abdul Rahman, N.; Idris, S.S.; Matali, S.; Abdul Manaf, S.F. Characterization of automotive paint sludge (APS) for chemical/energy recovery via microwave assisted pyrolysis. Adv. Mater. Res. 2015, 1113, 236–241. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Wu, M.; Gao, L.; Michel, F.C., Jr.; Dick, W.A. Windrow Composting of Waste Paint Sludge Containing Melamine Resins. Compost. Sci. Util. 2015, 23, 199–206. [Google Scholar] [CrossRef]
- Salihoglu, N.K.; Ucaroglu, S.; Salihoglu, G. Bioconversion of industrial wastes: Paint sludge from automotive manufacturing. J. Mater. Cycles Waste Manag. 2018, 20, 2100–2109. [Google Scholar] [CrossRef]
- Yenikaya, S.; Salihoglu, G.; Salihoglu, N.K.; Yenikaya, G. Microwave Drying of Automotive Industry Paint Sludge. J. Hazard. Toxic Radioact. Waste 2018, 22, 04018015. [Google Scholar] [CrossRef]
- Dalmazzo, D.; Vercelli, A.; Santagata, E.; Ruffino, B.; Zanetti, M.C. Rheological characterization and performance-related evaluation of paint sludge modified binders. Mater. Struct. 2017, 50, 74. [Google Scholar] [CrossRef]
- Available online: https://www.ideeeprodotti.it/Default.aspx#products (accessed on 10 January 2023).
- Available online: https://www.trucent.com/products/industrial-centrifuge-systems/two-phase-separation/ (accessed on 10 January 2023).
- Available online: https://www.flottweg.com/it/linea-di-prodotti/decanter/ (accessed on 10 January 2023).
- Available online: https://dolphincentrifuge.com/contact-for-alfa-laval-centrifuges/ (accessed on 10 January 2023).
- Available online: https://www.sludgeprocessing.com/process-and-operation/thickening-dewatering-drying/drying/belt-dryers/ (accessed on 10 January 2023).
- Available online: https://www.euroby.com/equipment/centrifuges/ (accessed on 10 January 2023).
- Amin, Z.; Salihoglu, N.K. Evaluation of free water removal from different sludge by solar energy utilization. Environ. Eng. Res. 2021, 26, 200088. [Google Scholar] [CrossRef]
- Amin, Z.; Salihoglu, N.K. Improvements to increase the efficiency of solar dryers for different waste sludge management. Int. J. Hum. Cap. Urban Manag. 2020, 5, 277–290. [Google Scholar] [CrossRef]
- Amin, Z.; Yuksel, G.; Salihoglu, G.; Salihoglu, N.K. Drying of Paint Sludge Using Paraffin in Solar Drying Systems. Düzce Üniversitesi Bilim Teknol. Derg. 2021, 9, 231–241. (In Turkish) [Google Scholar] [CrossRef]
- Bhatia, R.K.; Bhatia, V.K.; Vishnoi, A.S. Composition and Process for Conversion of Paint Sludge into Reusable Paint. WO2007072502A2, 13 July 2007. [Google Scholar]
- Johnson, J.C.; Slater, A. Method for Treating Waste Paint Sludge. U.S. Patent 4,980,030, 25 December 1990. [Google Scholar]
- Weinwurm, P. Method for Producing Insoluble Industrial Raw Material from Waste. U.S. Patent 5,087,375, 11 February 1992. [Google Scholar]
- Gerace, M.J.; Landaburu, Y.S.; Gamboa, S.C. Compounded Polymeric Compositions Utilizing Processed Paint Sludge as a Replacement for Polymeric Components. U.S. Patent 5,880,218, 9 March 1999. [Google Scholar]
- Gerace, M.J.; Gamboa, S.C.; Landaburu, Y.S. Method for Treating Paint Sludge. U.S. Patent 5,922,834, 13 July 1999. [Google Scholar]
- Gerace, M.J.; Landaburu, Y.S.; Klosterman, T.P. Roof Sealant Composition and Method of Applying. U.S. Patent 6,455,598 B1, 24 September 2002. [Google Scholar]
- St. Louis, D.M. Process for Producing Building Materials from Paint Sludge. U.S. Patent 5,573,587, 12 November 1996. [Google Scholar]
- Soroushian, P.; Okwuegbu, A.C. Shrinkage Compensating Concrete with Expansive Additive. U.S. Patent 5,489,333, 6 February 1996. [Google Scholar]
- Matheson, R.R.; Dixon, D.M.; Moore, J.R.; Fischer, D.A. Process for producing building materials from raw paint sludge. U.S. Patent 7,128,780, 31 October 2006. [Google Scholar]
- Tian, Y.; Chen, L.; Gao, L.; Michel, F.C., Jr.; Wan, C.; Li, Y.; Dick, W.A. Composting of waste paint sludge containing melamine resin as affected by nutrients and gypsum addition and microbial inoculation. Environ. Pollut. 2012, 162, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Ahmed, Z.B.; Ahmed, T. Feasibility of sludge generated in water-based paint industries as cement replacement material. Case Stud. Constr. Mater. 2022, 16, e01119. [Google Scholar] [CrossRef]
