Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like BPH/5 Mouse Prior to and During Pregnancy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Gene Expression Analysis
2.3. Protein Analysis
2.4. Statistical Analysis
3. Results
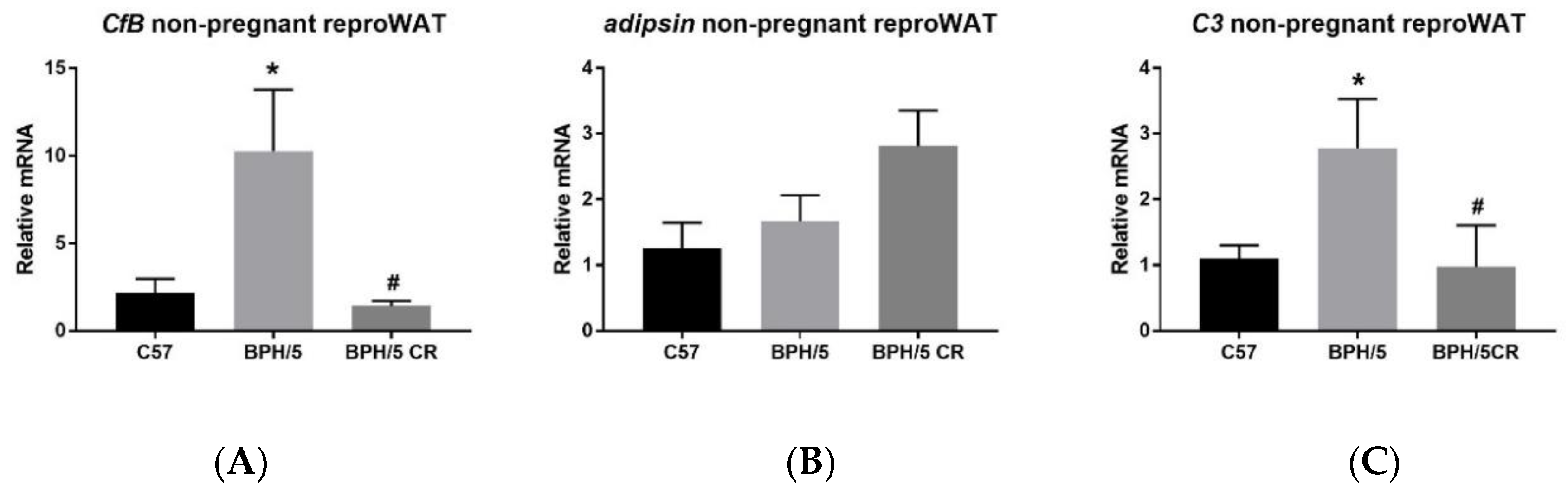
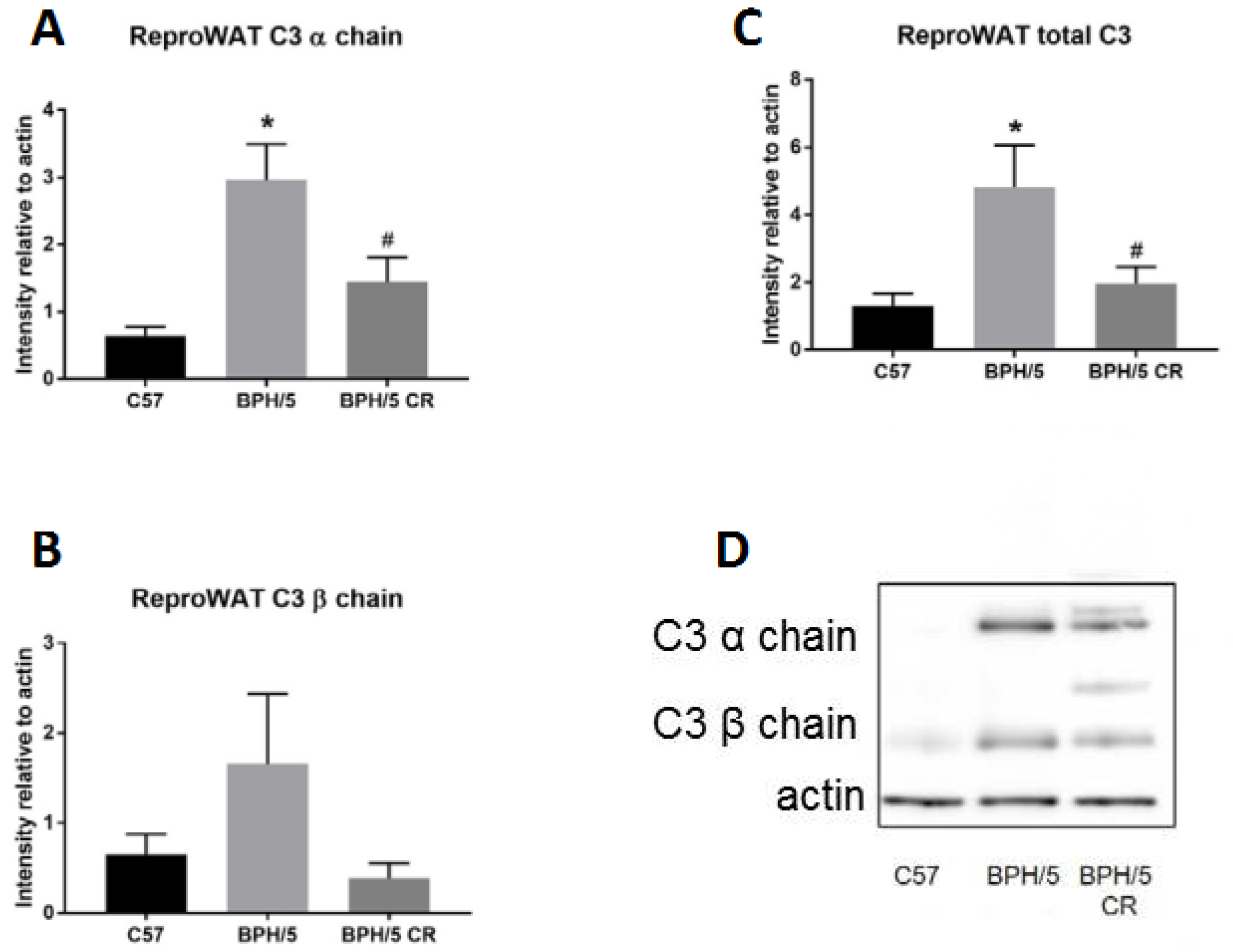
3.1. Complement Factor 3 mRNA is Upregulated in the Reproductive WAT of BPH/5 Females Prior to Pregnancy
3.2. Complement Factor 3 Dysregulation in Reproductive WAT Observed in Early BPH/5 Pregnancy and Can Be Attenuated by Reducing Adiposity
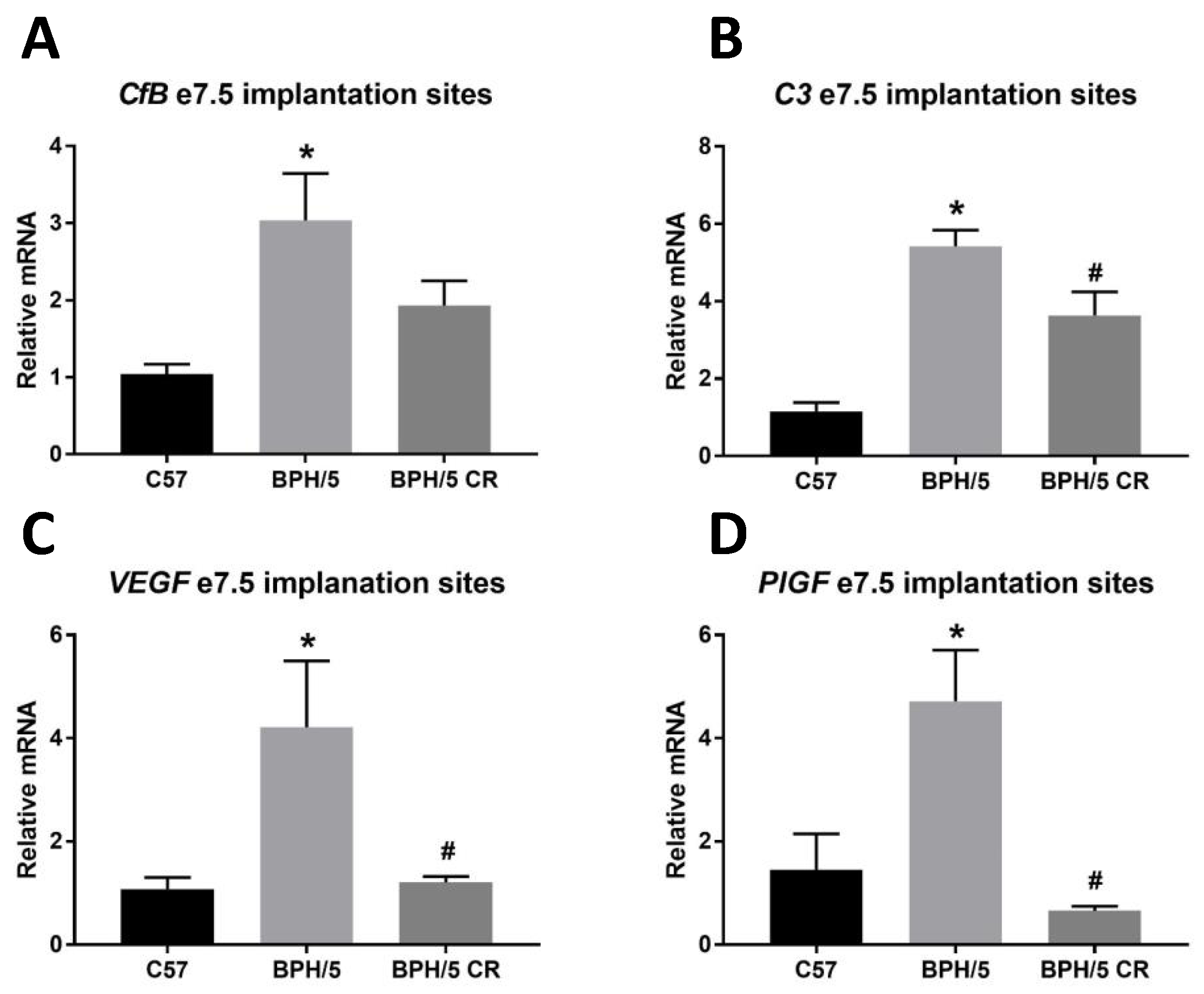
3.3. A Reduction in BPH/5 Maternal Adiposity Lowers C3 and Restores Angiogenic Balance at the Maternal–Fetal Interface in e7.5 BPH/5 Implantation Sites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef] [PubMed]
- Sones, J.L.; Cha, J.; Woods, A.K.; Bartos, A.; Heyward, C.Y.; Lob, H.E.; Isroff, C.E.; Butler, S.D.; Shapiro, S.E.; Dey, S.K.; et al. Decidual Cox2 inhibition improves fetal and maternal outcomes in a preeclampsia-like mouse model. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, K.N.; Redman, L.M.; Sones, J.L. Obesity “complements” preeclampsia. Physiol. Genom. 2019, 51, 73–76. [Google Scholar] [CrossRef]
- Lynch, A.; Eckel, R.H.; Murphy, J.R.; Gibbs, R.S.; West, N.A.; Giclas, P.C.; Salmon, J.E.; Holers, V.M. Prepregnancy obesity and complement system activation in early pregnancy and the subsequent development of preeclampsia. Am. J. Obstet. Gynecol. 2012, 206, 428.e1–428.e8. [Google Scholar] [CrossRef]
