New Concepts of Hydrogen Production and Storage in Arctic Region
Abstract
1. Introduction
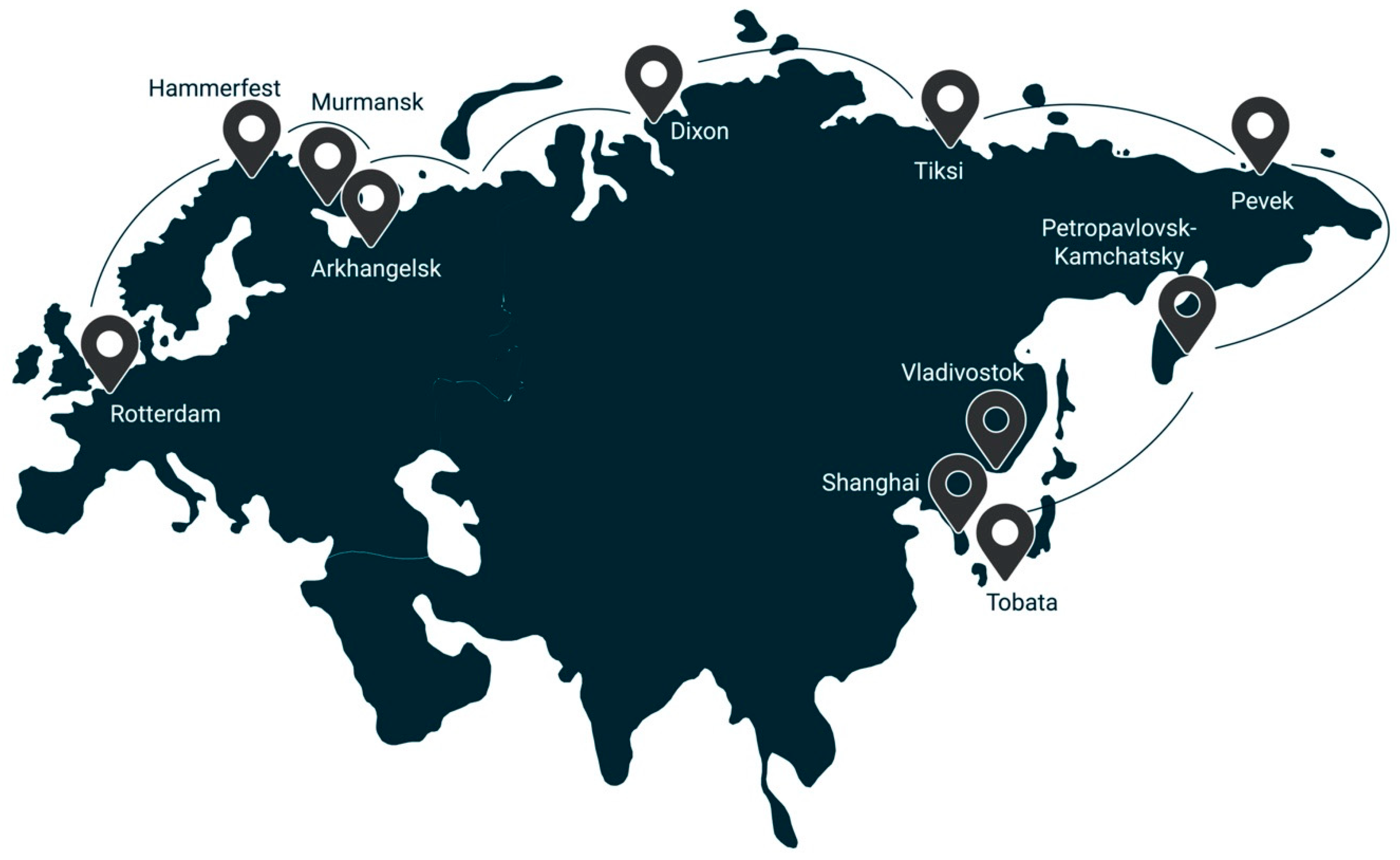
- The presence of the Northern Sea route (product-distribution routes, both to the countries of the EU and to the countries of the Asia-Pacific region);
- possibility of bound hydrogen transport in a solid form using dry cargo vessels and in liquid form by tankers.
