A Glance of p53 Functions in Brain Development, Neural Stem Cells, and Brain Cancer
Abstract
1. Introduction
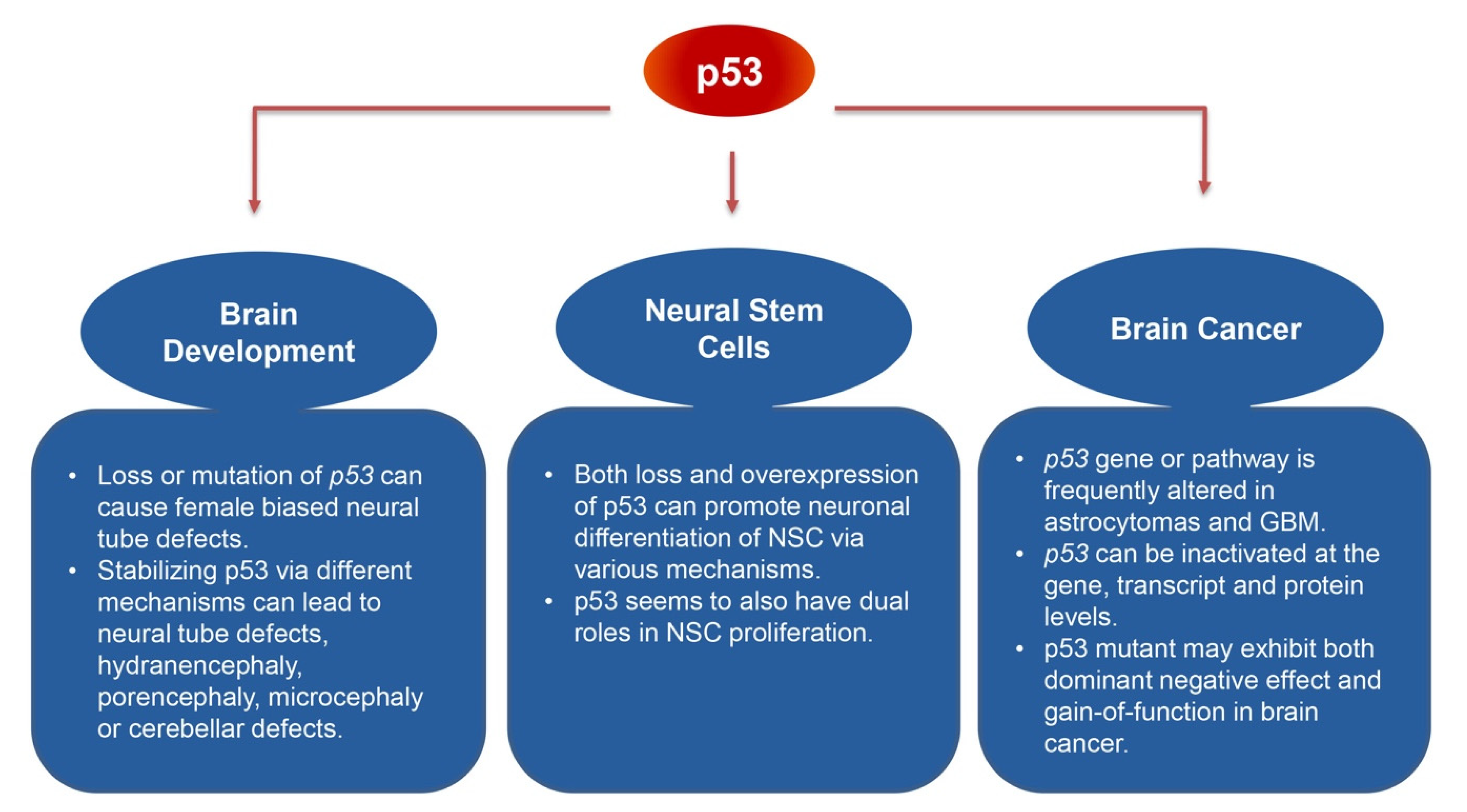
2. p53 and Brain Development
3. p53 and Neural Stem Cell Regulation
4. p53 and Brain Cancer
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kress, M.; May, E.; Cassingena, R.; May, P. Simian virus 40-transformed cells express new species of proteins precipitable by anti-simian virus 40 tumor serum. J. Virol. 1979, 31, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.P.; Crawford, L.V. T antigen is bound to a host protein in SV40-transformed cells. Nature 1979, 278, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Linzer, D.I.; Levine, A.J. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell 1979, 17, 43–52. [Google Scholar] [CrossRef]
- Kim, M.P.; Lozano, G. Mutant p53 partners in crime. Cell Death Differ. 2018, 25, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Levine, A. p53 Research: The past thirty years and the next thirty years. Cold Spring Harb. Perspect. Biol. 2010, 2, a000893. [Google Scholar] [CrossRef] [PubMed]
- Stein, Y.; Rotter, V.; Aloni-Grinstein, R. Gain-of-Function Mutant p53: All the Roads Lead to Tumorigenesis. Int. J. Mol. Sci. 2019, 20, 6197. [Google Scholar] [CrossRef]
- Levine, A.J. The many faces of p53: Something for everyone. J. Mol. Cell Biol. 2019, 11, 524–530. [Google Scholar] [CrossRef]
- Oren, M. Decision making by p53: Life, death and cancer. Cell Death Differ 2003, 10, 431–442. [Google Scholar] [CrossRef]
- Bowen, M.E.; Attardi, L.D. The role of p53 in developmental syndromes. J. Mol. Cell Biol. 2019, 11, 200–211. [Google Scholar] [CrossRef]
- Iwakuma, T.; Lozano, G. MDM2, an introduction. Mol. Cancer Res. 2003, 1, 993–1000. [Google Scholar]
- Montes de Oca Luna, R.; Wagner, D.S.; Lozano, G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995, 378, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Van Nostrand, J.L.; Brady, C.A.; Jung, H.; Fuentes, D.R.; Kozak, M.M.; Johnson, T.M.; Lin, C.Y.; Lin, C.J.; Swiderski, D.L.; Vogel, H.; et al. Inappropriate p53 activation during development induces features of CHARGE syndrome. Nature 2014, 514, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.F.; Kaufman, M.H.; Harrison, D.J.; Clarke, A.R. High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 1995, 5, 931–936. [Google Scholar] [CrossRef]
- Sah, V.P.; Attardi, L.D.; Mulligan, G.J.; Williams, B.O.; Bronson, R.T.; Jacks, T. A subset of p53-deficient embryos exhibit exencephaly. Nat. Genet. 1995, 10, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mendrysa, S.M.; Ghassemifar, S.; Malek, R. p53 in the CNS: Perspectives on Development, Stem Cells, and Cancer. Genes Cancer 2011, 2, 431–442. [Google Scholar] [CrossRef]
- Orr, B.A.; Clay, M.R.; Pinto, E.M.; Kesserwan, C. An update on the central nervous system manifestations of Li-Fraumeni syndrome. Acta Neuropathol. 2020, 139, 669–687. [Google Scholar] [CrossRef]
- Checler, F.; Alves da Costa, C. p53 in neurodegenerative diseases and brain cancers. Pharmacol. Ther. 2014, 142, 99–113. [Google Scholar] [CrossRef]
