Current Insights into Growing Microalgae for Municipal Wastewater Treatment and Biomass Generation
Abstract
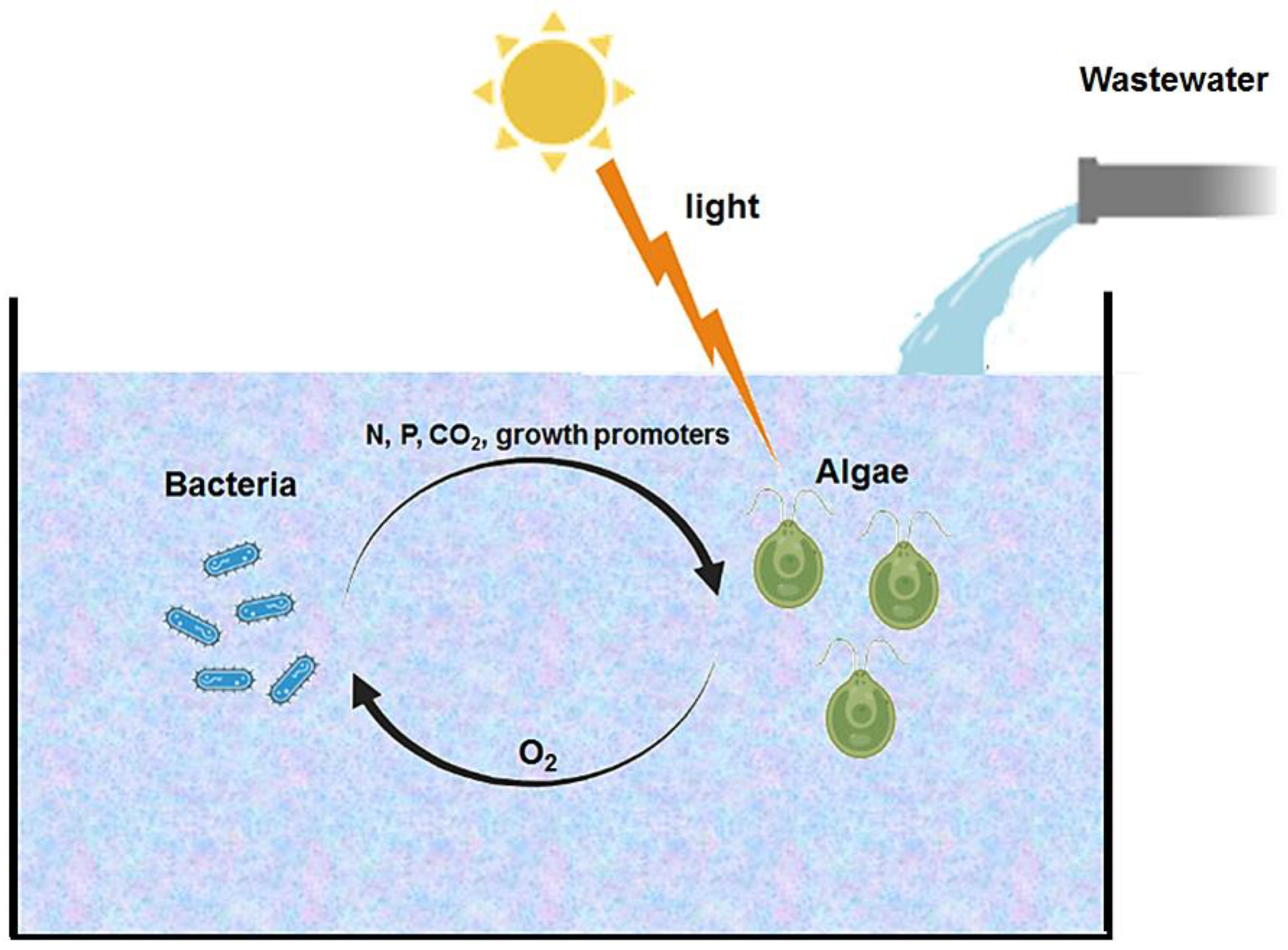
:1. Introduction
2. Municipal Wastewater Treatment and Microalgae
2.1. Municipal Wastewater Characterization
2.1.1. Municipal Wastewater Treatment Plants
| Treatment Technology | Description | Quality Parameters (Treated Water) | References |
|---|---|---|---|
| Activated Sludge | Aeration of wastewater in the presence of a microbial floc. | Total Organic Carbon (TOC): 20–40 mg·L−1, Total Nitrogen Kjeldahl (TNK): 10–20 mg·L−1, Total Phosphorus (TP): 1–5 mg·L−1, BOD: reduced to 10–20 mg·L−1, Chemical Oxygen Demand (COD): 50–100 mg·L−1, TSS: 10–30 mg·L−1 | [27,28] |
| Anaerobic Digestion | Biological breakdown in the absence of oxygen. | TOC: 100–130 mg·L−1, TNK: 40–50 mg·L−1, TP: 8–12 mg·L−1, BOD: reduced by approx. 80–90%, COD: 200–280 mg·L−1 | [29,30] |
| Trickling Filter | Wastewater is passed over a bed of media, promoting microbial growth. | TOC: 30–60 mg·L−1, TNK: 15–25 mg·L−1, TP: 2–6 mg·L−1, BOD: reduced to 15–30 mg·L−1, COD: 70–120 mg·L−1, TSS: 15–40 mg·L−1 | [31,32] |
| Constructed Wetlands | Use of plants and microbes to treat wastewater in a controlled environment. | TOC: 10–35 mg·L−1, TNK: 5–15 mg·L−1, TP: 0.5–3 mg·L−1, BOD: reduced to 5–20 mg·L−1, COD: 30–70 mg·L−1, TSS: 5–25 mg·L−1 | [33,34] |
| Membrane Bioreactor | Combination of activated sludge and membrane filtration. | TOC: 5–15 mg·L−1, TNK: 3–10 mg·L−1, TP: 0.5–2 mg·L−1, BOD: reduced to 2–10 mg·L−1, COD: 10–30 mg·L−1, TSS: <5 mg·L−1, NH4+: 0.5–3 mg·L−1 | [35,36] |
2.1.2. Microalgae
| Species | Proteins (%) | Carbohydrates (%) | Lipids (%) |
|---|---|---|---|
| Scenedesmus obliquus | 50–56 | 10–17 | 12–14 |
| Scenedesmus dimorphus | 8–18 | 21–52 | 16–40 |
| Chlamydomonas rheinhardii | 48 | 17 | 21 |
| Chlorella vulgaris | 51–58 | 12–17 | 14–22 |
| Chlorella pyrenoidosa | 57 | 26 | 2 |
| Spirogyra sp. | 6–20 | 33–64 | 11–21 |
| Dunaliella bioculata | 49 | 4 | 8 |
| Dunaliella salina | 57 | 32 | 6 |
| Euglena gracilis | 39–61 | 14–18 | 14–20 |
| Prymnesium parvum | 28–45 | 25–33 | 22–38 |
| Tetraselmis maculata | 52 | 15 | 3 |
| Porphyridium cruentum | 28–39 | 40–57 | 9–14 |
| Spirulina platensis | 46–63 | 8–14 | 4–9 |
| Spirulina maxima | 60–71 | 13–16 | 6–7 |
| Synechoccus sp. | 63 | 15 | 11 |
| Anabaena cylindrica | 43–56 | 25–30 | 4–7 |
2.2. Microalgae and Their Growth Factors
2.2.1. Nutrient Availability
2.2.2. pH
2.2.3. Temperature
2.2.4. Light Intensity
2.2.5. Aeration and Mixing
2.2.6. Type of Culture
2.2.7. Type of Cultivation System
2.3. Municipal Wastewater Treatment Plant Effluents and Microalgae Growth
2.3.1. Removal Mechanisms of Dominant Pollutants Using Microalgae
2.3.2. Reuse of Treated Municipal Wastewater
- Irrigation of parks and gardens located in public areas, centers, sports facilities, soccer fields, golf courses, school gardens and universities, lawns, decorative trees, and shrubs along avenues and highways;
- Irrigation of garden areas around public and residential buildings;
- Fire protection reserve;
- Dust control in earth movements;
- Aquatic decorative systems, such as fountains, mirrors, and waterfalls;
- Toilet flushing in public restrooms and commercial and industrial buildings;
- Washing of public trains and buses.
