Short-Term Effects of Early Menopause on Adiposity, Fatty Acids Profile and Insulin Sensitivity of a Swine Model of Female Obesity
Abstract
:1. Introduction
2. Experimental Section
2.1. Ethics Statement
2.2. Animals and Experimental Procedure
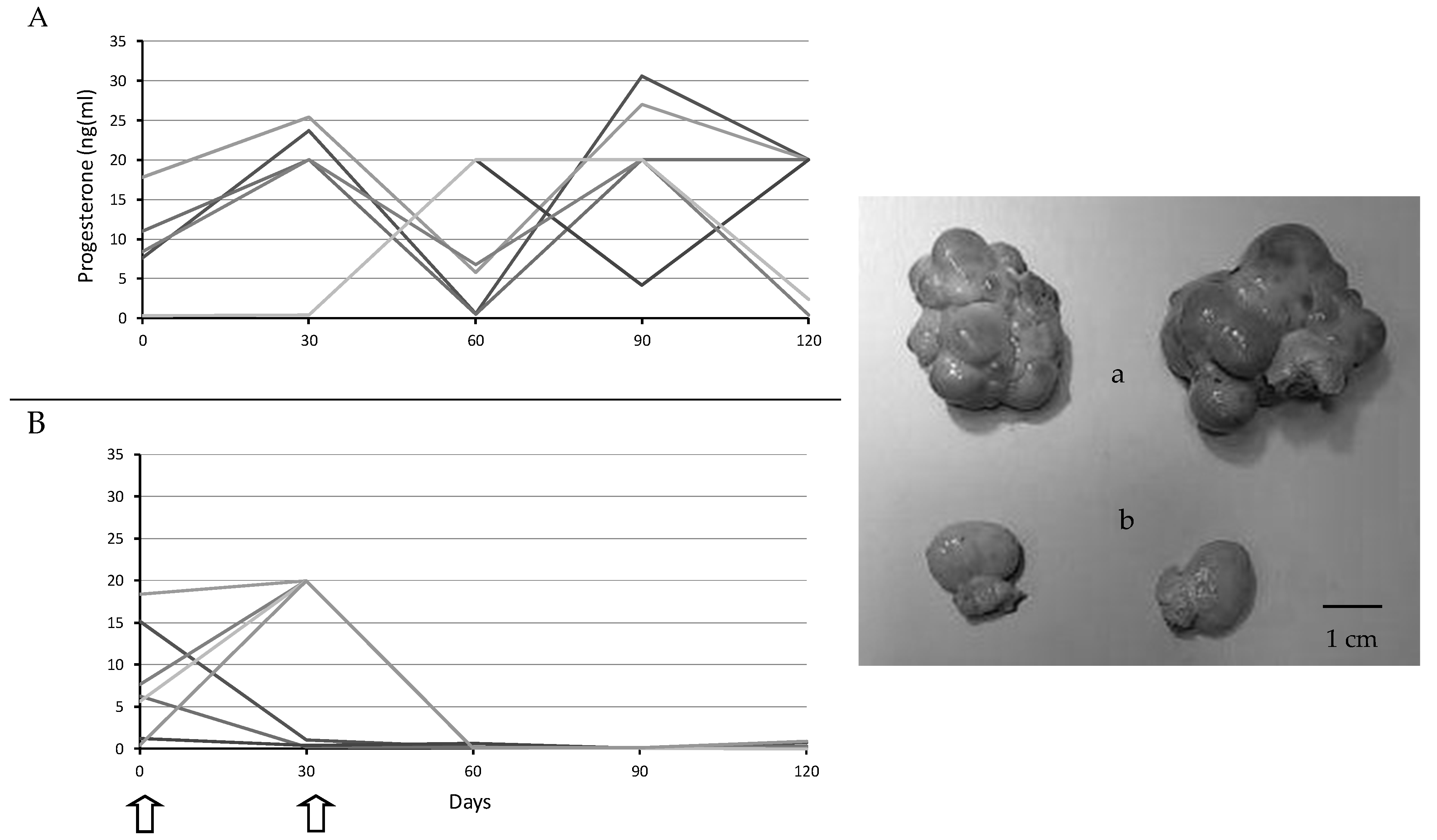
2.3. Assessment of Ovarian Activity
2.4. Evaluation of Metabolic Status
2.5. Evaluation of Fatty Acids Composition
2.6. Statistical Analysis
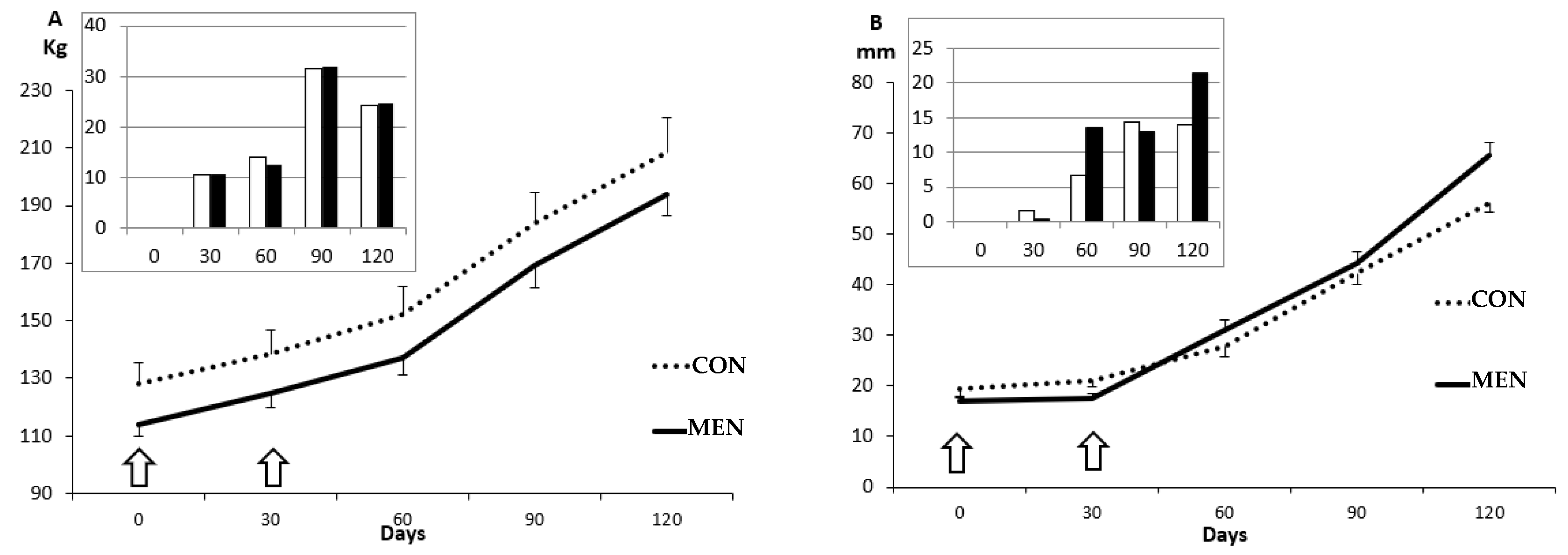
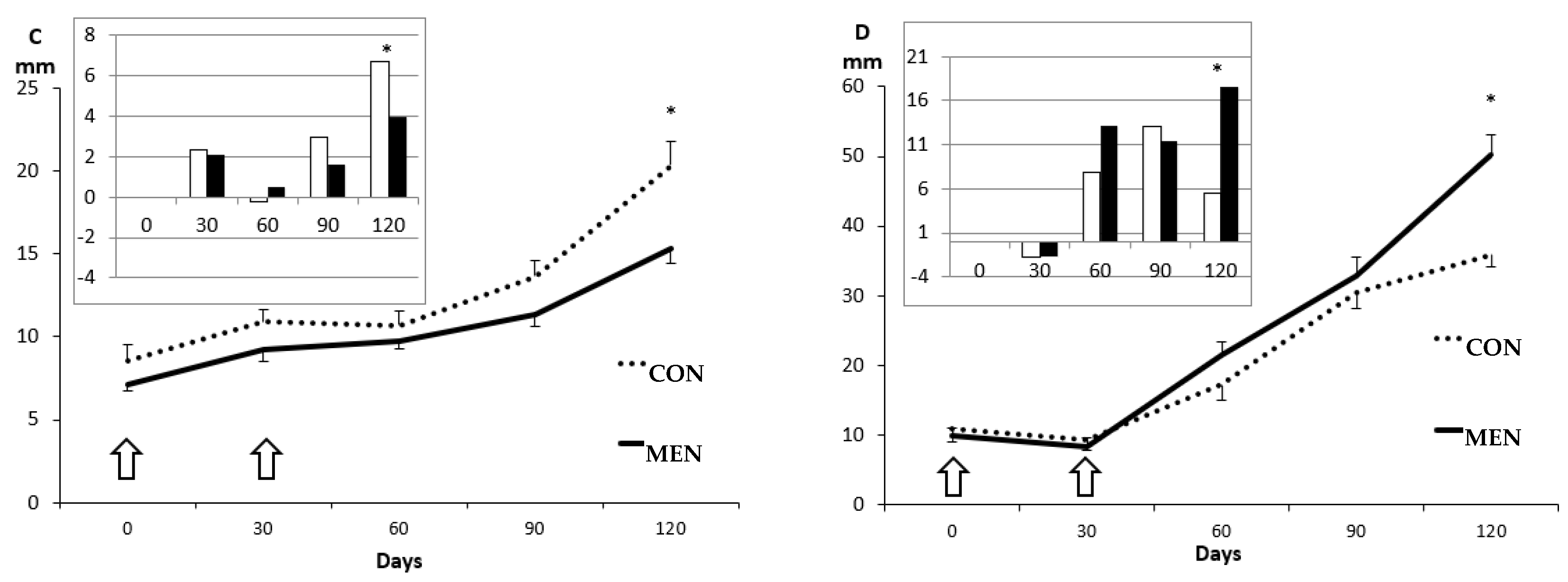
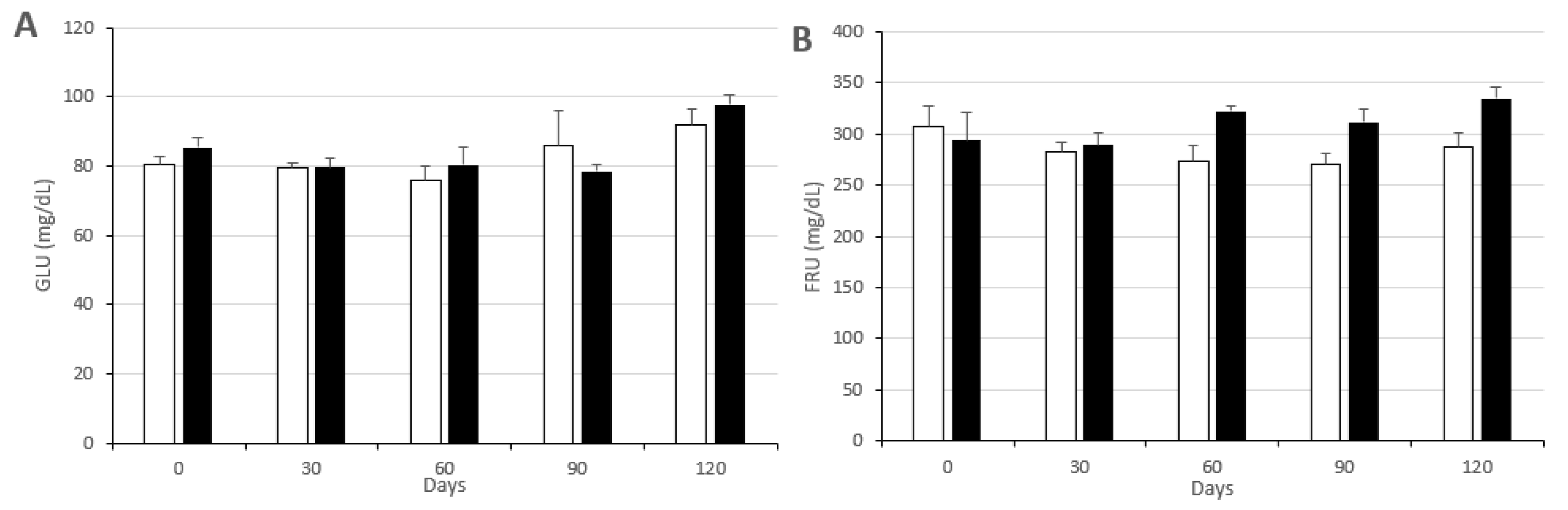
3. Results
3.1. Changes in Body Mass and Adiposity
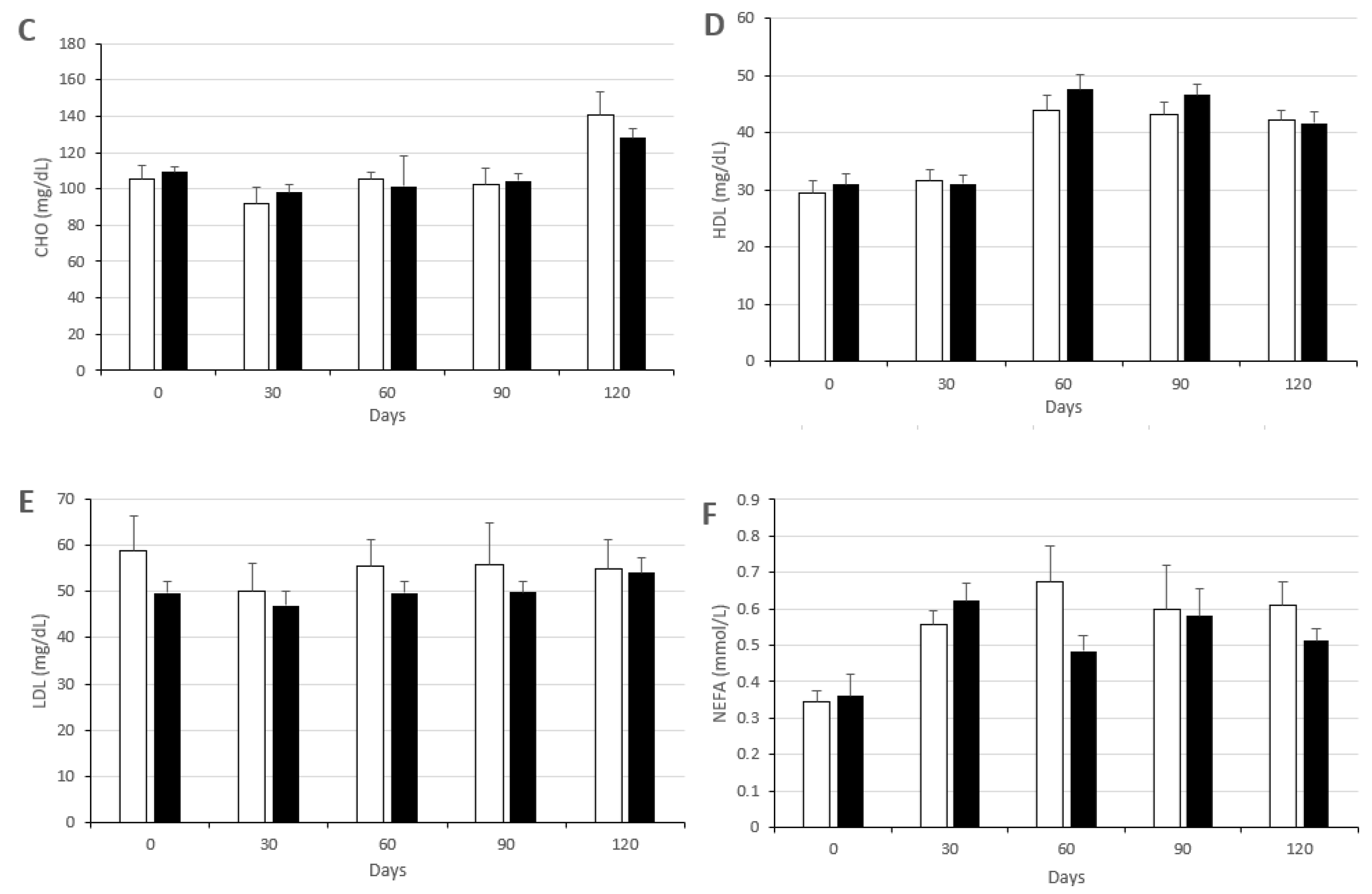
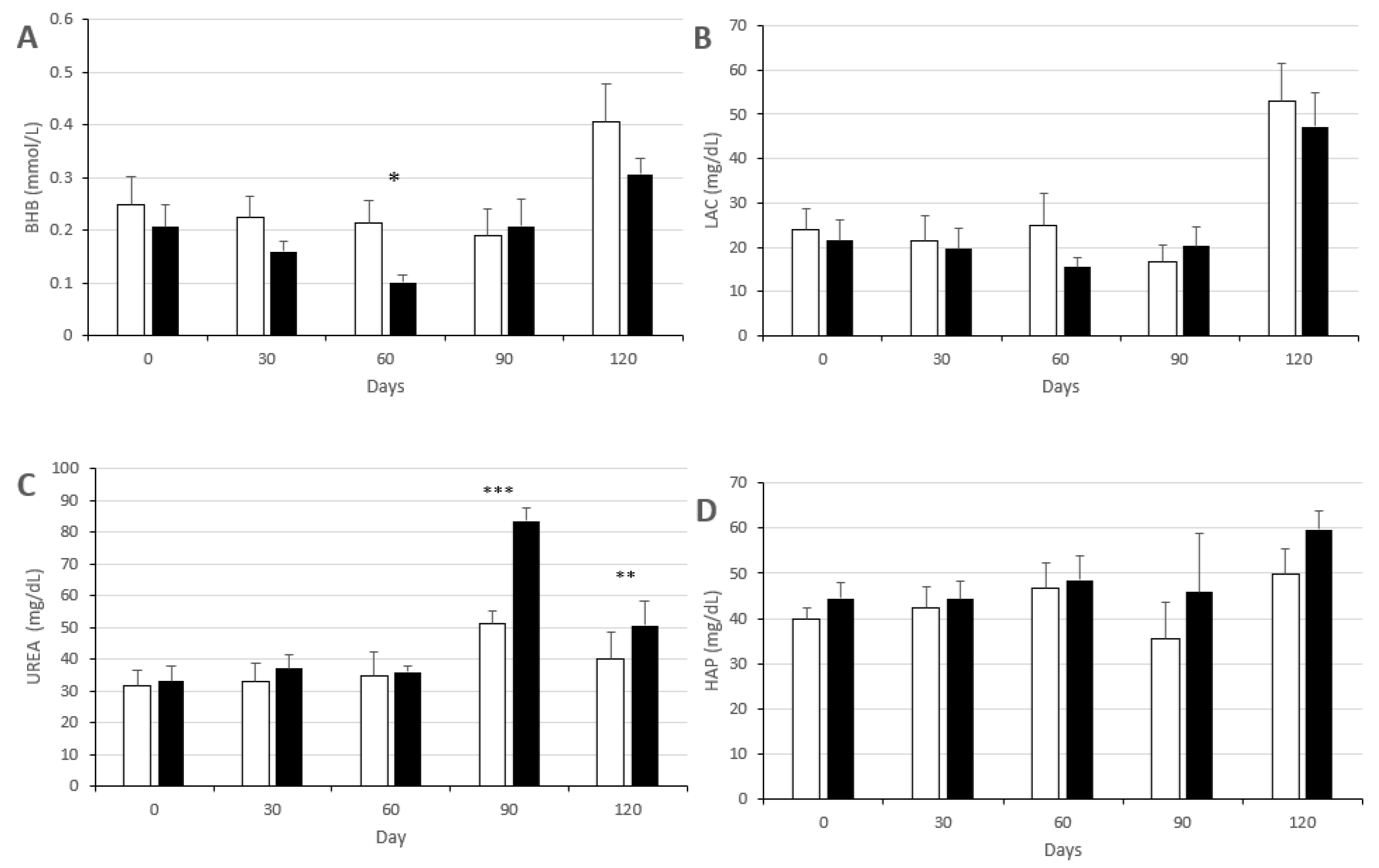
3.2. Changes in Metabolic Status
3.3. Changes in Fatty Acid Composition of Adipose Tissues (Subcutaneous and Visceral Fat)
3.4. Changes in Fatty Acid Composition of Non-Adipose Tissues (Muscle and Liver)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wake, R.; Yoshiyama, M. Gender differences in ischemic heart disease. Recent Pat. Cardiovasc. Drug Discov. 2009, 4, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.S.; Kelly, R.P.; Collins, P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc. Res. 2000, 46, 28–49. [Google Scholar] [CrossRef] [Green Version]
- Nuutila, P.; Knuuti, M.J.; Mäki, M.; Laine, H.; Ruotsalainen, U.; Teräs, M.; Haaparanta, M.; Solin, O.; Yki-Järvinen, H. Gender and insulin sensitivity in the heart and in skeletal muscles. Studies using positron emission tomography. Diabetes 1995, 44, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Cignarella, A.; Bolego, C. Mechanisms of estrogen protection in diabetes and metabolic disease. Horm. Mol. Biol. Clin. Investig. 