2. Promising Methods of Hydrogen Production
2.1. Blue Hydrogen
2.2. Turquoise Hydrogen
2.3. Green Hydrogen
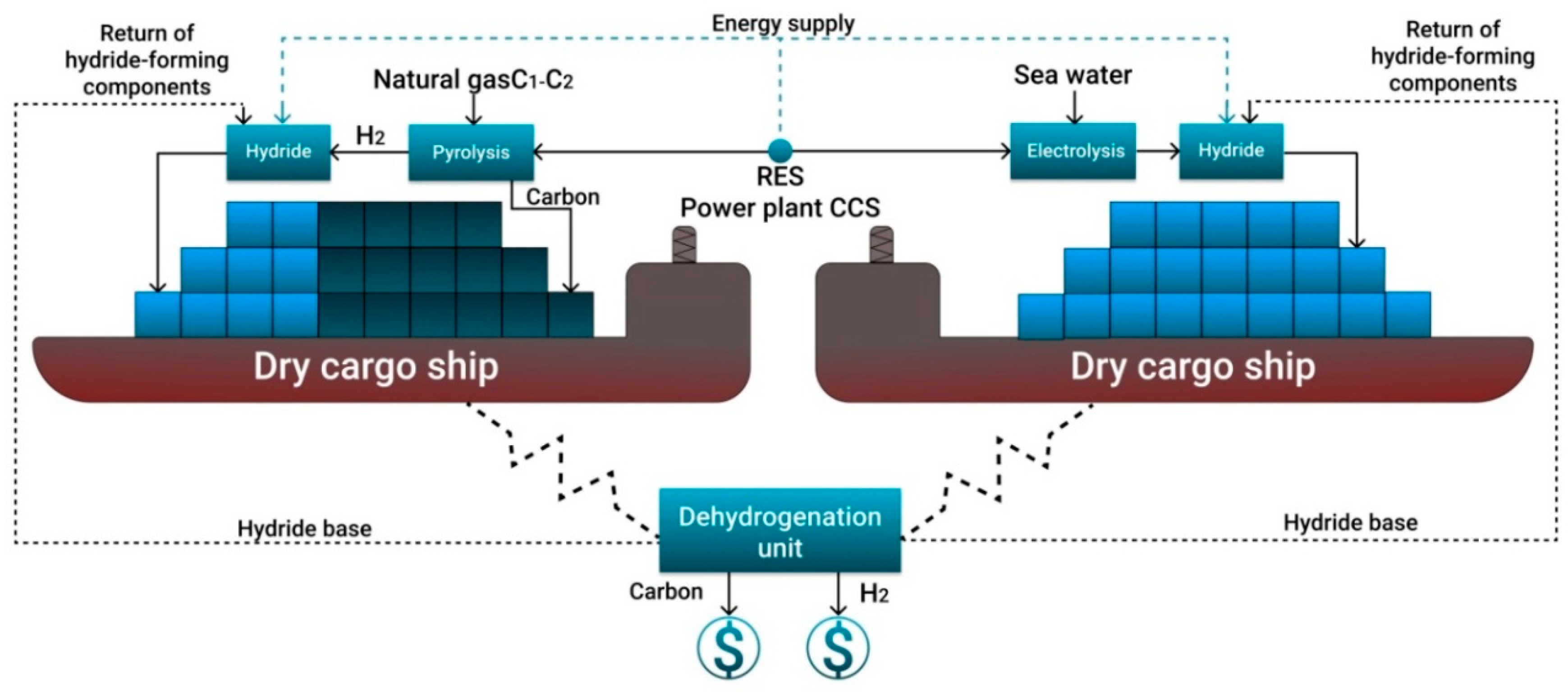
3. Concept of Transport and Storage of Hydrogen in a Bound Form
3.1. Ammonia
3.2. Methanol
3.3. Cyclohexane
3.4. Hydrides
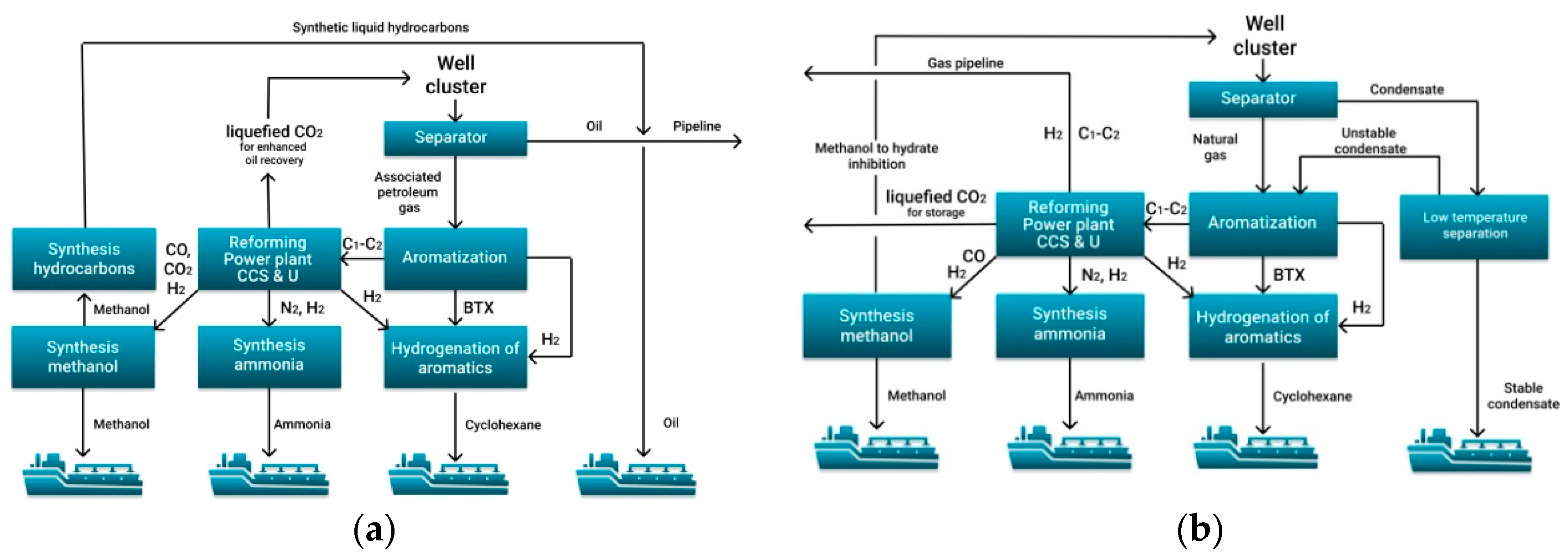
4. Concepts of Development of Oil and Gas Fields in the Arctic Shelf
4.1. Ammonia, Methanol and Cyclohexane as Hydrogen Carriers
4.2. Low Carbon Hydrogen Bound in Hydrates
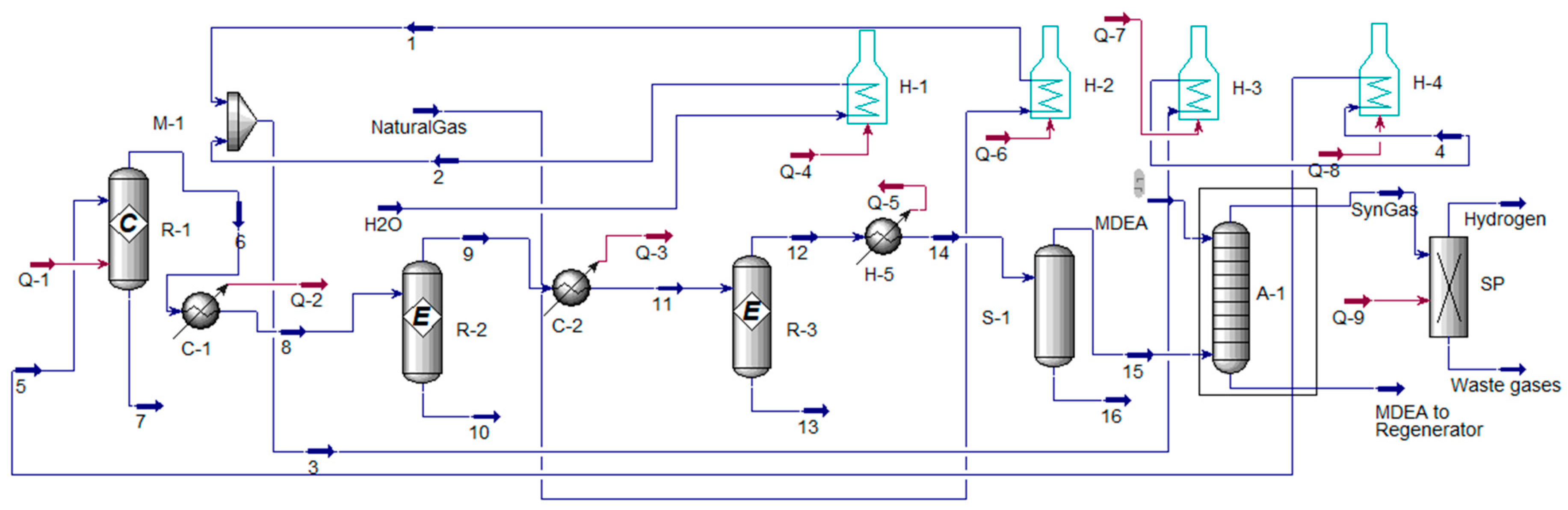
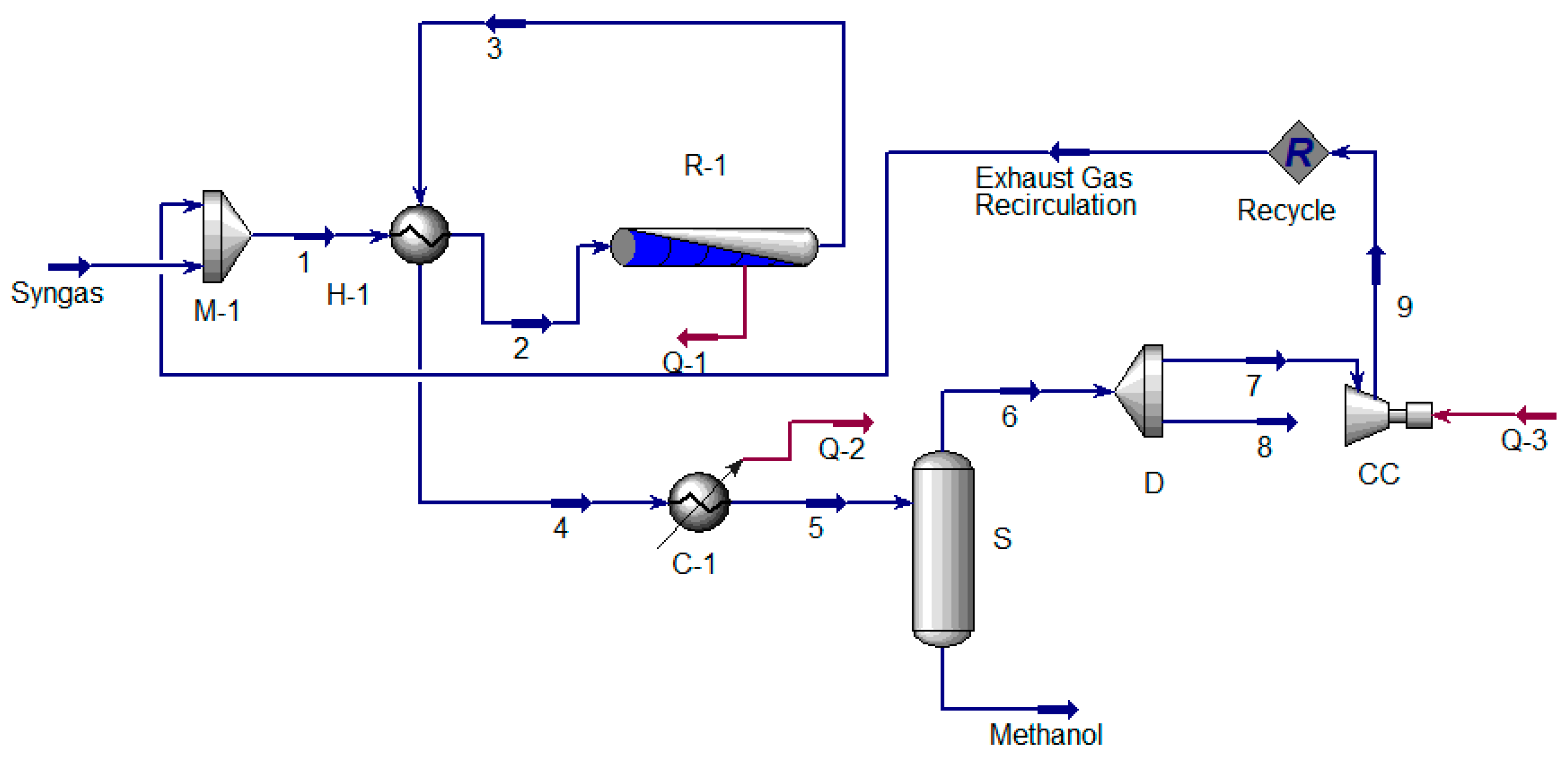
5. Extraction of Hydrogen from Ammonia, Methanol and Cyclohexane
5.1. Extraction of Hydrogen from Ammonia
5.2. Extraction of Hydrogen from Methanol
5.3. Extraction of Hydrogen from Cyclohexane
6. Discussion and Conclusions
- The most important are aromatization of C3+ hydrocarbon gases and unstable gas condensate to obtain benzene-toluene-xylene, as well as hydrogenation of benzene-toluene-xylene to produce cyclohexane.