- Fulci, G.; Van Meir, E.G. p53 and the CNS: Tumors and developmental abnormalities. Mol. Neurobiol. 1999, 19, 61–77. [Google Scholar] [CrossRef]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.; Copp, A.J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef]
- Hosako, H.; Francisco, L.E.; Martin, G.S.; Mirkes, P.E. The roles of p53 and p21 in normal development and hyperthermia-induced malformations. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 40–47. [Google Scholar] [CrossRef]
- Ikeda, S.; Hawes, N.L.; Chang, B.; Avery, C.S.; Smith, R.S.; Nishina, P.M. Severe ocular abnormalities in C57BL/6 but not in 129/Sv p53-deficient mice. Invest. Ophthalmol. Vis. Sci. 1999, 40, 1874–1878. [Google Scholar]
- Zhao, J.; Tian, Y.; Zhang, H.; Qu, L.; Chen, Y.; Liu, Q.; Luo, Y.; Wu, X. p53 Mutant p53(N236S) Induces Neural Tube Defects in Female Embryos. Int. J. Biol. Sci. 2019, 15, 2006–2015. [Google Scholar] [CrossRef]
- Zhang, Y.; Lozano, G. p53: Multiple Facets of a Rubik’s Cube. Annu. Rev. Cancer Biol. 2017, 1, 185–201. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Walker, N.; Bronson, R.; Kaghad, M.; Oosterwegel, M.; Bonnin, J.; Vagner, C.; Bonnet, H.; Dikkes, P.; Sharpe, A.; et al. p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature 2000, 404, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.G. Neural tube defects, sex ratios, and X inactivation. Lancet 1986, 2, 1334–1335. [Google Scholar] [CrossRef]
- Delbridge, A.R.D.; Kueh, A.J.; Ke, F.; Zamudio, N.M.; El-Saafin, F.; Jansz, N.; Wang, G.Y.; Iminitoff, M.; Beck, T.; Haupt, S.; et al. Loss of p53 Causes Stochastic Aberrant X-Chromosome Inactivation and Female-Specific Neural Tube Defects. Cell Rep. 2019, 27, 442–454.e5. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.V.; Martin, M.B.; Garcia-Morales, P.; Castro-Galache, M.D.; Ferragut, J.A.; Saceda, M. Regulation of estrogen receptor-alpha expression by the tumor suppressor gene p53 in MCF-7 cells. J. Endocrinol. 2004, 180, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Shirley, S.H.; Rundhaug, J.E.; Tian, J.; Cullinan-Ammann, N.; Lambertz, I.; Conti, C.J.; Fuchs-Young, R. Transcriptional regulation of estrogen receptor-alpha by p53 in human breast cancer cells. Cancer Res. 2009, 69, 3405–3414. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Schwartz, J.A.; Brooks, S.C. p53 down-regulates ER-responsive genes by interfering with the binding of ER to ERE. Biochem. Biophys. Res. Commun. 1999, 264, 359–364. [Google Scholar] [CrossRef]
- Berger, C.E.; Qian, Y.; Liu, G.; Chen, H.; Chen, X. p53, a target of estrogen receptor (ER) alpha, modulates DNA damage-induced growth suppression in ER-positive breast cancer cells. J. Biol. Chem. 2012, 287, 30117–30127. [Google Scholar] [CrossRef]
- Hutson, D.D.; Gurrala, R.; Ogola, B.O.; Zimmerman, M.A.; Mostany, R.; Satou, R.; Lindsey, S.H. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol. Sex Differ. 2019, 10, 4. [Google Scholar] [CrossRef]