2010, 4, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Louet, J.F.; LeMay, C.; Mauvais-Jarvis, F. Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Curr. Atheroscler. Rep. 2004, 6, 180–185. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [Green Version]
- Rhee, Y.; Paik, M.J.; Kim, K.R.; Ko, Y.G.; Kang, E.S.; Cha, B.S.; Lee, H.C.; Lim, S.K. Plasma free fatty acid level patterns according to cardiovascular risk status in postmenopausal women. Clin. Chim. Acta 2008, 392, 11–16. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Lin, F.; Grady, D.; Bittner, V.; Cauley, J.A.; Barrett-Connor, E. Heart and estrogen/progestin replacement study. Glycemic effects of postmenopausal hormone therapy: The heart and estrogen/progestin replacement study: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2003, 138, 1–9. [Google Scholar] [CrossRef]
- Margolis, K.L.; Bonds, D.E.; Rodabough, R.J.; Tinker, L.; Phillips, L.S.; Allen, C.; Bassford, T.; Burke, G.; Torrens, J.; Howard, B.V. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the Women’s Health Initiative Hormone Trial. Diabetologia 2004, 47, 1175–1187. [Google Scholar] [CrossRef]
- Yang, X.P.; Reckelhoff, J.F. Estrogen, hormonal replacement therapy and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2011, 20, 133–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schierbeck, L.L.; Rejnmark, L.; Tofteng, C.L.; Stilgren, L.; Eiken, P.; Mosekilde, L.; Køber, L.; Jensen, J.E. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: Randomised trial. BMJ 2012, 9, e6409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, E.F.; He, Y.; Black, D.M.; Brinton, E.A.; Budoff, M.J.; Cedars, M.I.; Hodis, H.N.; Lobo, R.A.; Manson, J.E.; Merriam, G.R.; et al. Self-reported menopausal symptoms, coronary artery calcification, and carotid intima-media thickness in recently menopausal women screened for the Kronos early estrogen prevention study (KEEPS). Fertil. Steril. 2013, 99, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Villiers, T.J.; Hall, J.E.; Pinkerton, J.V.; Cerdas Pérez, S.; Rees, M.; Yang, C.; Pierroz, D.D. Revised global consensus statement on menopausal hormone therapy. Climacteric 2016, 19, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Resistin: Yet another adipokine tells us that men are not mice. Diabetologia 2005, 48, 2203–2205. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.C.; Proctor, S.D. Small animal models of cardiovascular disease: Tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc. Pathol. 2006, 15, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Hamernik, D.L. Farm animals are important biomedical models. Anim. Front. 2019, 9, 3–5. [Google Scholar] [CrossRef] [Green Version]
- Lunney, J.K. Advances in swine biomedical model genomics. Int. J. Biol. Sci. 2007, 3, 179–184. [Google Scholar] [CrossRef]
- Douglas, W.R. Of pigs and men and research: A review of applications and analogies of the pig, sus scrofa, in human medical research. Space Life Sci. 1972, 3, 226–234. [Google Scholar] [CrossRef]
