Abstract
SARS-CoV-2, with an infection fatality rate between 0.5 and 1%, has spread to all corners of the globe and infected millions of people. While vaccination is essential to protect against the virus and halt community transmission, rapidly making and delivering safe and efficacious vaccines presents unique development, manufacturing, supply chain, delivery, and post-market surveillance challenges. Despite the large number of vaccines in or entering the clinic, it is unclear how many candidates will meet regulatory requirements and which vaccine strategy will most effectively lead to sustained, population-wide immunity. Interviews with experts from biopharmaceutical companies, regulatory and multilateral organizations, non-profit foundations, and academic research groups, complemented with extensive literature review, informed the development of a framework for understanding the factors leading to population-wide immunity against SARS-CoV-2, in particular considering the role of vaccines. This paper presents a systems-level modeling framework to guide the development of analytical tools aimed at informing time-critical decisions to make vaccines globally and equitably accessible. Such a framework can be used for scenario planning and evaluating tradeoffs across access strategies. It highlights the diverse and powerful ways in which data can be used to evaluate future risks and strategically allocate limited resources.
1. Introduction
The COVID-19 pandemic, caused by the spread of the severe acute respiratory syndrome (SARS) coronavirus 2 (CoV-2), was declared a ‘Public Health Emergency of International Concern’ by the World Health Organization (WHO) on 30 January 2020 and pandemic on 11 March 2020. SARS-CoV-2 is much more transmissible, though with a lower case fatality ratio, than related coronaviruses such as SARS and Middle East respiratory syndrome coronavirus (MERS) CoV [1,2]. As shown in Figure 1a, over 73 million people have been infected by and close to 1.6 million people have died from SARS-CoV-2 as of 15 December 2020 [3]. The impact of the pandemic has rippled through all aspects of society, where no place and no person has been left untouched. Although spread of the virus is slowing down in some countries as a result of persistent public health measures (e.g., quarantines and facial coverings), the pandemic is worsening in others and continues to be a health and security threat for all. A failure to address hotspots of infections in one part of the globe may quickly lead to a surge in cases elsewhere.
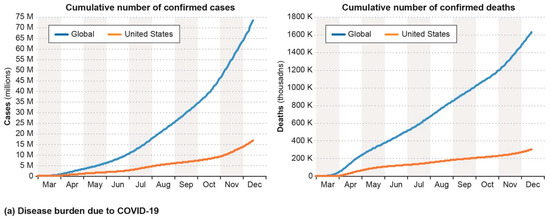
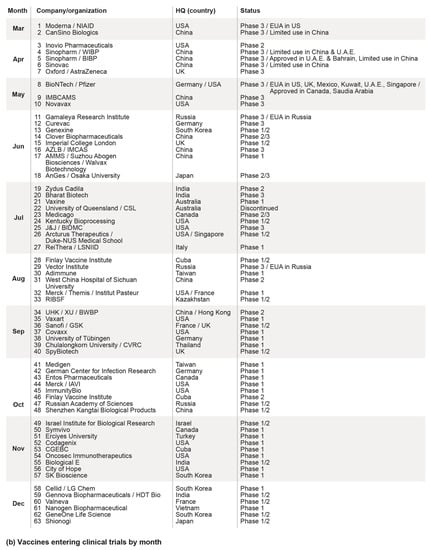
Figure 1.
(a) Cumulative number of confirmed cases (top) and deaths (bottom) due to SARS-CoV-2, from 1 March to 15 December 2020; (b) Vaccine candidates that have entered clinical trials as of mid-December 2020, including their approximate start time by month, the country of the respective lead entities, and clinical trial status. Data has been extracted from the New York Times Coronavirus Vaccine Tracker. HQ: Headquarters; EUA: Emergency Use Authorization; US: United States of America; UK: United Kingdom; U.A.E.: United Arab Emirates; NIAID: National Institute of Allergy and Infectious Diseases; WIBP: Wuhan Institute of Biological Products; BIBP: Beijing Institute of Biological Products; IMBCAMS: Institute of Medical Biology at the Chinese Academy of Medical Sciences; AZLB: Anhui Zhifei Longcom Biopharmaceutical; IMCAS: Institute of Microbiology of the Chinese Academy of Sciences; PLAAMS: People’s Liberation Army Academy of Military Sciences; J&J: Johnson & Johnson; BIDMC: Beth Israel Deaconess Medical Center; LSNIID: Lazzaro Spallanzani National Institute for Infectious Diseases; RIBSF: Research Institute for Biological Safety Problem; UHK: University of Hong Kong; XU: Xiamen University; BWBP: Beijing Wantai Biological Pharmacy; GSK: GlaxoSmithKline; CVRC: Chula Vaccine Research Center; IAVI: International AIDS Vaccine Initiative; CGEBC: Center for Genetic Engineering and Biotechnology of Cuba.
A vaccine that protects against the SARS-CoV-2 and halts community transmission is the most effective way to stop the pandemic today and prevent it in the future. While the body has an extensive array of defenses as part of its innate immune system, they are not specific to unique pathogenic agents. Vaccines complement these defenses through pathogen-specific responses that are adaptive in nature and become more effective after successive exposure. Such an immune response is characterized by T-cells recognizing antigen fragments from proteins predigested by dendritic cells (cell-mediated response) or when B-cells are stimulated to secrete antibodies specific to the antigen fragments (humoral response) [4]. Analysis of public health and economic benefits related to immunization programs demonstrates substantial returns on investments in vaccines [5]. Given the global scale and urgency of the crisis, rapidly making billions of safe and effective vaccine doses available within a compressed timescale presents unique development, manufacturing, supply chain, delivery, and post-market surveillance challenges [6].
