Essential Oils and Their Application on Active Packaging Systems: A Review
Abstract
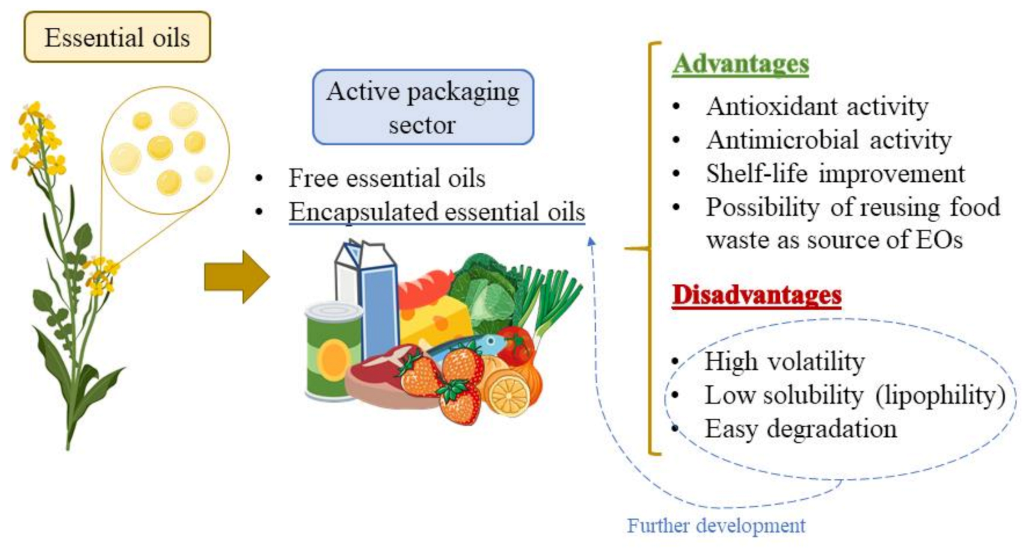
1. Introduction
2. Application of Essential Oils in Food Preservation and Packaging Sector
2.1. Free EOs Combined with Packaging Materials
2.2. Encapsulation of EOs
3. Current Trends on the Application of EOs in the Food Industry
3.1. Antimicrobial Activity of EOs in Food Systems
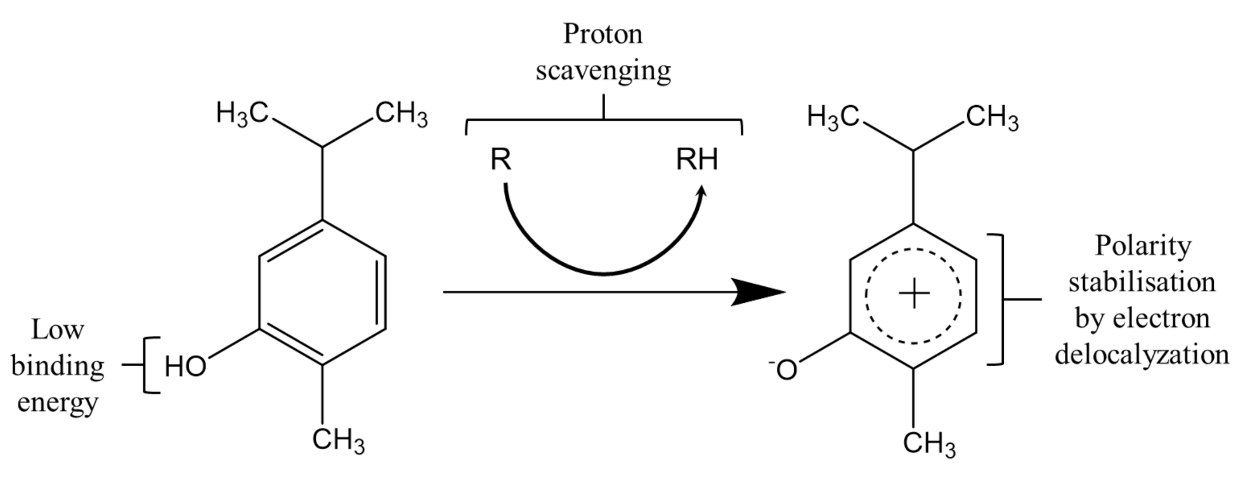
3.2. Antioxidant Activity of EOs in Food Systems
3.3. Flavors and Aromas Transference in Active Food Packaging
4. Legislation of EOs in Food
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Generic | |
| EO(s) | Essential oil(s) |
| ROS | Reactive oxygen species |
| UV | Ultraviolet |
| FDA | Food and Drug Administration |
| GRAS | Generally recognized as safe |
| MIC | Minimum inhibitory concentration |
| LAB | Lactic Acid Bacteria |
| Compounds | |
| PUFAs | Polyunsaturated fatty acids |
| HDPE | High density polyethylene |
| LDPE | Low density polyethylene |
| CMC | Carboxymethyl cellulose |
| BHT | Butylated hydroxytoluene |
| PLA | Polylactic acid |
| Techniques | |
| tPC | Total phenolic compounds |
| tFC | Total flavonoids content |
| FFA | Free fatty acid determination |
| POV | Peroxide value |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate free radical assay |
| TBARS | 2-thiobarbituric acid reductive value assay |
| FRAP | Ferric reducing antioxidant power assay |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic) acid assay |
References
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2020, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhao, Y.; Warner, R.D.; Johnson, S.K. Active and intelligent packaging in meat industry. Trends Food Sci. Technol. 2017, 61, 60–71. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; de Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Restuccia, D.; Spizzirri, U.G.; Parisi, O.I.; Cirillo, G.; Curcio, M.; Iemma, F.; Puoci, F.; Vinci, G.; Picci, N. New EU regulation aspects and global market of active and intelligent packaging for food industry applications. Food Control 2010, 21, 1425–1435. [Google Scholar] [CrossRef]
- European Parliament and the Concil of the European Union. Regulation (EC) No 1333/2008 of the European Parliament Ans of the Council of 16 December 2008 on Food Additives; European Parliament and the Concil of the European Union: Belgium, Brussel, 2008; pp. 16–33. [Google Scholar]
- European Parliament and Council. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Waste Framework); European Parliament and Council: Brussels, Belgium, 2008; pp. 3–30. [Google Scholar]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative stability of refined olive and sunflower oils supplemented with lycopene-rich oleoresin from tomato peels industrial by-product, during accelerated shelf-life storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citrus Wastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Settanni, L.; Palazzolo, E.; Guarrasi, V.; Aleo, A.; Mammina, C.; Moschetti, G.; Germanà, M.A. Inhibition of foodborne pathogen bacteria by essential oils extracted from citrus fruits cultivated in Sicily. Food Control 2012, 26, 326–330. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Cherrat, L.; Espina, L.; Lorán, S.; Rota, C.; Pagán, R. The antimicrobial activity of hydrophobic essential oil constituents acting alone or in combined processes of food preservation. Innov. Food Sci. Emerg. Technol. 2011, 12, 320–329. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, Protein and Lipid-Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, M.; Prabhakaran, M.P.; Ramakrishna, S. Edible polymers: An insight into its application in food, biomedicine and cosmetics. Trends Food Sci. Technol. 2020, 103, 248–263. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Blanco-Padilla, A.; Soto, K.M.; Hernández Iturriaga, M.; Mendoza, S. Food antimicrobials nanocarriers. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Zhu, G.; Xiao, Z.; Zhou, R.; Yi, F. Fragrance and flavor microencapsulation technology. Adv. Mater. Res. 2012, 2, 440–445. [Google Scholar] [CrossRef]
- Burgos, N.; Mellinas, A.C.; García-Serna, E.; Jiménez, A. Nanoencapsulation of Flavor and Aromas in Food Packaging; Elsevier Inc.: Philadelphia, PA, USA, 2017. [Google Scholar]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Martorell, M.; Rajkovic, J.; Ademiluyi, A.O.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Phytochemicals in Helicobacter pylori infections: What are we doing now? Int. J. Mol. Sci. 2018, 19, 2361. [Google Scholar] [CrossRef]