- Spurlock, M.E.; Gabler, N.K. The development of porcine models of obesity and the metabolic syndrome. J. Nutr. 2008, 138, 397–402. [Google Scholar] [CrossRef]
- LittenBrown, J.C.; Corson, A.M.; Clarke, L. Porcine models for the metabolic syndrome, digestive and bone disorders: A general overview. Animal 2010, 4, 899–920. [Google Scholar] [CrossRef] [Green Version]
- Cappai, M.G.; Dall’Aglio, C.; Sander, S.J.; Ratert, C.; Dimauro, C.; Pinna, W.; Kamphues, J. Different physical forms of one diet fed to growing pigs induce morphological changes in mandubular glands and local leptin (Ob) production and receptor (ObR) expression. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1067–1072. [Google Scholar] [CrossRef]
- Ovilo, C.; Fernández, A.; Noguera, J.L.; Barragán, C.; Letón, R.; Rodríguez, C.; Mercadé, A.; Alves, E.; Folch, J.M.; Varona, L.; et al. Fine mapping of porcine chromosome 6 QTL and LEPR effects on body composition inmultiple generations of an iberian by landrace intercross. Genet. Res. 2005, 85, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G.; Cowley, M.A.; Munzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Rovira, L.; Gonzalez-Anover, P.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Ovilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Effect of an obesogenic diet during the juvenile period on growth pattern, fatness and metabolic, cardiovascular and reproductive features of swine with obesity/leptin resistance. Endocr. Metab. Immune Disord. Drug Targets 2013, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rovira, L.; Astiz, S.; Caro, A.; Lopez-Bote, C.; Ovilo, C.; Pallares, P.; Perez-Solana, M.L.