- Ji, L.; Brkić, J.; Liu, M.; Fu, G.; Peng, C.; Wang, Y.-L. Placental trophoblast cell differentiation: Physiological regulation and pathological relevance to preeclampsia. Mol. Asp. Med. 2013, 34, 981–1023. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.C.; Sharkey, A.M.; Charnock-Jones, D.S.; Palmer, C.R.; Smith, S.K. VEGF mRNA levels in placentae from pregnancies complicated by pre-eclampsia. BJOG Int. J. Obstet. Gynaecol. 1996, 103, 1191–1196. [Google Scholar] [CrossRef]
- Sones, J.L.; Merriam, A.A.; Seffens, A.; Brown-Grant, D.-A.; Butler, S.D.; Zhao, A.M.; Xu, X.; Shawber, C.J.; Grenier, J.K.; Douglas, N.C. Angiogenic factor imbalance precedes complement deposition in placentae of the BPH/5 model of preeclampsia. FASEB J. 2018, 32, 2574–2586. [Google Scholar] [CrossRef] [Green Version]
- Teirilä, L.; Heikkinen-Eloranta, J.; Kotimaa, J.; Meri, S.; Lokki, A.I. Regulation of the complement system and immunological tolerance in pregnancy. Semin. Immunol. 2019, 45, 101337. [Google Scholar] [CrossRef]
- Choy, L.N.; Rosen, B.S.; Spiegelman, B.M. Adipsin and an endogenous pathway of complement from adipose cells. J. Biol. Chem. 1992, 267, 12736–12741. [Google Scholar]
- Li, K.; Sacks, S.; Zhou, W. The relative importance of local and systemic complement production in ischaemia, transplantation and other pathologies. Mol. Immunol. 2007, 44, 3866–3874. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, R.; Gao, L.; Wang, Y.; Song, C.; Gong, Y.; Jia, J.; Xiong, W.; Dai, L.; Zhang, L.; et al. Elevation of Urinary Adipsin in Preeclampsia. Hypertension 2014, 64, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Davisson, R.L.; Hoffmann, D.S.; Butz, G.M.; Aldape, G.; Schlager, G.; Merrill, D.C.; Sethi, S.; Weiss, R.M.; Bates, J.N. Discovery of a Spontaneous Genetic Mouse Model of Preeclampsia. Hypertension 2002, 39, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Reijnders, D.; Olson, K.N.; Liu, C.