- Mahomoodally, F.; Aumeeruddy-Elalfi, Z.; Venugopala, K.N.; Hosenally, M. Antiglycation, comparative antioxidant potential, phenolic content and yield variation of essential oils from 19 exotic and endemic medicinal plants. Saudi J. Biol. Sci. 2019, 26, 1779–1788. [Google Scholar] [CrossRef]
- Hassoun, A.; Carpena, M.; Prieto, M.A.; Simal-Gandara, J.; Özogul, F.; Özogul, Y.; Çoban, Ö.E.; Guðjónsdóttir, M.; Barba, F.J.; Marti-Quijal, F.J.; et al. Use of spectroscopic techniques to monitor changes in food quality during application of natural preservatives: A review. Antioxidants 2020, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Jugreet, B.S.; Suroowan, S.; Rengasamy, R.R.K.; Mahomoodally, M.F. Chemistry, bioactivities, mode of action and industrial applications of essential oils. Trends Food Sci. Technol. 2020, 101, 89–105. [Google Scholar] [CrossRef]
- Djilani, A.; Dicko, A. The Therapeutic Benefits of Essential Oils. In Nutrition, Well-Being and Health; Books on Demand: Norderstedt, Germany, 2012; pp. 155–178. [Google Scholar]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Trindade, H. Review on essential oil, extracts composition, molecular and phytochemical properties of Thymus species in Iran. Ind. Crops Prod. 2019, 134, 89–99. [Google Scholar] [CrossRef]
- Sarıcaoglu, F.T.; Turhan, S. Physicochemical, antioxidant and antimicrobial properties of mechanically deboned chicken meat protein films enriched with various essential oils. Food Packag. Shelf Life 2020, 25, 100527. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Ledward, D.A. Metmyoglobin Formation in Beef Stored in Carbon Dioxide Enriched and Oxygen Depleted Atmospheres. J. Food Sci. 1970, 35, 33–37. [Google Scholar] [CrossRef]
- Rukunudin, I.H.; White, P.J.; Bern, C.J.; Bailey, T.B. A modified method for determining free fatty acids from small soybean oil sample sizes. JAOCS J. Am. Oil Chem. Soc. 1998, 75, 563–568. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Robertson, T.R.; Hamza, M.F. Paper Products: Food Packages. Ref. Modul. Mater. Sci. Mater. Eng. 2016. [Google Scholar] [CrossRef]
- Buendía-Moreno, L.; Soto-Jover, S.; Ros-Chumillas, M.; Antolinos, V.; Navarro-Segura, L.; Sánchez-Martínez, M.J.; Martínez-Hernández, G.B.; López-Gómez, A. Innovative cardboard active packaging with a coating including encapsulated essential oils to extend cherry tomato shelf life. LWT 2019, 116, 108584. [Google Scholar] [CrossRef]
- Muratore, F.; Barbosa, S.E.; Martini, R.E. Development of bioactive paper packaging for grain-based food products. Food Packag. Shelf Life 2019, 20, 100317. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Chiralt, A.; González-Martínez, C.; Cháfer, M. Effect of essential oils on properties of film forming emulsions and films based on hydroxypropylmethylcellulose and chitosan. J. Food Eng. 2011, 105, 246–253. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Effect of essential oils and homogenization conditions on properties of chitosan-based films. Food Hydrocoll. 2012, 26, 9–16. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; de Lacey López, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef]
- Debiagi, F.; Kobayashi, R.K.T.; Nakazato, G.; Panagio, L.A.; Mali, S. Biodegradable active packaging based on cassava bagasse, polyvinyl alcohol and essential oils. Ind. Crops Prod. 2014, 52, 664–670. [Google Scholar] [CrossRef]
- Wen, P.; Zhu, D.-H.; Wu, H.; Zong, M.-H.; Jing, Y.-R.; Han, S.-Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Control 2016, 59, 366–376. [Google Scholar] [CrossRef]
- Zhou, Y.; Miao, X.; Lan, X.; Luo, J.; Luo, T.; Zhong, Z.; Gao, X.; Mafang, Z.; Ji, J.; Wang, H.; et al. Angelica Essential Oil Loaded Electrospun Gelatin Nanofibers for Active Food Packaging Application. Polymers 2020, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Fuenmayor, C.A.; Mascheroni, E.; Cosio, M.S.; Piergiovanni, L.; Benedetti, S.; Ortenzi, M.; Schiraldi, A.; Mannino, S. Encapsulation of R-(+)-limonene in edible electrospun nanofibers. Chem. Eng. Trans. 2013, 32, 1771–1776. [Google Scholar] [CrossRef]
- Joven, R.; Garcia, A.; Arias, A.; Medina, J. Development of an active thermoplastic film with oxygen scavengers made of activated carbon and sodium erythorbate. Packag. Technol. Sci. 2015, 28, 113–121. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Sanches-Silva, A. Application of encapsulated essential oils as antimicrobial agents in food packaging. Curr. Opin. Food Sci. 2017, 14, 78–84. [Google Scholar] [CrossRef]