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Diet-induced swine model with obesity/leptin resistance for the study of metabolic syndrome and type 2 diabetes. Sci. World J. 2012, 2012, 510149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Contreras, C.; Vazquez-Gomez, M.; Torres-Rovira, L.; Gonzalez, J.; Porrini, E.; Gonzalez-Colaço, M.; Isabel, B.; Astiz, S.; Gonzalez-Bulnes, A. Characterization of ageing- and diet-related swine models of sarcopenia and sarcopenic obesity. Int. J. Mol. Sci. 2018, 19, 823. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rodríguez, R.; González-Bulnes, A.; Garcia-Contreras, C.; Rodriguez-Rodriguez, A.E.; Astiz, S.; Vazquez-Gomez, M.; Pesantez, J.L.; Isabel, B.; Salido-Ruiz, E.; González, J.; et al. The Iberian pig fed with high-fat diet: A model of renal disease in obesity and metabolic syndrome. Int. J. Obes. 2020, 44, 457–465. [Google Scholar] [CrossRef]
- Chalvon-Demersay, T.; Blachier, F.; Tomé, D.; Blais, A. Animal models for the study of the relationships between diet and obesity: A focus on dietary protein and estrogen deficiency. Front. Nutr. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.W. Endocrinology of the Menopause First Consensus Meeting on Menopause in the East Asian Region. 2017. Available online: https://www.gfmer.ch/Books/bookmp/33.htm (accessed on 8 June 2020).