- The conversion of C1–C2 hydrocarbons for the further production of hydrogen, ammonia and methanol must combine processes with the addition of methane, water steam, oxygen and even carbon dioxide.
- All production chains must be harmoniously linked into one production process, achieving a synergistic effect. This effect can be achieved for the combined hydrogen-methanol-ammonia production.
- Due to the lack of fleet for shipping of compressed or liquefied hydrogen, it is suggested to use tankers and dry cargo vessels for the transport of bound hydrogen in liquid and solid form. For the existing gas transportation systems and port infrastructure, it is proposed to use C1–C2 pyrolysis to obtain turquoise hydrogen and solid carbon from renewable energy sources and to ship the compressed carbon and hydrogen bound into hydride.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alekseeva, M.B.; Bogachev, V.F.; Gorenburgov, M.A. Systemic Diagnostics of the Arctic Industry Development Strategy. J. Min. Inst. 2019, 238, 450–458. [Google Scholar] [CrossRef]
- Vasiltsov, V.S.; Vasiltsova, V.M. Strategic Planning of Arctic Shelf Development Using Fractal Theory Tools. J. Min. Inst. 2018, 234, 663–672. [Google Scholar] [CrossRef]
- Evangelopoulou, S.; De Vita, A.; Zazias, G.; Capros, P. Energy System Modelling of Carbon-Neutral Hydrogen as an Enabler of Sectoral Integration within a Decarbonization Pathway. Energies 2019, 12, 2551. [Google Scholar] [CrossRef]
- A Hydrogen Strategy for a Climate Neutral Europe. Available online: https://ec.europa.eu/commission/presscorner/detail/en/FS_20_1296 (accessed on 20 July 2020).
- Energy Strategy of the Russian Federation for the Period up to 2035. Available online: https://minenergo.gov.ru/node/1026 (accessed on 10 November 2020).
- Eckpunktepapier der Ostdeutschen Kohleländer zur Entwicklung Einer Regionalen Wasserstoffwirtschaft. Available online: https://www.medienservice.sachsen.de/medien/medienobjekte/130485/download (accessed on 13 November 2020).