The COVID-19 pandemic has spawned ambitious efforts to make vaccines available by the end of 2020 or early 2021. Such speed is unprecedented in the vaccine space, as exemplified by the first human clinical trials starting only 66 days after the DNA sequence for SARS-CoV-2 was published [7]. As shown in Figure 1b, over 60 vaccine candidates have entered clinical trials as of December 2020 to test their safety and efficacy at different dosage levels [8]. Early results from clinical trials are already becoming available, with emergency use authorization (EUA) serving as a possible alternative to the standard, full approval. Several vaccine candidates (e.g., Pfizer, Moderna, CanSino, Gamaleya, etc.) have already received approval for emergency or limited use, opening the door to community-wide vaccination campaigns. Other clinical trials are advancing steadily, with Phase 3 studies providing important information before conclusions can be made.
Vaccines differ in their ability to stimulate the innate immune system and both arms of the adaptive immune system, thus impacting the longevity, strength, and specificity of the immune protection. The benchmark set by the U.S. Food & Drug Administration for an EUA is disease prevention or a decrease in its severity in at least 50% of people who are vaccinated, followed by post-marketing studies to assess known or potential risks [9]. Despite the large number of candidate vaccines, it is unclear how many will meet the safety and efficacy requirements of regulatory agencies, on what timelines, as well as which vaccine strategy will most effectively lead to sustained, population-wide immunity.
Applications of systems thinking have shown to be important in the strengthening of healthcare operations. They allow for the development of tools that can help reveal the underlying and dynamic relationships of components within a system (e.g., where feedback loops and bottlenecks exist), tradeoffs across different types of investments, and which interventions lead to desired outcomes [10]. Effectively designing, deploying, and evaluating an immunization strategy involves the interaction of multiple stakeholders. Systems-based approaches facilitate the evaluation of both intended and unintended consequences of events in ways that can inform decision making. One such application has been understanding the barriers to and developing context-specific innovations for rapidly scaling-up routine childhood immunization against vaccine-preventable diseases in light of the COVID-19 pandemic’s toll on healthcare facilities [11]. Given the scale of the pandemic and its impact, leveraging systems thinking and related tools is important to design a vaccine strategy that can most effectively reach sustained population-wide immunity against SARS-CoV-2.
2. Methodology and Framework
This paper presents a systems-level modeling framework to guide the development of analytical tools aimed at informing time-critical decisions to make vaccines globally and equitably accessible. It highlights the diverse and powerful ways in which data, especially when collected in (near) real-time, can be used to evaluate future risks and strategically allocate limited resources. The paper also highlights some of the key challenges with making vaccines against SARS-CoV-2 globally accessible. It provides insights into the evolving landscape of the SARS-CoV-2 vaccine pipeline and important considerations that may hinder the ability to rapidly scale the development, manufacture, supply, and delivery of vaccines.
To inform the development of this framework, interviews were conducted with 25 experts from biopharmaceutical companies, regulatory and multilateral organizations, non-profit foundations, and academic research groups. The interviews were used to better define biological, manufacturing, logistical, and policy issues associated with making vaccines available and reaching sustained population-wide immunity. Interview questions were designed with a group of advisors, along with preparatory material to help guide the discussion with interviewees. Each interview lasted 45–60 min and has been kept anonymous. Interviews were complemented with a broad literature review into various topics and observations from the ongoing vaccine development process, such as vaccine immunology, vaccine manufacturing, supply chain management, as well as policy, financial, and regulatory considerations. Data used to generate the figures are publicly available. Microsoft Excel and R (Version 1.1.383—© 2009–2017 RStudio, Inc., Boston, MA, USA) were used to generate the plots.
The modeling framework presented takes a systems view of the different components influencing the ability to rapidly reach and sustain population-wide immunity. The dynamic nature of how a virus spreads points to the importance of building models that account for uncertainty, as well as embedding a learning system to promote continuous enhancement using the most recently available data. The framework, shown in Figure 2 has three components: (1) epidemiology (orange), (2) production and supply (blue), and (3) access and use (green) that interact with each other. Additional factors to consider for each of these modeling components can be found in Appendix A.
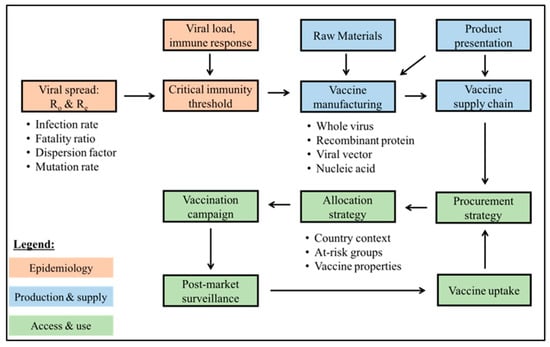
Figure 2.
High-level overview of key components contributing to sustained, population-wide immunity against SARS-CoV-2.
3. Results
3.1. Epidemiology
Understanding the spread of viral transmission is critical to design and evaluate the impact of public health interventions. Typically, viral transmission is modeled using a compartmentalized approach that labels each person as part of one of the following groups: susceptible (S), infected (X), or recovered (R), where the total population is n = S(t) + X(t) + R(t) [12]. Differential equations can be used to quantify the transitions from one state to the other for a given unit time. The R0 or reproduction number is a term that indicates the contagiousness of an infectious disease within a population that has not previously been infected or vaccinated. A similar value, the effective reproduction number (Re), reflects the variation in transmission intensity over time while the dispersion factor (k) describes the extent to which an infection clusters and thus the impact of individual variation in infectiousness on disease emergence [13].