- Hall, J.E. Endocrinology of the menopause. Endocrinol. Metab. Clin. N. Am. 2015, 44, 485–496. [Google Scholar] [CrossRef]
- Ueshiba, H.; Zerah, M.; New, M.I. Enzyme-linked immunosorbent assay (ELISA). Method for screening of nonclassical steroid 21-hydroxylase deficiency. Norm. Metab. Res. 1994, 26, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Añover, P.; Encinas, T.; Gomez-Izquierdo, E.; Sanz, E.; Letelier, C.A.; Torres-Rovira, L.; Pallares, P.; Sanchez-Sanchez, R.; Gonzalez-Bulnes, A. Advanced onset of puberty in gilts of thrifty genotype (Iberian pig). Reprod. Dom. Anim. 2010, 45, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.; Lopez-Bote, C.J. A laboratory efficient method for intramuscular fat analysis. Food Chem. 2014, 145, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Antequera, T.; Andres, A.I.; Petron, M.J.; Muriel, E. Improvement of a solid phase extraction method for analysis of lipid fractions in muscle foods. Anal. Chim. Acta 2004, 520, 201–205. [Google Scholar] [CrossRef]
- Daza, A.; Latorre, M.A.; Olivares, A.; López Bote, C.J. The effects of male and female immunocastration on growth performances and carcass and meat quality of pigs intended for dry-cured ham production: A preliminary study. Livest. Sci. 2016, 190, 20–26. [Google Scholar] [CrossRef]
- Lopez-Bote, C.; Rey, A.; Ruiz, J.; Isabel, B.; Sanz Arias, R. Effect of feeding diets high in monounsaturated fatty acids and α-tocopheryl acetate to rabbits on resulting carcass fatty acid profile and lipid oxidation. Anim. Sci. 1997, 64, 177–186. [Google Scholar] [CrossRef]
- Segura, J.; Escudero, R.; Romero de Avila, M.D.; Cambero, M.I.; Lopez-Bote, C.J. Effect of fatty acid composition and positional distribution within the triglyceride on selected physical properties of dry-cured ham subcutaneous fat. Meat Sci. 2015, 103, 90–95. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007, 87, 1175–1213. [Google Scholar] [CrossRef]
- Hulver, M.W.; Berggren, J.R.; Carper, M.J.; Miyazaki, M.; Ntambi, J.M.; Hoffman, E.P.; Thyfault, J.P.; Stevens, R.; Dohm, G.L.; Houmard, J.A.; et al. Elevated stearoyl-CoA desaturase-1 expression in skeletal muscle contributes to abnormal fatty acid partitioning in obese humans. Cell Metab. 2005, 2, 251–261. [Google Scholar] [CrossRef] [Green Version]
- Caruso, S.; Cianci, S.; Cariola, M.; Fava, V.; Rapisarda, M.C.; Cianci, A. Effects of nutraceuticals on quality of life and sexual function of perimenopausal women. J. Endocrinol. Investig. 2017, 40, 27–32. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Ma, J.; Jiang, A.; Tang, G.; Mai, M.; Zhu, L.; Bai, L.; Li, M.; Li, X. Gene expression profiling reveals distinct features of various porcine adipose tissues. Lipids Health Dis. 2013, 12, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wronska, A.; Kmiec, Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. 2012, 205, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P. Health consequences of visceral obesity. Ann. Med. 2001, 33, 534–541. [Google Scholar] [CrossRef]
- Lafontan, M.; Girard, J. Impact of visceral adipose tissue on liver metabolism. Part I: Heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008, 34, 317–327. [Google Scholar] [CrossRef]
- Bergen, W.G.; Mersmann, H.J. Comparative aspects of lipid metabolism: Impact on contemporary research and use of animal models. J. Nutr. 2005, 135, 2499–2502. [Google Scholar] [CrossRef] [Green Version]
- Roden, M.; Price, T.B.; Perseghin, G.; Petersen, K.F.; Rothman, D.L.; Cline, G.W.; Shulman, G.I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Investig. 1996, 97, 2859–2865. [Google Scholar] [CrossRef] [Green Version]
- Poudyal, H.; Brown, L. Stearoyl-CoA desaturase: A vital checkpoint in the development and progression of obesity. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 217–231. [Google Scholar] [CrossRef]
- Barbero, A.; Astiz, S.; Lopez-Bote, C.J.; Perez-Solana, M.L.; Ayuso, M.; Garcia-Real, I.; Gonzalez-Bulnes, A. Maternal malnutrition and offspring sex determine juvenile obesity and metabolic disorders in a swine model of leptin resistance. PLoS ONE 2013, 8, e78424. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Bulnes, A.; Astiz, S.; Ovilo, C.; Lopez-Bote, C.J.; Sanchez-Sanchez, R.; Perez-Solana, M.L.; Torres-Rovira, L.; Ayuso, M.; Gonzalez, J. Early-postnatal changes in adiposity and lipids profile by transgenerational developmental programming in swine with obesity/leptin resistance. J. Endocrinol. 2014, 223, M17–M29. [Google Scholar] [CrossRef] [Green Version]