-C.; Beckers, K.F.; Ghosh, S.; Redman, L.M.; Sones, J.L. Dyslipidemia and the role of adipose tissue in early pregnancy in the BPH/5 mouse model for preeclampsia. Am. J. Physiol. Integr. Comp. Physiol. 2019, 317, R49–R58. [Google Scholar] [CrossRef] [PubMed]
- Gelber, S.E.; Brent, E.; Redecha, P.; Perino, G.; Tomlinson, S.; Davisson, R.L.; Salmon, J.E. Prevention of Defective Placentation and Pregnancy Loss by Blocking Innate Immune Pathways in a Syngeneic Model of Placental Insufficiency. J. Immunol. 2015, 195, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Qing, X.; Redecha, P.B.; Burmeister, M.A.; Tomlinson, S.; D’Agati, V.D.; Davisson, R.L.; Salmon, J.E. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011, 79, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, E.F.; Lob, H.E.; Song, J.; Xia, Y.; Butler, S.D.; Liu, C.-C.; Redman, L.M.; Sones, J.L. Adverse metabolic phenotype of female offspring exposed to preeclampsia in utero: A characterization of the BPH/5 mouse in postnatal life. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R485–R491. [Google Scholar] [CrossRef] [PubMed]
- Del Zoppo, G.J. In stroke, complement will get you nowhere. Nat. Med. 1999, 5, 995–996. [Google Scholar] [CrossRef] [PubMed]
- Lundwall, A.; Wetsel, R.A.; Domdey, H.; Tack, B.F.; Fey, G.H. Structure of murine complement component C3. I. Nucleotide sequence of cloned complementary and genomic DNA coding for the beta chain. J. Biol. Chem. 1984, 259, 13851–13856. [Google Scholar]