2.3.3. Recovery of Microalgae Biomass
| Separation Process | Advantages | Disadvantages |
|---|---|---|
| Centrifugation | Fast | High cost |
| Easy | High energy consumption | |
| High efficiency | Cell damage | |
| Filtration | Small scale High efficiency | Slow process |
| Membrane fouling or clogging | ||
| Sedimentation | Large scale Low energetic demand Easy | Cost of coagulant Toxicity of biomass |
| Flotation | Large scale Low time of hydraulic detention Low cost Low required space | Use of coagulant Material stability Energetic demand for the generation of microbubbles |
2.3.4. Potential Applications of Algal Biomass
Biomass for Animal Feed and Soil Fertilization
Biomass for the Production of Bioenergy
Other Valuable Compounds
3. Challenges and Future Perspectives in Microalgal Municipal Wastewater Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, B.; Ran, T.; Liu, S.; Li, Q.; Cui, X.; Zhou, Y. Biofilm bioactivity affects nitrogen metabolism in a push-flow microalgae-bacteria biofilm reactor during aeration-free greywater treatment. Water Res. 2023, 244, 120461. [Google Scholar] [CrossRef]
- Zhou, Y.; Nguyen, B.T.; Zhou, C.; Straka, L.; Lai, Y.S.; Xia, S.; Rittmann, B.E. The distribution of phosphorus and its transformations during batch growth of Synechocystis. Water Res. 2017, 122, 355–362. [Google Scholar] [CrossRef]
- Zhou, Y.; Lai, Y.S.; Eustance, E.; Straka, L.; Zhou, C.; Xia, S.; Rittmann, B.E. How myristyltrimethylammonium bromide enhances biomass harvesting and pigments extraction from Synechocystis sp. PCC 6803. Water Res. 2017, 126, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Yu, Z.; Gao, M.; Liu, Q.; Wu, B.; Ren, T.; Zhou, Y. Aeration-free greywater treatment in a self-sustaining oxygenic photobiofilm: Performance and mechanisms. Chem. Eng. J. 2023, 454, 140336. [Google Scholar] [CrossRef]
- Zhou, Y.; Lai, Y.S.; Eustance, E.; Rittmann, B.E. Promoting Synechocystis sp. PCC 6803 harvesting by cationic surfactants: Alkyl-chain length and dose control for the release of extracellular polymeric substances and biomass aggregation. ACS Sustain. Chem. Eng. 2018, 7, 2127–2133. [Google Scholar] [CrossRef]
- Zhou, Y.; Marcus, A.K.; Straka, L.; Eustance, E.; Lai, Y.S.; Xia, S.; Rittmann, B.E. Uptake of phosphate by Synechocystis sp. PCC 6803 in dark conditions: Removal driving force and modeling. Chemosphere 2019, 218, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Eustance, E.; Straka, L.; Lai, Y.S.; Xia, S.; Rittmann, B.E. Quantification of heterotrophic bacteria during the growth of Synechocystis sp. PCC 6803 using fluorescence activated cell sorting and microscopy. Algal Res. 2018, 30, 94–100. [Google Scholar] [CrossRef]
- Lai, Y.S.; Zhou, Y.; Eustance, E.; Straka, L.; Wang, Z.; Rittmann, B.E. Cell disruption by cationic surfactants affects bioproduct recovery from Synechocystis sp. PCC 6803. Algal Res. 2018, 34, 250–255. [Google Scholar] [CrossRef]
- Von Sperling, M. Waste Stabilisation Ponds; IWA Publishing: London, UK, 2007. [Google Scholar]
- Burton, F.L.; Tchobanoglous, G. Wastewater Engineering: Treatment, Disposal, and Reuse; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Sidabutar, T.; Srimariana, E.; Wouthuyzen, S. The Potential Role of Eutrophication, Tidal and Climatic on the Rise of Algal Bloom Phenomenon in Jakarta Bay. In Proceedings of IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2020; p. 012021. [Google Scholar]
- Batista, A.M.; do Valle, D.; Dias, D.F.; Sinischachi, L.A.; Lopes, B.C.; von Sperling, M.; Figueredo, C.C.; Rossas Mota-Filho, C. Relationships between abiotic and biotic variables in a maturation pond and their influence on E. coli removal. Water Sci. Technol. 2021, 84, 2903–2912. [Google Scholar] [CrossRef]
- Azam, H.M.; Alam, S.T.; Hasan, M.; Yameogo, D.D.S.; Kannan, A.D.; Rahman, A.; Kwon, M.J. Phosphorous in the environment: Characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ. Sci. Pollut. Res. 2019, 26, 20183–20207. [Google Scholar] [CrossRef]
- Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Megharaj, M. Nutrient removal and biomass production: Advances in microalgal biotechnology for wastewater treatment. Crit. Rev. Biotechnol. 2018, 38, 1244–1260. [Google Scholar] [CrossRef] [PubMed]
- Slompo, N.D.M.; Quartaroli, L.; Fernandes, T.V.; da Silva, G.H.R.; Daniel, L.A. Nutrient and pathogen removal from anaerobically treated black water by microalgae. J. Environ. Manag. 2020, 268, 110693. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.; Tay, J.H. Microalgal-bacterial consortia: From interspecies interactions to biotechnological applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J. New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arévalo, T.; Lizarralde, I.; Fdz-Polanco, F.; Pérez-Elvira, S.I.; Garrido, J.M.; Puig, S.; Poch, M.; Grau, P.; Ayesa, E. Quantitative assessment of energy and resource recovery in wastewater treatment plants based on plant-wide simulations. Water Res. 2017, 118, 272–288. [Google Scholar] [CrossRef] [PubMed]
- El Wali, M.; Golroudbary, S.R.; Kraslawski, A. Circular economy for phosphorus supply chain and its impact on social sustainable development goals. Sci. Total Environ. 2021, 777, 146060. [Google Scholar] [CrossRef] [PubMed]
- Water, U. Progress on Change in Water-Use Efficiency: Global Status and Acceleration Needs for SDG Indicator 6.4.1, 2021; Food & Agriculture Org.: Rome, Italy, 2021. [Google Scholar]
- de Matos, A.T.; de Matos, M.P.; de Almeida Costa, R.; von Sperling, M. Influence factors in the adjustment of parameters of the modified first-order kinetics equation used to model constructed wetland systems. Acta Sci. Technol. 2019, 41, e36709. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, B.; Wester, A.E.; Chen, J.; He, F.; Chen, H.; Gao, B. Reclaiming phosphorus from secondary treated municipal wastewater with engineered biochar. Chem. Eng. J. 2019, 362, 460–468. [Google Scholar] [CrossRef]
- Lima, S.; Villanova, V.; Grisafi, F.; Caputo, G.; Brucato, A.; Scargiali, F. Autochthonous microalgae grown in municipal wastewaters as a tool for effectively removing nitrogen and phosphorous. J. Water Process Eng. 2020, 38, 101647. [Google Scholar] [CrossRef]
