1. Introduction
The issue of global warming is becoming a central focus of the research community. Global warming is mainly triggered by industry, urbanization, deforestation and over-population. The emission of CO
2 is especially problematic, as it contributes around 80% of the total greenhouse gas (GHG) emissions [
1] and its concentration in the environment reached almost 420 ppm in 2021 [
2].
Carbon capture and sequestration (CCS) is an effective method for mitigation of CO
2 levels. However, a major drawback of CCS is its economic penalty as it causes an increase in cost of electricity of up to 45–70% [
3] depending upon the type of power plant. Carbon capture and utilization (CCU) is another approach to CO
2 mitigation and shifting towards ‘clean and green energy’. In CCU, CO
2 being released from some conventional power plants is captured (and sometimes stored for a very short time) and then converted to some useful products aligned to market demands. Sustainable CO
2 utilization should recover the cost of CO
2 capture to some extent. Considering the environmental and economic concerns, CCU is an attractive option for CO
2 mitigation. However, CO
2 is a highly stable molecule, and it may therefore be difficult to convert it into some other product; a high energy input is required for CCU processes. Recently, adsorbents along with suitable catalysts have been used to capture CO
2 and convert it to some useful products [
4]. Other options for CO
2 mitigation include improving the efficiency of already existing systems [
5], e.g., co-generation along with CO
2 capture [
6,
7,
8], switching towards ‘clean and green fuels’ [
9] and use of low quality or waste energy [
10].
Possibilities for CO
2 utilization may be divided into physical and chemical utilization. Physical utilization of CO
2 includes its utilization in enhanced oil recovery (EOR), enhanced gas recovery (EGR), etc. [
11]. In chemical utilization, the CO
2 molecule loses its original identity and is converted to some other product. However, it may be emitted after utilization of the other product or some part of it, depending on the end use of the product. The chemical utilization of CO
2 involves its conversion into syngas, methanol, sugar, acetic acid, ethanol, dimethyl ether (DME), dimethyl carbonate (DMC), carbamates, etc. [
12]. The world is shifting from conventional fossil fuel consumption to alternative fuels to conserve natural resources and deal with global warming issues. The focus of CCU techniques is the conversion of CO
2 into liquid fuels, which is termed as ‘Power to Liquid’ (PtL).
Methanol is one of the most useful products of PtL since it can be used as a substitute for gasoline. It is also used as a feedstock for DME, DMC, and acetic acid. Methanol is used as a solvent in many industries [
13]. Traditionally, methanol is produced using syngas in the presence of Cu as catalyst [
14]. Another method of methanol production is by CO
2 hydrogenation employing Cu and Zn based catalysts [
15]. Methanol can be produced from CO
2 and hydrogen in two ways. It can be formed by direct hydrogenation of CO
2 as presented by Equation (1) or by a reverse water gas shift reaction (RWGS) in which CO is first produced, as in Equation (2), and then methanol is produced by reaction presented in Equation (3) [
16].
Van-Dal et al. [
17] simulated and analyzed the production of fuels including gasoline, methanol, and mixed alcohols by hydrogenation of CO
2. Lonis et al. [
18] identified that the major cost component is the cost of hydrogen production using water electrolysis techniques. Yousaf et al. [
19] carried out the techno-economic analysis of integrated hydrogen (by solid oxide electrolyzer (SOE) steam electrolysis process) and methanol production process (by hydrogenation of CO
2) using Aspen Plus
®. Use of the SOE resulted into a 22.3% reduction of the cost of hydrogen as cosoimpared to alkaline water electrolysis (AEL). This reduction in hydrogen cost along with configuration improvement and parametric analysis resulted into a methanol production cost of
$701.5/ton which was less than AEL based on a previous study (
$1063/ton) [
19]. However, further advances are required to match the current market price of methanol.
Production of dimethyl ether (DME) is another attractive option for PtL processes. DME is produced by the dehydration of methanol or is directly produced from synthesis gas. Conventional processes such as the gasification of coal, oil and natural gas emit CO
2 and syngas. The syngas is then converted to methanol and further to DME. Since in this study methanol is produced by the hydrogenation of CO
2, therefore the dehydration of methanol is adopted for DME production in the current work. Due to recent environmental issues, DME has gained much more attention as an alternative fuel for diesel, propane and liquefied petroleum gas in the automobile industry [
20]. It is also used for power generation and it is an efficient fuel since it has a high cetane number of 55–60 [
21]. DME is considered a ‘clean fuel’ since it has no sulfur and nitrous oxide emissions.
Hassan Babiker et al. [
22] simulated the DME production by dehydration of methanol in Aspen HYSYS
®. It was found that more than 80% overall conversion of methanol into DME can be achieved at a temperature range of 200–400 °C. Michael et al. [
23] simulated the dehydration of methanol for DME production using a nonrandom two-liquid (NRTL) property package. The methanol dehydration took place in the reactor producing water and DME, as presented by Equation (4). The purity of DME was 99%. It was mentioned that methanol and DME consume CO
2 and reduce the GHGs emissions by 82–86% since no sulfur and nitrogen contents are involved in their combustion. It was also reported that utilizing these fuels can reduce the consumption of fossil fuels by 82–91% [
23].
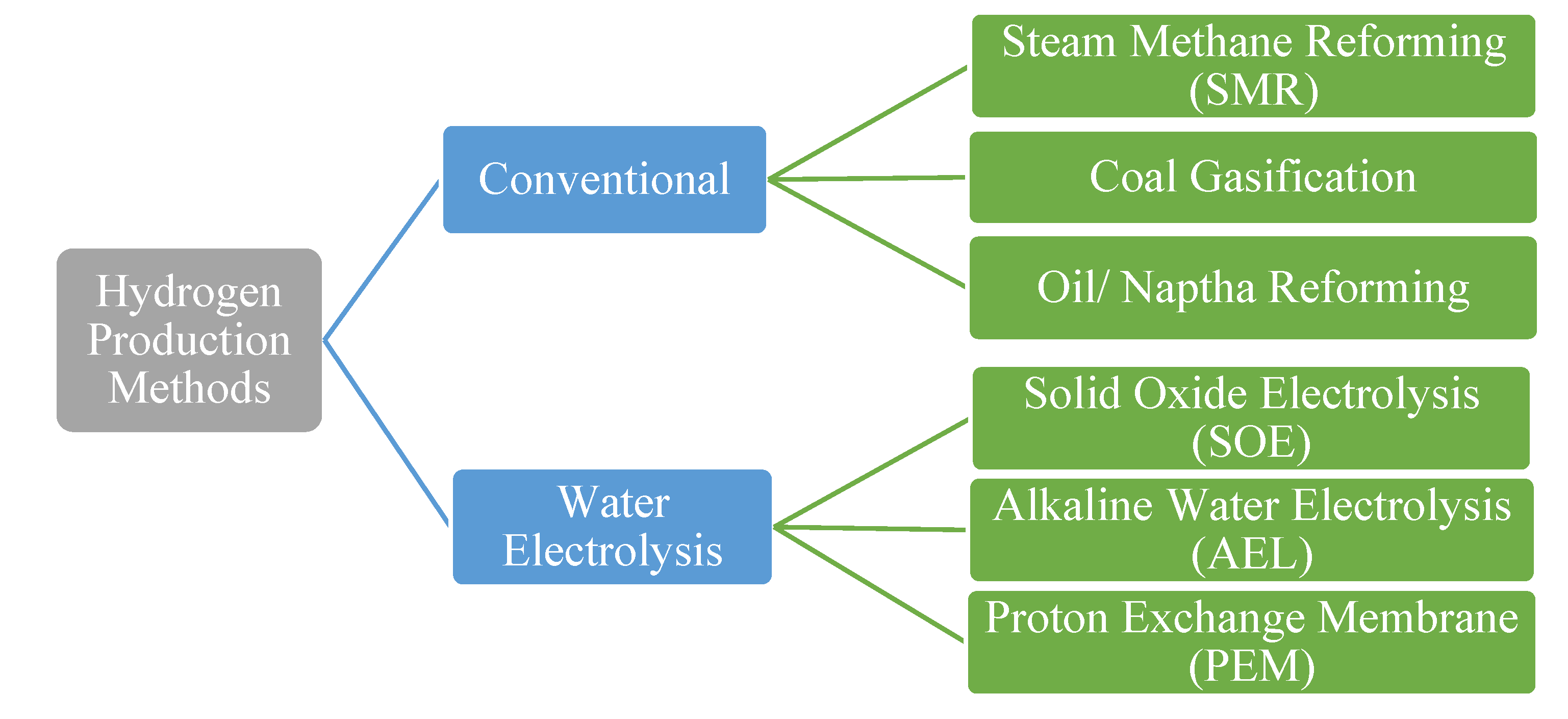
Hydrogen must be produced by some ‘clean and green source’ since the aim of this study is the mitigation of CO
2 emissions. Hydrogen is mainly produced by conventional and renewable techniques. Several methods of hydrogen production are shown in
Figure 1. Hydrogen produced by conventional processes utilizes fossil fuel energy in terms of coal, oil, and natural gas, and consequently emits huge amounts of CO
2. The second method involves the utilization of renewable energy (RE) for hydrogen production [
24]. The water electrolysis process for hydrogen production uses RE. The main challenge in the production of hydrogen by water electrolysis is the economics of the process.
