Separation of Magnesium and Lithium from Brine Water and Bittern Using Sodium Silicate Precipitation Agent
Abstract
:1. Introduction
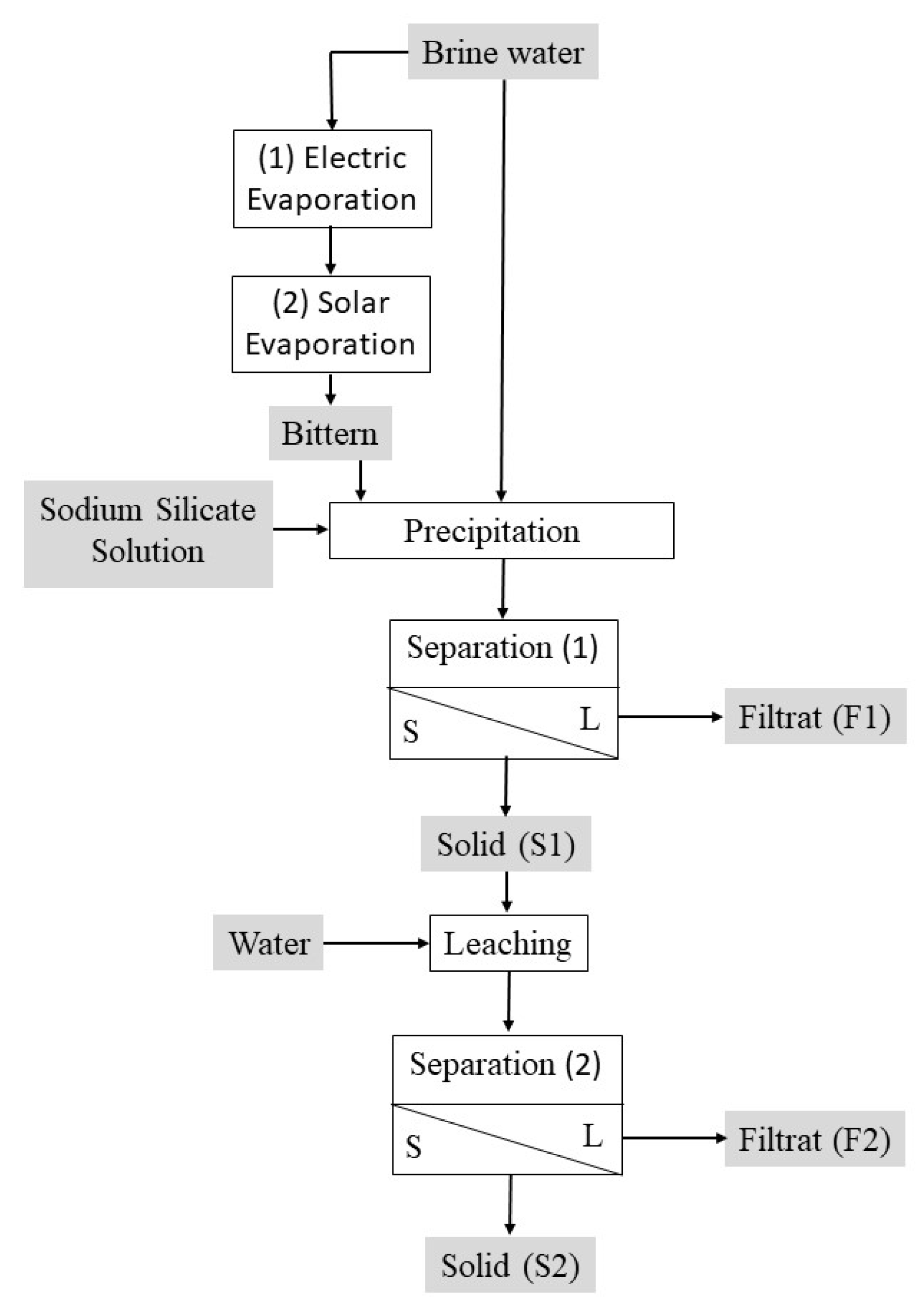
2. Materials and Method
2.1. Materials
2.2. Method
2.3. Characterization
3. Results and Discussion
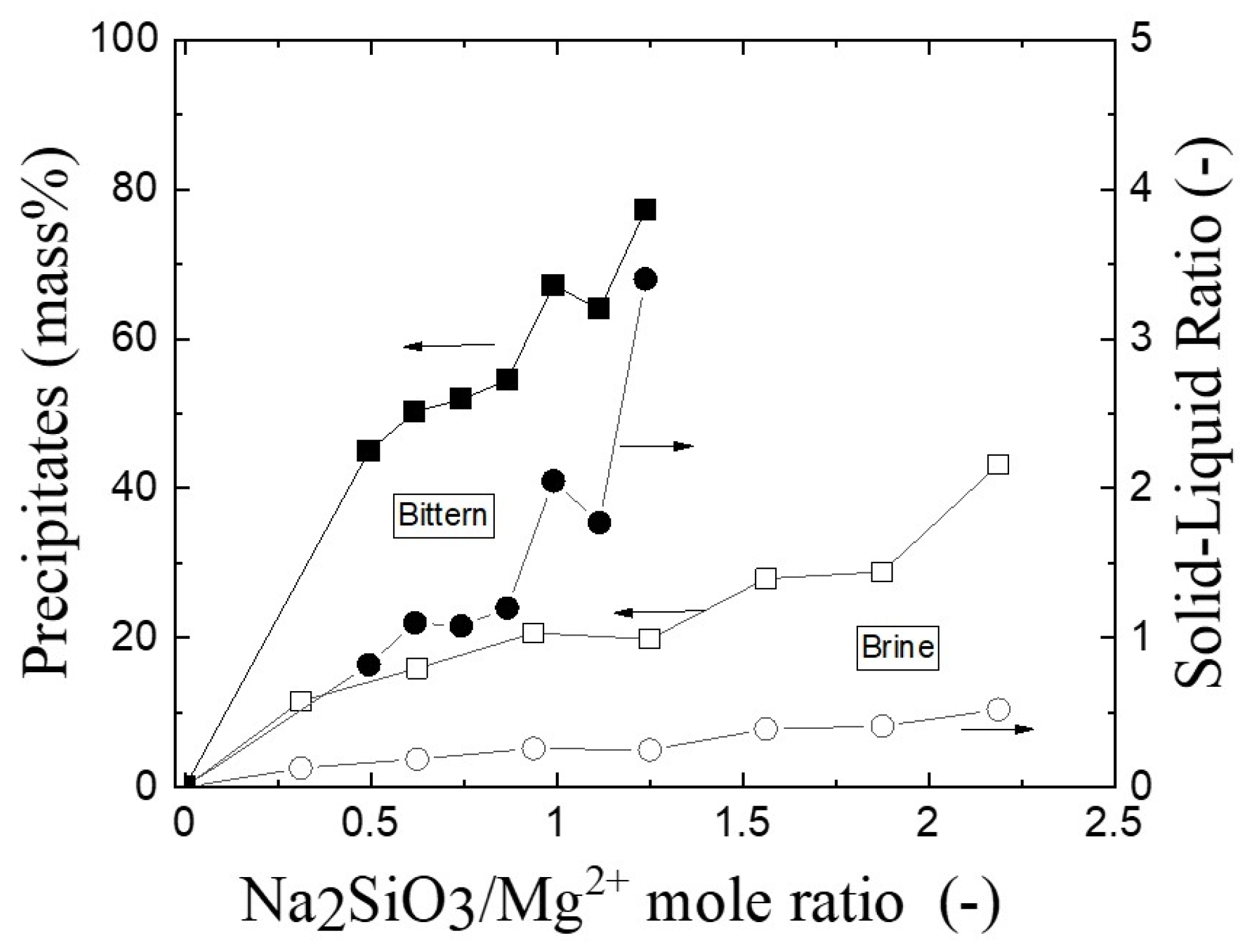
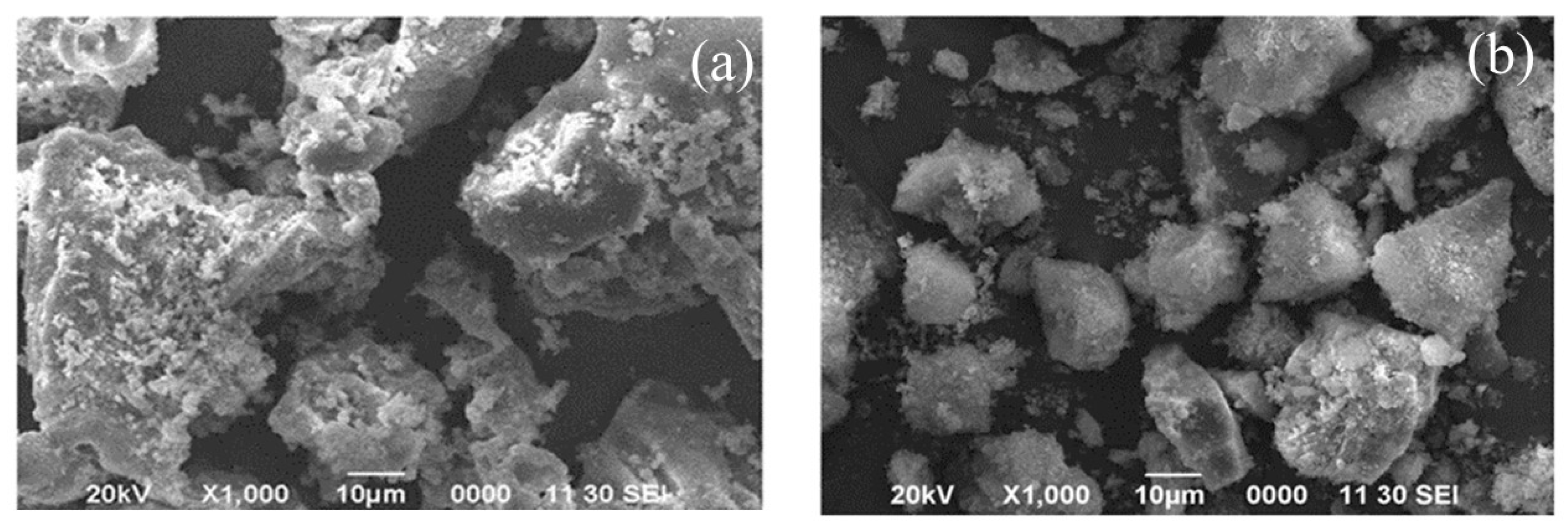
3.1. The Solid Products after Precipitation
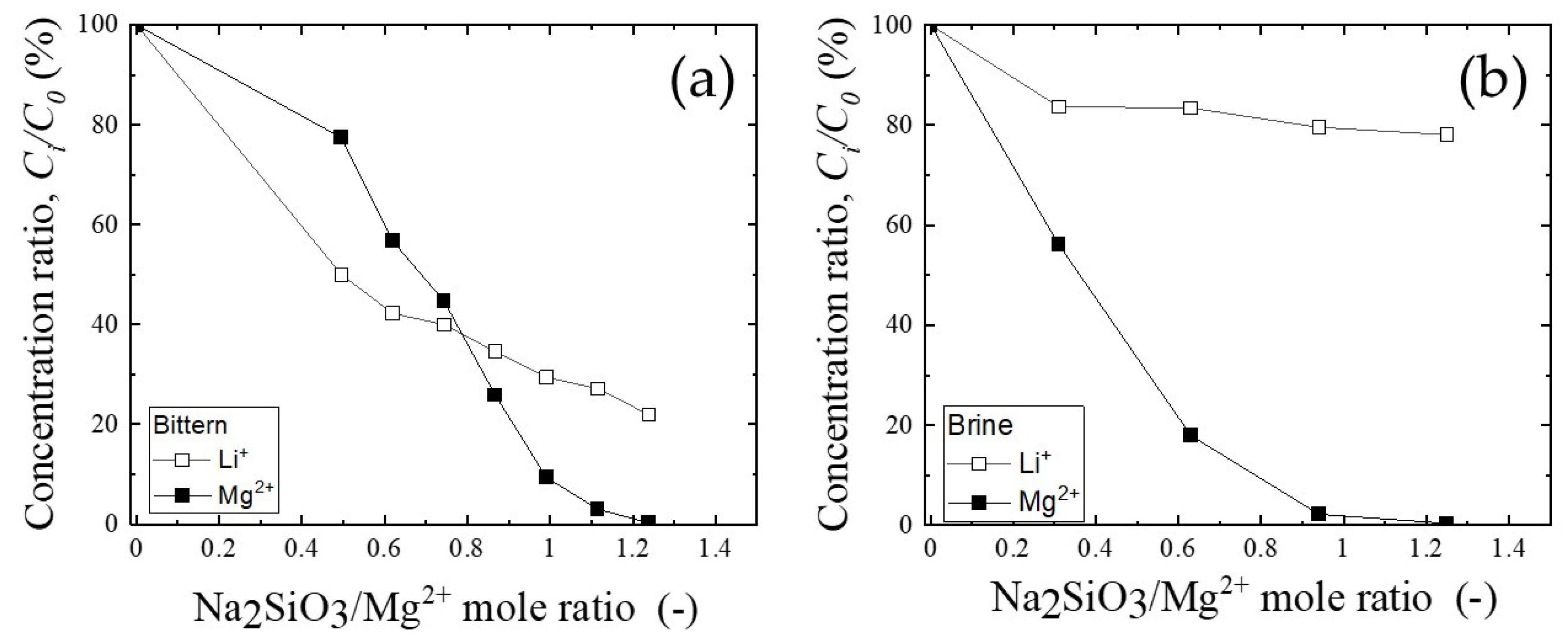
3.2. The Separation of Magnesium and Lithium in Filtrate after the Precipitation Process
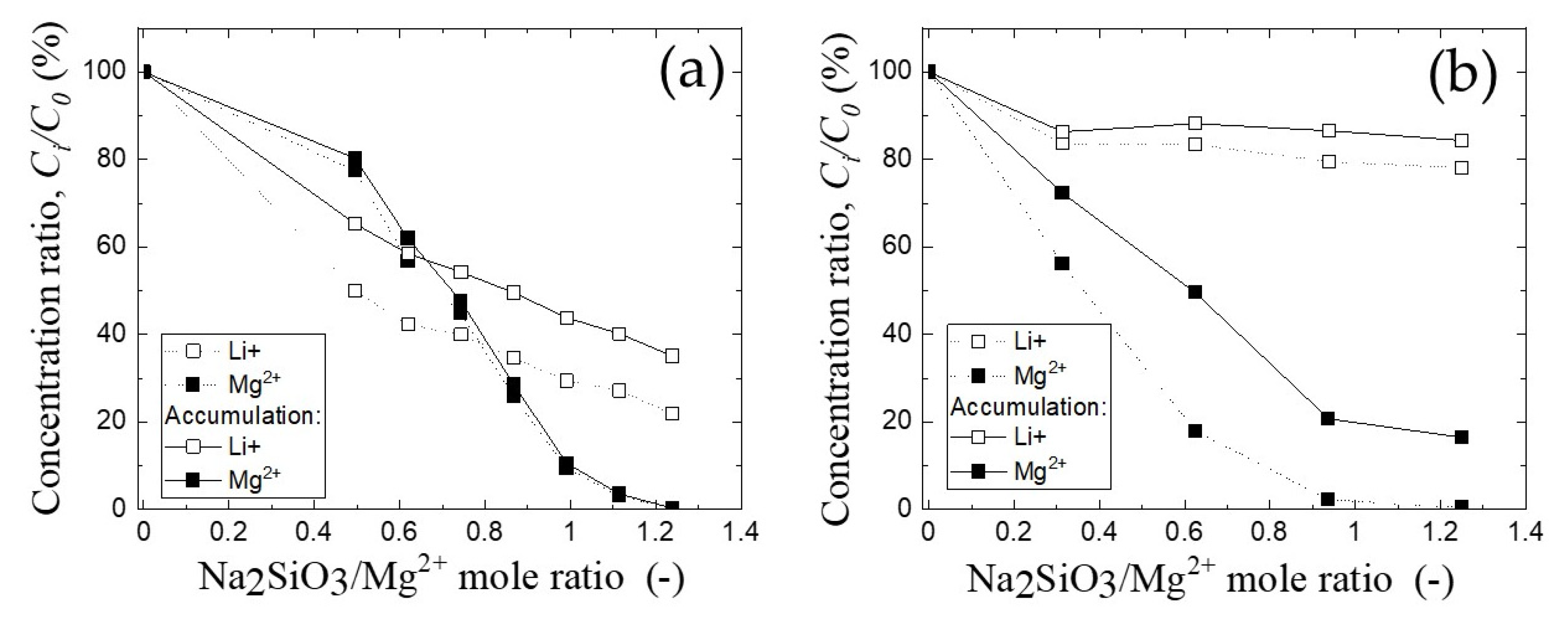
3.3. The Recovery of Lithium Ion from Solid Product by Water Leaching
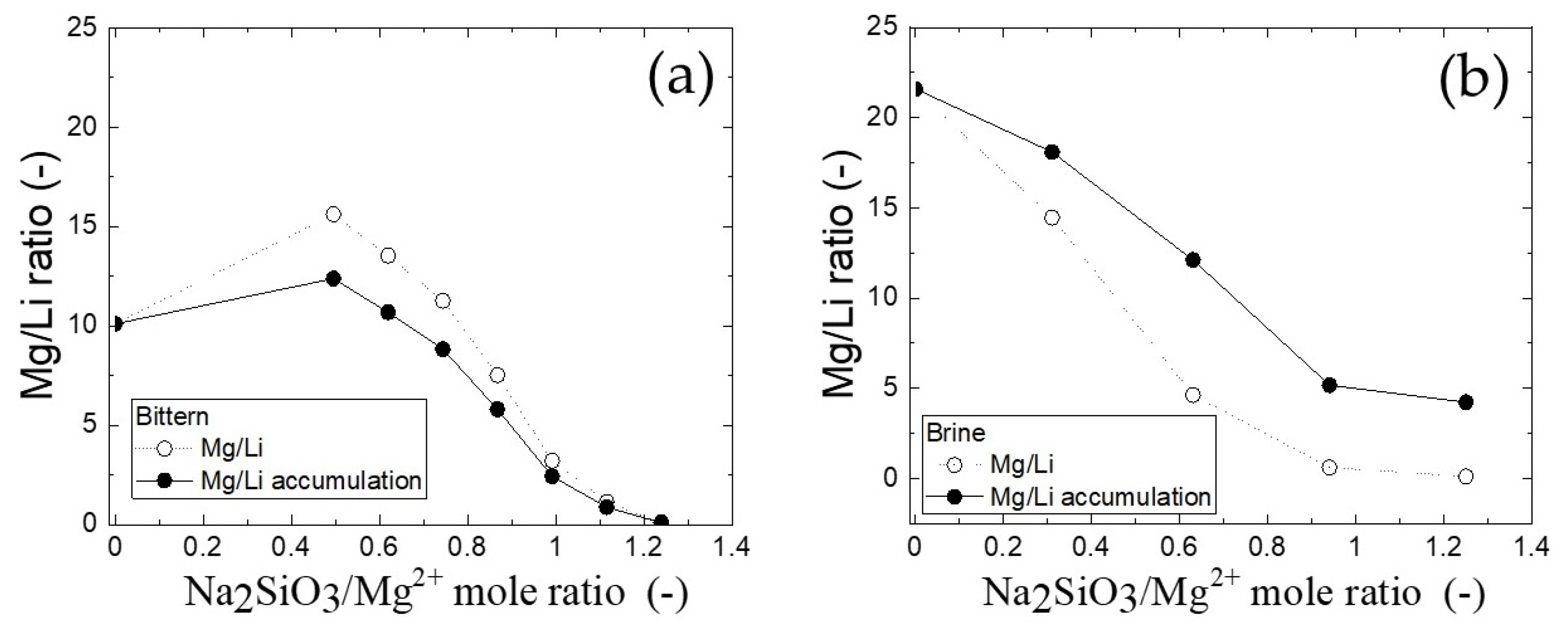
3.4. Mg/Li Ratio in Filtrate after Precipitation and Water Leaching
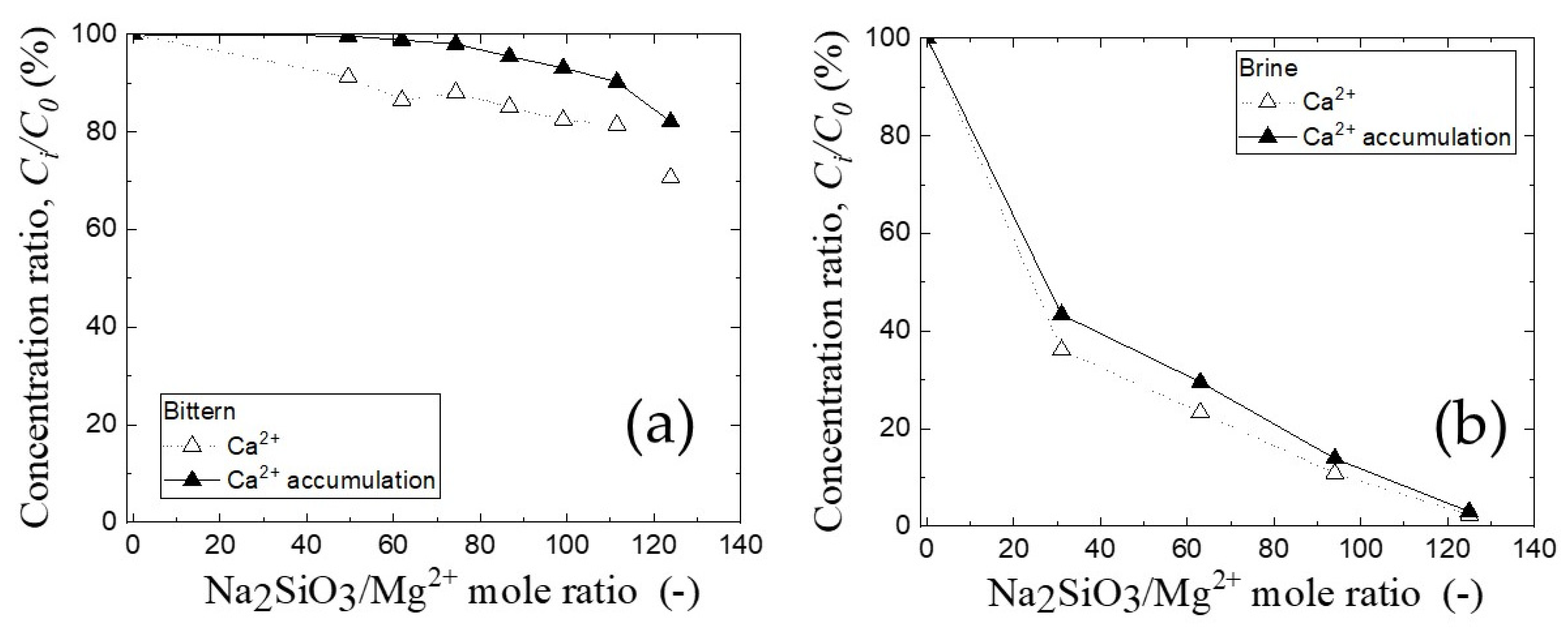
3.5. The Calcium Ion in Filtrate after Precipitation Process
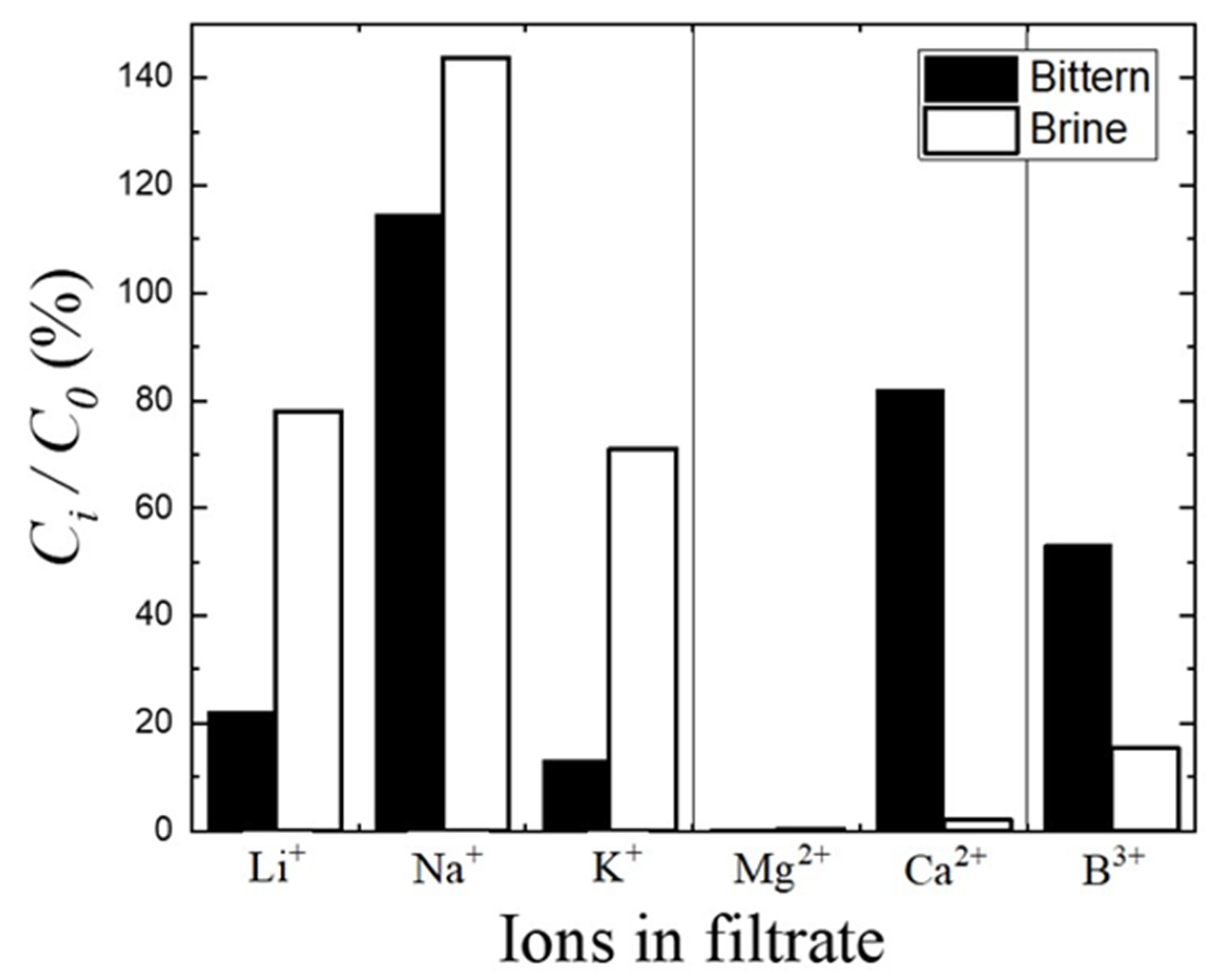
3.6. Concentration of Other Elements in the Filtrate after Precipitation