- Brack, W.; Dulio, V.; Ågerstrand, M.; Allan, I.; Altenburger, R.; Brinkmann, M.; Bunke, D.; Burgess, R.M.; Cousins, I.; Escher, B.I. Towards the review of the European Union Water Framework Directive: Recommendations for more efficient assessment and management of chemical contamination in European surface water resources. Sci. Total Environ. 2017, 576, 720–737. [Google Scholar] [CrossRef]
- Carvalho, F.; da Silva, F.T.; Szklo, A.; Portugal-Pereira, J. Potential for biojet production from different biomass feedstocks and consolidated technological routes: A georeferencing and spatial analysis in Brazil. Biofuels Bioprod. Biorefining 2019, 13, 1454–1475. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: A review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
- Lima, S.; Brucato, A.; Caputo, G.; Grisafi, F.; Scargiali, F. Inoculum of indigenous microalgae/activated sludge for optimal treatment of municipal wastewaters and biochemical composition of residual biomass for potential applications. J. Water Process Eng. 2022, 49, 103142. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Tian, J.; Pan, Z.; Chen, Y.; Ming, F.; Wang, R.; Wang, L.; Zhou, H.; Li, J. Co-cultivation of microalgae-activated sludge for municipal wastewater treatment: Exploring the performance, microbial co-occurrence patterns, microbiota dynamics and function during the startup stage. Bioresour. Technol. 2023, 374, 128733. [Google Scholar] [CrossRef] [PubMed]
- Kusmayadi, A.; Lu, P.-H.; Huang, C.-Y.; Leong, Y.K.; Yen, H.-W.; Chang, J.-S. Integrating anaerobic digestion and microalgae cultivation for dairy wastewater treatment and potential biochemicals production from the harvested microalgal biomass. Chemosphere 2022, 291, 133057. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, D.; Kusmayadi, A.; Yen, H.-W.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour. Technol. 2019, 289, 121718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, B.; Tian, J.; Liu, Y. Development, performance and microbial community analysis of a continuous-flow microalgal-bacterial biofilm photoreactor for municipal wastewater treatment. J. Environ. Manag. 2023, 338, 117770. [Google Scholar] [CrossRef]
- Sun, P.; Liu, C.; Li, A.; Ji, B. Using carbon dioxide-added microalgal-bacterial granular sludge for carbon-neutral municipal wastewater treatment under outdoor conditions: Performance, granule characteristics and environmental sustainability. Sci. Total Environ. 2022, 848, 157657. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, T.; Dang, B.; Guo, M.; Jin, M.; Li, C.; Hou, N.; Bai, S. Microalgae-based constructed wetland system enhances nitrogen removal and reduce carbon emissions: Performance and mechanisms. Sci. Total Environ. 2023, 877, 162883. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.O.; Felizzola, N.M.; Hickmann, E.V.; Konrad, O.; Lutterbeck, C.A.; Machado, Ê.L.; Rodrigues, L.R. Energy recovery by anaerobic digestion of algal biomass from integrated microalgae/constructed wetland wastewater treatment. Environ. Sci. Pollut. Res. 2023, 30, 13317–13326. [Google Scholar] [CrossRef]
- Ding, M.; Wang, C.; Bae, S.W.; Ng, H.Y. Enhanced nutrient removal and bioenergy production in microalgal photobioreactor following anaerobic membrane bioreactor for decarbonized wastewater treatment. Bioresour. Technol. 2022, 364, 128120. [Google Scholar] [CrossRef] [PubMed]
- Chaleshtori, S.N.; Shamskilani, M.; Babaei, A.; Behrang, M. Municipal wastewater treatment and fouling in microalgal-activated sludge membrane bioreactor: Cultivation in raw and treated wastewater. J. Water Process Eng. 2022, 49, 103069. [Google Scholar] [CrossRef]
- Mahmood, T.; Hussain, N.; Shahbaz, A.; Mulla, S.I.; Iqbal, H.; Bilal, M. Sustainable production of biofuels from the algae-derived biomass. Bioprocess. Biosyst. Eng. 2022, 46, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Aumeerun, S.; Soulange-Govinden, J.; Driver, M.F.; Ranga, R.A.; Ravishankar, G.A.; Hudaa, N. Macroalgae and microalgae: Novel sources of functional food and feed. In Handbook of Algal Technologies and Phytochemicals; CRC Press: Boca Raton, FL, USA, 2019; pp. 207–219. [Google Scholar]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Babaei, A.; Mehrnia, M.R.; Shayegan, J.; Sarrafzadeh, M.-H. Comparison of different trophic cultivations in microalgal membrane bioreactor containing N-riched wastewater for simultaneous nutrient removal and biomass production. Process Biochem. 2016, 51, 1568–1575. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of wastewaters by microalgae and the potential applications of the produced biomass—A review. Water 2020, 13, 27. [Google Scholar] [CrossRef]
- Saidu, H.; Mohammed Ndejiko, J.; Abdullahi, N.; Bello Mahmoud, A.; Eva Mohamad, S. Microalgae: A cheap tool for wastewater abatement and biomass recovery. Environ. Technol. Rev. 2022, 11, 202–225. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Ammar, E.E.; Aioub, A.A.; Elesawy, A.E.; Karkour, A.M.; Mouhamed, M.S.; Amer, A.A.; El-Shershaby, N.A. Algae as Bio-fertilizers: Between current situation and future prospective. Saudi J. Biol. Sci. 2022, 29, 3083–3096. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.Y.; Nghiem, L.; Ong, H.C. Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, G.; Chandel, H.; Shyam, K.; Thakur, S.; Vaswani, P.; Saxena, G. Microalgae in Wastewater Treatment and Biofuel Production: Recent Advances, Challenges, and Future Prospects. Omics Insights Environ. Bioremediation 2022, 8, 237–271. [Google Scholar]
- Johansen, M.N. Microalgae: Biotechnology, Microbiology, and Energy; Nova Science Publisher’s: Hauppauge, NY, USA, 2012. [Google Scholar]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.d.R.; Leite, M.d.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Chu, F.; Cheng, J.; Li, K.; Wang, Y.; Li, X.; Yang, W. Enhanced lipid accumulation through a regulated metabolic pathway of phosphorus luxury uptake in the microalga Chlorella vulgaris under nitrogen starvation and phosphorus repletion. ACS Sustain. Chem. Eng. 2020, 8, 8137–8147. [Google Scholar] [CrossRef]
- Jasni, J.; Arisht, S.N.; Yasin, N.H.M.; Abdul, P.M.; Lin, S.-K.; Liu, C.-M.; Wu, S.-Y.; Jahim, J.M.; Takriff, M.S. Comparative toxicity effect of organic and inorganic substances in palm oil mill effluent (POME) using native microalgae species. J. Water Process Eng. 2020, 34, 101165. [Google Scholar] [CrossRef]
- Vadlamani, A.; Viamajala, S.; Pendyala, B.; Varanasi, S. Cultivation of microalgae at extreme alkaline pH conditions: A novel approach for biofuel production. ACS Sustain. Chem. Eng. 2017, 5, 7284–7294. [Google Scholar] [CrossRef]