Estimates for the R0 can vary based on the mathematical method used to calculate it, geographic region considered, and period of time covered by available data. The time-dependent reproduction number can be estimated by the following equation: Re = R0S(t). In calculating viral spread, a challenge is accounting for the delay between onset of disease and presentation of symptoms (or diagnosis). Additionally, uncertainty around the impact of viral load on viral transmission and longevity of antibody protection for those naturally infected means that those in the R(t) state may transition back into the S(t) state after recovery and X(t) state if they are re-infected. If SARS-CoV-2 continues to be a risk in the long-term, people may need to receive seasonal or yearly vaccines to boost anti-SARS-CoV-2 antibody levels and maintain population-wide immunity. As a result, sustained manufacturing operations will be required to meet cyclical demand. More work is needed to determine the length of protection conferred by approved vaccines to proactively plan manufacturing and supply operations, as well as vaccination campaigns. Another challenge can arise if a mutated version of the SARS-CoV-2 becomes prevalent and vaccines currently in development fail to provide protection. Analysis of SARS-CoV-2 genome mutations from 28 countries shows correlations between specific genetic variants (e.g., S protein D614G and ORF1ab P4715L) and fatality rates [14]. Closely tracking the extent to which genetic drift of SARS-CoV-2 impacts the transmissibility of the virus and efficacy of vaccines for different strains will be important to plan future operations.
Various modeling tools provide historical estimates and future projections for the Re based on the daily number of reported cases across geographies [15]. This value can help estimate the proportion (P) of the population that needs to be vaccinated to reach population-wide immunity using the equation: P = 1 − (1/R0). In a review of 14 published studies, the value for R0 ranged from 1.4 to 6.49, with a mean of 3.28, a median of 2.79, and 95% CI (2.51, 4.05) [16]. Using this estimate of R0, we calculate the number of anti-SARS-CoV-2 vaccine doses (D) required to meet the critical population coverage P. Several factors need to be defined to estimate D: total population, proportion of the population that is susceptible, vaccine efficacy, and number of doses that need to be administered to confer long-term immunity. Figure 3 estimates the D required to reach P under varying vaccine efficacy levels (ranging from 50 to 90%), R0 values (ranging from 1.5 to 4), and dose regimens (single- or two-dose), assuming an S(t) of 90%, and population of 7.8 billion. Taking the worst case scenario, with a vaccine efficacy of 50%, R0 of 4, and each person needing a booster after initial administration, the estimated D required to reach P is over 22.5 billion. Under the best case scenario, with a vaccine efficacy level of 90% and R0 of 1.5, the estimated D required to reach P is approximately 2.25 billion for a one-dose regimen and 4.5 billion for a two-dose regimen. These estimates provide a scale for the public health need to be met by vaccine manufacturers, regulators, and providers alike. It also provides insights into features of a vaccine that can be optimized during the design and development process to reduce the estimated D.
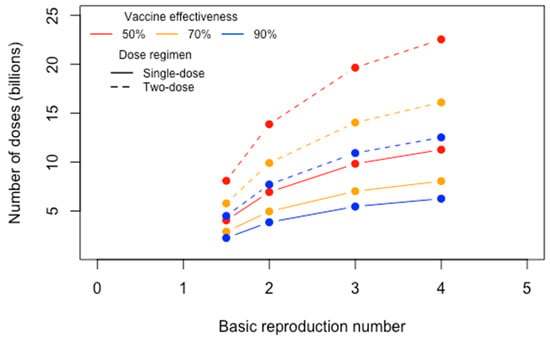
Figure 3.
Product volume (number of vaccines doses) needed to reach coverage necessary for sustained, population-wide immunity under various scenarios (e.g., rate of viral spread, vaccine efficacy, dose regimen).
3.2. Production and Supply
Estimates for the D required to reach P highlight the unique challenge associated with scaling production and effectively supplying vaccines in a compressed timeline. There are numerous vaccine candidates being explored, both based on established platforms as well as novel technologies. Each brings unique opportunities and challenges to the goal of designing safe, effective vaccines for widespread use across geographies. Four primary platforms are: whole virus, recombinant protein-based/subunit, viral vector, and nucleic acid vaccines. Figure 4 compares these vaccine platforms across key preferred product characteristics (PPCs), optimal product properties that can help meet strategic public health goals and subsequently inform more specific target product profiles. For example, low dose size, high production yield, temperature stability, and easy injection, among other factors, are ideal for global delivery. While PPCs are informative to compare vaccine platforms to the ideal product design, data from ongoing clinical trials will be crucial to better define the specific characteristics across different anti-SARS-CoV-2 vaccines. Whole virus and protein-based vaccines are considered safer as they have a long history as regulated and commercially marketed products. Live-attenuated vaccines most effectively induce a strong immune response, while other vaccine platforms rely on various adjuvants and product-specific properties to tune immunogenicity. All vaccine platforms, expect live-attenuated vaccines and some inactivated vaccines, have a high dose regimen as they usually require multiple doses for long-term immunity. The use of replication-defective viruses, both as antigens in vaccines and as vectors for transporting them, is ideal to reduce the risk of reverting to a virulent form after successive mutations that can occur when the virus replicates [17]. Despite the relative novelty of viral vector and nucleic acid vaccine platforms, they may be faster to scale-up manufacturing compared to traditional platforms. Nucleic acid vaccines, for example, can made through catalytic chemical reactions rather than the typical, resource-intensive production in eggs or mammalian cells. Of the 200+ programs under development, whole virus vaccines are the least frequent while protein-based vaccines are most common [18].