- Ilinova, A.A.; Romasheva, N.V.; Stroykov, G.A. Prospects and social effects of carbon dioxide sequestration and utilization projects. J. Min. Inst. 2020, 244, 493–502. [Google Scholar] [CrossRef]
- Tcvetkov, P.S.; Cherepovitsyn, A.E.; Fedoseev, S.V. The Changing Role of CO2 in the Transition to a Circular Economy: Review of Carbon Sequestration Projects. Sustainability 2019, 11, 5834. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline. Networks: A Review of Key Issues. 2013. Available online: https://www.nrel.gov/docs/fy13osti/51995.pdf (accessed on 5 October 2020).
- Litvinenko, V. The Role of Hydrocarbons in the Global Energy Agenda: The Focus on Liquefied Natural Gas. Resources 2020, 9, 59. [Google Scholar] [CrossRef]
- Saphin, A.K. Production of Hydrogen Plant Sand Construction of Hydrogen Infrastructure in Industrialized Countries. Technical and Investment Indicators of Installation Sand Hydrogen Stations; Issue 2; LLC Prima-Khimmash: St. Petersburg, Russia, 2015; p. 226. [Google Scholar]
- Salikhov, K.M.; Stoyanov, N.D.; Stoyanova, T.V. Using Optical Activation to Create Hydrogen and Hydrogen-Containing Gas Sensors. Key Eng. Mater. 2020, 854, 87–93. [Google Scholar] [CrossRef]
- Parkinson, B.; Tabatabaei, M.; Upham, D.C.; Ballinger, B.; Greig, C.; Smart, S.; McFarland, E. Hydrogen production using methane: Techno economics of decarbonizing fuels and chemicals. Int. J. Hydrogen Energy 2018, 43, 2540–2555. [Google Scholar] [CrossRef]
- Susmozas, A.; Iribarren, D.; Dufour, J. Assessing the Life-Cycle Performance of Hydrogen Production via Biofuel Reforming in Europe. Resources 2015, 4, 398–411. [Google Scholar] [CrossRef]
- Dahl, P.Y.; Christensen, T.S.; Winter-Madsen, S. Autothermal Reforming Technology for modern large-capacity methanol plants. In Proceedings of the International Conference Nitrogen and Syngas, Paris, France, 24–27 February 2014; p. 14. [Google Scholar]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrog. Energy 2019, 7, 107–154. [Google Scholar] [CrossRef]
- Liu, K.; Song, C.; Subraman, V. Hydrogen and Syngas Production and Purification Technologies; John Wiley & Sons: Hoboken, NJ, USA, 2010; p. 564. [Google Scholar] [CrossRef]
- Machlin, V.A.; Cetaruk, J.R. Modern technologies of producing synthesis gas from natural and associated gas. Sci. Tech. J. Chem. Ind. Today 2010, 3, 6–17. [Google Scholar]
- Indarto, A.; Palguandi, J. Syngas Production, Application and Environmental Impact; Nova Science Publishers: New York, NY, USA, 2013; p. 365. [Google Scholar]
- Zagashvili, U.V.; Levain, A.A.; Kuzmin, A.M.; Aniskevich, Y.V.; Vasilieva, O.V. Technology of hydrogen production using small transportable units based on high-temperature syngas generators. Sci. Tech. J. Vopr. Mater. Edeniya 2017, 2, 92–109. [Google Scholar]
- Zagashvili, U.V.; Levain, A.A.; Kuzmin, A.M. Principles of design of three-component gas synthesis gas. Oil and gas. LLC Obrakademnauka 2017, 4, 9–16. [Google Scholar]
- Buslaev, G.V. Technology of associated petroleum gas processing at remote Arctic oil and gas facilities to produce synthetic liquid hydrocarbons. In Proceedings of the Plenary Report at the XIX International Youth Scientific Conference SeverGeoEcoTech-2018, Ukhta, Russia, 21–23 March 2018. [Google Scholar]
- Abalaev, A.V.; Del Toro Fonseca, D.A.; Lewiner, I.I.; Vyatkin, Y.L. Optimization of the operating mode of shelf reactors with a fixed layer in the process of aromatization of hydrocarbons C5. Scientific service on the Internet: Search for new solutions. In Proceedings of the International Supercomputing Conference, Hamburg, Germany, 17–21 June 2012; pp. 273–277. [Google Scholar]
- Azhazha, V.M.; Tikhonovskiy, M.A.; Shepelev, A.G.; Kurilo, Y.P.; Ponomarenko, T.A.; Vinogradov, D.V. Materials for Hydrogen Storage: Analysis of Development Trends Based on Data on Information Flows. Questions of Nuclear Science and Technology. Series: Vacuum, Pure Materials, Superconductors; National Scientific Center, Kharkiv Institute of Physics and Technology: Kharkov, Ukraine, 2006; pp. 145–153. Available online: https://vant.kipt.kharkov.ua/ARTICLE/VANT_2006_1/article_2006_1_145.pdf (accessed on 10 July 2020).