- Reddy, R.C.; Estill, C.T.; Meaker, M.; Stormshak, F.; Roselli, C.E. Sex differences in expression of oestrogen receptor alpha but not androgen receptor mRNAs in the foetal lamb brain. J. Neuroendocrinol. 2014, 26, 321–328. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pani, L.; Horal, M.; Loeken, M.R. Rescue of neural tube defects in Pax-3-deficient embryos by p53 loss of function: Implications for Pax-3- dependent development and tumorigenesis. Genes Dev. 2002, 16, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, J.; Niswander, L. Zinc deficiency causes neural tube defects through attenuation of p53 ubiquitylation. Development 2018, 145. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Van Pelt, C.S.; Elizondo-Fraire, A.C.; Liu, G.; Lozano, G. Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proc. Natl. Acad. Sci. USA 2006, 103, 3226–3231. [Google Scholar] [CrossRef]
- Frappart, P.O.; Tong, W.M.; Demuth, I.; Radovanovic, I.; Herceg, Z.; Aguzzi, A.; Digweed, M.; Wang, Z.Q. An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nat. Med. 2005, 11, 538–544. [Google Scholar] [CrossRef]
- Houlihan, S.L.; Feng, Y. The scaffold protein Nde1 safeguards the brain genome during S phase of early neural progenitor differentiation. Elife 2014, 3, e03297. [Google Scholar] [CrossRef]
- Marjanovic, M.; Sanchez-Huertas, C.; Terre, B.; Gomez, R.; Scheel, J.F.; Pacheco, S.; Knobel, P.A.; Martinez-Marchal, A.; Aivio, S.; Palenzuela, L.; et al. CEP63 deficiency promotes p53-dependent microcephaly and reveals a role for the centrosome in meiotic recombination. Nat. Commun. 2015, 6, 7676. [Google Scholar] [CrossRef]
- Breuss, M.; Fritz, T.; Gstrein, T.; Chan, K.; Ushakova, L.; Yu, N.; Vonberg, F.W.; Werner, B.; Elling, U.; Keays, D.A. Mutations in the murine homologue of TUBB5 cause microcephaly by perturbing cell cycle progression and inducing p53-associated apoptosis. Development 2016, 143, 1126–1133. [Google Scholar] [CrossRef]
- Mao, H.; McMahon, J.J.; Tsai, Y.H.; Wang, Z.; Silver, D.L. Haploinsufficiency for Core Exon Junction Complex Components Disrupts Embryonic Neurogenesis and Causes p53-Mediated Microcephaly. PLoS Genet 2016, 12, e1006282. [Google Scholar] [CrossRef]
- Bianchi, F.T.; Tocco, C.; Pallavicini, G.; Liu, Y.; Verni, F.; Merigliano, C.; Bonaccorsi, S.; El-Assawy, N.; Priano, L.; Gai, M.; et al. Citron Kinase Deficiency Leads to Chromosomal Instability and TP53-Sensitive Microcephaly. Cell Rep. 2017, 18, 1674–1686. [Google Scholar] [CrossRef] [PubMed]
- Little, J.N.; Dwyer, N.D. p53 deletion rescues lethal microcephaly in a mouse model with neural stem cell abscission defects. Hum. Mol. Genet. 2019, 28, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Garcia, I.; Crowther, A.J.; Li, S.; Stewart, A.; Liu, H.; Lough, K.J.; O’Neill, S.; Veleta, K.; Oyarzabal, E.A.; et al. Aspm sustains postnatal cerebellar neurogenesis and medulloblastoma growth in mice. Development 2015, 142, 3921–3932. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Terzian, T.; Xiong, S.; Van Pelt, C.S.; Audiffred, A.; Box, N.F.; Lozano, G. The p53-Mdm2 network in progenitor cell expansion during mouse postnatal development. J. Pathol. 2007, 213, 360–368. [Google Scholar] [CrossRef]