- Bokisch, V.A.; Dierich, M.P.; Müller-Eberhard, H.J. Third component of complement (C3): Structural properties in relation to functions. Proc. Natl. Acad. Sci. USA 1975, 72, 1989–1993. [Google Scholar] [CrossRef] [Green Version]
- Langer, H.F.; Chung, K.-J.; Orlova, V.V.; Choi, E.Y.; Kaul, S.; Kruhlak, M.J.; Alatsatianos, M.; DeAngelis, R.A.; Roche, P.A.; Magotti, P.; et al. Complement-mediated inhibition of neovascularization reveals a point of convergence between innate immunity and angiogenesis. Blood 2010, 116, 4395–4403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girardi, G.; Yarilin, D.; Thurman, J.M.; Holers, V.M.; Salmon, J.E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 2006, 203, 2165–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, F.W.-N.; Lee, Y.L.; Wong, P.-C.; Chung, M.-K.; Lee, K.-F.; Yeung, W.S.B. Complement 3 deficiency impairs early pregnancy in mice. Mol. Reprod. Dev. 2009, 76, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Rai, A.; Kambham, N.; Sung, J.F.; Singh, N.; Petitt, M.; Dhal, S.; Agrawal, R.; Sutton, R.E.; Druzin, M.L.; et al. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J. Clin. Investig. 2014, 124, 4941–4952. [Google Scholar] [CrossRef] [Green Version]
- Reijnders, D.; Liu, C.-C.; Xu, X.; Zhao, A.M.; Olson, K.N.; Butler, S.D.; Douglas, N.C.; Sones, J.L. Celecoxib restores angiogenic factor expression at the maternal-fetal interface in the BPH/5 mouse model of preeclampsia. Physiol. Genom. 2018, 50, 385–392. [Google Scholar] [CrossRef]
- Charles, M.A.; Delpierre, C.; Bréant, B. Developmental origin of health and adult diseases (DOHaD): Evolution of a concept over three decades. Med. Sci. 2016, 32, 15–20. [Google Scholar]