- Baraban, A.P.; Gabis, I.E.; Dmitriev, V.A.; Dobrotvorskii, M.A.; Kuznetsov, V.G.; Matveeva, O.P.; Titov, S.A.; Voyt, A.P.; Yelets, D.I. Luminescent properties of aluminum hydride. J. Lumin. 2015, 166, 162–166. [Google Scholar] [CrossRef]
- Bazhin, V.Y.; Trushnikov, V.E.; Suslov, A.P. Simulation of partial oxidation of natural gas in a resource-saving reactor mixer. IOP Conf. Ser. Mater. Sci. Eng. 2020, 862, 862. [Google Scholar] [CrossRef]
- Belousova, O.Y.; Kutepov, B.I. Textbook of Aromatization of Hydrocarbons on Pentasyl-Containing Catalysts; Khimiya: Moscow, Russia, 2000; p. 95. [Google Scholar]
- Bilera, I.V. Education of Fine Soot When Receiving the Synthesis Gas under Conditions of Combustion of Methane. In The Gas Chemistry; Billera, I.V., Borisov, A.A., Borunova, A.B., Kolbanoskiy, Y.A., Korolev, Y.M., Rossokhin, I.V., Troshin, I.V., Eds.; Metaprocess Ltd.: Saint-Brice-Courcelles, France, 2010; Volume 3, pp. 72–78. [Google Scholar]
- Cooper, H.; Donnis, B.B.L. Aromatic saturation of distillates: An overview. Appl. Catal. A Gen. 1996, 137, 203–223. [Google Scholar] [CrossRef]
- Abanades, A.; Ruiz, E.; Ferruelo, E.M.; Hernández, F.; Cabanillas, A.; Martínez-Val, J.M.; Rubio, J.A.; López, C.; Gavela, R.; Barrera, G.; et al. Experimental analysis of direct thermal methane cracking. Int. J. Hydrog. Energy 2011, 36, 12877–12886. [Google Scholar] [CrossRef]
- Abbas, H.F.; Wan Daud, W.M.A. Hydrogen production by methane decomposition: A review. Int. J. Hydrog. Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Bode, A.; Anderlohr, C.; Bernnat, J.; Flick, F.; Glenk, F.; Klingler, D.; Kolios, G.; Scheiff, F.; Wechsung, A.; Hensmann, M.; et al. Feste und Fluide Produkte ausGas—FfPaG, Schlussbericht; BMBF: Bonn, Germany, 2018. [Google Scholar]
- Geißler, T.; Plevan, M.; Abánades, A.; Heinzel, A.; Mehravaran, K.; Rathnam, R.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stuckrad, S.; et al. Experimental investigation and thermo-chemical modeling of methane pyrolysis in a liquid metal bubble column reactor with a packed bed. Int. J. Hydrog. Energy 2015, 40, 14134–14146. [Google Scholar] [CrossRef]
- Moliner, R.; Suelves, I.; Lazaro, M.; Moreno, O. Thermocatalytic decomposition of methane over activated carbons: Influence of textural properties and surface chemistry. Int. J. Hydrog. Energy 2005, 30, 293–300. [Google Scholar] [CrossRef]
- Muradov, N.; Chen, Z.; Smith, F. Fossil hydrogen with reduced CO2 emission: Modeling thermocatalytic decomposition of methane in a fluidized bed of carbon particles. Int. J. Hydrog. Energy 2005, 30, 1149–1158. [Google Scholar] [CrossRef]
- Muradov, N.; Smith, F.; Huang, C.; T-Raissi, A. Autothermal catalytic pyrolysis of methane as a new route to hydrogen production with reduced CO2 emissions. Catal. Today 2006, 116, 281–288. [Google Scholar] [CrossRef]
- Plevan, M.; Geißler, T.; Abanades, A.; Mehravaran, K.; Rathnam, R.; Rubbia, C.; Salmieri, D.; Stoppel, L.; Stückrad, S.; Wetzel, T. Thermal cracking of methane in a liquid metal bubble column reactor: Experiments and kinetic analysis. Int. J. Hydrog. Energy 2015, 40, 8020–8033. [Google Scholar] [CrossRef]
- Schultz, I.; Agar, D.W. Decarbonization of fossil energy via methane pyrolysis using two reactor concepts: Fluid wall flow reactor and molten metal capillary reactor. Int. J. Hydrog. Energy 2015, 40, 11422–11427. [Google Scholar] [CrossRef]
- Osman, A.I.; Farrell, C.; Al-Muhtaseb, A.H.; Harrison, J.; Rooney, D.W. The production and application of carbon nanomaterials from high alkali silicate herbaceous biomass. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Ostadi, M.; Paso, K.G.; Rodriguez-Fabia, S.; Oi, L.E.; Manenti, F.; Hillestad, M. Process Integration of Green Hydrogen: Decarbonization of Chemical Industries. Energies 2020, 13, 4859. [Google Scholar] [CrossRef]
- Olivier, P.; Bourasseau, C.; Bouamama, P. Low-temperature electrolysis system modelling: A review. Renew. Sustain. Energy Rev. 2017, 78, 280–300. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S.; et al. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, C.; Fu, Y.; Gao, J.; Huang, H.; Liu, Y.; Kang, Z. Construction of CDs/CdS photocatalysts for stable and efficient hydrogen production in water and seawater. Appl. Catal. B Environ. 2019, 242, 178–185. [Google Scholar] [CrossRef]
- Hung, W.-H.; Xue, B.-Y.; Lin, T.-M.; Lu, S.-Y.; Tsao, I.-Y. A highly active selenized nickel-iron electrode with layered double hydroxide for electrocatalytic water splitting in saline electrolyte. Mater. Today Energy 2021, 19, 100575. [Google Scholar] [CrossRef]
- Jamesh, M.I.; Harb, M. Recent advances on hydrogen production through seawater electrolysis. Mater. Sci. Energy Technol. 2020, 3, 780–807. [Google Scholar] [CrossRef]
- Materials of SKOLKOVO Energy Centre, Moscow School of Management SKOLKOVO. Available online: https://energy.skolkovo.ru/downloads/documents/SEneC/Research/SKOLKOVO_EneC_Hydrogen-ecomy_Eng.pdf (accessed on 20 June 2019).