- Binnal, P.; Babu, P.N. Optimization of environmental factors affecting tertiary treatment of municipal wastewater by Chlorella protothecoides in a lab scale photobioreactor. J. Water Process Eng. 2017, 17, 290–298. [Google Scholar] [CrossRef]
- Thomas, P.K.; Dunn, G.P.; Good, A.R.; Callahan, M.P.; Coats, E.R.; Newby, D.T.; Feris, K.P. A natural algal polyculture outperforms an assembled polyculture in wastewater-based open pond biofuel production. Algal Res. 2019, 40, 101488. [Google Scholar] [CrossRef]
- Masojídek, J.; Prášil, O. The development of microalgal biotechnology in the Czech Republic. J. Ind. Microbiol. Biotechnol. 2010, 37, 1307–1317. [Google Scholar] [CrossRef]
- González-Camejo, J.; Ferrer, J.; Seco, A.; Barat, R. Outdoor microalgae-based urban wastewater treatment: Recent advances, applications, and future perspectives. Wiley Interdiscip. Rev. Water 2021, 8, e1518. [Google Scholar] [CrossRef]
- Sathinathan, P.; Parab, H.; Yusoff, R.; Ibrahim, S.; Vello, V.; Ngoh, G. Photobioreactor design and parameters essential for algal cultivation using industrial wastewater: A review. Renew. Sustain. Energy Rev. 2023, 173, 113096. [Google Scholar] [CrossRef]
- Park, J.; Kumar, G.; Bakonyi, P.; Peter, J.; Nemestóthy, N.; Koter, S.; Kujawski, W.; Bélafi-Bakó, K.; Pientka, Z.; Muñoz, R. Comparative evaluation of CO2 fixation of microalgae strains at various CO2 aeration conditions. Waste Biomass Valori 2021, 12, 2999–3007. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Badruddin, I.A.; Khan, T.Y.; Ong, H.C. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef] [PubMed]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Noguez, I.Y.; Ledesma, M.T.O. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019, 151, 107319. [Google Scholar] [CrossRef]
- Razzak, S.A.; Hossain, M.M.; Lucky, R.A.; Bassi, A.S.; De Lasa, H. Integrated CO2 capture, wastewater treatment and biofuel production by microalgae culturing—A review. Renew. Sustain. Energy Rev. 2013, 27, 622–653. [Google Scholar] [CrossRef]
- Moreno-Garcia, L.; Gariépy, Y.; Barnabé, S.; Raghavan, V. Factors affecting the fatty acid profile of wastewater-grown-algae oil as feedstock for biodiesel. Fuel 2021, 304, 121367. [Google Scholar] [CrossRef]
- Abdelfattah, A.; Ali, S.S.; Ramadan, H.; El-Aswar, E.I.; Eltawab, R.; Ho, S.-H.; Elsamahy, T.; Li, S.; El-Sheekh, M.M.; Schagerl, M. Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environ. Sci. Ecotechnol. 2022, 13, 100205. [Google Scholar] [CrossRef]
- Sarwer, A.; Hamed, S.M.; Osman, A.I.; Jamil, F.; Al-Muhtaseb, A.a.H.; Alhajeri, N.S.; Rooney, D.W. Algal biomass valorization for biofuel production and carbon sequestration: A review. Environ. Chem. Lett. 2022, 20, 2797–2851. [Google Scholar] [CrossRef]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single cell protein: Sources, mechanism of production, nutritional value and its uses in aquaculture nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Yaakob, M.A.; Mohamed, R.M.S.R.; Al-Gheethi, A.; Aswathnarayana Gokare, R.; Ambati, R.R. Influence of nitrogen and phosphorus on microalgal growth, biomass, lipid, and fatty acid production: An overview. Cells 2021, 10, 393. [Google Scholar] [CrossRef]
- Sells, M.D.; Brown, N.; Shilton, A.N. Determining variables that influence the phosphorus content of waste stabilization pond algae. Water Res. 2018, 132, 301–308. [Google Scholar] [CrossRef] [PubMed]
- KHALIL, M.; El-Baz, A.A.A.; Ficara, E. Integration of microalgae culture as a natural-based solution for wastewater treatment. Egypt. J. Eng. Sci. Technol. 2021, 35, 16–26. [Google Scholar] [CrossRef]
- Ala’a, H.; Jamil, F.; Sarwer, A.; Al-Maawali, S. Valorization of microalgal biomass for food. In Valorization of Microalgal Biomass and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 81–112. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Varjani, S.; Jeevanantham, S.; Yaashikaa, P.; Thamarai, P.; Abirami, B.; George, C.S. A review on algal-bacterial symbiotic system for effective treatment of wastewater. Chemosphere 2021, 271, 129540. [Google Scholar] [CrossRef] [PubMed]
- Pahazri, N.F.; Mohamed, R.; Al-Gheethi, A.; Kassim, A.H.M. Production and harvesting of microalgae biomass from wastewater: A critical review. Environ. Technol. Rev. 2016, 5, 39–56. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Xu, X.; Gu, X.; Wang, Z.; Shatner, W.; Wang, Z. Progress, challenges and solutions of research on photosynthetic carbon sequestration efficiency of microalgae. Renew. Sustain. Energy Rev. 2019, 110, 65–82. [Google Scholar] [CrossRef]
- Huang, J.; Hankamer, B.; Yarnold, J. Design scenarios of outdoor arrayed cylindrical photobioreactors for microalgae cultivation considering solar radiation and temperature. Algal Res. 2019, 41, 101515. [Google Scholar] [CrossRef]
- Sforza, E.; Ramos-Tercero, E.A.; Gris, B.; Bettin, F.; Milani, A.; Bertucco, A. Integration of Chlorella protothecoides production in wastewater treatment plant: From lab measurements to process design. Algal Res. 2014, 6, 223–233. [Google Scholar] [CrossRef]
- Binnal, P.; Babu, P.N. Statistical optimization of parameters affecting lipid productivity of microalga Chlorella protothecoides cultivated in photobioreactor under nitrogen starvation. S. Afr. J. Chem. Eng. 2017, 23, 26–37. [Google Scholar] [CrossRef]
- Rinanti, A.; Fachrul, M.F.; Hadisoebroto, R.; Desty, S.; Rahmadhania, R.; Widyaningrum, D.A.; Saad, N.A. Heavy metal pollutant sorption in aquatic environment by microalgae consortium. Indones. J. Urban Environ. Technol. 2021, 5, 51–71. [Google Scholar] [CrossRef]
- Yeo, U.-H.; Lee, I.-B.; Seo, I.-H.; Kim, R.-W. Identification of the key structural parameters for the design of a large-scale PBR. Biosys. Eng. 2018, 171, 165–178. [Google Scholar] [CrossRef]
- Gonzalez-Camejo, J.; Aparicio, S.; Ruano, M.; Borrás, L.; Barat, R.; Ferrer, J. Effect of ambient temperature variations on an indigenous microalgae-nitrifying bacteria culture dominated by Chlorella. Bioresour. Technol. 2019, 290, 121788. [Google Scholar] [CrossRef] [PubMed]
- Behrens, P.W. Photobioreactors and fermentors: The light and dark sides of growing algae. Algal Cult. Tech. 2005, 1, 189–204. [Google Scholar]
- Pedruzi, G.O.; Amorim, M.L.; Santos, R.R.; Martins, M.A.; Vaz, M.G. Biomass accumulation-influencing factors in microalgae farms. Rev. Bras. Eng. Agric. AMB 2019, 24, 134–139. [Google Scholar] [CrossRef]