The electrolysis of water needs energy for splitting water into hydrogen and oxygen. Energy is supplied to water in terms of thermal and electrical energy. Worldwide, electrolysis of water contributes only a 4% share of total hydrogen production while the remainder of hydrogen production still relies on conventional sources [
25]. The contribution of water electrolysis is less due to its high production cost for hydrogen compared to conventional sources. The main challenges in water electrolysis include maintenance, minimization of energy consumption, increasing durability and safety [
26]. The hydrogen produced by water electrolysis techniques is highly pure and can directly be supplied for utilization in some cases [
27]. The purity of the hydrogen gas produced depends upon the nature of the electrolysis technique used. In this work, hydrogen is produced using water electrolysis techniques and then used as a feed stock for methanol production utilizing captured CO
2. The methanol produced using hydrogen from water electrolysis is finally converted into DME by a dehydration reaction.
The most well-known green hydrogen production technologies based on water electrolysis are alkaline water electrolysis (AEL), proton exchange membrane (PEM) and solid oxide electrolyzer (SOE) [
14]. AEL and PEM are low temperature technologies, while SOE is a high temperature method for producing hydrogen from water electrolysis. AEL and PEM have an operating temperature range of 60–95 °C while SOE operates at around 800 °C. AEL and PEM are mature technologies compared to SOE. The main challenge associated with SOE is the selection of material, but this technology seems to have future potential due to its improved economic feasibility [
27]. The major difference in these processes lies in terms of thermodynamics since the operating temperature of SOE is high as compared to AEL and PEM so it requires less use of electricity. In past few years, hydrogen as a fuel has been revolutionized due to its negligible emissions and its production from REs [
26].
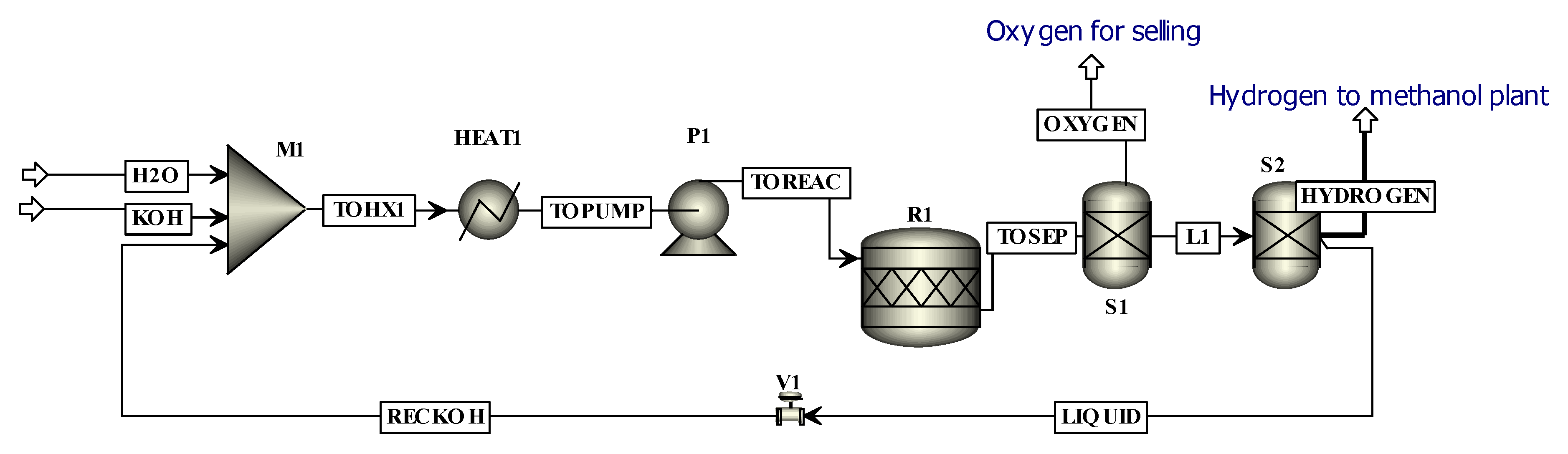
AEL is the most mature technique for hydrogen production. It uses an alkaline solution (35% KOH) as the electrolyte and electricity is supplied for the electrolysis. The water electrolysis reaction occurs in the stack to produce gaseous hydrogen and oxygen. Hydrogen is produced at the cathode while oxygen is produced at the anode. The reversible cell potential is determined through the Nernst equation, which predicts the minimum electrical potential required for the electrolysis of water at a particular location in the cell with a specific temperature and gas concentration. The Nernst voltage is calculated using the Gibbs free energy by the following relation.
Here,
F is the Faraday’s constant, and its value is 96,485 C/mol and
= 2 i.e., two electrons are transferred in the electrolysis process of water. ∆
G is the Gibb’s free energy of the reaction in kJ/mol [
27]. Hence,
Here,
is the total energy demand,
is the electrical energy demand and
represents the thermal energy demand. If the thermal energy is supplied to the system, the electrical energy requirements will decrease; i.e., if the electrolysis occurs at high temperature as in SOE, the total electricity requirement is reduced [
19]. The actual voltage required for the cell is greater than the reversible voltage due to the irreversibility in the system [
27].
Jingang et al. [
28] reported that among hydrogen production methods including biogas steam reforming, fluidized bed biomass steam gasification and AEL, AEL has highest efficiency (i.e., 66%). The investment cost of AEL is less compared to other technologies but operating cost is high. Schmidt et al. [
29] compared AEL, SOE and PEM based on the different operating conditions, costs and life-cycles. The comparison was based on previous literature. It was reported that the capital cost of AEL was the least while that for SOE was greatest. In another study, Christian et al. [
25] reported AEL to be an environmentally friendly technique if the source is REs while AEL presented maximum environmental impact if coal was used for power production instead of REs. Sanchez et. el [
30] made an experimental setup for AEL as well as simulating the AEL process. It was reported that the stack of AEL consumed 92% of the power and the stack power was the highest contributing factor in the overall cost of AEL.