- Feng, E.; Sun, J.; Feng, L. Regeneration of paint sludge and reuse in cement concrete. E3S Web Conf. 2018, 38, 02021. [Google Scholar] [CrossRef]
- Burande, B.C. Utilisation of paint sludge from automotive industries into valuable products. Int. J. Recent Trends Eng. Res. 2017, 3, 513–519. [Google Scholar] [CrossRef]
- Salihoglu, G.; Salihoglu, N.K. Paint Sludge from Automotive Industries: Recycling as Construction Material. In Proceedings of the EurAsia Waste Management Symposium 2016, Istanbul, Turkey, 2–4 May 2016; pp. 244–250. [Google Scholar]
- Avci, H.; Ghorbanpoor, H.; Topcu, I.B.; Nurbase, M. Investigation and recycling of paint sludge with cement and lime for producing lightweight construction mortar. J. Environ. Chem. Eng. 2017, 5, 861–869. [Google Scholar] [CrossRef]
- Martínez-González, G.M.; Castro-Agüero, A.; Quintana-Hernández, P.A. Use of automotive paint sludge as filler in asphaltic mixtures. J. Solid Waste Technol. Manag. 1998, 25, 193–196. [Google Scholar]
- Ruffino, B.; Farina, A.; Dalmazzo, D.; Blengini, G.; Zanetti, M.; Santagata, E. Cost analysis and environmental assessment of recycling paint sludge in asphalt pavements. Environ. Sci. Pollut. Res. 2021, 28, 24628–24638. [Google Scholar] [CrossRef]
- Zanetti, M.C.; Ruffino, B.; Vercelli, A.; Dalmazzo, D.; Santagata, E. Reuse of paint sludge in road pavements: Technological and environmental issues. Waste Manag. Res. 2018, 36, 11. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.B. Pyrolysis Process and Apparatus. U.S. Patent 5,129,995, 14 July 1992. [Google Scholar]
- Agarwal, K.B. Pyrolysis Process and Apparatus. U.S. Patent 5,198,018, 30 March 1993. [Google Scholar]
- Narula, C.K.; Kim, B.R.; Salmeen, I.T. Pyrolytic conversion of paint sludge to useful materials. U.S. Patent 5,543,367, 6 August 1996. [Google Scholar]
- Nakouzi, S.; Mielewski, D.; Ball, J.; Kim, B.; Salemeen, I.; Bauer, D.; Narula, C. A novel approach to paint sludge recycling: Reclaiming of paint sludge components as ceramic composites and their applications in reinforcement of metals and polymers. J. Mater. Res. 1998, 13, 53–60. [Google Scholar] [CrossRef]
- Kim, B.R.; Kalis, E.M.; Salmeen, T.; Kruse, C.W.; Demir, I.; Carlson, S.L.; Rostam-Abadi, M. Evaluating paint-sludge chars for adsorption of selected paint solvents. J. Environ. Eng. 1996, 122, 532–539. [Google Scholar] [CrossRef]
- Kim, B.R.; Kalis, E.M.; Adams, J.A. Integrated emissions management for automotive painting operations. Pure Appl. Chem. 2001, 73, 1277–1280. [Google Scholar] [CrossRef]
- Li, S.; Feng, J.; Tian, S.; Lan, S.; Fan, C.; Liu, X.; Xiong, Y. Tuning role and mechanism of paint sludge for characteristics of sewage sludge carbon: Paint sludge as a new macro-pores forming agent. J. Hazard. Mater. 2018, 344, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.S.; Nasrul Bojy, M.; Januri, Z. Conversion of automotive paint sludge to activated carbon via microwave pyrolysis technique for supercapacitor application. Malays. J. Chem. Eng. Technol. 2020, 3, 35–43. [Google Scholar] [CrossRef]
- Muniz, L.A.R.; Costa, A.R.; Steffani, E.; Zattera, A.J.; Hofsetz, K.; Bossardi, K.; Valentini, L. A study of paint sludge deactivation by pyrolysis reactions. Braz. J. Chem. Eng. 2003, 20, 63–68. [Google Scholar] [CrossRef]
- Rosli, N.L.; Abd Rahman, N.; Kadri, A. Methane synthesis from automotive paint sludge via microwave assisted pyrolysis. IOP Conf. Ser. Mater. Sci. Eng. 2018, 358, 012029. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Gao, L.; Michel, F.C., Jr.; Keener, H.M.; Klingman, M.; Dick, W.A. Composting of waste paint sludge containing melamine resin and the compost’s effect on vegetable growth and soil water quality. J. Hazard. Mater. 2012, 243, 28–36. [Google Scholar] [CrossRef]
- An, H.; Li, X.; Yang, Q.; Wang, D.; Xie, T.; Zhao, J.; Xu, Q.; Chen, F.; Zhong, Y.; Yuan, Y.; et al. The behavior of melamine in biological wastewater treatment system. J. Hazard. Mater. 2017, 322, 445–453. [Google Scholar] [CrossRef]
- Ghomi Avili, R.; Takdastan, A.; Atabi, F.; Ali Omrani, G. Feasibility study of chromium removal from paint sludge with biological sludge, using vermicomposting by Esenia fetida (Case study: Saipa Automotive Industry). Jundishapur J. Health Sci. 2018, 10, e78891. [Google Scholar] [CrossRef]