- Buyanov, S. Prospects for the construction of ships for Russian shipowners. In Proceedings of the Current State and Prospects for Development of Russian Bunker Services Market, Saint Petersburg, Russia, 27–28 June 2019; Available online: http://cniimf.ru/press-tsentr/news/870/ (accessed on 2 July 2019).
- Litvinenko, V.S.; Tsvetkov, P.S.; Dvoynikov, M.V.; Buslaev, G.V. Barriers to implementation of hydrogen initiatives in the context of global energy sustainable development. J. Min. Inst. 2020, 244, 421–431. [Google Scholar] [CrossRef]
- Anhydrous Liquefied Ammonia. Specifications; Russian Government Standard GOST 6221-90; Standartinform: Moscow, Russia, 2011; Available online: http://www.gostrf.com/normadata/1/4294823/4294823189.pdf (accessed on 15 September 2020).
- Production of Ammonia, Mineral Fertilizers and Inorganic Acids. Information and Technical Directory (ITD) 2019. Available online: http://docs.cntd.ru/document/564068887 (accessed on 13 November 2020).
- Samuel, S.A.; Vincenzo, L.; Xiaoti, C.; Na, L.; Jimin, Z.; Simon, L.S.; Søren, H.J.; Mads, P.N.; Søren, K.K. A Review of The Methanol Economy: The Fuel Cell Route. Energies 2020, 13, 1–32. [Google Scholar]
- Yurieva, T.M.; Plyasova, L.; Makarova, O.; Krieger, T. Mechanisms for hydrogenation of acetone to isopropanol and of carbon oxides to methanol over copper-containing oxide catalysts. J. Mol. Catal. A Chem. 1996, 113, 455–468. [Google Scholar] [CrossRef]
- Chen, W.H.; Chen, C.Y. Water gas shift reaction for hydrogen production and carbon dioxide capture: A review. Appl. Energy 2020, 258, 114078. [Google Scholar] [CrossRef]
- Angell, V.W.; Graham, H.J.; Post, G.J. Case History: Ice Island Drilling Application and Well Considerations in Alaskan Beaufort Sea. Soc. Pet. Eng. 1990. [Google Scholar] [CrossRef]
- Bolotov, V.A.; Cheremisina, O.V.; Ponomareva, M.A.; Alferova, D.A. Prospects for the use of the sorbent for purification of gases from sulfur-containing components on the basis of manganese ore. Key Eng. Mater. 2020, 836, 13–18. [Google Scholar] [CrossRef]
- Vyatkin, Y.L.; Lishchiner, I.I.; Sinitsyn, S.A.; Kuz’min, A.M. Perspective directions of chemical processing of hydrocarbon raw materials. Neftegaz 2020, 4, 114–118. [Google Scholar]
- Rusman, N.A.A.; Dahari, M.A. Review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrog. Energy 2016, 12108–12126. [Google Scholar] [CrossRef]
- Tarasov, B.P. Problems and prospects of creating materials for hydrogen storage in a bound state. Int. Sci. J. Altern. Energy Ecol. 2006, 2, 11–17. [Google Scholar]
- Tarasov, B.P.; Burnasheva, V.V.; Lototsky, M.V.; Yartys, V.A. Methods of hydrogen storage and the possibility of using metallohydrides. Int. Sci. J. Altern. Energy Ecol. 2005, 12, 14–37. [Google Scholar]
- Tarasov, B.P.; Lototsky, M.V.; Yartys, V.A. The Problem of hydrogen storage and prospects for using hydrides for hydrogen storage. Russ. Chem. J. 2006, 6, 34–48. [Google Scholar]
- McKay, M. Hydrogen Compounds of Metals; Mir: Moscow, Russia, 1968; p. 244. [Google Scholar]
- York, A.P.E.; Xiao, T.; Green, M.L.H. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Schlapbach, L. Hydrogen as a fuel and its storage for mobility and transport. MRS Bull. 2002, 675–679. [Google Scholar] [CrossRef]
- Kalashnikov, J.A. Physical Chemistry of Substances at High Pressures; Higher School: Moscow, Russia, 1987; p. 237. [Google Scholar]
- Hydride Hydrogen Storage System. Available online: https://lektsia.com/2x8693.html (accessed on 13 November 2020).