- Fettah, N.; Derakhshandeh, M.; Tezcan Un, U.; Mahmoudi, L. Effect of light on growth of green microalgae Scenedesmus quadricauda: Influence of light intensity, light wavelength and photoperiods. Int. J. Energy Environ. Eng. 2022, 13, 703–712. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, L.; Ji, B.; Fan, S.; Xiao, Y.; Ma, Y. Reactivation of frozen stored microalgal-bacterial granular sludge under aeration and non-aeration conditions. Water 2021, 13, 1974. [Google Scholar] [CrossRef]
- Wang, M.; Shi, L.-D.; Lin, D.-X.; Qiu, D.-S.; Chen, J.-P.; Tao, X.-M.; Tian, G.-M. Characteristics and performances of microalgal-bacterial consortia in a mixture of raw piggery digestate and anoxic aerated effluent. Bioresour. Technol. 2020, 309, 123363. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- de Castro Gonçalves, G.; Carraro Borges, A.; de Freitas Souza Ramos, N.; Daiane de Souza, T.; Teixeira de Matos, A.; de Fátima Souza, C. Performance of a hybrid anaerobic reactor in the treatment of swine wastewater. Rev. Cienc. Agron. 2021, 52, 1–8. [Google Scholar]
- González-González, L.M.; de-Bashan, L.E. Toward the enhancement of microalgal metabolite production through microalgae–bacteria consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Alam, M.A.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, J.; Chen, P.; Ji, C.; Kang, Q.; Lu, B.; Li, K.; Liu, J.; Ruan, R. Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1163–1175. [Google Scholar] [CrossRef]
- Galès, A.; Bonnafous, A.; Carré, C.; Jauzein, V.; Lanouguère, E.; Le Floc’h, E.; Pinoit, J.; Poullain, C.; Roques, C.; Sialve, B. Importance of ecological interactions during wastewater treatment using high rate algal ponds under different temperate climates. Algal Res. 2019, 40, 101508. [Google Scholar] [CrossRef]
- Baldisserotto, C.; Demaria, S.; Accoto, O.; Marchesini, R.; Zanella, M.; Benetti, L.; Avolio, F.; Maglie, M.; Ferroni, L.; Pancaldi, S. Removal of nitrogen and phosphorus from thickening effluent of an urban wastewater treatment plant by an isolated green microalga. Plants 2020, 9, 1802. [Google Scholar] [CrossRef]
- Moreno-García, A.F.; Neri-Torres, E.E.; Mena-Cervantes, V.Y.; Altamirano, R.H.; Pineda-Flores, G.; Luna-Sánchez, R.; García-Solares, M.; Vazquez-Arenas, J.; Suastes-Rivas, J.K. Sustainable biorefinery associated with wastewater treatment of Cr (III) using a native microalgae consortium. Fuel 2021, 290, 119040. [Google Scholar] [CrossRef]
- Mantovani, M.; Marazzi, F.; Fornaroli, R.; Bellucci, M.; Ficara, E.; Mezzanotte, V. Outdoor pilot-scale raceway as a microalgae-bacteria sidestream treatment in a WWTP. Sci. Total Environ. 2020, 710, 135583. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Hernandez, J.; Rodriguez Miranda, E.; Guzman Sanchez, J.L.; Acien Fernandez, F.G.; Visioli, A. Temperature optimization in microalgae raceway reactors by depth regulation. Rev. Iberoam. Automática Informática Ind. 2022, 19, 164–173. [Google Scholar]
- Rodríguez-Miranda, E.; Guzmán, J.; Acién, F.; Berenguel, M.; Visioli, A. Indirect regulation of temperature in raceway reactors by optimal management of culture depth. Biotechnol. Bioeng. 2021, 118, 1186–1198. [Google Scholar] [CrossRef]
- Rodríguez-Miranda, E.; Acién, F.G.; Guzmán, J.L.; Berenguel, M.; Visioli, A. A new model to analyze the temperature effect on the microalgae performance at large scale raceway reactors. Biotechnol. Bioeng. 2021, 118, 877–889. [Google Scholar] [CrossRef]
- Hernández, J.G.; Miranda, E.R.; Sánchez, J.L.G.; Fernández, F.G.A.; Visioli, A. Optimización de temperatura en reactores raceway para la producción de microalgas mediante regulación de nivel. Rev. Iberoam. Automática Informática Ind. 2022, 19, 164–173. [Google Scholar] [CrossRef]
- Satyanarayana, K.; Mariano, A.; Vargas, J. A review on microalgae, a versatile source for sustainable energy and materials. Int. J. Energy Res. 2011, 35, 291–311. [Google Scholar] [CrossRef]
- Ahmad, I.; Abdullah, N.; Koji, I.; Yuzir, A.; Muhammad, S.E. Evolution of Photobioreactors: A Review based on Microalgal Perspective. In Proceedings of IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2020; p. 012004. [Google Scholar]
- Belohlav, V.; Uggetti, E.; García, J.; Jirout, T.; Kratky, L.; Díez-Montero, R. Assessment of hydrodynamics based on Computational Fluid Dynamics to optimize the operation of hybrid tubular photobioreactors. J. Environ. Chem. Eng. 2021, 9, 105768. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, C.; Feng, C.; Ren, Y.; Wu, H.; Preis, S. Advances in characteristics analysis, measurement methods and modelling of flow dynamics in airlift reactors. Chem. Eng. Process. Process Intensif. 2019, 144, 107633. [Google Scholar] [CrossRef]
- Dasa, Y.; Lam, M.-K.; Yusup, S.; Lim, J.-W.; Ho, Y.; Show, P.; Tan, I.-S.; Kiew, P. Microalgae Cultivation in Open Pond and Photobioreactor; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2021; pp. 27–49. [Google Scholar]
- Salguero-Rodríguez, Y.; Gómez-Pérez, C.; Arango-Restrepo, J.P.; Espinosa, J. Static mixer proposal for tubular photobioreactors to reduce mixing energy consumption and enhance light–dark cycles. J. Chem. Technol. Biotechnol. 2021, 96, 113–124. [Google Scholar] [CrossRef]
- Ojah, A.; Sabri, L.S.; Aldahhan, M.H. Local volumetric mass transfer coefficient estimation for Scenedesmus microalgae culture in a cylindrical airlift photobioreactor. J. Chem. Technol. Biotechnol. 2021, 96, 764–774. [Google Scholar] [CrossRef]
- Shadpour, S.; Pirouzi, A.; Nosrati, M.; Hamze, H. Chlorella vulgaris Cultivation Using Triangular External Loop Airlift Photobioreactor: Hydrodynamic and Mass Transfer Studies. J. Renew. Energy Environ. 2022, 9, 93–105. [Google Scholar]
- Yaqoubnejad, P.; Rad, H.A.; Taghavijeloudar, M. Development a novel hexagonal airlift flat plate photobioreactor for the improvement of microalgae growth that simultaneously enhance CO2 bio-fixation and wastewater treatment. J. Environ. Manag. 2021, 298, 113482. [Google Scholar] [CrossRef]
- Oswald, W.J.; Gotaas, H.B. Photosynthesis in sewage treatment. Trans. Am. Soc. Civ. Eng. 1957, 122, 73–105. [Google Scholar] [CrossRef]