PEM is termed as ‘polymer exchange membrane’ since it is made of polymeric membranes as electrolyte. In PEM, the membrane lies between the electrodes [
28]. PEM is a mature technique and has gained much attention in the previous years due to its high performance. The hydrogen produced using this technology is clean since it has no CO
2 or other GHGs emission, if a carbon free source of electricity is used. The membrane of PEM operates at a temperature of almost 70–80 °C [
28]. PEM uses a relatively thin membrane. The membrane splits the water and, since it is proton conducting, it only allows the proton or hydrogen ions to pass through [
29]. The investment cost of PEM is high as highly expensive materials such as platinum, titanium and iridium are used [
31]. The thickness of PEM is only 0.2 mm [
32]. The membranes of PEM must have high ionic conductivity, excellent chemical stability, mechanical strength and be relatively less expensive [
28]. The electrolysis of water demands a higher voltage, while PEM has typical cell voltages ranging from 1.5 V to 2 V [
31].
Julio et al. [
33] reported that although the investment cost of PEM is high compared to other technologies, it can operate with high efficiency in MW ranges, unlike AEL which usually operates in kW ranges. Ibrahim et al. [
34] mentioned that Nafion
® has excellent properties and chemical stability. The main challenge is to maintain the humidification level inside the membranes which may deteriorate the membrane if it is not sufficient, and the chemical degradation of membrane might occur. A comparison was made between different membranes used for PEM such as Nafion
® (Solution Technology Inc., Mendenhall, PA), organic and inorganic membranes and non-fluorinated membranes. It was reported that Nafion
® membranes with fillers were the best.
Methanol and DME are among the best fossil fuel alternatives for the abatement of CO
2 considering the global concerns over GHG emissions. Lonis et al. [
18] simulated the production of methanol using both AEL and SOE based hydrogen production processes in Aspen Plus
®. The efficiency of the AEL based process was less as compared to the SOE based process, since AEL required more power. Moreover, it was also reported that the capital cost of SOE was higher than AEL. Hydrogen production using different water electrolysis methods is considered clean, if a carbon free energy source is used. However, mostly experimental work is done on water electrolysis techniques and very limited simulation work is available on their detailed thermodynamic and economic performance. These water electrolysis techniques are usually compared based on experimental