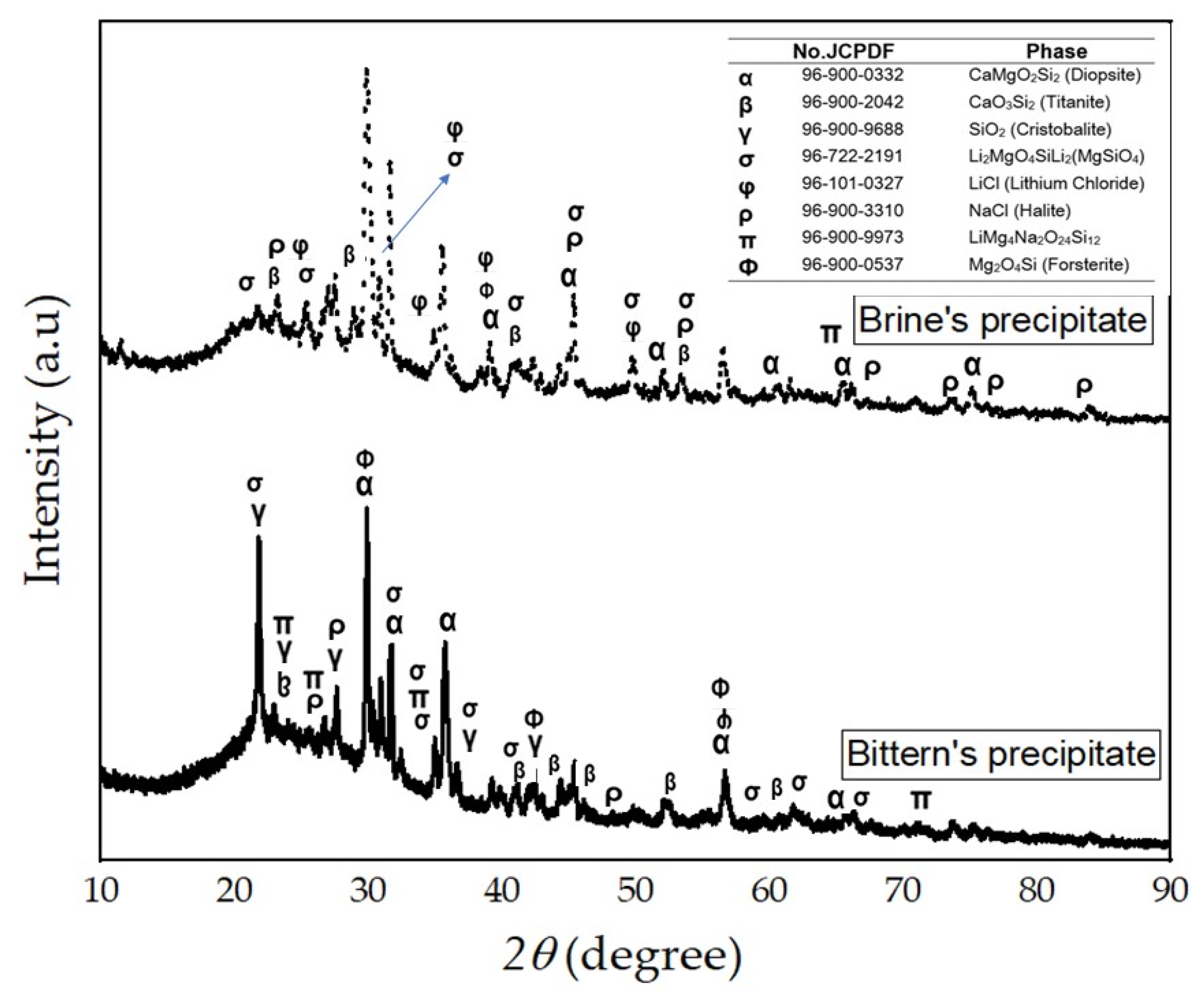
3.7. Solid Product of the Precipitation Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vikstrom, H.; Davidson, S.; Hock, M. Lithium Availability, and Future-Production Outlooks. Appl. Energy 2019, 110, 252–266. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Hock, M.; Berterteau, M. Lithium Market Research, Global Supply, future demand, and price development. Energy Storage Mater. 2017, 171–179. [Google Scholar] [CrossRef]
- Li, X.H.; Mo, Y.H.; Qing, W.; Shao, S.; Ye, C.; Li, J.X. Membrane-based technologies for lithium recovery from water lithium resources: A Review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankkhand, T.R. Extraction from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–206. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.W.; Gaherman, A. Novel Approaches for lithium extraction from salt lake brines A Review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Giurco, D.; Mohr, S.; Mudd, G.; Mason, L.; Prior, T. Resource