- Garcia-Cao, I.; Garcia-Cao, M.; Martin-Caballero, J.; Criado, L.M.; Klatt, P.; Flores, J.M.; Weill, J.C.; Blasco, M.A.; Serrano, M. “Super p53” mice exhibit enhanced DNA damage response, are tumor resistant and age normally. EMBO J. 2002, 21, 6225–6235. [Google Scholar] [CrossRef]
- Tollini, L.A.; Jin, A.; Park, J.; Zhang, Y. Regulation of p53 by Mdm2 E3 ligase function is dispensable in embryogenesis and development, but essential in response to DNA damage. Cancer Cell 2014, 26, 235–247. [Google Scholar] [CrossRef]
- Beattie, R.; Hippenmeyer, S. Mechanisms of radial glia progenitor cell lineage progression. FEBS Lett. 2017, 591, 3993–4008. [Google Scholar] [CrossRef]
- Liu, H.; Jia, D.; Li, A.; Chau, J.; He, D.; Ruan, X.; Liu, F.; Li, J.; He, L.; Li, B. p53 regulates neural stem cell proliferation and differentiation via BMP-Smad1 signaling and Id1. Stem. Cells Dev. 2013, 22, 913–927. [Google Scholar] [CrossRef]
- Forsberg, K.; Wuttke, A.; Quadrato, G.; Chumakov, P.M.; Wizenmann, A.; Di Giovanni, S. The tumor suppressor p53 fine-tunes reactive oxygen species levels and neurogenesis via PI3 kinase signaling. J. Neurosci. 2013, 33, 14318–14330. [Google Scholar] [CrossRef]
- Marin Navarro, A.; Pronk, R.J.; van der Geest, A.T.; Oliynyk, G.; Nordgren, A.; Arsenian-Henriksson, M.; Falk, A.; Wilhelm, M. p53 controls genomic stability and temporal differentiation of human neural stem cells and affects neural organization in human brain organoids. Cell Death Dis. 2020, 11, 52. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Skamagki, M.; Khodadadi-Jamayran, A.; Zhang, W.; Kong, D.; Chang, C.W.; Feng, J.; Han, X.; Townes, T.M.; et al. Elevated p53 Activities Restrict Differentiation Potential of MicroRNA-Deficient Pluripotent Stem Cells. Stem. Cell Reports 2017, 9, 1604–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, W.; Chen, X. p53 is required for nerve growth factor-mediated differentiation of PC12 cells via regulation of TrkA levels. Cell Death Differ. 2006, 13, 2118–2128. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Lu, X.; Wang, Y.; Duan, Y.; Cheng, C.; Shen, A. SCYL1BP1 modulates neurite outgrowth and regeneration by regulating the Mdm2/p53 pathway. Mol. Biol. Cell 2012, 23, 4506–4514. [Google Scholar] [CrossRef]
- Glazova, M.B. Role of p53 in the regulation of neuronal differentiation. Ross Fiziol Zh Im I M Sechenova 2015, 101, 633–646. [Google Scholar] [CrossRef]
- Stavridis, M.P.; Lunn, J.S.; Collins, B.J.; Storey, K.G. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development 2007, 134, 2889–2894. [Google Scholar] [CrossRef]
- Gil-Perotin, S.; Marin-Husstege, M.; Li, J.; Soriano-Navarro, M.; Zindy, F.; Roussel, M.F.; Garcia-Verdugo, J.M.; Casaccia-Bonnefil, P. Loss of p53 induces changes in the behavior of subventricular zone cells: Implication for the genesis of glial tumors. J. Neurosci. 2006, 26, 1107–1116. [Google Scholar] [CrossRef]