- Pathak, C.; Vaidya, F.U.; Pandey, S.M. Mechanism for Development of Nanobased Drug Delivery System. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–67. [Google Scholar]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef]
- Peng, X.Q.; Wei, M.J.; Wang, L.; Gu, L.P. Study on microcrystalline chitin cinnamon nanoemulsion. Appl. Mech. Mater. 2014, 525, 53–57. [Google Scholar] [CrossRef]
- Severino, R.; Ferrari, G.; Vu, K.D.; Donsì, F.; Salmieri, S.; Lacroix, M. Antimicrobial effects of modified chitosan based coating containing nanoemulsion of essential oils, modified atmosphere packaging and gamma irradiation against Escherichia coli O157:H7 and Salmonella Typhimurium on green beans. Food Control 2015, 50, 215–222. [Google Scholar] [CrossRef]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and antimicrobial properties of starch-carboxy methyl cellulose film containing rosemary essential oils encapsulated in chitosan nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef]
- Yao, Z.-C.; Chang, M.-W.; Ahmad, Z.; Li, J.-S. Encapsulation of rose hip seed oil into fibrous zein films for ambient and on demand food preservation via coaxial electrospinning. J. Food Eng. 2016, 191, 115–123. [Google Scholar] [CrossRef]
- Sapper, M.; Wilcaso, P.; Santamarina, M.P.; Roselló, J.; Chiralt, A. Antifungal and functional properties of starch-gellan films containing thyme (Thymus zygis) essential oil. Food Control 2018, 92, 505–515. [Google Scholar] [CrossRef]
- Silva, F.; Caldera, F.; Trotta, F.; Nerín, C.; Domingues, F.C. Encapsulation of coriander essential oil in cyclodextrin nanosponges: A new strategy to promote its use in controlled-release active packaging. Innov. Food Sci. Emerg. Technol. 2019, 56, 102177. [Google Scholar] [CrossRef]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009, 82, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Coelho, P.M.; Corona, B.; ten Klooster, R.; Worrell, E. Sustainability of reusable packaging—Current situation and trends. Resour. Conserv. Recycl. X 2020, 6, 100037. [Google Scholar] [CrossRef]
- Jeya Jeevahan, J.; Chandrasekaran, M.; Venkatesan, S.P.; Sriram, V.; Britto Joseph, G.; Mageshwaran, G.; Durairaj, R.B. Scaling up difficulties and commercial aspects of edible films for food packaging: A review. Trends Food Sci. Technol. 2020, 100, 210–222. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 450/2009 of 29 May 2009 on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Commun. 2009, 135, 3–11. [Google Scholar]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Stephansen, K.; van der Kruijs, J.; Guadix, A.; Guadix, E.M.; Chronakis, I.S.; Jacobsen, C. Encapsulation of fish oil in nanofibers by emulsion electrospinning: Physical characterization and oxidative stability. J. Food Eng. 2016, 183, 39–49. [Google Scholar] [CrossRef]
- Adel, A.M.; Ibrahim, A.A.; El-Shafei, A.M.; Al-Shemy, M.T. Inclusion complex of clove oil with chitosan/β-cyclodextrin citrate/oxidized nanocellulose biocomposite for active food packaging. Food Packag. Shelf Life 2019, 20, 100307. [Google Scholar] [CrossRef]
- Alparslan, Y.; Yapici, H.H.; Metin, C.; Baygar, T.; Günlü, A.; Baygar, T. Quality assessment of shrimps preserved with orange leaf essential oil incorporated gelatin. LWT Food Sci. Technol. 2016, 72, 457–466. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Ghavi, F.F. Effect of Fish Gelatin Coating Enriched with Oregano Essential Oil on the Quality of Refrigerated Rainbow Trout Fillet. J. Aquat. Food Prod. Technol. 2016, 25, 835–842. [Google Scholar] [CrossRef]
- Azarifar, M.; Ghanbarzadeh, B.; Sowti khiabani, M.; Akhondzadeh basti, A.; Abdulkhani, A. The effects of gelatin-CMC films incorporated with chitin nanofiber and Trachyspermum ammi essential oil on the shelf life characteristics of refrigerated raw beef. Int. J. Food Microbiol. 2020, 318, 108493. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zhang, C.; Show, P.L.; Du, A.; Fu, J.C.; Ashokkumar, V. Preparation and characterization of curdlan/polyvinyl alcohol/ thyme essential oil blending film and its application to chilled meat preservation. Carbohydr. Polym. 2020, 247, 116670. [Google Scholar] [CrossRef]