results in terms of capital cost and operating parameters such as temperature, pressure, voltages and current densities. Detailed thermodynamic and economic analysis of the water electrolyzed hydrogen production processes is required to evaluate and compare the performance using different hydrogen production methods.
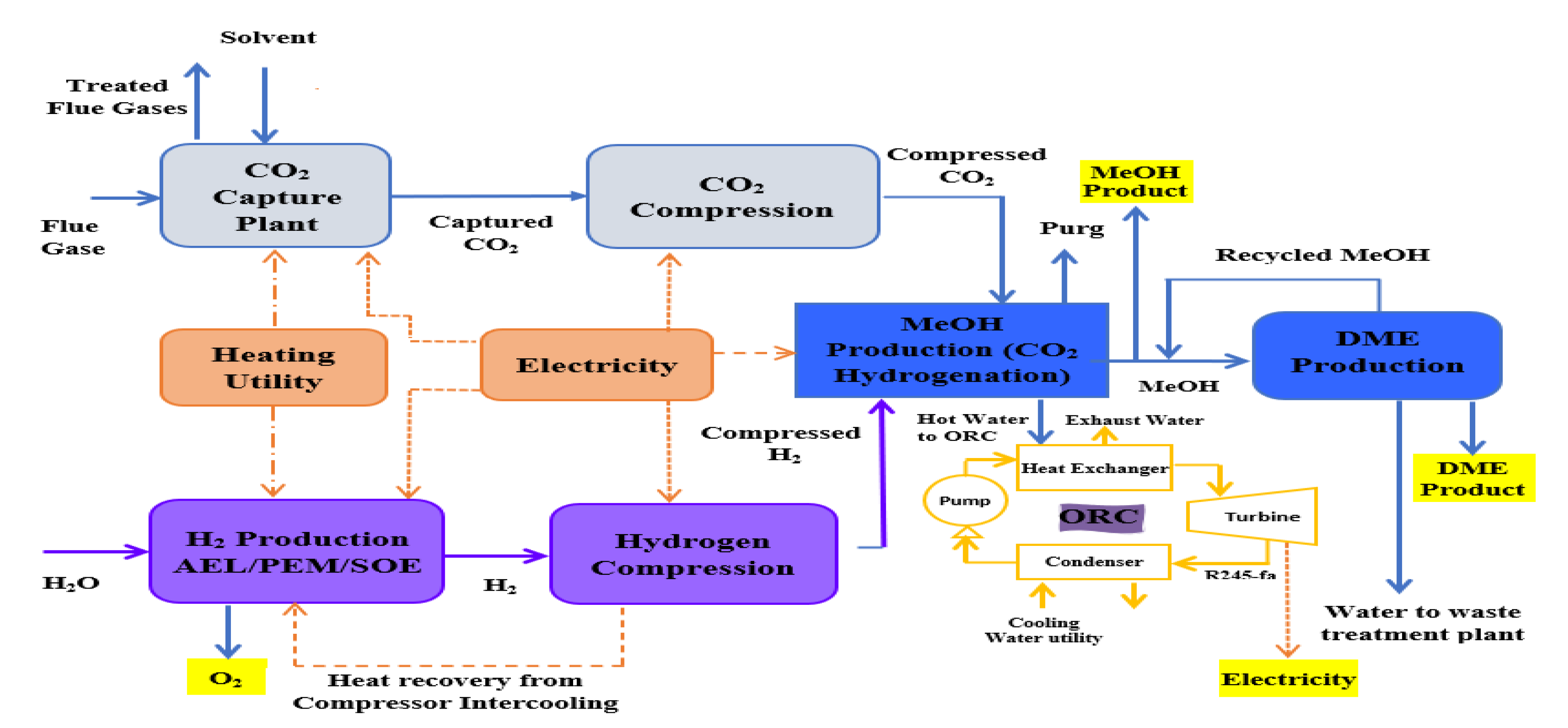
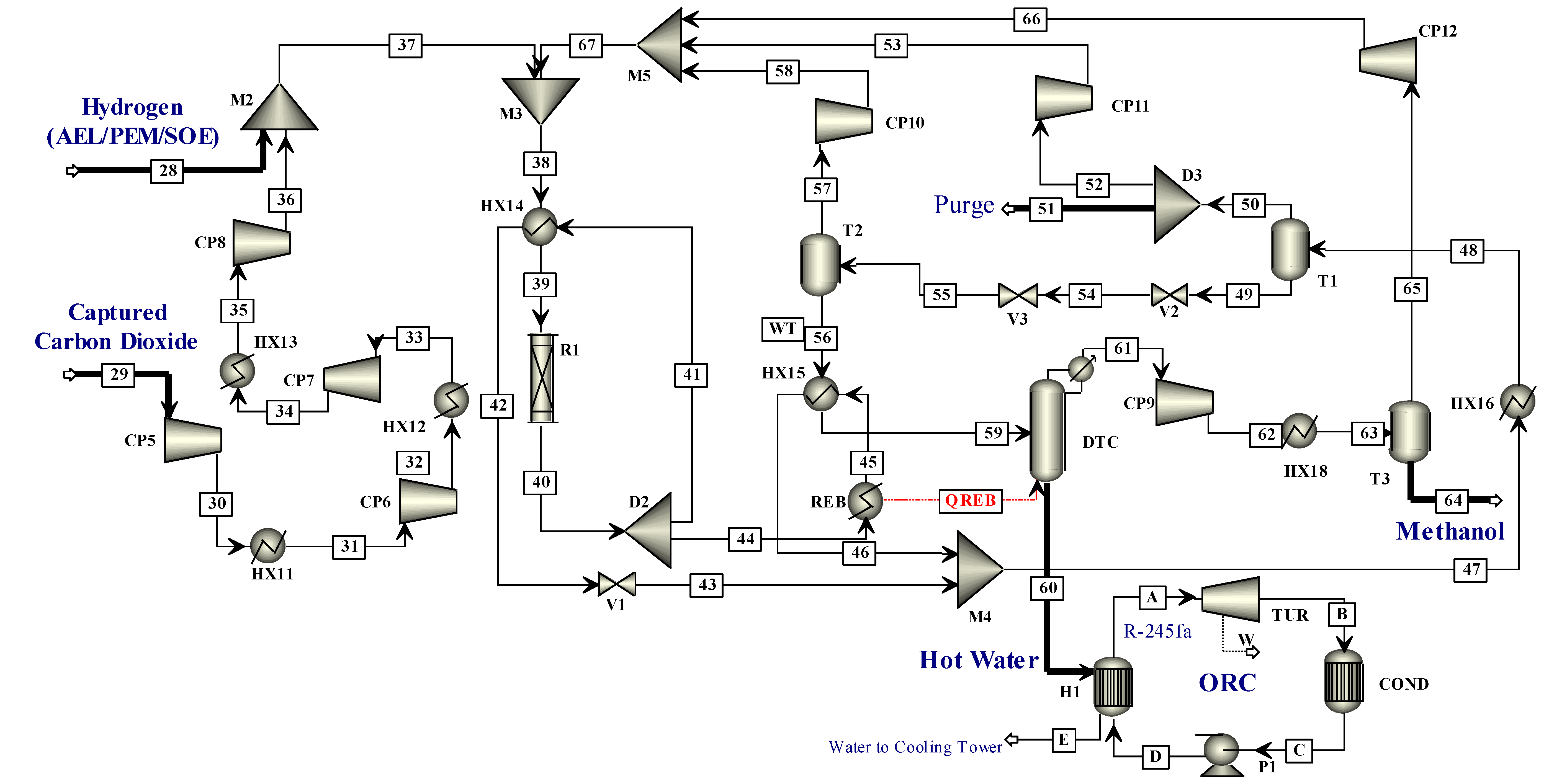
This study has some significant features such as attempting to cover the research gap by presenting different routes for DME and methanol production from CO2 and hydrogen. In this study, hydrogenation of CO2 to methanol was integrated with a methanol dehydration plant for DME production. Instead of conventional processes of hydrogen production such as steam methane reforming, coal gasification and oil reforming that release CO2 into the environment, the hydrogen used is produced by clean technology, i.e., water electrolysis that utilizes REs to disassociate water molecules. The CO2 captured from a cement plant was used for hydrogenation to methanol. This process has two advantages, firstly it utilizes the captured CO2 that would be otherwise released into the environment and, secondly, hydrogen is produced by water electrolysis instead of conventional processes; hence, this process prevents the further release of CO2 into the