- Rockowitz, S.; Zheng, D. Significant expansion of the REST/NRSF cistrome in human versus mouse embryonic stem cells: Potential implications for neural development. Nucleic Acids Res. 2015, 43, 5730–5743. [Google Scholar] [CrossRef]
- Gupta, A.; Dwivedi, T. A Simplified Overview of World Health Organization Classification Update of Central Nervous System Tumors 2016. J. Neurosci. Rural. Pract. 2017, 8, 629–641. [Google Scholar] [CrossRef]
- Hill, J.R.; Kuriyama, N.; Kuriyama, H.; Israel, M.A. Molecular genetics of brain tumors. Arch. Neurol. 1999, 56, 439–441. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, C.; Gibert, M., Jr.; Cruickshanks, N.; Wang, B.; Coughlan, M.; Yang, Y.; Setiady, I.; Deveau, C.; Saoud, K.; et al. The p53 Pathway in Glioblastoma. Cancers 2018, 10, 297. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Uno, M.; Oba-Shinjo, S.M.; de Aguiar, P.H.; Leite, C.C.; Rosemberg, S.; Miura, F.K.; Junior, R.M.; Scaff, M.; Nagahashi Marie, S.K. Detection of somatic TP53 splice site mutations in diffuse astrocytomas. Cancer Lett. 2005, 224, 321–327. [Google Scholar] [CrossRef]
- Jesionek-Kupnicka, D.; Szybka, M.; Malachowska, B.; Fendler, W.; Potemski, P.; Piaskowski, S.; Jaskolski, D.; Papierz, W.; Skowronski, W.; Och, W.; et al. TP53 promoter methylation in primary glioblastoma: Relationship with TP53 mRNA and protein expression and mutation status. DNA Cell Biol. 2014, 33, 217–226. [Google Scholar] [CrossRef]
- Simeonova, I.; Huillard, E. In vivo models of brain tumors: Roles of genetically engineered mouse models in understanding tumor biology and use in preclinical studies. Cell Mol. Life Sci. 2014, 71, 4007–4026. [Google Scholar] [CrossRef]
- Nakamura, M.; Watanabe, T.; Klangby, U.; Asker, C.; Wiman, K.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. p14ARF deletion and methylation in genetic pathways to glioblastomas. Brain Pathol. 2001, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Liu, L.; Ichimura, K.; Schmidt, E.E.; Collins, V.P. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993, 53, 2736–2739. [Google Scholar]
- Riemenschneider, M.J.; Buschges, R.; Wolter, M.; Reifenberger, J.; Bostrom, J.; Kraus, J.A.; Schlegel, U.; Reifenberger, G. Amplification and overexpression of the MDM4 (MDMX) gene from 1q32 in a subset of malignant gliomas without TP53 mutation or MDM2 amplification. Cancer Res. 1999, 59, 6091–6096. [Google Scholar]
- Tao, W.; Levine, A.J. P19(ARF) stabilizes p53 by blocking nucleo-cytoplasmic shuttling of Mdm2. Proc. Natl. Acad. Sci. USA 1999, 96, 6937–6941. [Google Scholar] [CrossRef]
- Tao, W.; Levine, A.J. Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53. Proc. Natl. Acad. Sci. USA 1999, 96, 3077–3080. [Google Scholar] [CrossRef]
- Wade, M.; Wang, Y.V.; Wahl, G.M. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010, 20, 299–309. [Google Scholar] [CrossRef]