- Frazão, G.G.S.; Blank, A.F.; de Aquino Santana, L.C.L. Optimisation of edible chitosan coatings formulations incorporating Myrcia ovata Cambessedes essential oil with antimicrobial potential against foodborne bacteria and natural microflora of mangaba fruits. LWT Food Sci. Technol. 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Dini, H.; Fallah, A.A.; Bonyadian, M.; Abbasvali, M.; Soleimani, M. Effect of edible composite film based on chitosan and cumin essential oil-loaded nanoemulsion combined with low-dose gamma irradiation on microbiological safety and quality of beef loins during refrigerated storage. Int. J. Biol. Macromol. 2020, 164, 1501–1509. [Google Scholar] [CrossRef]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Pongsapakworawat, T.; Bangmo, P.; Sutthasupa, S. Effect of chitosan beads incorporated with lavender or red thyme essential oils in inhibiting Botrytis cinerea and their application in strawberry packaging system. LWT Food Sci. Technol. 2016, 74, 14–20. [Google Scholar] [CrossRef]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef] [PubMed]
- Seydim, A.C.; Sarikus-Tutal, G.; Sogut, E. Effect of whey protein edible films containing plant essential oils on microbial inactivation of sliced Kasar cheese. Food Packag. Shelf Life 2020, 26, 100567. [Google Scholar] [CrossRef]
- Alizadeh Sani, M.; Ehsani, A.; Hashemi, M. Whey protein isolate/cellulose nanofibre/TiO2 nanoparticle/rosemary essential oil nanocomposite film: Its effect on microbial and sensory quality of lamb meat and growth of common foodborne pathogenic bacteria during refrigeration. Int. J. Food Microbiol. 2017, 251, 8–14. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of oregano essential oil and resveratrol nanoemulsion loaded pectin edible coating on the preservation of pork loin in modified atmosphere packaging. Food Control 2020, 114, 107226. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of alginate-based edible coatings enriched with essential oils constituents on Arbutus unedo L. fresh fruit storage. Postharvest Biol. Technol. 2015, 100, 226–233. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The effect of edible coating based on Arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2018, 245, 820–828. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Jafari, S.M.; Salehabadi, A.; Nafchi, A.M.; Uthaya Kumar, U.S.; Khalil, H.P.S.A. Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci. Technol. 2020, 100, 262–277. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Mandrell, R.E. Bactericidal activities of plant essential oils and some of their isolated constituents against Campylobacter jejuni, Escherichia coli, Listeria monocytogenes, and Salmonella enterica. J. Food Prot. 2002, 65, 1545–1560. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Carrión-Granda, X.; Maté, J.I. Antimicrobial efficiency of edible coatings on the preservation of chicken breast fillets. Food Control 2014, 36, 69–75. [Google Scholar] [CrossRef]
- Friedman, M.; Henika, P.R.; Levin, C.E.; Mandrell, R.E. Antibacterial activities of plant essential oils and their components against Escherichia coli O157:H7 and Salmonella enterica in apple juice. J. Agric. Food Chem. 2004, 52, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Otero, V.; Becerril, R.; Santos, J.A.; Rodríguez-Calleja, J.M.; Nerín, C.; García-López, M.L. Evaluation of two antimicrobial packaging films against Escherichia coli O157: H7 strains invitro and during storage of a Spanish ripened sheep cheese (Zamorano). Food Control 2014, 42, 296–302. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Shankar, K.; Mehendale, H.M. Oxidative Stress. Encycl. Toxicol. Third Ed. 2014, 735–737. [Google Scholar] [CrossRef]
- Lee, D.S. Antioxidative Packaging System; Elsevier Ltd.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- European Commission. Proposal for a Directive of the European Parliament and of the Council on the Reduction of the Impact of Certain Plastic Products on the Environment; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Kumar, A.; Singh, P.; Gupta, V.; Prakash, B. Application of Nanotechnology to Boost the Functional and Preservative Properties of Essential Oils; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Dini, I. Use of Essential Oils in Food Packaging; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Properties and antioxidant activity of fish skin gelatin film incorporated with citrus essential oils. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef]
- Wang, L.; Heising, J.; Fogliano, V.; Dekker, M. Fat content and storage conditions are key factors on the partitioning and activity of carvacrol in antimicrobial packaging. Food Packag. Shelf Life 2020, 24, 100500. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Pepping, L.; Dekker, M.; Fogliano, V.; Heising, J. Using particle size and fat content to control the release of Allyl isothiocyanate from ground mustard seeds for its application in antimicrobial packaging. Food Chem. 2020, 308, 125573. [Google Scholar] [CrossRef]