- Laganà, A.S.; Vitale, S.G.; Nigro, A.; Sofo, V.; Salmeri, F.M.; Rossetti, P.; Rapisarda, A.M.; La Vignera, S.; Condorelli, R.A.; Rizzo, G.; et al. Pleiotropic actions of peroxisome proliferator-activated receptors (PPARs) in dysregulated metabolic homeostasis, inflammation and cancer: Current evidence and future perspectives. Int. J. Mol. Sci. 2016, 17, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardif, N.; Salles, J.; Landrier, J.F.; Mothe-Satney, I.; Guillet, C.; BoueVaysse, C.; Combaret, L.; Giraudet, C.; Patrac, V.; Bertrand-Michel, J.; et al. Oleate enriched diet improves insulin sensitivity and restores muscle protein synthesis in old rats. Clin. Nutr. 2011, 30, 799–806. [Google Scholar] [CrossRef]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbernagel, G.; Kovarova, M.; Cegan, A.; Machann, J.; Schick, F.; Lehmann, R.; Häring, H.U.; Stefan, N.; Schleicher, E.; Fritsche, A.; et al. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. J. Clin. Endocrinol. Metab. 2012, 97, E2288–E2292. [Google Scholar] [CrossRef] [PubMed]
- Coll, T.; Eyre, E.; Rodríguez-Calvo, R.; Palomer, X.; Sánchez, R.M.; Merlos, M.; Laguna, J.C.; Vázquez-Carrera, M. Oleate reverses palmitate-induced insulin resistance and inflammation in skeletal muscle cells. J. Biol. Chem. 2008, 283, 11107–11116. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Griffiths, H.R.; Bailey, C.J. Oleate protects against palmitate-induced insulin resistance in L6 myotubes. Br. J. Nutr. 2009, 102, 1557–1563. [Google Scholar] [CrossRef] [Green Version]
- Martínez-García, C.; Izquierdo-Lahuerta, A.; Vivas, Y.; Velasco, I.; Yeo, T.K.; Chen, S.; Medina-Gomez, G. Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS ONE 2015, 10, e0142291. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Lee, A.; Yoo, H.J.; Kim, M.; Kim, M.; Shin, D.Y.; Lee, J.H. Association between increased visceral fat area and alterations in plasma fatty acid profile in overweight subjects: A crosssectional study. Lipids Health Dis. 2017, 16, 248. [Google Scholar] [CrossRef] [Green Version]
- Arany, I.; Clark, J.; Reed, D.; Juncos, L.; Dixit, M. The role of p66shc in renal toxicity of oleic acid. Am. J. Nephrol. 2013, 38, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Puglisi, M.J.; Hasty, A.H.; Saraswathi, V. The role of adipose tissue in mediating the beneficial effects of dietary fish oil. J. Nutr. Biochem. 2011, 22, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Carpentier, Y.A.; Portois, L.; Malaisse, W.J. N-3 fatty acids and the metabolic syndrome. Am. J. Clin. Nutr. 2006, 83, 1499S–1504S. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. (n − 3) Fatty acids and cardiovascular health: Are effects of EPA and DHA shared or complementary? J. Nutr. 2012, 142, 614S–625S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.Y.; Jacobson, T.A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: A systematic review and meta-analysis. Curr. Atheroscler. Rep. 2011, 13, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Chen, Y.J.; Yang, J.T.; Chen, C.Y.; Ajuwon, K.M.; Chen, S.E.; Su, N.W.; Chen, Y.S.; Mersmann, H.J.; Ding, S.T. Docosahexaenoic acid increases accumulation of adipocyte triacylglycerol through up-regulation of lipogenic gene expression in pigs. Lipids Health Dis. 2017, 16, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murali, G.; Desouza, C.V.; Clevenger, M.E.; Ramalingam, R.; Saraswathi, V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3 T3-L1 preadipocytes. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Del Cornò, M.; D’Archivio, M.; Conti, L.; Scazzocchio, B.; Varì, R.; Donninelli, G.; Varano, B.; Giammarioli, S.; De Meo, S.; Silecchia, G.; et al. Visceral fat adipocytes from obese and colorectal cancer subjects exhibit distinct secretory and ω6 polyunsaturated fatty acid profiles and deliver immunosuppressive signals to innate immunity cells. Oncotarget 2016, 7, 63093–63105. [Google Scholar] [CrossRef]
- Horas, H.; Nababan, S.; Nishiumi, S.; Kawano, Y.; Kobayashi, T.; Yoshida, M.; Azuma, T. Adrenic acid as an inflammation enhancer in non-alcoholic fatty liver disease. Arch. Biochem. Biophys. 2017, 623–624, 64–75. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J.P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Borkman, M.; Storlien, L.H.; Pan, D.A.; Jenkins, A.B.; Chisholm, D.J.; Campbell, L.V. The relation between insulin sensitivity and the fatty acid composition of skeletal-muscle phospholipids. N. Engl. J. Med. 1993, 328, 238–244. [Google Scholar] [CrossRef]
- Clore, J.N.; Li, J.; Gill, R.; Gupta, S.; Spencer, R.; Azzam, A.; Zuelzer, W.; Rizzo, W.B.; Blackard, W.G. Skeletal muscle phosphatidylcholine fatty acids and insulin sensitivity in normal humans. Am. J. Physiol. Endocrinol. Metab. 1998, 275, E665–E670. [Google Scholar] [CrossRef]