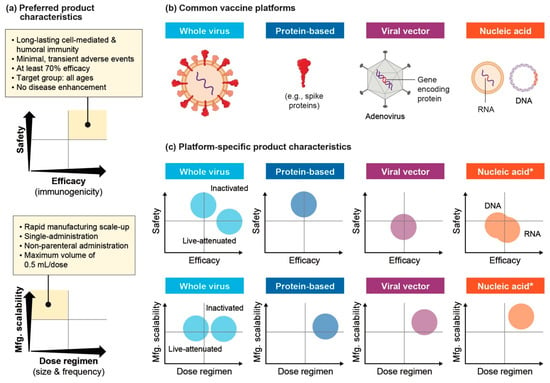
Figure 4.
(a) Preferred product characteristics for rapid manufacturing scale-up and global distribution of vaccines against SARS-CoV-2 based on WHO guidelines [19]; (b) Biologic structure of common vaccine platforms; (c) Relative performance of different product characteristics across primary vaccine platforms. * No approved products, so it is, therefore, difficult to assert characteristics from limited real-world evidence.
Regardless of the platform utilized, the vaccine development timeline from clinical trials through patient access is traditionally five years or longer. Given the current situation, the shared aim among manufacturers, governments, and other stakeholders is to work towards delivering vaccines within an expedited timeline of 12–18 months from when COVID-19 was declared a pandemic. This translates to an ambitious launch of prospective vaccines between Q4 of 2020 and Q2 of 2021. A significant challenge is the need for product and process development, clinical testing, and large-scale manufacturing to move forward in parallel rather than in sequence. This non-traditional approach to vaccine development introduces significant tradeoffs as decisions that would have been delayed until data becomes available are now being made at-risk.
The potential for significantly reducing the timeline for vaccine delivery can be attributed to a few factors [20]: (i) pre-existing work to understand coronaviruses such as SARS and MERS, whose genomic and structural similarity to SARS-CoV-2 can help shorten discovery of vaccines and animal studies; (ii) re-prioritization of efforts and investments to support vaccine development (especially from governments, foundations, and the biotechnology industry); (iii) risk-taking by expediting the start of clinical phases despite limited knowledge about SARS-CoV-2 and simultaneously investing in large scale production capacity before safety and efficacy results are available; (iv) innovative trial designs; (v) using emergency provisions to expedite regulatory review; (vi) diversifying the portfolio of vaccine candidates, including novel platforms that have no regulatory precedence but may be faster to produce than traditional vaccines; (vii) increased levels of collaboration across stakeholders (e.g., government, industry, academia, foundations, etc.) to better understand the virus, develop the necessary production capacity, and make vaccines. To adequately demonstrate the effects of a vaccine, an adequate number of clinical trial volunteers are needed and clinical trial sites with sufficient transmission rates need to be selected. Innovative clinical trial designs have been suggested, such as the NIH-launched COVID-19 Prevention Trials Network, which allows comparison of protection across multiple vaccine trials in 100+ sites across the globe [21].
The production of vaccines from raw materials, to drug substance, and eventual drug product is a time- and resource-intensive process, with each vaccine platform adopting unique manufacturing processes. Raw materials need to be qualified and in adequate supply to avoid bottlenecks in manufacturing. The risk of supply interruptions increases when raw materials are sourced from one country that may prioritize national use over exportation or when manufacturing equipment needs to be customized to specific manufacturing processes. Building in redundancy by having multiple qualified vendors from whom materials can be purchased can abate some of this risk to avoid delays in the production and eventual distribution of vaccines. Scale-up of raw materials supply chains for COVID-19 vaccines, especially critical components such as glass for vials and single-dose syringes, rubber stoppers, plastic bags for single-use systems, nucleotides, lipids, resins, filters, media, and freezer systems, among others, are a prerequisite to continuous, large scale manufacturing [22]. There have been a growing number of bilateral agreements made between governments and vendors to secure raw materials in anticipation of a successful vaccine candidate. In all cases, demonstrating quality is essential to avoid variability in raw material properties that may eventually lead to deviations from a vaccine’s target product profile.
The production volume required to manufacture D can be estimated based on the expected dose size of the vaccine. Ongoing clinical trials play an important role in validating the ideal dose size that elicits the desired immunogenic response. Another important consideration is the ability to rapidly scale up manufacturing to produce billions of doses in the shortest timeframe. Bioprocess modeling can be used to determine the product volume per batch and per unit time based on a range of factors (e.g., cell line productivity, run duration, time between runs, batch success rate, production yield, titer, bioreactor number, volumetric capacity). Under normal conditions, there is a long lead time required to design, build, and validate new facilities that have unique production processes in place. In pandemic response, however, vaccine developers are exploring a combination of alternative options to rapidly secure the adequate manufacturing capacity to meet D: (i) retrofitting existing, unused facilities, (ii) optimizing scheduling in existing facilities, (iii) outsourcing less time-critical products to contract manufacturing organizations, (iv) scaling-out by initiating production in multiple sites in parallel, and (v) collaborating with foundations and governments to rapidly develop new capacity. Each of these has tradeoffs around cost, time to market, and risk that need to be considered before investments are made.
Formulation of the finished product should also be designed in such a way that enables easy distribution, as well as efficient delivery at the point-of-care. An important choice is the drug presentation, specifically whether the finished product is a liquid or lyophilized freeze-dried powder. For liquid formulation, single-dose or multi-dose containers can be filled with the drug product and sealed with sterile stoppers. Alternatively, single-dose glass syringes can be pre-filled ahead of distribution. In most cases, liquid formulation requires a cold chain that keeps products at a low temperature in order to maintain potency during transportation and storage. Lyophilization provides thermal stability and can help extend shelf-life, however, there is an elevated risk of deviations in product quality and efficacy when transitioning between liquid and solid states. For pandemic response, to reduce supply chain footprint and as a result of anticipated shortages in filling capacity, multi-dose vials are the preferred option although they may require an effective bacteriostatic agent to reduce risks of contamination that could lead to wastage [23]. Ultimately, the choice of formulation will have substantial impact on the downstream product supply chain and performance of immunization programs. More evidence is needed on the impact of formulation choice, for example dose per vaccine, on factors such as operational and product costs, timely coverage, and safety [24].