- Ghomi Avili, R.; Takdastan, A.; Atabi, F.; Ali Omrani, G. Investigating the reduction of BTEX in automotive paint sludge combined with biological sludge by vermicomposting process using Eisenia fetida. Environ. Health Eng. Manag. J. 2021, 8, 87–94. [Google Scholar] [CrossRef]
- Honarjooy Barkusaraey, F.H.; Mafigholami, R.; Faezi Ghasemi, M.; Khayati, G. Optimization of zinc bioleaching from paint sludge using Acidithiobacillus thiooxidans based on response surface methodology. J. Environ. Sci. Health Part A 2021, 56, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Honarjooy Barkusaraey, F.H.; Mafigholami, R.; Faezi Ghasemi, M.; Khayati, G. Zn bio extraction from a zinc rich paint sludge by indigenous Pseudomonas aeruginosa. Chem. Eng. Commun. 2022, 209, 1397–1412. [Google Scholar] [CrossRef]
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current nature-based biological practices for rare earth elements extraction and recovery: Bioleaching and biosorption. Renew. Sustain. Energy Rev. 2023, 173, 113099. [Google Scholar] [CrossRef]
- Liapun, V.; Motola, M. Current overview and future perspective in fungal biorecovery of metals from secondary sources. J. Environ. Manag. 2023, 332, 117345. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, D.; Yang, J.; Wang, W.; Chen, P.; Zhang, S.; Yan, L. Acidithiobacillus thiooxidans and its potential application. Appl. Microbiol. Biotechnol. 2019, 103, 7819–7833. [Google Scholar] [CrossRef] [PubMed]
- Piemonte, V.; Francioni, F.; Capocelli, M.; Prisciandaro, M. Biodegradation of acrylic paints: Process modelling of biocide effect on biomass growth at different temperatures. Braz. J. Chem. Eng. 2017, 34, 557–566. [Google Scholar] [CrossRef]
- Honarjooy Barkusaraey, F.; Mafigholami, R.; Faezi Ghasemi, M.; Khayati, G. Isolation and identification of resistant microorganisms from automotive paint sludge. Jundishapur J. Health Sci. 2020, 12, e101226. [Google Scholar] [CrossRef]
- Vaccarezza, V.; Anderson, C. Using automotive paint sludge as a binder for pelletizing magnetite ore. In Proceedings of the 2022 IBA, International Briquetting and Agglomeration Conference, Denver, CO, USA, 18–21 September 2022. [Google Scholar]
- Arce, R.; Galán, B.; Coz, A.; Andrés, A.; Viguri, J.R. Stabilization/solidification of an alkyd paint waste by carbonation of waste-lime based formulations. J. Hazard. Mater. 2010, 177, 428–436. [Google Scholar] [CrossRef]
- Hoang, D.T.; Vu, B.D. A study of paint sludge treatment by solidification method. In Proceedings of the 4th AUN/SEED-Net Regional Conference on Global Environment and Seminar of NRCT-JSPS Asian Core Program, Bangkok, Thailand, 18–19 January 2012; Available online: https://www.researchgate.net/publication/356429607_The_4_th_AUNSEED-Net_Regional_Conference_on_Global_Environment_and_Seminar_of_NRCT-JSPS_Asian_Core_Program_A_Study_of_Paint_Sludge_Treatment_by_Solidification_Method (accessed on 2 March 2023).
- Leppänen, T.; Rönkkö, P.; Haapasalo, H.; Tervonen, P. The role of logistics in industrial side stream utilization—Case: Aqueous paint sludge. Int. J. Manag. Knowl. Learn. 2022, 11, 17–32. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruffino, B.; Campo, G.; Idris, S.S.; Salihoğlu, G.; Zanetti, M. Automotive Paint Sludge: A Review of Pretreatments and Recovery Options. Resources 2023, 12, 45. https://doi.org/10.3390/resources12040045
Ruffino B, Campo G, Idris SS, Salihoğlu G, Zanetti M. Automotive Paint Sludge: A Review of Pretreatments and Recovery Options. Resources. 2023; 12(4):45. https://doi.org/10.3390/resources12040045
Chicago/Turabian StyleRuffino, Barbara, Giuseppe Campo, Siti Shawalliah Idris, Güray Salihoğlu, and Mariachiara Zanetti. 2023. "Automotive Paint Sludge: A Review of Pretreatments and Recovery Options" Resources 12, no. 4: 45. https://doi.org/10.3390/resources12040045
APA StyleRuffino, B., Campo, G., Idris, S. S., Salihoğlu, G., & Zanetti, M. (2023). Automotive Paint Sludge: A Review of Pretreatments and Recovery Options. Resources, 12(4), 45. https://doi.org/10.3390/resources12040045