criticality and commodity production projector. Resources 2012, 1, 23–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.H.; Wang, L.; Sun, W. Systematic review of lithium extraction from salt lake brines via precipitation approaches. Miner. Eng. 2019, 139, 105868. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Wang, Y.; Sha, Z. Review on the electrochemical extraction of lithium from seawater/brine. J. Electroanal. Chem. 2019, 850, 11133894. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, M.; Chen, X.Y.; Zhao, Z.W. Separating lithium and magnesium in brine by aluminum-based materials. Hydrometallurgy 2018, 176, 73–77. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, M.; Chen, X.Y.; Li, J.; He, L.; Zhao, Z.W. Enriching lithium and separating lithium to magnesium from sulfate type salt lake brine. Hydrometallurgy 2020, 192, 105247. [Google Scholar] [CrossRef]
- Tran, K.T.; Luong, T.V.; An, J.W.; Kang, D.J.; Kim, M.J.; Tran, T. Recovery of magnesium from Uyuni salar brine as high purity magnesium oxalate. Hydrometallurgy 2013, 130, 93–99. [Google Scholar] [CrossRef]
- Sulistiyono, E.; Lalasari, L.H.; Mayangsari, W.; Prasetyo, A.B. Study of lithium extraction from brine water Bledug Kuwu Indonesia by precipitation series of oxalic acid and carbonate sodium. AIP Conf. Proc. 2018, 1964, 020007. [Google Scholar]
- He, L.; Xu, W.; Song, Y.; Liu, X.; Zhao, Z.W. Selective removal of magnesium from a lithium-concentrated anolyte by magnesium ammonium phosphate precipitation. Sep. Purif. Technol. 2017, 187, 124–220. [Google Scholar] [CrossRef]
- Zhan, Y.; Hu, Y.; Sun, N.; Khoso, S.A.; Wang, L.; Sun, W. A Novel precipitant for separating lithium from magnesium in high Mg/Li ratio brine. Hydrometallurgy 2019, 187, 125–133. [Google Scholar] [CrossRef]