environment. The methanol and DME can be used as gasoline substitutes; less hazardous emissions are expected from methanol and DME because they do not have nitrogen and sulfur compounds associated with them as compared to conventional fuels. Hence, the environment can be saved from further emissions of CO2 and pollution to some extent, by implementing this technique.
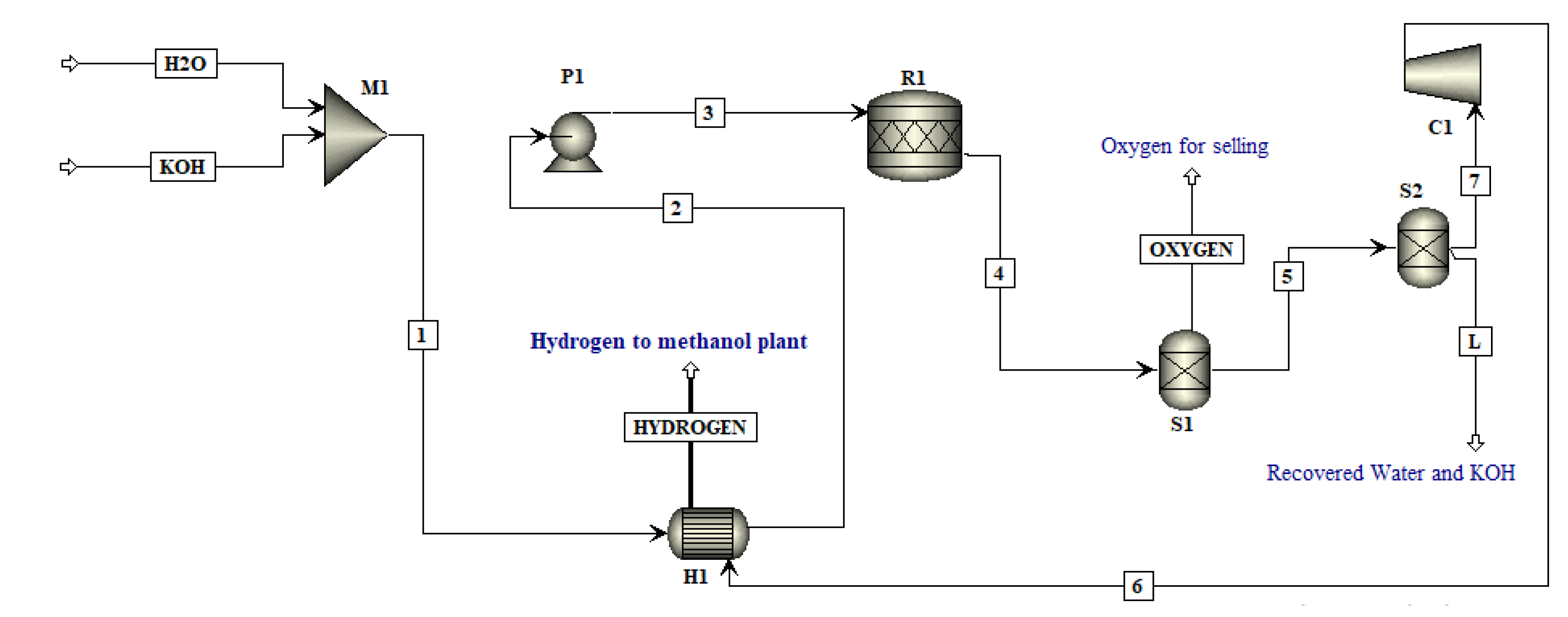
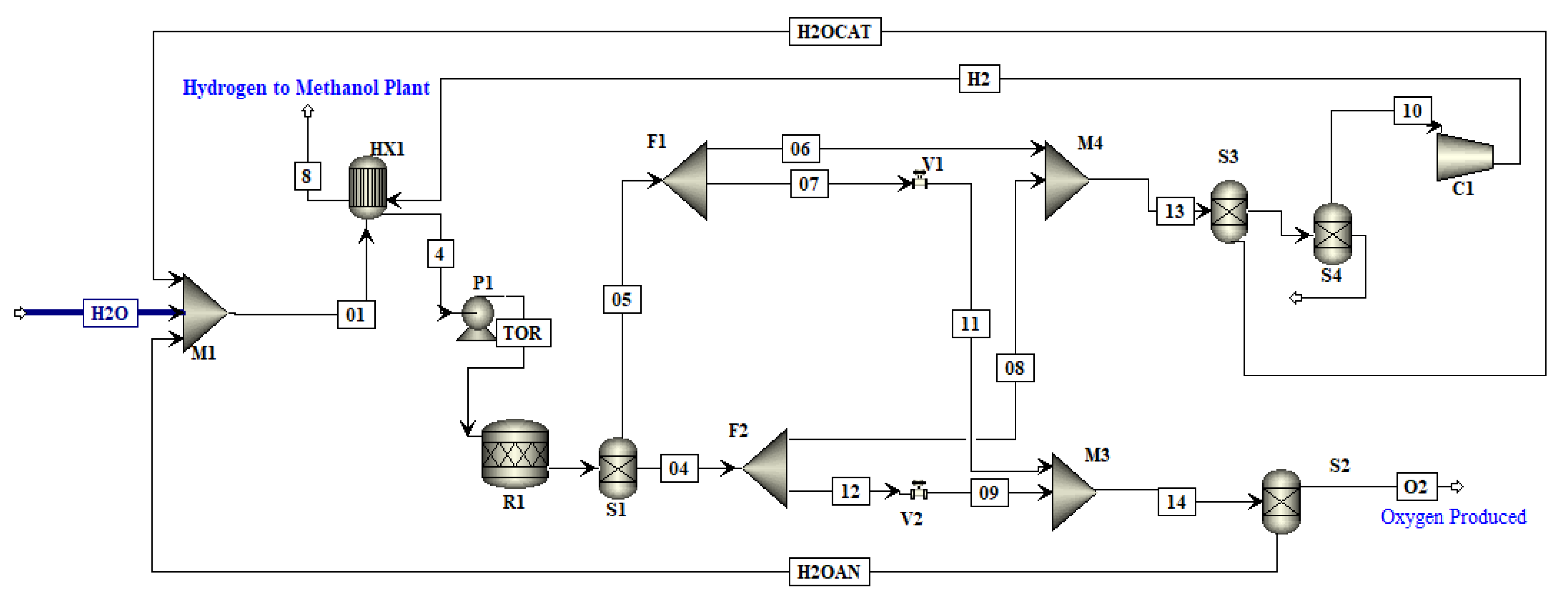
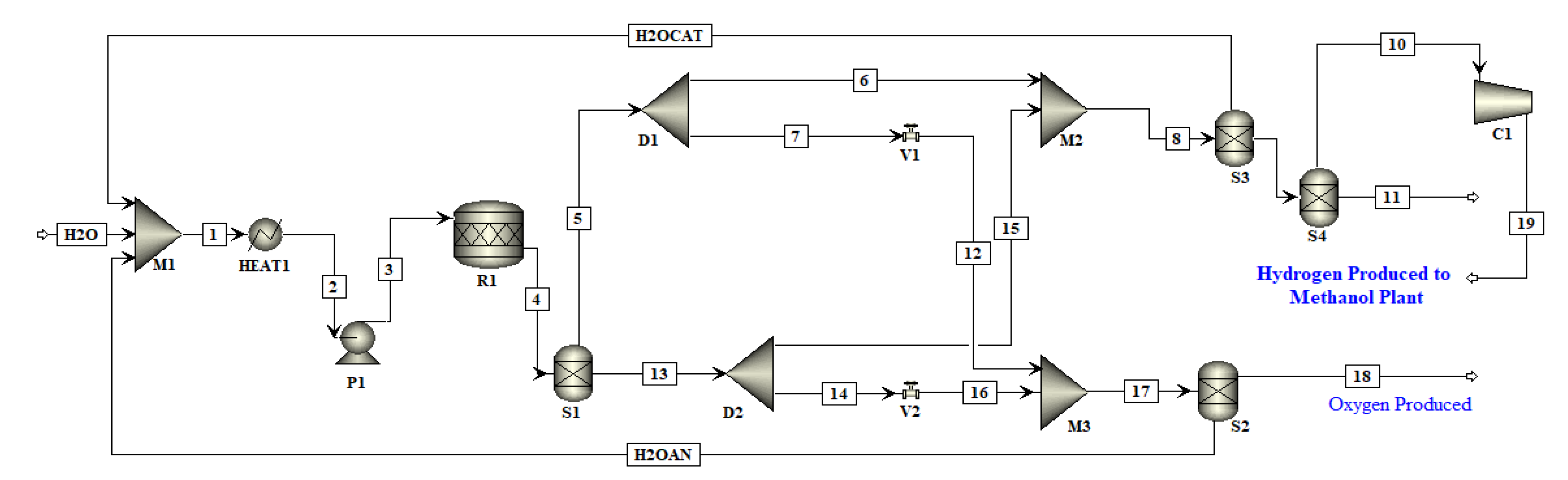
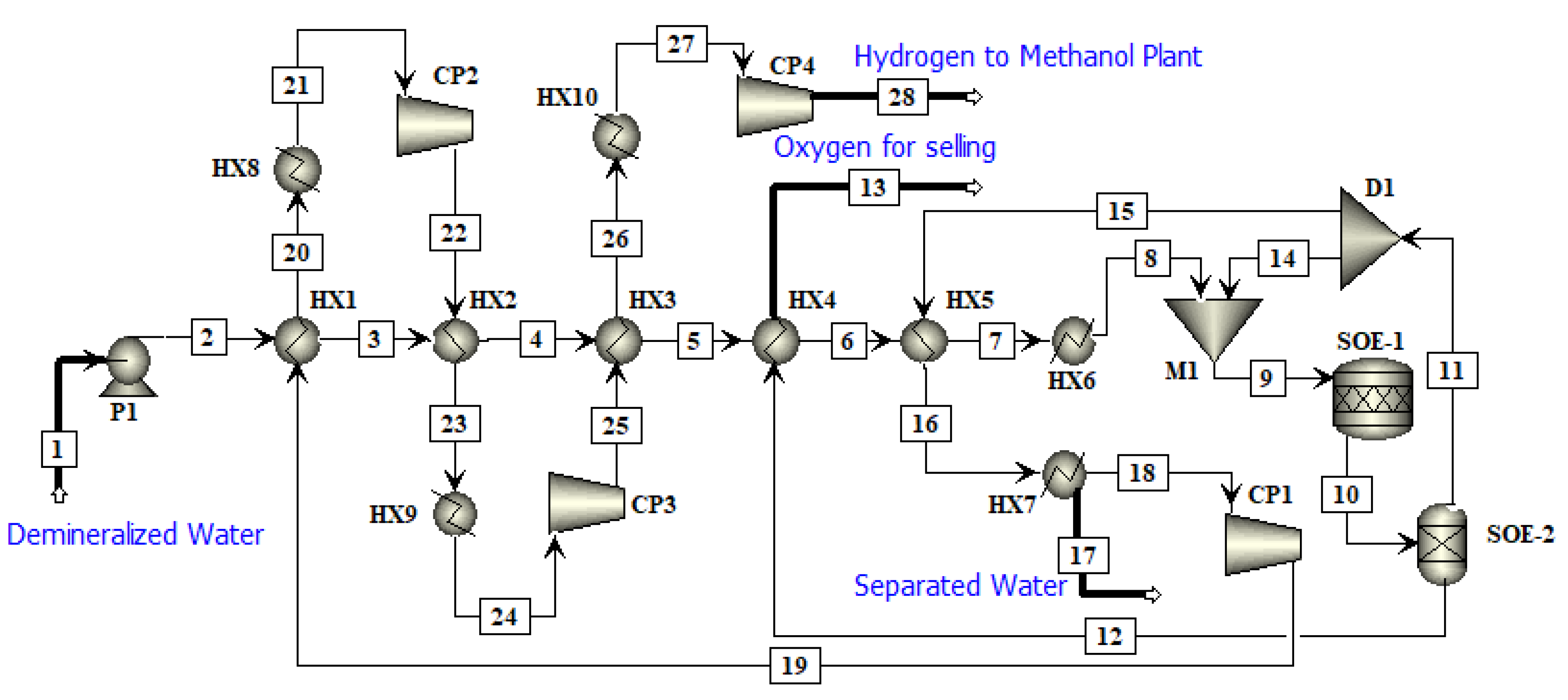
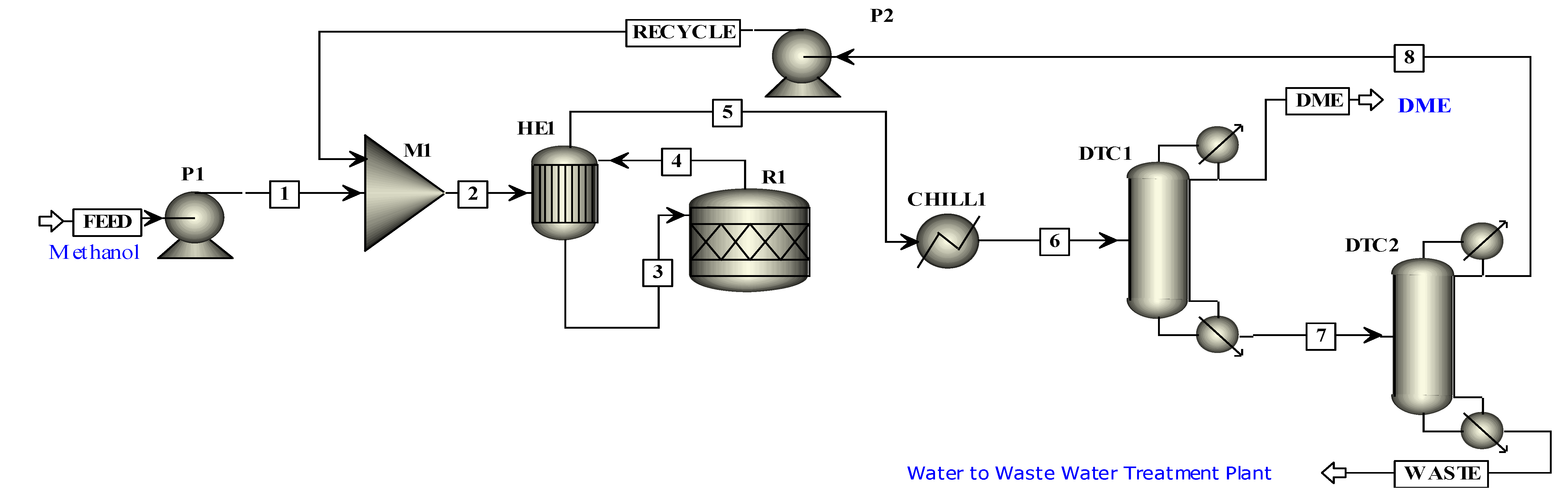
In this work, an integrated process is proposed to produce methanol and DME by using hydrogen from water electrolysis and CO
2 from a post-combustion capture plant. The schematic of the proposed integrated process is presented in
Figure 2. Simulations of the CO
2 capture process are not performed in this study since extensive data are available on it. The captured CO
2 from a cement plant was used in this study. The captured CO
2 was compressed and sent to methanol production. Hydrogen produced by electrolysis of water, i.e., AEL, PEM or SOE was fed to the compression unit. The energy for compressors was provided externally. The compressed hydrogen was then fed to the methanol production unit. The amount of hydrogen produced by each water electrolysis process was