- Camo, J.; Beltrán, J.A.; Roncalés, P. Extension of the display life of lamb with an antioxidant active packaging. Meat Sci. 2008, 80, 1086–1091. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Kodal Coşkun, B.; Çalikoǧlu, E.; Karagöz Emiroǧlu, Z.; Candoǧan, K. Antioxidant active packaging with soy edible films and oregano or thyme essential oils for oxidative stability of ground beef patties. J. Food Qual. 2014, 37, 203–212. [Google Scholar] [CrossRef]
- Romani, V.P.; Prentice-Hernández, C.; Martins, V.G. Active and sustainable materials from rice starch, fish protein and oregano essential oil for food packaging. Ind. Crops Prod. 2017, 97, 268–274. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Montero, P.; Giménez, B.; Gómez-Guillén, M.C. Effect of functional edible films and high pressure processing on microbial and oxidative spoilage in cold-smoked sardine (Sardina pilchardus). Food Chem. 2007, 105, 511–520. [Google Scholar] [CrossRef]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A. Quince seed mucilage films incorporated with oregano essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Food Hydrocoll. 2014, 36, 9–19. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Pires, C.; Ramos, C.; Batista, I.; Saraiva, J.A.; Nunes, M.L. Characterization of fish protein films incorporated with essential oils of clove, garlic and origanum: Physical, antioxidant and antibacterial properties. LWT Food Sci. Technol. 2014, 59, 533–539. [Google Scholar] [CrossRef]
- Sebranek, J.G.; Sewalt, V.J.H.; Robbins, K.L.; Houser, T.A. Comparison of a natural rosemary extract and BHA/BHT for relative antioxidant effectiveness in pork sausage. Meat Sci. 2005, 69, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of chitosan-montmorillonite bionanocomposites incorporated with rosemary essential oil: From in vitro assays to application in fresh poultry meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Choulitoudi, E.; Ganiari, S.; Tsironi, T.; Ntzimani, A.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Edible coating enriched with rosemary extracts to enhance oxidative and microbial stability of smoked eel fillets. Food Packag. Shelf Life 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Kykkidou, S.; Giatrakou, V.; Papavergou, A.; Kontominas, M.G.; Savvaidis, I.N. Effect of thyme essential oil and packaging treatments on fresh Mediterranean swordfish fillets during storage at 4 °C. Food Chem. 2009, 115, 169–175. [Google Scholar] [CrossRef]
- Jouki, M.; Mortazavi, S.A.; Yazdi, F.T.; Koocheki, A. Characterization of antioxidant-antibacterial quince seed mucilage films containing thyme essential oil. Carbohydr. Polym. 2014, 99, 537–546. [Google Scholar] [CrossRef]
- Sharma, H.; Mendiratta, S.K.; Agrawal, R.K.; Gurunathan, K.; Kumar, S.; Singh, T.P. Use of various essential oils as bio preservatives and their effect on the quality of vacuum packaged fresh chicken sausages under frozen conditions. LWT Food Sci. Technol. 2017, 81, 118–127. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Leyva, J.M.; Ortega-Ramírez, L.A.; Carrazco-Lugo, D.K.; Pérez-Carlón, J.J.; Melgarejo-Flores, B.G.; González-Aguilar, G.A.; Miranda, M.R.A. Pectin-cinnamon leaf oil coatings add antioxidant and antibacterial properties to fresh-cut peach. Flavour Fragr. J. 2013, 28, 39–45. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilled pork. LWT Food Sci. Technol. 2015, 63, 519–526. [Google Scholar] [CrossRef]
- Xing, Y.; Li, X.; Xu, Q.; Yun, J.; Lu, Y.; Tang, Y. Effects of chitosan coating enriched with cinnamon oil on qualitative properties of sweet pepper (Capsicum annuum L.). Food Chem. 2011, 124, 1443–1450. [Google Scholar] [CrossRef]
- Andevari, G.T.; Rezaei, M. Effect of gelatin coating incorporated with cinnamon oil on the quality of fresh rainbow trout in cold storage. Int. J. Food Sci. Technol. 2011, 46, 2305–2311. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Sanches-Silva, A.; Motta, J.F.G.; Andrade, M.; de Neves, I.A.; Teófilo, R.F.; de Carvalho, M.G.; de Melo, N.R. Combined use of essential oils applied to protein base active food packaging: Study in vitro and in a food simulant. Eur. Polym. J. 2017, 93, 75–86. [Google Scholar] [CrossRef]
- Xuntao, Z.; Schaich, K.; Chen, X.; Yam, K. Antioxidant Effects of Sesamol Released from Polymeric Films on Lipid Oxidation in Linoleic Acid and Oat Cereal. Packag. Technol. Sci. 2013, 29, 399–412. [Google Scholar] [CrossRef]
- Moosavy, M.H.; Hassanzadeh, P.; Mohammadzadeh, E.; Mahmoudi, R.; Khatibi, S.A.; Mardani, K. Antioxidant and antimicrobial activities of essential oil of lemon (Citrus limon) peel in vitro and in a food model. J. Food Qual. Hazards Control 2017, 4, 42–48. [Google Scholar]