- Hai, C.X.; Zhou, Y.; Fuji, M.; Shirai, T.; Ren, X.; Zeng, J.; Li, X. Method for Preparing High Specific Surface Area Porous Magnesium Silicate Lithium Powder by Using Salt Lake Brine after Potassium Extraction. China Patent CN-106006653-A, 29 May 2018. [Google Scholar]
- Pambudi, N.A.; Itoi, R.; Yamashiro, R.; Yoseph, B.C.S.S.; Alam, S.; Tusara, L.; Jalilinnastrabady, S.; Khasani, J. The Behavior of silica in geothermal brine from Dieng geothermal power plant Indonesia. Geothermic 2015, 54, 109–114. [Google Scholar] [CrossRef]
- Claverie, M.; Dumas, A.; Careme, C.; Poirier, M.; Roux, C.L.; Micoud, P.; Martin, F.; Aymonier, C. Synthetic Talc and Talc like structure: Preparation, features and application. Chem. Eur. J. 2018, 24, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, D.D.; Vajgand, V.J.V. Studies of the mechanism of formation of calcium silicate compounds by titration based on emission and atomic absorption spectroscopy. Spectrochem. Acta 1984, 389, 767–775. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review. Resources 2018, 7, 57. [Google Scholar] [CrossRef]
- Ponka, M.A.; Sahdarani, D.N.; Kurniadi, D.T.; Yoga, D.A.; Sihombing, F.M.H.; Supriyanto, S. Hydrogeochemical model of Ciseeng geothermal field, Bogor, West java. In Life and Environmental Science Academic Forum 2019; IOP Conference Series: Earth and Environmental Science 2020; IOP Publishing: Bristol, UK, 2020; Volume 538, p. 012029. [Google Scholar]
- Xu, Z.H.; Zhang, H.; Wang, R.; Gu Wi Liu, G.; Yang, Y. Systemic and Direct Production of Battery Grade Lithium Carbonate from a saline lake, Industrial and Engineering Chemistry Research. Ind. Eng. Chem. Res. 2014, 53, 16502–16507. [Google Scholar] [CrossRef]
- Kent, D.B.; Kaster, M. Mg2+-amorphous SiO2-H2O by adsorption and Mg-Hydrosilicate precipitation. Geochim. Cosmochim. Acta 1985, 49, 1123–1136. [Google Scholar] [CrossRef]
| Raw Material | Concentration (ppm) | Ratio | |||||
|---|---|---|---|---|---|---|---|
| Li | Mg | Na | K | Ca | B | Mg/Li | |