- Landrum, L.; Holland, M.M. Extremes become routine in an emerging new Arctic. Nat. Clim. Chang. 2020, 10, 1108–1115. [Google Scholar] [CrossRef]
- Fateev, V.N.; Alekseeva, O.K.; Korobtsev, S.V.; Seregina, E.A.; Fateeva, T.V.; Grigoriev, A.S.; Aliev, A.S. Problems of hydrogen accumulation and hydrogen storage. KimyaProblemleri Baku Aliyev Akif Shihanoglu 2018, 4, 453–483. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Kornev, A.V.; Barkan, M.S. Prospects for the use of associated gas of oil development as energy product. Int. J. Energy Econ. Policy 2017, 7, 374–383. [Google Scholar]
- Kozʼmenko, S.Y.; Masloboev, V.A.; Matviishin, D.A. Justification of Economic Benefits of Arctic LNG Transportation by Sea. J. Min. Inst. 2018, 233, 554–560. [Google Scholar] [CrossRef]
- Buslaev, G.; Morenov, V.; Konyaev, Y.; Kraslawski, A. Reduction of carbon footprint of the production and field transport of high-viscosity oils in the Arctic region. Chem. Eng. Process. Process Intensif. 2020, 108189. [Google Scholar] [CrossRef]
- Morenov, V.A.; Leusheva, E.L.; Buslaev, G.V.; Gudmestad, O.T. System of comprehensive energy-efficient utilization of associated petroleum gas with reduced carbon footprint in the field conditions. Energies 2020, 13, 4921. [Google Scholar] [CrossRef]
- Bode, A.; Agar, D.W.; Buker, K.; Göke, V.; Hensmann, M.; Janhsen, U.; Klingler, D.; Schlichting, J.; Schunk, S.A. Research cooperation develops innovative technology for environmentally sustainable syngas production from carbon dioxide and hydrogen. In Proceedings of the 20th World Hydrogen Energy Conference, Gwangju, Korea, 15–20 June 2014. [Google Scholar]
- Church, J.A.; Clark, P.U.; Cazenave, A.; Gregory, J.M.; Jevrejeva, S.; Levermann, A.; Merrifield, M.A.; Milne, G.A.; Nerem, R.S.; Nunn, P.D.; et al. Sea Level Change. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Climate Change 2013: The Physical Science Basis; Stocker, T.F.D., Qin, G.-K., Plattner, M., Tignor, S.K., Allen, J., Boschung, A., Nauels, Y., Xia, V.B., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Accelerating the Uptake of CCS: Industrial Use of Captured Carbon Dioxide; Global CCS Institute: Melbourne, Australia, 2011.
- Kirsanova, N.Y.; Lenkovets, O.M.; Nikulina, A.Y. The Role and Future Outlook for Renewable Energy in the Arctic Zone of Russian Federation. Eur. Res. Stud. J. 2018, 21, 356–368. [Google Scholar]
- Wang, M.; Li, J.; Chen, L.; Lu, Y. Miniature NH3 cracker based on microfibrous entrapped Ni-CeO2/Al2O3 catalyst monolith for portable fuel cell power supplies. Int. J. Hydrog. Energy 2009, 34, 1710–1716. [Google Scholar] [CrossRef]
- Mukhovikova, N.K.; Nepomnyakschikh, Y.V. Preparation of Formaldehyde by Oxidative Dehydration of Methanol. In Proceedings of the XI All-Russian Scientific-Practical Conference of Young Scientists «YOUNG RUSSIA», Moscow, Russia, 2–4 October 2019; Available online: http://science.kuzstu.ru/wp-content/Events/Conference/RM/2019/RM19/pages/Articles/70207.pdf (accessed on 22 October 2020).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvoynikov, M.; Buslaev, G.; Kunshin, A.; Sidorov, D.; Kraslawski, A.; Budovskaya, M. New Concepts of Hydrogen Production and Storage in Arctic Region. Resources 2021, 10, 3. https://doi.org/10.3390/resources10010003
Dvoynikov M, Buslaev G, Kunshin A, Sidorov D, Kraslawski A, Budovskaya M. New Concepts of Hydrogen Production and Storage in Arctic Region. Resources. 2021; 10(1):3. https://doi.org/10.3390/resources10010003
Chicago/Turabian StyleDvoynikov, Mikhail, George Buslaev, Andrey Kunshin, Dmitry Sidorov, Andrzej Kraslawski, and Margarita Budovskaya. 2021. "New Concepts of Hydrogen Production and Storage in Arctic Region" Resources 10, no. 1: 3. https://doi.org/10.3390/resources10010003
APA StyleDvoynikov, M., Buslaev, G., Kunshin, A., Sidorov, D., Kraslawski, A., & Budovskaya, M. (2021). New Concepts of Hydrogen Production and Storage in Arctic Region. Resources, 10(1), 3. https://doi.org/10.3390/resources10010003