- Sanchez-Gonzalez, L.; Pastor, C.; Vargas, M.; Chiralt, A.; Gonzalez-Martinez, C.; Chafer, M. Effect of hydroxypropylmethylcellulose and chitosan coatings with and without bergamot essential oil on quality and safety of cold-stored grapes. Postharvest Biol. Technol. 2011, 60, 57–63. [Google Scholar] [CrossRef]
- Hari, N.; Francis, S.; Rajendran Nair, A.G.; Nair, A.J. Synthesis, characterization and biological evaluation of chitosan film incorporated with β-Carotene loaded starch nanocrystals. Food Packag. Shelf Life 2018, 16, 69–76. [Google Scholar] [CrossRef]
- Assis, R.Q.; Pagno, C.H.; Costa, T.M.H.; Flôres, S.H.; de Rios, A.O. Synthesis of biodegradable films based on cassava starch containing free and nanoencapsulated β-carotene. Packag. Technol. Sci. 2018, 31, 157–166. [Google Scholar] [CrossRef]
- López De Dicastillo, C.; Ares Pernas, A.; Castro López, M.D.M.; López Vilariño, J.M.; González Rodríguez, M.V. Enhancing the release of the antioxidant tocopherol from polypropylene films by incorporating the natural plasticizers lecithin, olive oil, or sunflower oil. J. Agric. Food Chem. 2013, 61, 11848–11857. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Pereira, L.; Cruz, J.M.; Sendón, R.; Rodríguez Bernaldo de Quirós, A.; Ares, A.; Castro-López, M.; Abad, M.J.; Maroto, J.; Paseiro-Losada, P. Development of antioxidant active films containing tocopherols to extend the shelf life of fish. Food Control 2013, 31, 236–243. [Google Scholar] [CrossRef]
- Zhu, X.; Lee, D.S.; Yam, K.L. Release property and antioxidant effectiveness of tocopherol-incorporated LDPE/PP blend films. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 461–468. [Google Scholar] [CrossRef]
- Otero-Pazos, P.; Sendón, R.; Blanco-Fernandez, B.; Blanco-Dorado, S.; Alvarez-Lorenzo, C.; Concheiro, A.; Angulo, I.; Paseiro-Losada, P.; Rodríguez-Bernaldo de Quirós, A. Preparation of antioxidant active films based on chitosan: Diffusivity study of α-tocopherol into food simulants. J. Food Sci. Technol. 2016, 53, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.Y.; Summanen, P.H.; Lee, R.P.; Huang, J.; Henning, S.M.; Heber, D.; Finegold, S.M.; Li, Z. Prebiotic Potential and Chemical Composition of Seven Culinary Spice Extracts. J. Food Sci. 2017, 82, 1807–1813. [Google Scholar] [CrossRef]
- Carvalho, C.B.; Madrona, G.S.; Mitcha, J.G.; Valero, M.V.; Guerrero, A.; Da Silva Scapim, M.R.; Yamashita, F.; Do Prado, I.N. Effect of active packaging with oregano oil on beef burgers with low sodium content. Acta Sci. Technol. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Camo, J.; Lorés, A.; Djenane, D.; Beltrán, J.A.; Roncalés, P. Display life of beef packaged with an antioxidant active film as a function of the concentration of oregano extract. Meat Sci. 2011, 88, 174–178. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y.; Zhang, P.; Ye, L.; Wu, L.; He, S. Physical Characterization and Pork Packaging Application of Chitosan Films Incorporated with Combined Essential Oils of Cinnamon and Ginger. Food Bioprocess Technol. 2017, 10, 503–511. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Y.; Critzer, F.; Davidson, P.M.; Zivanovic, S.; Zhong, Q. Physical, mechanical, and antimicrobial properties of chitosan films with microemulsions of cinnamon bark oil and soybean oil. Food Hydrocoll. 2015, 52, 533–542. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Jia, H.; Liu, Y.X.; Zhang, H.; He, Z.; Ni, Y.H. Effects of Cellulose Nanofibers Filling and Palmitic Acid Emulsions Coating on the Physical Properties of Fish Gelatin Films. Food Biophys. 2017, 12, 23–32. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food applications of emulsion-based edible films and coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1130/2011 of 11 November 2011 amending Annex III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council on food additives by establishing a Union list of food additives approved for use in food additives, foodenzymes, food flavourings and nutrients. Off. J. Eur. Commun. 2011, 295, 178–204. [Google Scholar]
- European Commission. Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91. Off. J. Eur. Commun. 2008, 354, 34–50. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334. Off. J. Eur. Commun. 2012, 267, 1–161. [Google Scholar]
- European Food Safety Authority. European Compendium of botanicals reported to contain naturally occuring substances of possible concern for human health when used in food and food supplements. EFSA J. 2012. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Commun. 2002, 31, 1–24. [Google Scholar]