| Brine Water | 22.06 | 475.30 | 4476 | 960.50 | 2127 | 95.48 | 21.55 |
| Bittern | 1342 | 13,527 | 7574 | 28,816 | 24,222 | 2514 | 10.08 |
| Reagent | Concentration Ion (ppm) | |||||
|---|---|---|---|---|---|---|
| Si | Mg | Na | K | Ca | B | |
| Sodium Silicate | 16,731 | 18 | 25,695 | 189 | 27 | 8 |
| Stages | Bittern (%) | Brine Water (%) | |||
|---|---|---|---|---|---|
| Mg2+ | Li+ | Mg2+ | Li+ | ||
| Precipitation | [F1] | 0.2 | 22 | 0.4 | 78 |
| [S1] | 99.8 | 78 | 99.6 | 22 | |
| Water leaching (accumulation) | [F1] + [F2] | 0.3 | 35 | 17 | 84 |
| [S1] + [S2] | 99.7 | 65 | 83 | 16 | |
| Phase | Solid Precipitates of (wt%.) | |
|---|---|---|
| Bittern | Brine Water | |
| Diopside (CaMgO6Si2) | 42.7 | 60.4 |
| Li2MgO4SiLi2(MgSiO4) | 10.0 | 4.1 |
| LiMg4Na3O30Si12 | 4.2 | - |
| Titanite (CaSi2O5) | 18.7 | 12.3 |
| Halite (NaCl) | 8.7 | 9.9 |
| Lithium Chloride (LiCl) | - | 8.4 |
| Forsterite (Mg2SiO4) | 2.3 | 5.0 |
| Cristobalite (SiO2) | 13.5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulistiyono, E.; Harjanto, S.; Lalasari, L.H. Separation of Magnesium and Lithium from Brine Water and Bittern Using Sodium Silicate Precipitation Agent. Resources 2022, 11, 89. https://doi.org/10.3390/resources11100089
Sulistiyono E, Harjanto S, Lalasari LH. Separation of Magnesium and Lithium from Brine Water and Bittern Using Sodium Silicate Precipitation Agent. Resources. 2022; 11(10):89. https://doi.org/10.3390/resources11100089
Chicago/Turabian StyleSulistiyono, Eko, Sri Harjanto, and Latifa Hanum Lalasari. 2022. "Separation of Magnesium and Lithium from Brine Water and Bittern Using Sodium Silicate Precipitation Agent" Resources 11, no. 10: 89. https://doi.org/10.3390/resources11100089
APA StyleSulistiyono, E., Harjanto, S., & Lalasari, L. H. (2022). Separation of Magnesium and Lithium from Brine Water and Bittern Using Sodium Silicate Precipitation Agent. Resources, 11(10), 89. https://doi.org/10.3390/resources11100089