- Vesco, K.K.; Karanja, N.; King, J.C.; Gillman, M.W.; Leo, M.C.; Perrin, N.; McEvoy, C.T.; Eckhardt, C.L.; Smith, K.S.; Stevens, V.J. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: A randomized trial. Obesity 2014, 22, 1989–1996. [Google Scholar] [CrossRef]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.W.; Panda, S. Time-restricted feeding prevents besity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2018, 29, 303–319. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olson, K.N.; Reijnders, D.; Gomes, V.C.L.; Hebert, R.C.; Liu, C.-C.; Stephens, J.M.; Redman, L.M.; Douglas, N.C.; Sones, J.L. Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like BPH/5 Mouse Prior to and During Pregnancy. Biology 2020, 9, 304. https://doi.org/10.3390/biology9090304
Olson KN, Reijnders D, Gomes VCL, Hebert RC, Liu C-C, Stephens JM, Redman LM, Douglas NC, Sones JL. Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like BPH/5 Mouse Prior to and During Pregnancy. Biology. 2020; 9(9):304. https://doi.org/10.3390/biology9090304
Chicago/Turabian StyleOlson, Kelsey N., Dorien Reijnders, Viviane C. L. Gomes, R. Caitlin Hebert, Chin-Chi Liu, Jacqueline M. Stephens, Leanne M. Redman, Nataki C. Douglas, and Jennifer L. Sones. 2020. "Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like BPH/5 Mouse Prior to and During Pregnancy" Biology 9, no. 9: 304. https://doi.org/10.3390/biology9090304
APA StyleOlson, K. N., Reijnders, D., Gomes, V. C. L., Hebert, R. C., Liu, C.-C., Stephens, J. M., Redman, L. M., Douglas, N. C., & Sones, J. L. (2020). Complement in Reproductive White Adipose Tissue Characterizes the Obese Preeclamptic-Like BPH/5 Mouse Prior to and During Pregnancy. Biology, 9(9), 304. https://doi.org/10.3390/biology9090304