- European Commission. Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on materials and articles intended to come into contact with food and repealing Directives 80/590/EEC and 89/109/EEC. Off. J. Eur. Commun. 2004, 338, 4–17. [Google Scholar]
- European Commission. Regulation (EC) No 2023/2006 of 22 December 2006 on good manufacturing practice for materials and articles intended to come into contact with food. Off. J. Eur. Commun. 2006, 50, 75–78. [Google Scholar]
- Es, I.; Khaneghah, A.M.; Akbariirad, H. Global Regulation of Essential Oils. In Essential Oils in Food Processing: Chemistry, Safety and Applications; John Wiley & Sons: New York, NY, USA, 2017; pp. 327–338. [Google Scholar]
- Food and Drug Administration. Code of Federal Regulations. Part 182: Substances Generally Recognized as Safe; Food and Drug Administration: Washington, DC, USA, 2019; p. 3. [Google Scholar]
| Chemical Class | Characteristics | Examples | Example of Chemical Structure |
|---|---|---|---|
| Hydrocarbons | - Their basic structures are 5-carbon-based units (isoprenes) - They act as major compounds (90%) | Citronellol, limonene, α-pinene, camphor, myrcene, E-β-Ocimene | 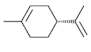 (Limonene) |
| Phenols | - They include phenolic terpenoids - They are aromatic components among the most reactive | Carvacrol, eugenol, thymol, chavicol | 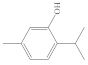 (Thymol) |
| Esters | - Pleasant smell | Eugenol acetate, geranyl acetate, linalyl acetate, bornyl acetate | 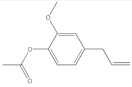 (Eugenol acetate) |
| Alcohols | - Pleasant aromas and no reported contraindications | Linalool, menthol, borneol, santalol, nerool, citronellol, generaniol, terpineol, pinocarveol | 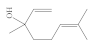 (Linallol) |
| Volatile compounds | - They include aromatic compounds | p-cymene, γ-terpinene, camphene, etc. | 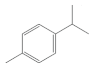 (p-cymene) |
| Aldehydes | - They are unstable and are oxidized easily - Derived from spices and fruits (aromatic compounds) | Benzaldehyde, citronellal, cinnamaldehyde, myrtenal, citral, citronellal | 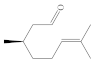 (Citronellal) |
| Film | EOs | Food | Main Results | Ref. |
|---|---|---|---|---|
| Gelatin | Orange (Citrus sinensis (L.) Osbeck) EO | Pink shrimp (Parapenaeus longirostris) | Antioxidant and antimicrobial activity Extension of shelf-life of nearly 10 days | [69] |
| Gelatin- | Oregano EO | Refrigerated Rainbow Trout Fillets | Lowering of total volatile basic nitrogen, peroxide value, thiobarbituric acid and microbial growth | [70] |
| Gelatin-carboxymethyl cellulose-chitin nanofibers- | Trachyspermum ammi EO (Ajowan) | Refrigerated raw beef | Growth inhibition of Pseudomonas spp., S. aureus, lactic acid bacteria (LAB), molds and yeasts. Stability of the chemical profile, color and sensory properties | [71] |
| Curdlan-PVA | Thyme EO | Chilled meat | Improvement of antioxidant activity and extension of the shelf life | [72] |
| Chitosan-cassava starch | Myrcia ovata Cambessedes EOs | Mangaba fruits | Antimicrobial effect against Bacillus cereus, B. subtilis and Serratia marcescens | [73] |
| Chitosan | Cumin EO nanoemulsion | Refrigerated beef loins | Growth inhibition of mesophilic and psychrophilic bacteria, Enterobacteriaceae and LAB. Antioxidant effects. | [74] |
| Chitosan | Clove EO | Cooked pork sausages | Microbial growth inhibition, retarded lipid oxidation and shelf-life extension when refrigerated storage | [75] |
| Chitosan beads | Lavender or red thyme EOs | Strawberry (clamshell) | Antifungal effect against Botrytis cinerea. Maintenance of appearance, color and firmness but odor, flavor and overall acceptability decrease | [76] |
| Chitosan and whey protein | Garlic EO nanoencapsulated | Vacuum-packed sausages | Retarded lipid oxidation and growth inhibition of main spoilage bacterial | [77] |
| Whey protein isolate | Oregano and garlic EOs | Kasar cheese slices | Antimicrobial effect against Escherichia coli, Salmonella enteritidis, Listeria monocytogenes, Staphylococcus aureus and Penicillium spp. | [78] |
| Whey protein isolate-cellulose nanofibers + TiO2 nanoparticles | Rosemary EO | Refrigerated lamb meat | Increase of the shelf life and antimicrobial effect against Pseudomonas spp., Enterobacteriaceae, LAB, S. aureus, L. monocytogenes and E. coli | [79] |
| Pectin | Oregano EO + resveratrol nanoemulsion | Fresh pork loin | Lowering pH effect, color change, retarded lipid and protein oxidation, microbial growth inhibition under high oxygen modified atmosphere packaging | [80] |