- Makut, B.B.; Das, D.; Goswami, G. Production of microbial biomass feedstock via co-cultivation of microalgae-bacteria consortium coupled with effective wastewater treatment: A sustainable approach. Algal Res. 2019, 37, 228–239. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.; Gu, X.; Lin, H.; Melis, A. Engineering microalgae: Transition from empirical design to programmable cells. Crit. Rev. Biotechnol. 2021, 41, 1233–1256. [Google Scholar] [CrossRef] [PubMed]
- Alazaiza, M.Y.; Albahnasawi, A.; Ahmad, Z.; Bashir, M.J.; Al-Wahaibi, T.; Abujazar, M.S.S.; Amr, S.S.A.; Nassani, D.E. Potential use of algae for the bioremediation of different types of wastewater and contaminants: Production of bioproducts and biofuel for green circular economy. J. Environ. Manag. 2022, 324, 116415. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Camejo, J.; Aparicio, S.; Jiménez-Benítez, A.; Pachés, M.; Ruano, M.; Borrás, L.; Barat, R.; Seco, A. Improving membrane photobioreactor performance by reducing light path: Operating conditions and key performance indicators. Water Res. 2020, 172, 115518. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Gonzalez-Camejo, J.; Jiménez-Benítez, A.; Ruano, M.; Robles, A.; Barat, R.; Ferrer, J. Optimising an outdoor membrane photobioreactor for tertiary sewage treatment. J. Environ. Manag. 2019, 245, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Ansari, F.A.; Shriwastav, A.; Sahoo, N.K.; Rawat, I.; Bux, F. Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J. Clean. Prod. 2016, 115, 255–264. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- AlMomani, F.A.; Örmeci, B. Performance of Chlorella vulgaris, Neochloris oleoabundans, and mixed indigenous microalgae for treatment of primary effluent, secondary effluent and centrate. Ecol. Eng. 2016, 95, 280–289. [Google Scholar] [CrossRef]
- Arias, D.M.; Rueda, E.; García-Galán, M.J.; Uggetti, E.; García, J. Selection of cyanobacteria over green algae in a photo-sequencing batch bioreactor fed with wastewater. Sci. Total Environ. 2019, 653, 485–495. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Zhu, S.-F.; Yu, Y.; Shi, X.-J.; Wu, G.-X.; Hu, H.-Y. Mixed cultivation as an effective approach to enhance microalgal biomass and triacylglycerol production in domestic secondary effluent. Chem. Eng. J. 2017, 328, 665–672. [Google Scholar] [CrossRef]
- Pachés, M.; Martínez-Guijarro, R.; Gonzalez-Camejo, J.; Seco, A.; Barat, R. Selecting the most suitable microalgae species to treat the effluent from an anaerobic membrane bioreactor. Environ. Technol. 2020, 41, 267–276. [Google Scholar] [CrossRef]
- Ledda, C.; Villegas, G.R.; Adani, F.; Fernández, F.A.; Grima, E.M. Utilization of centrate from wastewater treatment for the outdoor production of Nannochloropsis gaditana biomass at pilot-scale. Algal Res. 2015, 12, 17–25. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Álvarez-Díaz, P.; Garrido-Pérez, C.; Perales, J.A. Capability of different microalgae species for phytoremediation processes: Wastewater tertiary treatment, CO2 bio-fixation and low cost biofuels production. Water Res. 2014, 49, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, C.; Fernández, C.; García-Encina, P.A.; Muñoz, R. Mixotrophic metabolism of Chlorella sorokiniana and algal-bacterial consortia under extended dark-light periods and nutrient starvation. Appl. Microbiol. Biotechnol. 2015, 99, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Villegas, G.R.; Fiamengo, M.; Fernández, F.A.; Grima, E.M. Outdoor production of microalgae biomass at pilot-scale in seawater using centrate as the nutrient source. Algal Res. 2017, 25, 538–548. [Google Scholar] [CrossRef]
- González, I.; Ekelhof, A.; Herrero, N.; Siles, J.Á.; Podola, B.; Chica, A.F.; Ángeles Martín, M.; Melkonian, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Ren, N. Hydrogen and lipid production from starch wastewater by co-culture of anaerobic sludge and oleaginous microalgae with simultaneous COD, nitrogen and phosphorus removal. Water Res. 2015, 85, 404–412. [Google Scholar] [CrossRef]
- Qin, L.; Wang, Z.; Sun, Y.; Shu, Q.; Feng, P.; Zhu, L.; Xu, J.; Yuan, Z. Microalgae consortia cultivation in dairy wastewater to improve the potential of nutrient removal and biodiesel feedstock production. Environ. Sci. Pollut. Res. 2016, 23, 8379–8387. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef]
- Gonçalves, A.; Abreu, A.; Coqueiro, A.; Gaspar, A.; Borges, F.; Choi, Y.; Pires, J.; Simoes, M. Co-cultivation of Synechocystis salina and Pseudokirchneriella subcapitata under varying phosphorus concentrations evidences an allelopathic competition scenario. RSC Adv. 2016, 6, 56091–56100. [Google Scholar] [CrossRef]
- Ruan, Y.; Wu, R.; Lam, J.C.; Zhang, K.; Lam, P.K. Seasonal occurrence and fate of chiral pharmaceuticals in different sewage treatment systems in Hong Kong: Mass balance, enantiomeric profiling, and risk assessment. Water Res. 2019, 149, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef]
- Espinosa, M.F.; Verbyla, M.E.; Vassalle, L.; Rosa-Machado, A.T.; Zhao, F.; Gaunin, A.; Mota, C.R. Reduction and partitioning of viral and bacterial indicators in a UASB reactor followed by high rate algal ponds treating domestic sewage. Sci. Total Environ. 2021, 760, 144309. [Google Scholar] [CrossRef] [PubMed]
- Marchello, A.E.; Lombardi, A.T.; Dellamano-Oliveira, M.J.; de Souza, C.W. Microalgae population dynamics in photobioreactors with secondary sewage effluent as culture medium. Braz. J. Microbiol. 2015, 46, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ruas, G.; Serejo, M.; Farias, S.; Scarcelli, P.; Boncz, M. Removal of pathogens from domestic wastewater by microalgal-bacterial systems under different cultivation conditions. Int. J. Environ. Sci. Technol. 2022, 19, 10177–10188. [Google Scholar] [CrossRef]
- Gutiérrez-Alfaro, S.; Rueda-Márquez, J.J.; Perales, J.A.; Manzano, M.A. Combining sun-based technologies (microalgae and solar disinfection) for urban wastewater regeneration. Sci. Total Environ. 2018, 619, 1049–1057. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Sci. Total Environ. 2020, 698, 134154. [Google Scholar] [CrossRef]
- Helmecke, M.; Fries, E.; Schulte, C. Regulating water reuse for agricultural irrigation: Risks related to organic micro-contaminants. Environ. Sci. Eur. 2020, 32, 4. [Google Scholar] [CrossRef]
- Ramírez-García, R.; Gohil, N.; Singh, V. Recent advances, challenges, and opportunities in bioremediation of hazardous materials. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 517–568. [Google Scholar]