Another important consideration is maintaining integrity of the supply chain by preventing counterfeit (falsified) medicines, including both finished products (e.g., 2012 case of bevacizumab) or individual raw materials and active ingredients (e.g., 2008 case of heparin). The complex, multi-stage, and often opaque pharmaceutical supply chain provides ample opportunities for illicit activities and makes it difficult to effectively track-and-trace the source of problems. In response to the growing threat of falsified medicines, the WHO launched the Global Surveillance and Monitoring System in 2013 to encourage governments to more systematically report incidents in a common, structured manner. Additionally, various methods have been developed to detect falsified medicines: analytical chemistry and spectroscopy technologies (e.g., detecting chemical signatures of ingredients), as well as digital anti-counterfeit technologies (e.g., package-level barcodes and radio frequency identification). Reports of cyberattacks against the COVID-19 vaccine distribution chain, especially cold-chain companies, as well as online fraud linked to the marketing of fake products are emerging as vaccines start to receive regulatory authorization [25].
The large manufacturing volume required to meet public health needs continues to be a rate-limiting step, meaning that typical operations may need to be displaced for some time. Looking forward, an important consideration is how manufacturing decisions impact the production timelines of other products, as well as strategically anticipating how investments made as part of a pandemic response can promote agility, business continuity, and proactive manufacturing systems in the future to better respond to unmet needs and new threats. Manufacturing and logistic models play an important role in promoting effective operations, especially considering the production scale, complex temperature requirements of most vaccines, and the impact product presentation can have on downstream transportation, storage, and delivery. Accounting for risks across the product value-chain is crucial to anticipate the time to reach the product volume D under different scenarios.
3.3. Access and Use
Closing the mismatch between supply and demand requires dynamic, responsive systems that integrate information around production, storage, transportation, and procurement. To make vaccines globally and equitably accessible, especially in initial periods of scarcity, clear allocation frameworks are also needed. A key performance indicator of vaccine supply chains is the continuous and uninterrupted availability of affordable products at the point of administration, especially when a large proportion of the population needs to be vaccinated. However, shortages or stockouts continue to jeopardize the delivery of many vaccines. In most cases, stockouts can be attributed to country-specific factors such as delays in government funding that restrict procurement, poor forecasting, and inefficient stock management. Understanding the root-cause of these stockouts can help anticipate potential barriers in the timely delivery of sufficient vaccine doses against SARS-CoV-2 when they become available. The primary objective of modeling efforts around the procurement and allocation of vaccines is determining the optimal strategy that will help reach P in the shortest time to meet public health goals.
In June, the WHO released a preliminary vaccine allocation framework to inform distribution within and between countries based on epidemiological and other country-specific factors [26]. Such a framework aims to identify priority populations and inform the optimal use of scarce resources with the goal of reducing mortality and protecting health systems. Data can be used to further highlight geographies where the virus is most prevalent and health systems are most vulnerable. The initial allocation of products can then be fine-tuned based on the unique product characteristics of vaccines that become available over time, as they may only be suitable for certain sub-populations and geographies. Continuous learning based on available data is crucial to proactively and effectively plan vaccine operations across the value-chain.
An ideal global access program would allow all countries to engage in a shared mechanism for the procurement of products in a way that pools risks for both the manufacturers and governments. Such a system has been established in the form of an advanced market commitment (AMC) for vaccines, where investments from governments incentivize manufacturers to establish the necessary manufacturing capacity and produce substantial volumes of vaccines [27]. This AMC, named ‘COVAX’, is co-led by Gavi (The Vaccine Alliance), the Coalition for Epidemic Preparedness Innovations (CEPI) and WHO, as part of a broader effort called Access to COVID-19 Tools (ACT) Accelerator. The financing framework provides volume guarantees to specific manufacturers for vaccine candidates before they are licensed. Since governments are likely able to substantially invest in only one or a few vaccine programs, each of which has an average success rate of 7% while in the pre-clinical phase (compared to 17% in Phase I, 21% in Phase II, and 67% in Phase III trials), the ability to invest in a portfolio of vaccine candidates provides insurance against the risk of failures [28]. Although there has been extensive support for the AMC, there are a growing number of bilateral purchasing agreements between a single government (e.g., Operation Warp Speed in the US) or regional group (e.g., European Union) and manufacturers, leading to a risk of vaccine monopolies or a hoarding of vaccine supply in ways that delay access for low- and middle-income countries [29]. As of December 2020, high-income countries held 3.9 billion doses and middle-income countries held 2.7 billion doses from confirmed bilateral agreements, while low-income countries continue to be entirely reliant on doses provided by COVAX [30].
Figure 5a shows how various data-driven modeling tools can help evaluate the impact of different vaccine strategies based on production, procurement, allocation, and distribution behaviors of vaccines. For example, tools can be developed to stratify a population into risk groups based on various features (e.g., age, comorbidities, etc.). An assignment algorithm could then be built to allocate the most optimal vaccines based on country characteristics and product properties to the appropriate population sub-group.