the same. The thermo-economic comparison of methanol and DME integrated processes employing three different hydrogen production processes was performed in this work. The simulation flow sheets and the results of the SOE method for hydrogen production were taken from the work of Yousaf et al. [
19]. This work is the extension of the published results of Yousaf et al. [
19], where methanol production is simulated using hydrogen from SOE. In the already published results of Yousaf et al. [
18], hydrogen produced by SOE was integrated with methanol production. However, the temperature of SOE is almost 800°C for electrolysis and the materials needed for SOE operation are still under research as they need to tolerate a high temperature. In the present study, the low temperature technologies (below 100 °C) such as AEL and PEM are used for simulation of hydrogen production. The AEL and PEM water electrolysis processes were modelled and integrated with the methanol production process to assess the technological and economic impacts of these processes on methanol production. The methanol flowsheet is used with some slight modification in the feed stream of hydrogen and then methanol was converted into DME to assess another PtL process for CO
2 mitigation. Process flow sheets for hydrogen production were simulated using Aspen Plus
® V11. The economic analysis was carried out using model equations and the Aspen Process Economic Analyzer
® V11 (APEA).
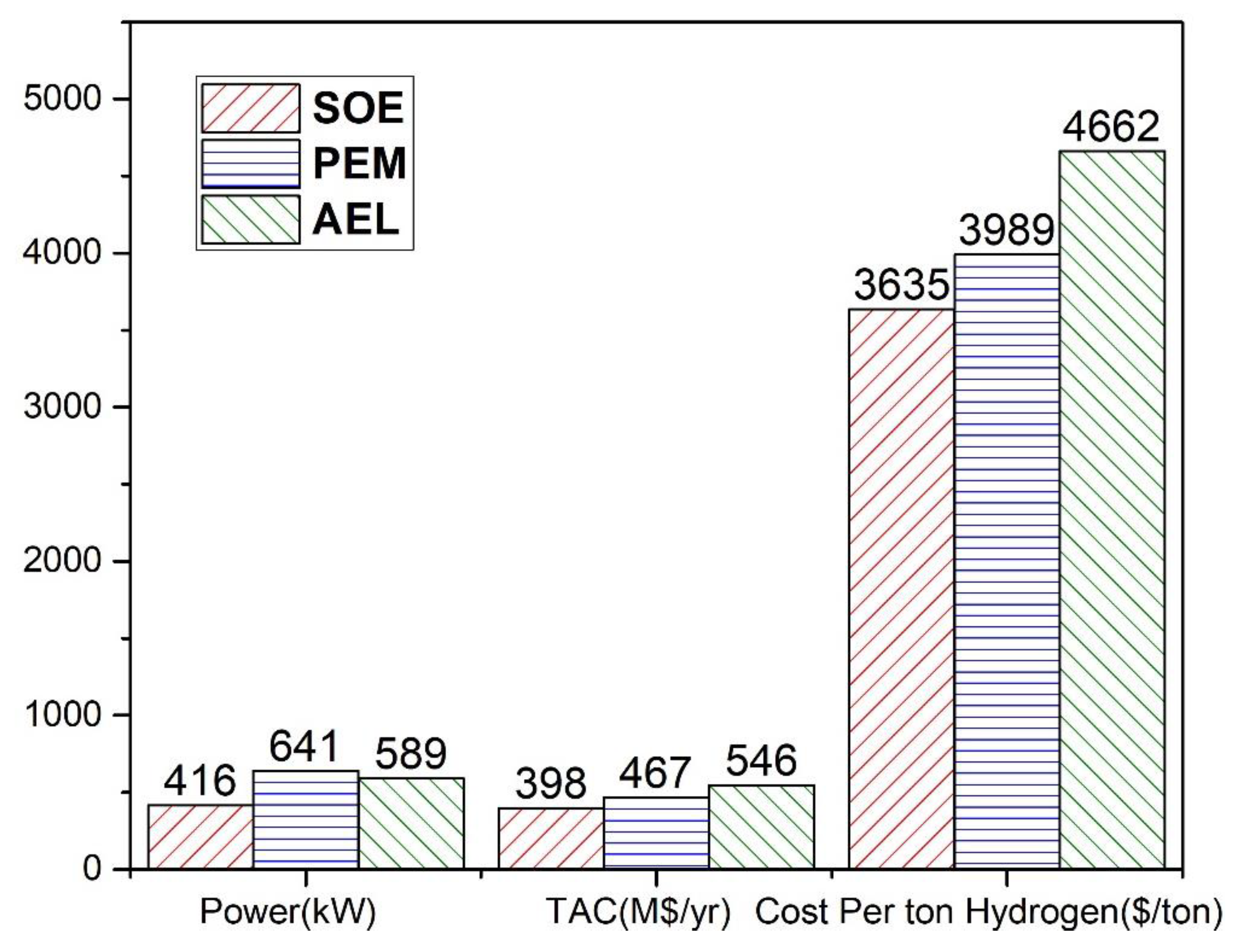
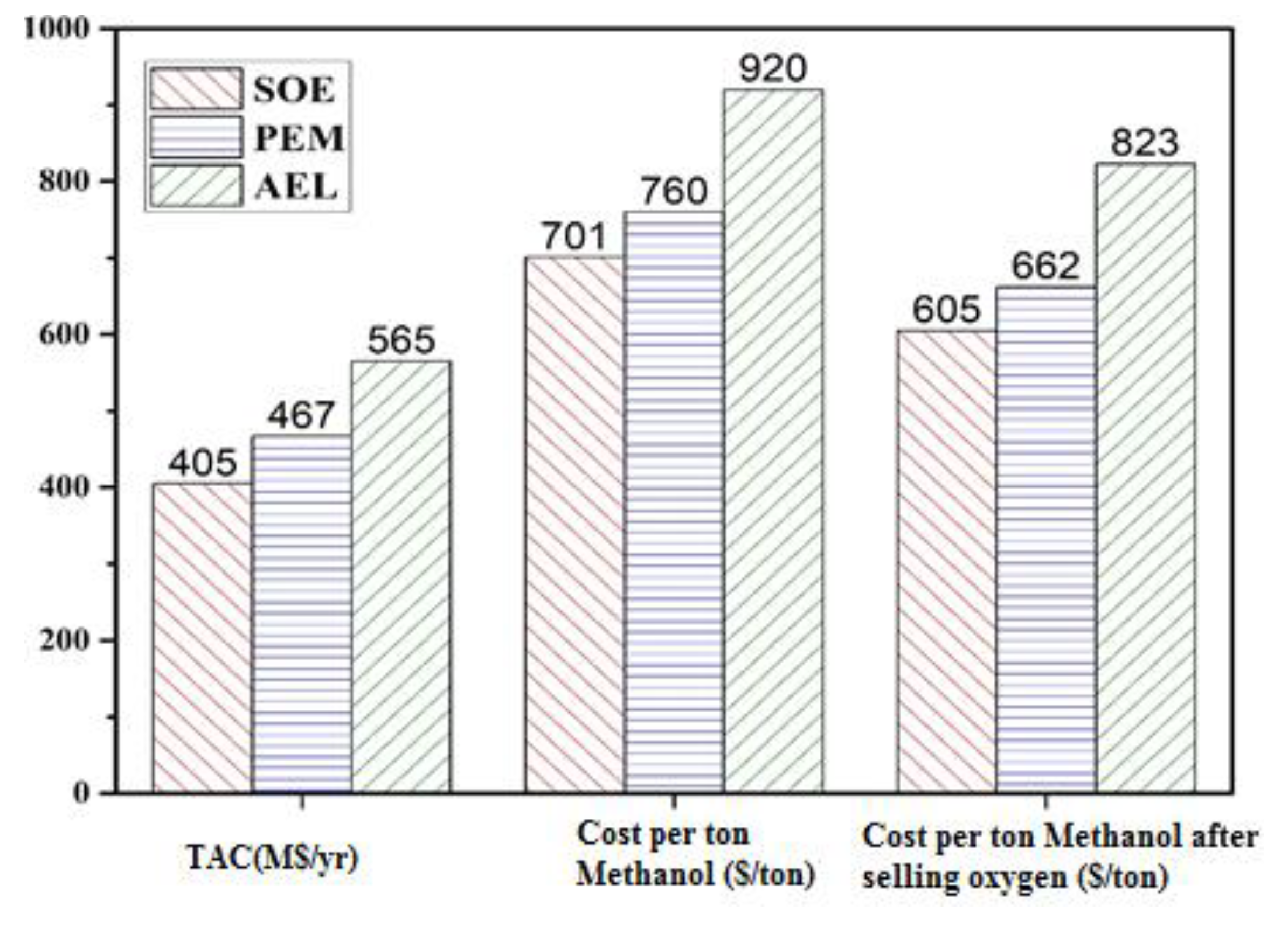
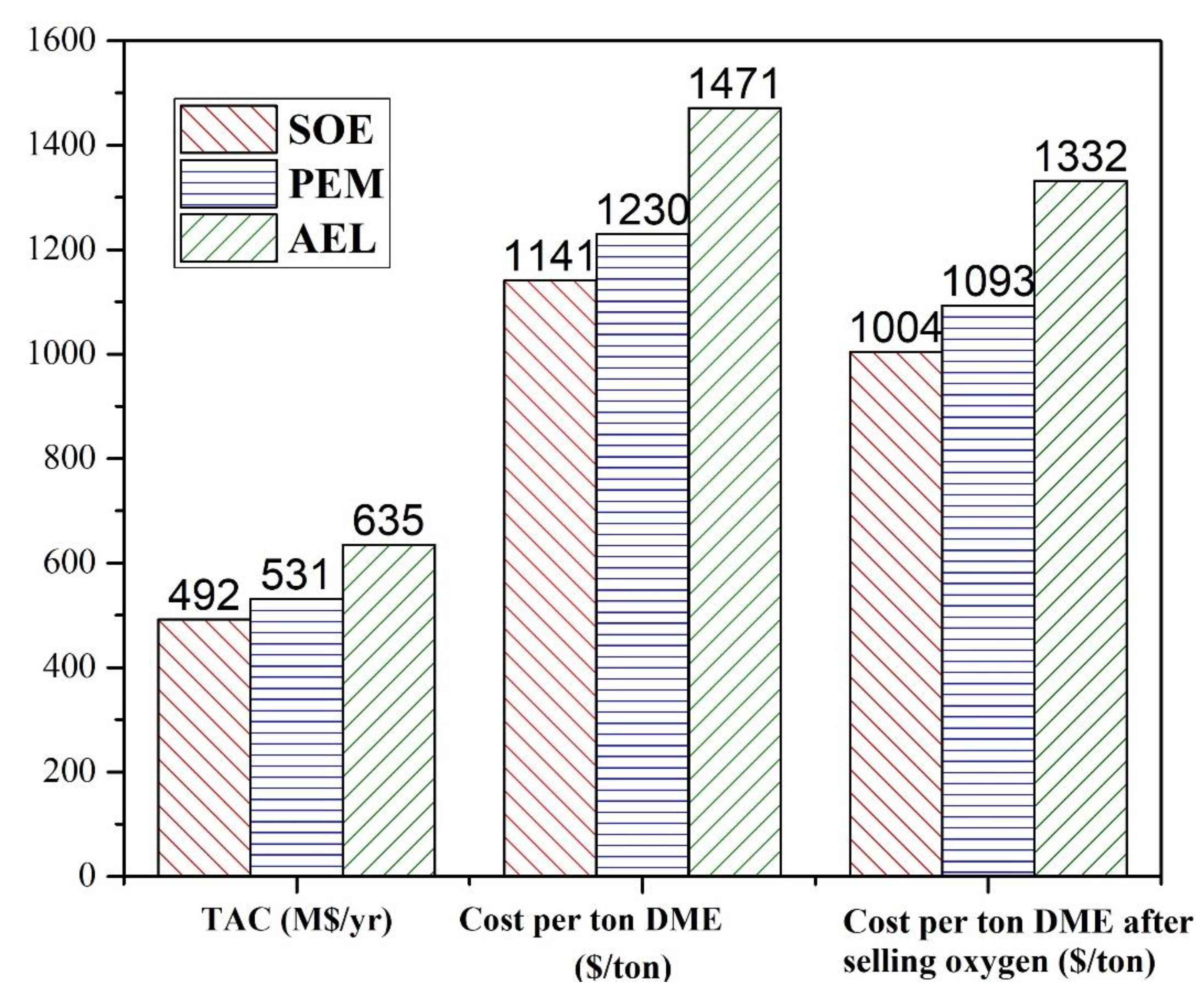
In this work, water electrolyzed hydrogen production techniques, i.e., AEL and PEM, were simulated in Aspen Plus
® and compared with the SOE [
19]. The simulated water electrolyzed processes were compared to verify their economic feasibility in terms of total annual cost (TAC), power and cost per ton of hydrogen. The hydrogen production methods integrated with methanol production are compared in terms of TAC, cost per ton of methanol and cost per ton of methanol after selling oxygen. The methanol produced by the water electrolysis process is converted to DME and the cost per ton of DME is evaluated. The net emissions of CO
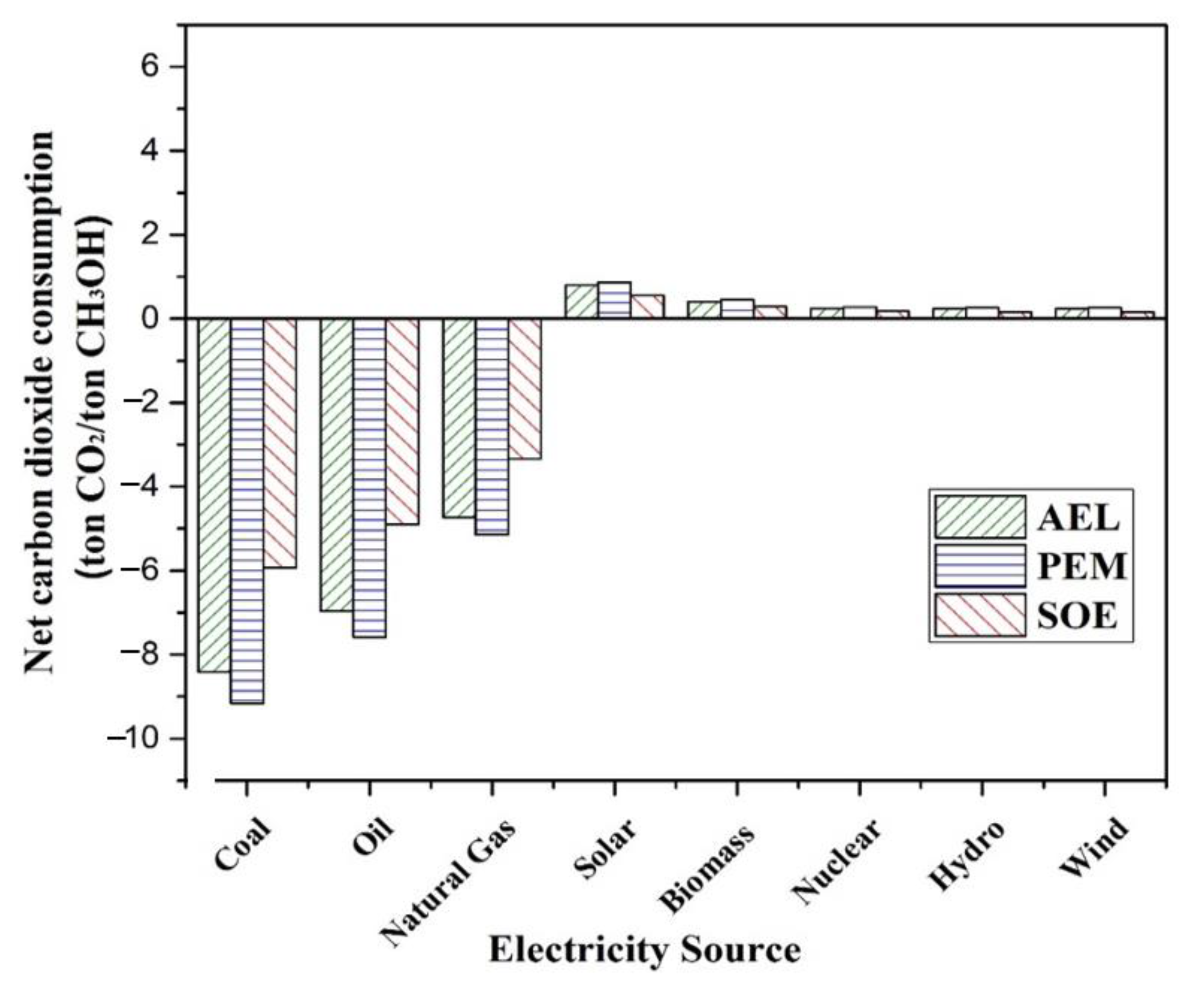
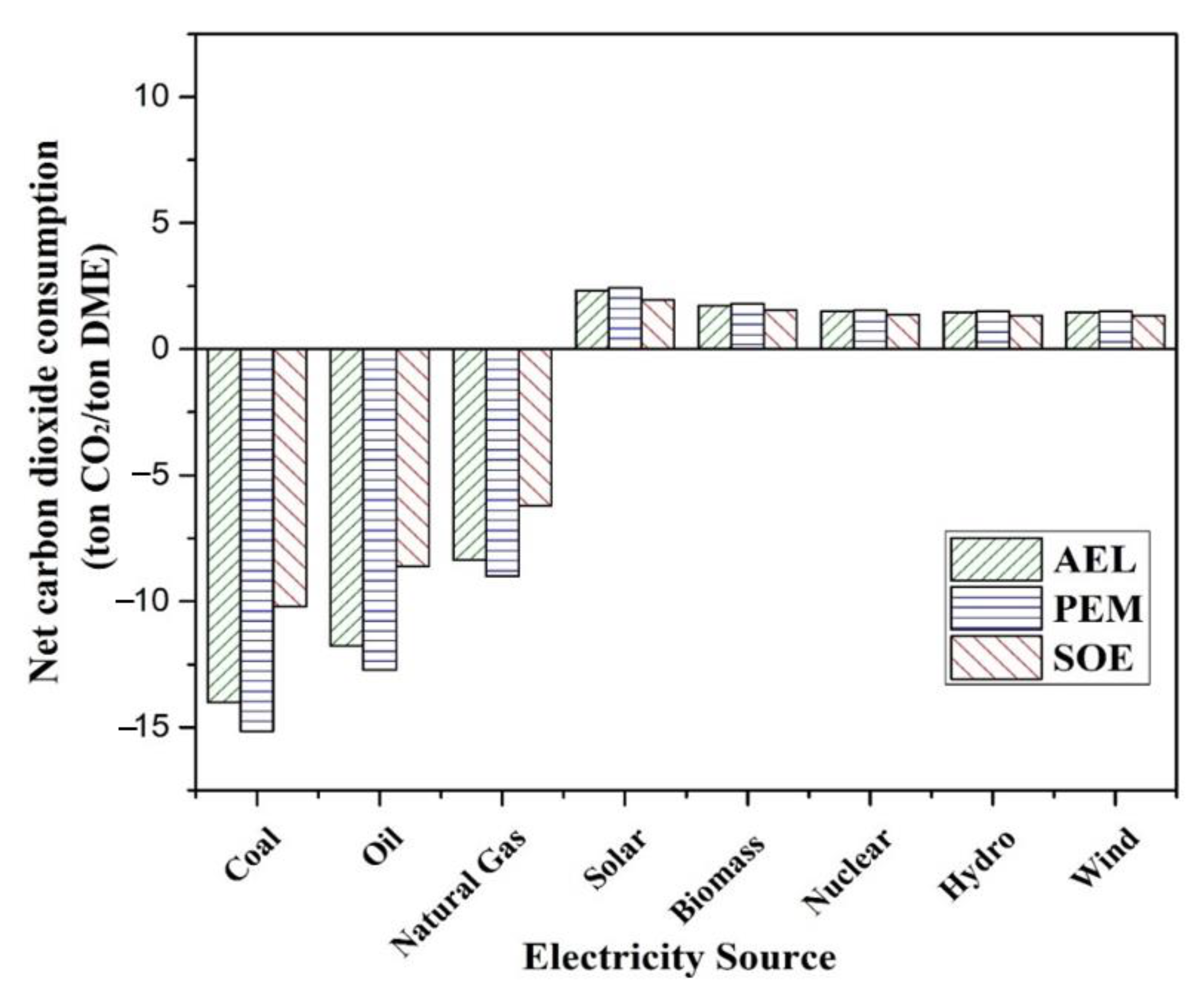
2 by each integrated process are also assessed. The comparison shows that the cost for hydrogen production by AEL is the highest, and by SOE is lowest. Similarly, the cost of methanol and DME production is highest for AEL, and lowest for SOE. PEM has the highest power consumption among these three techniques, which may lead to high net emissions of CO
2 if non-renewal energy is used.