- Uggetti, E.; Sialve, B.; Trably, E.; Steyer, J.P. Integrating microalgae production with anaerobic digestion: A biorefinery approach. Biofuels Bioprod. Biorefining 2014, 8, 516–529. [Google Scholar] [CrossRef]
- Moghaddam, A.; Khayatan, D.; Esmaeili Fard Barzegar, P.; Ranjbar, R.; Yazdanian, M.; Tahmasebi, E.; Alam, M.; Abbasi, K.; Esmaeili Gouvarchin Ghaleh, H.; Tebyaniyan, H. Biodegradation of pharmaceutical compounds in industrial wastewater using biological treatment: A comprehensive overview. Int. J. Environ. Sci. Technol. 2023, 20, 5659–5696. [Google Scholar] [CrossRef]
- Viswanaathan, S.; Perumal, P.K.; Sundaram, S. Integrated approach for carbon sequestration and wastewater treatment using algal–bacterial consortia: Opportunities and challenges. Sustainability 2022, 14, 1075. [Google Scholar] [CrossRef]
- Abdella, B.; Mahmoud, N.H.; Mohamed, J.H.; Moffit, S.M.; Elsherbiny, B.A.; El-Sheekh, M.M. Bioremediation of Organic and Heavy Metal Co-contaminated Environments. In Industrial Wastewater Reuse: Applications, Prospects and Challenges; Springer: Berlin/Heidelberg, Germany, 2023; pp. 393–420. [Google Scholar]
- Inuwa, A.; Pervez, A.; Nazir, R. Microalgae-based wastewater treatment system: Current state, antibiotic resistant bacteria and antibiotic resistance genes reduction potentials. Int. J. Environ. Sci. Technol. 2023, 1–20. [Google Scholar] [CrossRef]
- Mathew, M.M.; Khatana, K.; Vats, V.; Dhanker, R.; Kumar, R.; Dahms, H.-U.; Hwang, J.-S. Biological approaches integrating algae and bacteria for the degradation of wastewater contaminants—A review. Front. Microbiol. 2022, 12, 801051. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.H.; Rawindran, H.; Ameen, F.; Alam, M.M.; Chai, Y.H.; Ho, Y.C.; Lam, M.K.; Lim, J.W.; Tong, W.-Y.; Bashir, M.J. Advancements of microalgal upstream technologies: Bioengineering and application aspects in the paradigm of circular bioeconomy. Chemosphere 2023, 339, 139699. [Google Scholar] [CrossRef]
- Solé-Bundó, M.; Cucina, M.; Folch, M.; Tàpias, J.; Gigliotti, G.; Garfí, M.; Ferrer, I. Assessing the agricultural reuse of the digestate from microalgae anaerobic digestion and co-digestion with sewage sludge. Sci. Total Environ. 2017, 586, 1–9. [Google Scholar] [CrossRef]
- Roy, M.; Mohanty, K. A comprehensive review on microalgal harvesting strategies: Current status and future prospects. Algal Res. 2019, 44, 101683. [Google Scholar] [CrossRef]
- Ortiz, A.; García-Galán, M.J.; García, J.; Diez-Montero, R. Optimization and operation of a demonstrative full scale microalgae harvesting unit based on coagulation, flocculation and sedimentation. Sep. Purif. Technol. 2021, 259, 118171. [Google Scholar] [CrossRef]
- Lv, J.; Liu, G.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. Harvesting biomass of an oil-rich microalga Parachlorella kessleri TY02 by ferric chloride: Effects on harvesting efficiency, lipid production and municipal wastewater treatment. J. Environ. Manag. 2020, 273, 111128. [Google Scholar] [CrossRef]
- Niemi, C.; Gentili, F.G. The use of natural organic flocculants for harvesting microalgae grown in municipal wastewater at different culture densities. Physiol. Plant. 2021, 173, 536–542. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley Online Library: New York, NY, USA, 2004; Volume 577. [Google Scholar]
- Urbina-Suarez, N.A.; Ayala-González, D.D.; Rivera-Amaya, J.D.; Barajas-Solano, A.F.; Machuca-Martínez, F. Evaluation of the Light/Dark Cycle and Concentration of Tannery Wastewater in the Production of Biomass and Metabolites of Industrial Interest from Microalgae and Cyanobacteria. Water 2022, 14, 346. [Google Scholar] [CrossRef]
- Teixeira, M.S.; Speranza, L.G.; da Silva, I.C.; Moruzzi, R.B.; Silva, G.H.R. Tannin-based coagulant for harvesting microalgae cultivated in wastewater: Efficiency, floc morphology and products characterization. Sci. Total Environ. 2022, 807, 150776. [Google Scholar] [CrossRef]
- Ruggeri, M.V.R.; Godoy, R.F.B.; Arroyo, P.A.; Trevisan, E. Evaluation of natural flocculant efficiency in the harvest of microalgae Monoraphidium contortum. SN Appl. Sci. 2021, 3, 1–9. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Goswami, S. Microalgae–A green multi-product biorefinery for future industrial prospects. Biocatal. Agric. Biotechnol. 2020, 25, 101580. [Google Scholar] [CrossRef]
- Geada, P.; Moreira, C.; Silva, M.; Nunes, R.; Madureira, L.; Rocha, C.M.; Pereira, R.N.; Vicente, A.A.; Teixeira, J.A. Algal proteins: Production strategies and nutritional and functional properties. Bioresour. Technol. 2021, 332, 125125. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Khan, S.; Chaudhary, A.K.; AbdulQuadir, M.; Thaher, M.I.; Al-Jabri, H. Potential applications of algae-based bio-fertilizer. Biofertil. Sustain. Agric. Environ. 2019, 41–65. [Google Scholar] [CrossRef]
- Elleuch, J.; Hmani, R.; Drira, M.; Michaud, P.; Fendri, I.; Abdelkafi, S. Potential of three local marine microalgae from Tunisian coasts for cadmium, lead and chromium removals. Sci. Total Environ. 2021, 799, 149464. [Google Scholar] [CrossRef] [PubMed]
- Dammak, M.; Hlima, H.B.; Tounsi, L.; Michaud, P.; Fendri, I.; Abdelkafi, S. Effect of heavy metals mixture on the growth and physiology of Tetraselmis sp.: Applications to lipid production and bioremediation. Bioresour. Technol. 2022, 360, 127584. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jung, J.-M.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Lin, K.-Y.A.; Choi, Y.-E.; Kwon, E.E. Biodiesel from microalgae: Recent progress and key challenges. Prog. Energy Combust. Sci. 2022, 93, 101020. [Google Scholar] [CrossRef]
- Xiaogang, H.; Jalalah, M.; Jingyuan, W.; Zheng, Y.; Li, X.; Salama, E.-S. Microalgal growth coupled with wastewater treatment in open and closed systems for advanced biofuel generation. Biomass Convers. Biorefinery 2022, 12, 1939–1958. [Google Scholar] [CrossRef]
- Srimongkol, P.; Sangtanoo, P.; Songserm, P.; Watsuntorn, W.; Karnchanatat, A. Microalgae-based wastewater treatment for developing economic and environmental sustainability: Current status and future prospects. Front. Bioeng. Biotechnol. 2022, 10, 904046. [Google Scholar]

| Factors | Literature Findings | References |
|---|---|---|
| Nutrient availability | Nutrients, vitamins, and trace metals are essential for optimal microalgae growth. Nitrogen and phosphorus play crucial roles in cell synthesis and energy storage. | [49,50] |