- Weber, J.D.; Taylor, L.J.; Roussel, M.F.; Sherr, C.J.; Bar-Sagi, D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat. Cell Biol. 1999, 1, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Squatrito, M.; Brennan, C.W.; Helmy, K.; Huse, J.T.; Petrini, J.H.; Holland, E.C. Loss of ATM/Chk2/p53 pathway components accelerates tumor development and contributes to radiation resistance in gliomas. Cancer Cell 2010, 18, 619–629. [Google Scholar] [CrossRef]
- Bagchi, A.; Papazoglu, C.; Wu, Y.; Capurso, D.; Brodt, M.; Francis, D.; Bredel, M.; Vogel, H.; Mills, A.A. CHD5 is a tumor suppressor at human 1p36. Cell 2007, 128, 459–475. [Google Scholar] [CrossRef]
- Viotti, J.; Duplan, E.; Caillava, C.; Condat, J.; Goiran, T.; Giordano, C.; Marie, Y.; Idbaih, A.; Delattre, J.Y.; Honnorat, J.; et al. Glioma tumor grade correlates with parkin depletion in mutant p53-linked tumors and results from loss of function of p53 transcriptional activity. Oncogene 2014, 33, 1764–1775. [Google Scholar] [CrossRef]
- Jiang, B.; Zhao, W.; Shi, M.; Zhang, J.; Chen, A.; Ma, H.; Suleman, M.; Lin, F.; Zhou, L.; Wang, J.; et al. IDH1 Arg-132 mutant promotes tumor formation through down-regulating p53. J. Biol. Chem. 2018, 293, 9747–9758. [Google Scholar] [CrossRef]
- Lee, J.S.; Xiao, J.; Patel, P.; Schade, J.; Wang, J.; Deneen, B.; Erdreich-Epstein, A.; Song, H.R. A novel tumor-promoting role for nuclear factor IA in glioblastomas is mediated through negative regulation of p53, p21, and PAI1. Neuro Oncol. 2014, 16, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, W.; Zeng, A.; Nie, E.; Jin, X.; Yu, T.; Zhi, T.; Jiang, K.; Wang, Y.; Zhang, J.; et al. MicroRNA-141-3p promotes glioma cell growth and temozolomide resistance by directly targeting p53. Oncotarget 2017, 8, 71080–71094. [Google Scholar] [CrossRef]
- Stegh, A.H.; Brennan, C.; Mahoney, J.A.; Forloney, K.L.; Jenq, H.T.; Luciano, J.P.; Protopopov, A.; Chin, L.; Depinho, R.A. Glioma oncoprotein Bcl2L12 inhibits the p53 tumor suppressor. Genes Dev. 2010, 24, 2194–2204. [Google Scholar] [CrossRef]
- Stegh, A.H.; Kim, H.; Bachoo, R.M.; Forloney, K.L.; Zhang, J.; Schulze, H.; Park, K.; Hannon, G.J.; Yuan, J.; Louis, D.N.; et al. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes Dev. 2007, 21, 98–111. [Google Scholar] [CrossRef]
- Fukaya, R.; Ohta, S.; Yaguchi, T.; Matsuzaki, Y.; Sugihara, E.; Okano, H.; Saya, H.; Kawakami, Y.; Kawase, T.; Yoshida, K.; et al. MIF Maintains the Tumorigenic Capacity of Brain Tumor-Initiating Cells by Directly Inhibiting p53. Cancer Res. 2016, 76, 2813–2823. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Graziano, V.; Galavotti, S.; Henriquez, N.V.; Betts, J.; Saxena, J.; Minieri, V.; Deli, A.; Karlsson, A.; Martins, L.M.; et al. Inhibition of oxidative metabolism leads to p53 genetic inactivation and transformation in neural stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Oren, M.; Rotter, V. Mutant p53 gain-of-function in cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a001107. [Google Scholar] [CrossRef] [PubMed]