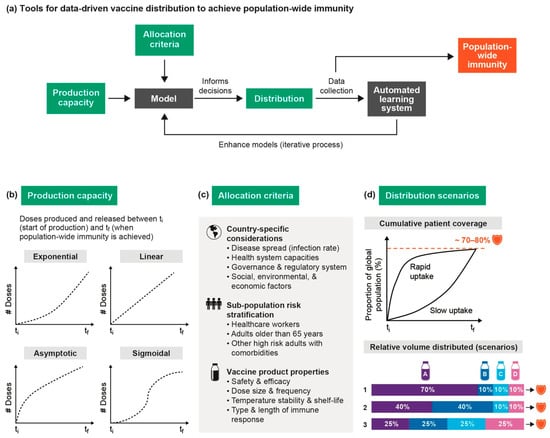
Figure 5.
Detailed schematic for the potential flow of vaccines under various production, procurement and allocation strategies. (a) Overall framework demonstrating how data-driven tools can be used to evaluate the impact of different scenarios on vaccine distribution and the time needed to reach population-wide immunity; (b) Modeling the supply behavior for different vaccine products; (c) Criteria to inform allocation of vaccines between and within countries; (d) Demonstrating the impact of vaccine uptake on reaching critical population coverage and how various distribution scenarios can lead to population-wide immunity.
Integrating real-world data into an automated learning system is particularly important to continually enhance models and inform decisions using evidence generated over time. While safety and efficacy will need to be demonstrated prior to regulatory approval, regardless of the vaccine, there is always some risk of an acceptable level of adverse events. Therefore, safety and efficacy across diverse populations needs to be demonstrated as large-scale vaccination campaigns begin. Adverse events following immunization can be categorized based on the severity (mild, moderate, and serious), the type of reaction they elicit (product-related reaction, quality defect-related reaction, immunization error-related reaction, immunization anxiety-related reaction, coincidental event), and frequency [31]. To better understand the risks posed by a vaccine, differentiating coincidental events from those caused by a reaction to a vaccine can be done by comparing the background rate (determined prior to vaccination or simultaneously in non-vaccinated people) with the observed rate of an event.
Vaccination at the point-of-care also requires special attention. Depending on the vaccine presentation, the product may need to be stored at a specific temperature to maintain high quality throughout its shelf-life. The route of administration, the path by which a vaccine is brought into contact with the body, is particularly important since it may affect the flow of patients and skills needed by healthcare professionals to effectively deliver the vaccine. Parenteral dosage forms, the most traditional among vaccines, are administered as a needle-based injection in one of three following ways: intramuscular (especially common for vaccines containing adjuvants to avoid adverse effects), subcutaneous (between the muscle and skin), and intradermal. Most vaccines against SARS-CoV-2 have been designed for intramuscular injection. Non-parenteral vaccines can be administered orally, via intranasal spray, or through the skin using pressure by gas or electrophoresis, thus eliminating the need for needles and incentivizing great patient compliance due to self-administration [32]. These delivery routes are appealing as mucosal surfaces can induce both mucosal antibodies (IgA) and cell-mediated immune responses, while still producing a systemic antibody response (IgG), though they are less efficacious and thus seldom used [33].
4. Scenario Planning and Discussion
Once various analytical tools have been developed for different components of the modeling framework, the next step is defining realistic scenarios of vaccine production and distribution, as well as better evaluating their economic implications and public health impact. Various choices across the development, manufacture, supply, and distribution of vaccines can have substantial impact on cost, availability, affordability, equity, and public health outcomes. An important dimension to account for is how the considerations outlined above may evolve over time and change the ability to optimally manufacture, supply, and distribute vaccines.
The dynamic nature of virus propagation highlights the importance of stochastic rather than deterministic models. Figure 5b demonstrates potential variations in the cumulative supply of vaccines between the time of initial production (ti) until sustained population-wide immunity is reached (tf). Various factors can influence the number of doses made available for supply per unit time, for example cell-line productivity and biomanufacturing capacity. A linear trend indicates no variation in the rate at which doses are produced and supplied over time. Despite the relative novelty of viral vector and nucleic acid vaccine platforms, they may be faster to scale-up manufacturing compared to traditional platforms and so could follow an exponential supply curve. In the case of anti-SARS-CoV-2 vaccines, manufacturing capacity is constrained by available facility space and the rate of production depends on the vaccine platform used. Additionally, some raw materials may become scarce during the production process, creating a bottleneck.
Based on the available supply of vaccines, Figure 5c outlines different layers of information that can be considered when designing an allocation algorithm that can optimize the distribution of vaccines. The WHO’s allocation framework identifies three priority groups: healthcare workers, adults who are older than 65 years, and other high-risk adults (with comorbidities), accounting for 1%, 8%, and 15% of the global population, respectively. Initially, if only one vaccine is available then the allocation is based on country considerations and priority groups. However, as more vaccines become available, especially on safety and efficacy across different demographic groups, product properties can be matched with features of population clusters in such a way that will lead to greatest immunity.
Figure 5d shows how the same portfolio of approved vaccines (i.e., A, B, C, D) can lead to population-wide coverage in different times depending on the uptake of vaccines. Many elements will impact whether the uptake of vaccines is slow or rapid, with great variation across sub-groups of the population. These include the initial vaccine cost, support from national regulatory authorities, vaccination campaigns and schedules, and public perceptions, among others [34]. Even if safety and efficacy are not compromised by the expedited nature at which vaccines are expected to enter the market, misconceptions can lead to lower rates of vaccination that jeopardize the ability to reach population-wide immunity. In a survey on vaccine hesitancy conducted in 19 countries, 71.5% of participants reported that they would likely to take a COVID-19 vaccine, while 61.4% reported that they would accept their employer’s recommendation to do so [35].