| pH level | pH can influence metabolic activity, with optimal growth observed close to neutral conditions. Photosynthetic activity can lead to pH fluctuations. | [51,52] |
| Temperature | Optimal temperatures are species-specific and can influence metabolic activity, photosynthesis, and cellular tolerance. | [53,54] |
| Light intensity | Light intensity and photoperiod are crucial for photosynthetic activity, affecting biomass production and growth. | [55,56] |
| Aeration and mixing | Proper aeration and mixing prevent sedimentation, enhance nutrient contact, and aid in removing unwanted gases. However, excessive mixing can damage cells. | [57,58] |
| Type of culture | Choosing between pure cultures or consortia can influence adaptability, resilience, and efficiency in wastewater treatment. Native cultures may offer advantages in adaptability and cost-effectiveness. | [59,60] |
| Type of cultivation system | Open systems are cost-effective but susceptible to environmental fluctuations. Closed systems (photobioreactors) offer more control but can be costlier. | [61,62] |
| Microorganisms | Effluent | System and Mode of Operation | Time of Operation (Days) | Nitrogen | Phosphorus | References | ||
|---|---|---|---|---|---|---|---|---|
| Ci § (mg·L−1) | NRE § (%) | Ci (mg·L−1) | PRE (%) | |||||
| Indoor cultivation | ||||||||
| Scenedesmus obliquus | Raw effluent | PBR (2 L), L = 80 μmol·m−2·s−1, L/D = 16/8, T = 22 °C, pH = 7 | 15 | 52.6 | 99 | 8.5 | 98 | [116] |
| Chlorella sorokiniana | Raw effluent | PBR (2 L), L = 80 μmol·m−2·s−1, L/D = 16/8, T = 22 °C, pH = 7 | 15 | 52.6 | 87 | 8.5 | 68 | [116] |
| Consortium of microalgae | Primary effluent | PBR (200 mL), L = 250 μmol·m−2·s−1, L/D = 12/12, T = 15 °C, pH = 8 | 8 | 49.4 | 83 | 3.1 | 100 | [117] |
| Chlorella vulgaris | Secondary effluent | PBR (500 mL), batch, L = 213 μmol·m−2·s−1, L/D = 24/0, T = 20 °C | 28 | 66.9 | 56 | 26.0 | 12 | [118] |
| Neochloris oleoabundans | Secondary effluent | PBR (500 mL), batch, L = 213 μmol·m−2·s−1, L/D = 24/0, T = 20 °C | 28 | 66.9 | 57 | 26.0 | 6 | [118] |
| Consortium of native microalgae | Secondary effluent | L = 213 μmol·m−2·s−1, L/D = 24/0, T = 20 °C | 28 | 66.9 | 67 | 26.0 | 31 | [118] |
| Cyanobacteria and Scenedesmus sp. | Secondary effluent | PBR (2.5 L), L = 220 μmol·m−2·s−1, L/D = 12/12, T = 27 °C, pH = 8.5 | 30 | 71.6 | 58 | 20.0 | 83 | [119] |
| Scenedesmus sp. | Secondary effluent | PBR (500 mL), L = 200 μmol·m−2·s−1, L/D = 14/10, T = 25 °C, pH = 7.8 | 13 | 27.4 | 72 | 2.3 | ~100 | [120] |
| Scenedesmus sp. and Haematococcus pluvialis | Secondary effluent | PBR (500 mL), L = 200 μmol·m−2·s−1, L/D = 14/10, T = 25 °C, pH = 7.8 | 13 | 27.4 | 85 | 2.3 | ~100 | [120] |
| Chlorella vulgaris | Anaerobic membrane bioreactor effluent | PBR (2 L), L = 250 μmol·m−2·s−1, L/D = 14/10, T = 30–35 °C, pH = 7.5 | 13 | 48.7 | 85 | 5.4 | 100 | [121] |
| Scenedesmus obliquus | Anaerobic membrane bioreactor effluent | PBR (2 L), L = 250 μmol·m−2·s−1, L/D = 14/10, T = 20–25 °C, pH = 7.5 | 8 | 68 | 97 | 4.6 | 100 | [121] |
| Chlorella sp. | Porcine effluent diluted in distilled water | PBR (500 mL), L = 150 μmol·m−2·s−1, L/D = 24/0, pH = 8, aeration 0.3 L.min−1 | 15 | 60 | 95 | 18.1 | 85 | [122] |
| Outdoor cultivation | ||||||||
| Scenedemus obliquus | Secondary effluent | PBR tubular (533 L), mixture = 0.2–0.3 m∙s−1, pH = 8 | 3.1–4.6 | 20.46 | 74–82 | 2.14 | 70–90 | [123] |
| The native consortium of microalga-bacteria | Domestic effluent | PBR (1.9 L), pH = 9 | 7 | 50 | 99.8 | 7 | 99.8 | [124] |
| Chlorella sp., Scenedesmus sp. and Chlamydomonas sp. | Tertiary effluent after anaerobic digestion | Raceway pond (1200 L) | 10 | 244 | 86 | 5.7 | 71 | [95] |
| Nannochloropsis gaditana | Effluent after anaerobic digestion diluted in seawater | PBR tubular (340 L), T = 25 °C, pH = 8 | Fed-batch | 6.55 | 95 | 14.1 | 95 | [125] |
| Consortium of native microalgae | Anaerobic membrane bioreactor effluent | Flat-panel (14 L), L = 300 μmol·m−2·s−1, T = 16 °C, pH = 7.5 | 25–35 | 45 | 85 | 4.7 | 99 | [126] |
| Chlorella sorokiniana | Anaerobically treated blackwater | PBR (50 L), L = 196 μmol·m−2·s−1, L/D = 12/12 | 7 | 111.6 | 66 | 15.5 | 74 | [15] |
| Scenedesmus sp. and native bacteria from anaerobic sludge | Starch wastewater | PBR (0.06 L), L = 70.4 μmol·m−2·s−1 | 5 | 30–50 | 88.7 | 54 | 80.1 | [127] |
| Chlorella sp., Scenedesmus sp. and Chlorella zofingiensis | Dairy effluent | PBR (0.4 L), L = 150 μmol·m−2·s−1 T = 25 °C | 7 | 176 | 87.0–91.0 | 39.6 | 91.2–96.0 | [128] |
| Consortium of microalga–bacteria | Synthetic medium | High-rate lagoon (500 L) | 6 | 17.3 | 60 | 3.9 | 66 | [17] |
| Synechocystis salina and Chlorella vulgaris | Synthetic medium | PBR (0.45 L), T = 25 °C, L = 120 μmol·m−2·s−1, atmospheric air aeration | 7 | 45 | 84.5 | 10 | 85.9 | [129] |
| Synechocystis salina and Microcystis aeruginosa | Synthetic medium | PBR (0.45 L), L = 120 μmol·m−2·s−1, T = 25 °C, atmospheric air aeration | 7 | 45 | 77.7 | 10 | 97.2 | [130] |
| Consortium of natives microalgae–bacteria from activated sludge | Synthetic medium | PBR (2.7 L), L = 400 μmol·m−2·s−1, T = 24 °C, pH = 7.8 | 2–20 | 120 | 75–96 | - | - | [124] |
| Synechocystis salina and Pseudokirchneriella subcapitata | Synthetic medium | PBR (0.45 L), L = 120 μmol·m−2·s−1, T = 25 °C, atmospheric air aeration | 7 | 45 | 72 | 10 | 91.8 | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dammak, I.; Fersi, M.; Hachicha, R.; Abdelkafi, S. Current Insights into Growing Microalgae for Municipal Wastewater Treatment and Biomass Generation. Resources 2023, 12, 119. https://doi.org/10.3390/resources12100119
Dammak I, Fersi M, Hachicha R, Abdelkafi S. Current Insights into Growing Microalgae for Municipal Wastewater Treatment and Biomass Generation. Resources. 2023; 12(10):119. https://doi.org/10.3390/resources12100119
Chicago/Turabian StyleDammak, Ilyes, Mariem Fersi, Ridha Hachicha, and Slim Abdelkafi. 2023. "Current Insights into Growing Microalgae for Municipal Wastewater Treatment and Biomass Generation" Resources 12, no. 10: 119. https://doi.org/10.3390/resources12100119
APA StyleDammak, I., Fersi, M., Hachicha, R., & Abdelkafi, S. (2023). Current Insights into Growing Microalgae for Municipal Wastewater Treatment and Biomass Generation. Resources, 12(10), 119. https://doi.org/10.3390/resources12100119