- Pedrote, M.M.; Motta, M.F.; Ferretti, G.D.S.; Norberto, D.R.; Spohr, T.; Lima, F.R.S.; Gratton, E.; Silva, J.L.; de Oliveira, G.A.P. Oncogenic Gain of Function in Glioblastoma Is Linked to Mutant p53 Amyloid Oligomers. iScience 2020, 23, 100820. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.W.; Jeon, H.Y.; Jin, X.; Kim, E.J.; Kim, J.K.; Shin, Y.J.; Lee, Y.; Kim, S.H.; Lee, S.Y.; Seo, S.; et al. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ. 2019, 26, 409–425. [Google Scholar] [CrossRef]
- Hegi, M.E.; Klein, M.A.; Ruedi, D.; Chene, P.; Hamou, M.F.; Aguzzi, A. p53 transdominance but no gain of function in mouse brain tumor model. Cancer Res. 2000, 60, 3019–3024. [Google Scholar]
- Klein, M.A.; Ruedi, D.; Nozaki, M.; Dell, E.W.; Diserens, A.C.; Seelentag, W.; Janzer, R.C.; Aguzzi, A.; Hegi, M.E. Reduced latency but no increased brain tumor penetrance in mice with astrocyte specific expression of a human p53 mutant. Oncogene 2000, 19, 5329–5337. [Google Scholar] [CrossRef]
| Mouse Models | p53-Dependent Brain Developmental Phenotypes | p53 Status |
|---|---|---|
| p53−/− [13,14] | Female-specific exencephaly, spina bifida, retinal dysplasia | p53 deletion |
| p53N236S/N236S [22] | Female specific exencephaly and spina bifida | p53 missense mutation |
| p53−/−; Bim+/− [26] | 100% penetrate, female-exclusive exencephaly | p53 deletion |
| Pax3Sp/Sp [33] | Exencephaly | p53 stabilization |
| Mdm2FM/+; Nestin-Cre [35] | Hydranencephaly | p53 stabilization |
| Mdm4FX/+; Nestin-Cre [35] | Porencephaly | p53 stabilization |
| Nbnflox/flox; Nestin-Cre [36] | Microcephaly | p53 stabilization |
| Nde1−/− [37] | Microcephaly | p53 stabilization |
| Cep63T/T [38] | Microcephaly | p53 stabilization |
| Tubb5E401K/E401K; Nestin-Cre [39] Tubb5flox/+; Nestin-Cre [39] | Microcephaly | p53 stabilization |
| Eif4a3flox/+; Emx1-Cre [40] | Microcephaly | p53 stabilization |
| Rbm8aflox/+; Emx1-Cre [40] | Microcephaly | p53 stabilization |
| Magohflox/+; Emx1-Cre [40] | Microcephaly | p53 stabilization |
| CitK−/− [41] | Microcephaly | p53 stabilization |
| Kif20bm/m [42] | Microcephaly | p53 stabilization |
| AspmSA/SA [43] Aspmflox/flox; Math1-Cre [43] | Microcephaly (hypoplastic cerebellum) | p53 stabilization |
| p53515C/515C; Mdm2−/− [44] | Cerebellar defects | Mutant p53 stabilization |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Zhang, Y.; Xiong, S.; Williams-Villalobo, A.E. A Glance of p53 Functions in Brain Development, Neural Stem Cells, and Brain Cancer. Biology 2020, 9, 285. https://doi.org/10.3390/biology9090285
Xiong Y, Zhang Y, Xiong S, Williams-Villalobo AE. A Glance of p53 Functions in Brain Development, Neural Stem Cells, and Brain Cancer. Biology. 2020; 9(9):285. https://doi.org/10.3390/biology9090285
Chicago/Turabian StyleXiong, Yuqing, Yun Zhang, Shunbin Xiong, and Abie E. Williams-Villalobo. 2020. "A Glance of p53 Functions in Brain Development, Neural Stem Cells, and Brain Cancer" Biology 9, no. 9: 285. https://doi.org/10.3390/biology9090285
APA StyleXiong, Y., Zhang, Y., Xiong, S., & Williams-Villalobo, A. E. (2020). A Glance of p53 Functions in Brain Development, Neural Stem Cells, and Brain Cancer. Biology, 9(9), 285. https://doi.org/10.3390/biology9090285