- Pan, D.A.; Lillioja, S.; Milner, M.R.; Kriketos, A.D.; Baur, L.A.; Bogardus, C.; Storlien, L.H. Skeletal muscle membrane lipid composition is related to adiposity and insulin action. J. Clin. Investig. 1995, 96, 2802–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storlien, L.H.; Pan, D.A.; Kriketos, A.D.; O’Connor, J.; Caterson, I.D.; Cooney, G.J.; Jenkins, A.B.; Baur, L.A. Skeletal muscle membrane lipids and insulin resistance. Lipids 1996, 31, S261–S265. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Zhang, X.; Yi, Z.; Cichello, S. Skeletal intramyocellular lipid metabolism and insulin resistance. Biophys. Rep. 2015, 1, 90–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattullo, K.M.; Kidney, B.A. Exploring fructosamine beyond diabetes mellitus. J. Am. Vet. Med. Assoc. 2014, 244, 1268–1277. [Google Scholar] [CrossRef]
- Armbruster, D.A. Fructosamine: Structure, analysis and clinical usefulness. Clin. Chem. 1987, 33, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Carenini, A.; Zoppi, F.; Carpinelli, A.; Banfi, G.; Ceriotti, F.; Bonini, P.; Pozza, G. Plasma protein glycation as measured by fructosamine assay. Clin. Chem. 1987, 33, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, R.E. Nonenzymatically glycated proteins. Adv. Clin. Chem. 1987, 26, 1–78. [Google Scholar] [PubMed]
- Lovejoy, J.; Newby, F.D.; Gebhart, S.S.; DiGirolamo, M. Insulin resistance in obesity is associated with elevated basal lactate levels and diminished lactate appearance following intravenous glucose and insulin. Metabolism 1992, 41, 22–27. [Google Scholar] [CrossRef]
- Schutz, Y. Protein turnover, ureagenesis and gluconeogenesis. Int. J. Vitam. Nutr. Res. 2011, 81, 101–107. [Google Scholar] [CrossRef]
- Chang, A.R.; Zafar, W.; Grams, M.E. Kidney function in obesity-challenges in indexing and estimation. Adv. Chronic Kidney Dis. 2018, 25, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Curry, A.S. Biochemistry of Women: Clinical Concepts; CRC Press: Boca Raton, FL, USA, 2018; p. 187. [Google Scholar]
- Atapattu, P.M. Obesity at menopause: An expanding problem. J. Pat. Care 2015, 1, 103. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.C.; Verdin, E. β-hydroxybutyrate: A signaling metabolite. Annu. Rev. Nutr. 2017, 37, 51–76. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Di Roma, P.; Paoli, G.; Guida, P.; Pannacciulli, N.; Giorgino, R. Haptoglobin serum levels are independently associated with insulinemia in overweight and obese women. J. Endocrinol. Investig. 2007, 30, 399–403. [Google Scholar] [CrossRef] [PubMed]
| Tissue | Layer/Fraction | Variable (g/100 g) | CON | MEN |
|---|---|---|---|---|
| SCF | Out | C16:1n-9 | 0.34 a ± 0.02 | 0.30 b ± 0.01 |
| C22:4n-6 | 0.06 a ± 0.00 | 0.07 b ± 0.00 | ||
| C22:6n-3 | 0.01 c ± 0.00 | 0.02 d ± 0.00 | ||
| DN6 | 0.01 a ± 0.00 | 0.01 b ± 0.00 | ||
| In | C16:1n-7 | 2.28 c ± 0.12 | 1.87 d ± 0.12 | |
| C20:5n-3 | 0.01 a ± 0.00 | 0.01 b ± 0.00 | ||
| C22:4n-6 | 0.05 c ± 0.00 | 0.06 d ± 0.00 | ||
| C22:6n-3 | 0.01 c ± 0.00 | 0.02 d ± 0.00 | ||
| DN3 | 0.02 a ± 0.00 | 0.02 b ± 0.00 | ||
| VF | C14:0 | 1.43 c ± 0.06 | 1.21 d ± 0.06 | |
| C16:0 | 28.0 c ± 0.36 | 26.8 d ± 0.45 | ||
| C16:1n-9 | 0.38 c ± 0.02 | 0.31 d ± 0.02 | ||
| C16:1n-7 | 1.94 a ± 0.14 | 1.54 b ± 0.12 | ||
| C18:1n-9 | 36.7 c ± 0.33 | 38.4 d ± 0.54 | ||
| C18:2n-6 | 10.7 a ± 0.41 | 9.82 b ± 0.26 | ||
| MUFA | 43.4 c ± 0.21 | 44.5 d ± 0.52 | ||
| LD | Polar | MUFA | 23.5 c ± 0.20 | 22.5 d ± 0.34 |
| Liver | Neutral | C18:1n-7 | 1.72 a ± 0.16 | 2.11 b ± 0.06 |
| C20:5n-3 | 0.18 a ± 0.02 | 0.14 b ± 0.01 | ||
| C22:6n-3 | 0.85 e ± 0.08 | 1.24 f ± 0.03 | ||
| ∑n3 | 1.89 a ± 0.17 | 2.23 b ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heras-Molina, A.; Pesantez-Pacheco, J.L.; Vazquez-Gomez, M.; Garcia-Contreras, C.; Astiz, S.; Isabel, B.; Gonzalez-Bulnes, A. Short-Term Effects of Early Menopause on Adiposity, Fatty Acids Profile and Insulin Sensitivity of a Swine Model of Female Obesity. Biology 2020, 9, 284. https://doi.org/10.3390/biology9090284
Heras-Molina A, Pesantez-Pacheco JL, Vazquez-Gomez M, Garcia-Contreras C, Astiz S, Isabel B, Gonzalez-Bulnes A. Short-Term Effects of Early Menopause on Adiposity, Fatty Acids Profile and Insulin Sensitivity of a Swine Model of Female Obesity. Biology. 2020; 9(9):284. https://doi.org/10.3390/biology9090284
Chicago/Turabian StyleHeras-Molina, Ana, José Luis Pesantez-Pacheco, Marta Vazquez-Gomez, Consolacion Garcia-Contreras, Susana Astiz, Beatriz Isabel, and Antonio Gonzalez-Bulnes. 2020. "Short-Term Effects of Early Menopause on Adiposity, Fatty Acids Profile and Insulin Sensitivity of a Swine Model of Female Obesity" Biology 9, no. 9: 284. https://doi.org/10.3390/biology9090284
APA StyleHeras-Molina, A., Pesantez-Pacheco, J. L., Vazquez-Gomez, M., Garcia-Contreras, C., Astiz, S., Isabel, B., & Gonzalez-Bulnes, A. (2020). Short-Term Effects of Early Menopause on Adiposity, Fatty Acids Profile and Insulin Sensitivity of a Swine Model of Female Obesity. Biology, 9(9), 284. https://doi.org/10.3390/biology9090284