| Sodium alginate | Citral and Eugenol EOs | Arbutus unedo L. Fresh fruit | Improvement of postharvest quality attributes during storage. Reduction of microbial growth | [81] |
| Sodium caseinate | Ginger EO nanoemulsion | Chicken breast fillets | Growth inhibition of total aerobic psychrophilic bacteria | [82] |
| Arabic gum- sodium caseinate | Cinnamon or lemongrass EO | Guava fruit | Browning and related enzymes decrease, higher acceptability, antioxidant activity and high content of phenolic compounds | [83] |
| EOs/Components | Bacterial Species | ||||
|---|---|---|---|---|---|
| CJ | EC | LM | SE | SA | |
| Cinnamaldehyde, thymol, carvacrol, perillaldehyde, eugenol, estragole | + | + | + | + | |
| Oregano, cinnamon, thyme, bay leaf, allspice, clove bud oils | + | + | + | ||
| Palmarosa oil, salicylaldehyde, geraniol, isoeugenol | + | + | |||
| Ginger root, marigold, jasmine, carrot seeds, celery seeds, mugwort, spikenard, orange bitter oil, benzaldehyde, citral, carvone R, geranyl acetate | + | ||||
| Lemongrass oil, citral | + | ||||
| Patchouli, gardenia, cedarwood oils | + | + | |||
| Citral, geraniol, carvone S, salicylaldehyde | + | ||||
| Marjoram oil, terpineol | + | ||||
| Lemon EO | + | + | + | ||
| Melissa and lemon oils, terpineol, geraniol, linalool | + | ||||
| Rosemary EO | + | + | + | ||
| Oregano and garlic EOs | + | + | + | + | |
| Food | Film Polymers | Identified Compounds | Source of EOs | Antioxidant Assays | Ref. |
|---|---|---|---|---|---|
| Lamb | Polyethylene/polyamide | - | Oregano, rosemary | TBARS, metmyoglobin | [98] |
| Foal | Polyethylene | r-cymene, d-limonene, camphor, borneol, thymol, carvacrol, […] | Oregano | TBARS, metmyoglobin | [99] |
| Beef | Soy protein | - | Oregano, thyme | DPPH, TBARS, Rancimat | [100] |
| - | Rice starch/fish muscle protein | - | Oregano | DPPH | [101] |
| Smoked sardine | Gelatin (pigskin)/chitosan | tPC | Oregano, rosemary | FRAP, FFA | [102] |
| - | Quince seed mucilage | tPC | Oregano | DPPH | [103] |
| - | Hake muscle protein | Propenyldisulfide, thymol, carvacrol […] | Oregano, clove, garlic | DPPH | [104] |
| Pork sausage | - | - | Rosemary | TBARS | [105] |
| Poultry | Chitosan/montmorillonite | - | Rosemary | TBARS | [106] |
| Eel | CMC | tPC, rosmarinic acid, isorhamnetin-3-O- hexoside […] | Rosemary | DPPH | [107] |
| Swordfish | LDPE/polyamide | Carvacrol, thymol | Rosemary | TBARS […] | [108] |
| - | Quince seed mucilage | tPC | Thyme | DPPH | [109] |
| Chicken sausage | LPDE/nylon | tPC | Thyme, clove | DPPH, TBARS | [110] |
| Cut peach | Pectin | Eugenol, cinnamaldehyde […] | Cinnamon | DPPH | [111] |
| Pork | Chitosan/LDPE | - | Cinnamon | POV, TBARS | [112] |
| Sweet pepper | Chitosan | - | Cinnamon | TBARS, catalase and peroxidase activity | [113] |
| Rainbow trout | Gelatin | - | Cinnamon | FFA, TBARS | [114] |
| Rainbow trout | Chitosan | - | Cinnamon | POV, TBARS | [115] |
| - | Whey protein | tPC, tFC | Rosemary, cinnamon, basil | ABTS, β-carotene bleaching | [116] |
| Oat, linoleic acid | HDPE, LDPE, EVA | Sesamol | Sesame | Hexanal retention | [117] |
| Barley soup | - | DL-Limonene, γ-terpinene, tri-cyclene […] | Lemon peel | DPPH | [118] |
| Grapes | Chitosan/glycerol | tPC | Bergamot | DPPH | [119] |
| - | Chitosan/gelatin | β-carotene | Commercial isolate | DPPH | [120] |
| Sunflower oil | Cassava starch, LDPE | β-carotene | Carrots | POV | [121] |
| - | Polyethylene/polypropylene | α-tocopherol | Commercial isolate | DPPH, ABTS | [122] |
| Salmon | LDPE | α, γ, δ-tocopherols | Commercial isolate | DPPH, TBARS | [123] |
| - | LDPE/polypropylene | α, γ, δ-tocopherol | Soybean | Conjugated dienes | [124] |
| Butter | Chitosan/glycerol | α-tocopherol | Commercial isolate | DPPH | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. https://doi.org/10.3390/resources10010007
Carpena M, Nuñez-Estevez B, Soria-Lopez A, Garcia-Oliveira P, Prieto MA. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources. 2021; 10(1):7. https://doi.org/10.3390/resources10010007
Chicago/Turabian StyleCarpena, Maria, Bernabe Nuñez-Estevez, Anton Soria-Lopez, Paula Garcia-Oliveira, and Miguel A. Prieto. 2021. "Essential Oils and Their Application on Active Packaging Systems: A Review" Resources 10, no. 1: 7. https://doi.org/10.3390/resources10010007
APA StyleCarpena, M., Nuñez-Estevez, B., Soria-Lopez, A., Garcia-Oliveira, P., & Prieto, M. A. (2021). Essential Oils and Their Application on Active Packaging Systems: A Review. Resources, 10(1), 7. https://doi.org/10.3390/resources10010007