There are numerous scenarios under which a portfolio of approved vaccines can meet sustained population-wide immunity. In each scenario, the relative volumetric contribution of different vaccine products to the overall global supply can differ. Assuming a portfolio of four approved vaccines, scenario 1 demonstrates a monopoly-like market where one manufacturer is able to fulfill 70% of the required supply while the three other entities fill the remaining gap. In scenario 2, two vaccines share a majority of the market, while all four vaccines are equally distributed in scenario 3. Quantitative simulations can be valuable to compare these divergent scenarios, as well as better anticipate their implication on various performance metrics (e.g., equity, cost, time to first dose, time to last dose).
The ability to simulate various scenarios can also help proactively point to areas that could jeopardize the ability to make vaccines globally accessible. In turn, this can be used to direct investments and innovations. How a vaccine is designed and where it is introduced could significantly influence its efficacy. For example, live-attenuated vaccines most effectively induce a strong immune response with one dose, so may be more efficient for older populations and other groups who would be difficult to reach for follow-on boosters. Additionally, since production timelines for vaccines will not be aligned, not all vaccines will be equally available, as the rate of replenishment after initial vaccine allocation may differ. In some cases, interruption in the raw material supply chain or a manufacturing issue may limit production for some time, thus making only a few vaccine options available for distribution. Accounting for the dynamic nature of the complex considerations outlined will be important to better understand underlying risks and tradeoffs across different scenarios in order to effectively inform decisions.
5. Conclusions
The modeling framework and considerations outlined in this paper serve as a guide to not only respond to the current health emergency but also proactively anticipate and prepare for emerging and future (still unknown) pandemics. Gaining foresight into the potential consequences of different investments can be valuable to navigate an increasingly complex environment with numerous interconnected time-critical decisions and uncertainties. To accomplish this, innovations are needed across the biopharmaceutical value chain. Decision-making processes, while involving actors from multiple sectors with similar end-goals, will need to overcome misalignments to promote pre-competitive activities that are focused on maximizing public health impact. Ensuring access to SARS-CoV-2 vaccines, as well as complementary diagnostics and treatments, is an imperative. Modeling frameworks support risk-based systems analysis to inform decisions, anticipate challenges, evaluate risks, and shorten timeframes towards equitable access of safe, effective vaccines. Moreover, the framework presented can provide knowledge and information flows across traditionally siloed parts of vaccine strategies, including data on epidemiology, production, supply, procurement, distribution, access, and use.
Author Contributions
D.G., A.J.S. and S.L.S. contributed equally to the preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the MIT-IBM Watson AI Lab.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the interviewees for generously providing their time to inform the development of this paper, the MIT CBI team for their valuable review of the manuscript, and Betsy Skrip for illustrating the figures.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Factors to consider when modeling different components of the framework.
Table A1.
Factors to consider when modeling different components of the framework.
| Component | Important Values to Model | Factors to Consider |
|---|---|---|
| Epidemiology | Reproduction number (R0, Re) | Size of the total and susceptible population, number of confirmed cases, duration of contagiousness after infection, likelihood of transmission, contact rate, public health interventions. |
| Dispersion factor (k) | Reproduction number, contact tracing and transmission chain data. | |
| Population coverage (P) | Time-dependent reproduction number. | |
| Doses (D) | Population coverage, vaccine efficacy, number of administrations per person. | |
| Production and supply | Production volume per batch | Number of bioreactors, bioreactor volume, titer, yield. |
| Total volume to reach P | Doses, dose size. | |
| Time to manufacture D | Production volume per batch, total volume to reach P, run time, batch success rate. | |
| Time to reach supplier | Mode of transportation, cold chain and storage requirements, vaccine presentation, customs, environmental conditions. | |
| Access and use | Procurement | Financing, contractual agreements. |
| Allocation | Country-specific factors, risk thresholds within populations, vaccine properties (e.g., safety and efficacy profile). | |
| Uptake | Review from regulatory authorities, public perceptions, cost, vaccination campaigns. |
References
- Schröder, I. COVID-19: A Risk Assessment Perspective. J. Chem. Health Saf. 2020, 27, 160–169. [Google Scholar] [CrossRef]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; Khalili, S.A.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef]
- Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering. Available online: https://coronavirus.jhu.edu/map.html (accessed on 15 December 2020).
- Siegrist, C.-A. Vaccine immunology. In Vaccines, 6th ed.; Elsevier Saunders: Philadelphia, USA, 2013; pp. 14–32. [Google Scholar]
- Sim, S.Y.; Watts, E.; Constenla, D.; Brenzel, L.; Patenaude, B.N. Return on Investment from Immunization against 10 Pathogens In 94 Low- and Middle-Income Countries, 2011–2030. Health Aff. 2020, 39, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Mullard, A. COVID-19 vaccine development pipeline gears up. Lancet 2020, 395, 1751–1752. [Google Scholar] [CrossRef]
- The New York Times. Coronavirus Vaccine Tracker. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed on 18 December 2020).
- U.S. Food & Drug Administration. Coronavirus (COVID-19) Update: FDA Takes Action to Help Facilitate Timely Development of Safe, Effective COVID-19 Vaccines. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-action-help-facilitate-timely-development-safe-effective-covid (accessed on 1 November 2020).
- De Savigny, D.; Adam, T. (Eds.) Systems Thinking for Health Systems Strengthening; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Adamu, A.A.; Jalo, R.I.; Habonimana, D.; Wiysonge, C.S. COVID-19 and routine childhood immunization in Africa: Leveraging systems thinking and implementation science to improve immunization system performance. Int. J. Inf. Dis. 2020, 68, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lu, P.E.; Chang, C.S.; Liu, T.H. A Time-dependent SIR model for COVID-19 with Undetectable Infected Persons. 2020. Available online: https://arxiv.org/abs/2003.00122 (accessed on 18 December 2020).
- Kupferschmidt, K. Why do some COVID-19 patients infect many others, whereas most don’t spread the virus at all? Science 2020. [Google Scholar] [CrossRef]
- Toyoshima, Y.; Nemoto, K.; Matsumoto, S.; Nakamura, Y.; Kiyotani, K. SARS-CoV-2 genomic variations associated with mortality rate of COVID-19. J. Hum. Genet. 2020, 65, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.; Hellewell, J.; Thompson, R.N.; Sherratt, K.; Gibbs, H.P.; Bosse, N.I.; Munday, J.D.; Meakin, S.; Doughty, E.L.; Chun, J.Y.; et al. Estimating the time-varying reproduction number of SARS-CoV-2 using national and subnational case counts [version 1; peer review: Awaiting peer review]. Wellcome Open Res. 2020, 5, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Gayle, A.A.; Wilder-Smith, A.; Rocklöv, J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Travel Med. 2020, 27, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Dudek, T.; Knipe, D.M. Replication-defective viruses as vaccines and vaccine vectors. Virology 2006, 344, 230–239. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines, 3 November 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 1 November 2020).
- World Health Organization. WHO Target Product Profiles for COVID-19 Vaccines, 9 April 2020. Available online: https://www.who.int/blueprint/priority-diseases/key-action/WHO_Target_Product_Profiles_for_COVID-19_web.pdf (accessed on 1 November 2020).
- The New York Times. How Long Will a Vaccine Really Take? Available online: https://www.nytimes.com/interactive/2020/04/30/opinion/coronavirus-covid-vaccine.html (accessed on 1 November 2020).
- U.S. Department of Health and Human Services. National Institutes of Health: NIH Launches Clinical Trials Network to Test COVID-19 Vaccines and Other Prevention Tools. Available online: https://www.nih.gov/news-events/news-releases/nih-launches-clinical-trials-network-test-covid-19-vaccines-other-prevention-tools (accessed on 1 November 2020).
- Vox Recode. How Quickly Can the US Distribute a Covid-19 Vaccine? Here Are the Four Biggest Logistical Challenges. Available online: https://www.vox.com/recode/22151473/vaccine-covid-19-pfizer-glass-syringes-needles-freezers (accessed on 15 December 2020).
- Lee, B.Y.; Norman, B.A.; Assi, T.M.; Chen, S.I.; Bailey, R.R.; Rajgopal, J.; Brown, S.T.; Wiringa, A.E.; Burke, D.S. Single versus multi-dose vaccine vials: An economic computational model. Vaccine 2010, 28, 5292–5300. [Google Scholar] [CrossRef] [PubMed]
- Heaton, A.; Krudwig, K.; Lorenson, T.; Burgess, C.; Cunningham, A.; Steinglass, R. Doses per vaccine vial container: An understated and underestimated driver of performance that needs more evidence. Vaccine 2017, 35, 2272–2278. [Google Scholar] [CrossRef] [PubMed]
- International Criminal Police Organization. INTERPOL Warns of Organized Crime Threat to COVID-19 Vaccines. Available online: https://www.interpol.int/en/News-and-Events/News/2020/INTERPOL-warns-of-organized-crime-threat-to-COVID-19-vaccines (accessed on 15 December 2020).
- World Health Organization. A Global Framework to Ensure Equitable and Fair Allocation of COVID-19 Products: And Potential Implications for COVID-19 Vaccines. Available online: https://www.who.int/docs/default-source/coronaviruse/who-covid19-vaccine-allocation-final-working-version-9sept.pdf?sfvrsn=e1d0376_6&download=true (accessed on 1 November 2020).
- Gavi (The Vaccine Alliance). The GAVI COVAX AMC: An Investment Opportunity. Available online: https://www.gavi.org/sites/default/files/2020-06/Gavi-COVAX-AMC-IO.pdf (accessed on 1 November 2020).
- Pronker, E.S.; Pronker, T.C.; Commandeur, H.; Claassen, E.H.; Osterhaus, A.D. Risk in vaccine research and development quantified. PLoS ONE 2013, 8, e57755. [Google Scholar] [CrossRef] [PubMed]
- Third World Network. COVID-19 Vaccines: EU Prioritises Preferential Access, Paying Lip-Service to Global Solidarity. Available online: https://twn.my/title2/intellectual_property/info.service/2020/ip200603.htm (accessed on 1 November 2020).
- Duke Global Health Innovation Center. Mapping COVID-19 Vaccine Pre-Purchase across the Globe. Available online: https://launchandscalefaster.org/COVID-19 (accessed on 18 December 2020).
- Causality Assessment of an Adverse Event Following Immunization (AEFI): User Manual for the Revised WHO AEFI Causality Assessment Classification, 2nd ed.; World Health Organization: Geneva, Switzerland, 2020.
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J. Vaccine hesitancy: An overview. Hum. Vaccin. Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.; Zhang, X.; Qian, F. Intranasal and oral vaccination with protein-based antigens: Advantages, challenges and formulation strategies. Protein Cell 2015, 6, 480–503. [Google Scholar] [CrossRef] [PubMed]
- Determann, D.; Korfage, I.J.; Lambooij, M.S.; Bliemer, M.; Richardus, J.H.; Steyerberg, E.W.; de Bekker-Grob, E.W. Acceptance of vaccinations in pandemic outbreaks: A discrete choice experiment. PLoS ONE 2014, 9, e102505. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A global survey of potential acceptance of a COVID-19 vaccine. Nat. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).