General Microbiota of the Soft Tick Ornithodoros turicata Parasitizing the Bolson Tortoise (Gopherus flavomarginatus) in the Mapimi Biosphere Reserve, Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
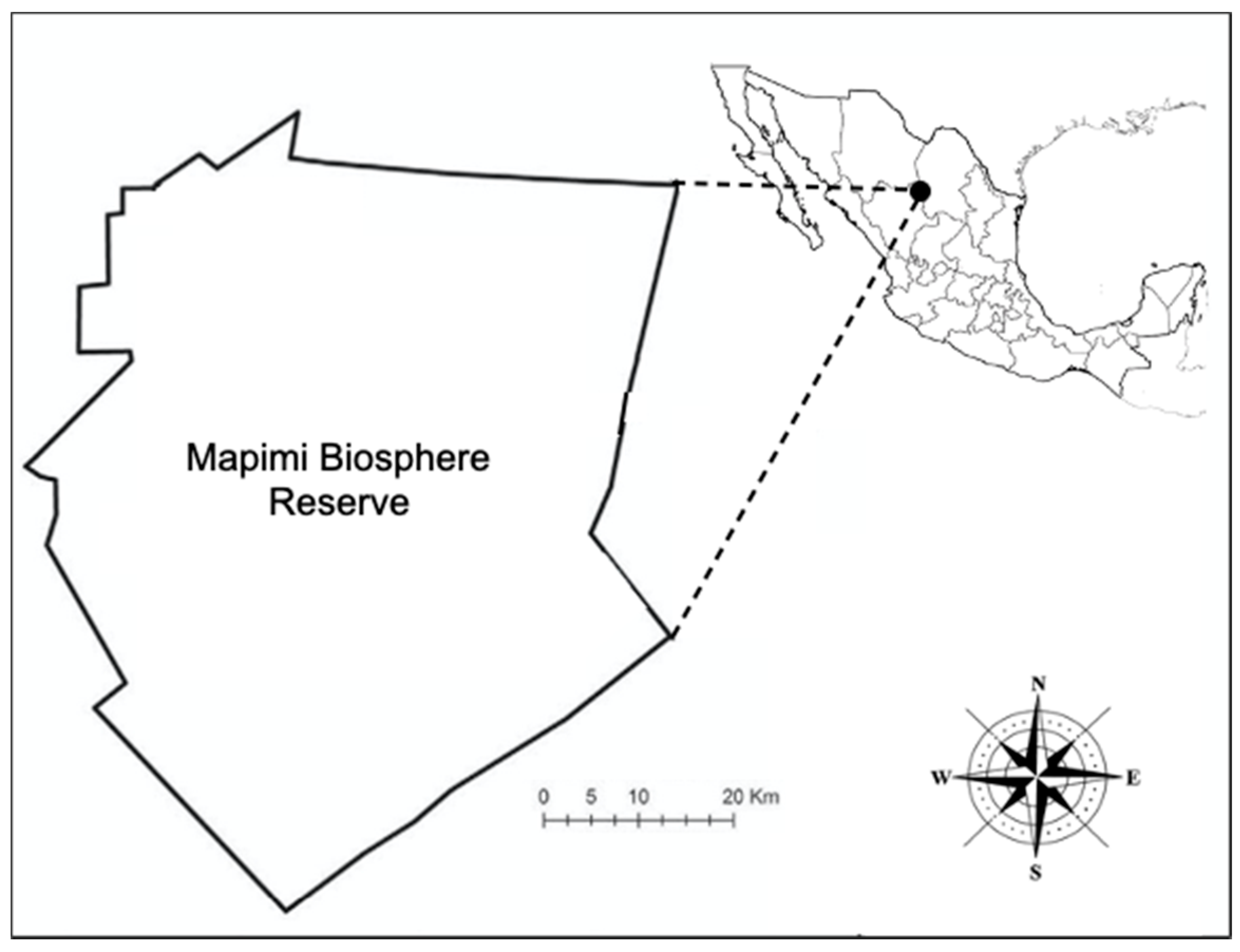
2.2. Location and Environmental Conditions
2.3. Field Work
2.4. DNA Extraction, Visualization, and Quantification
2.5. Bioinformatics Analyses
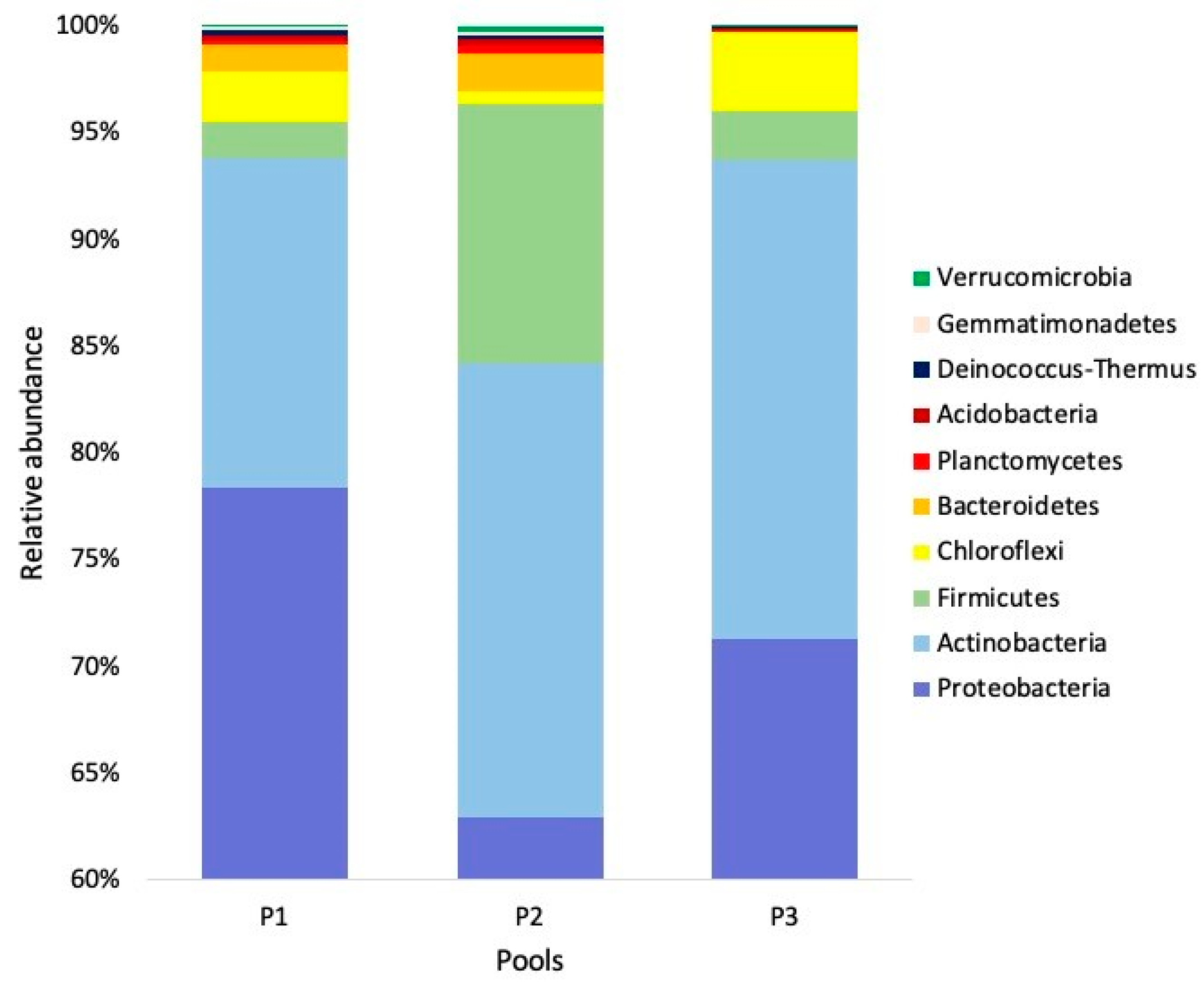
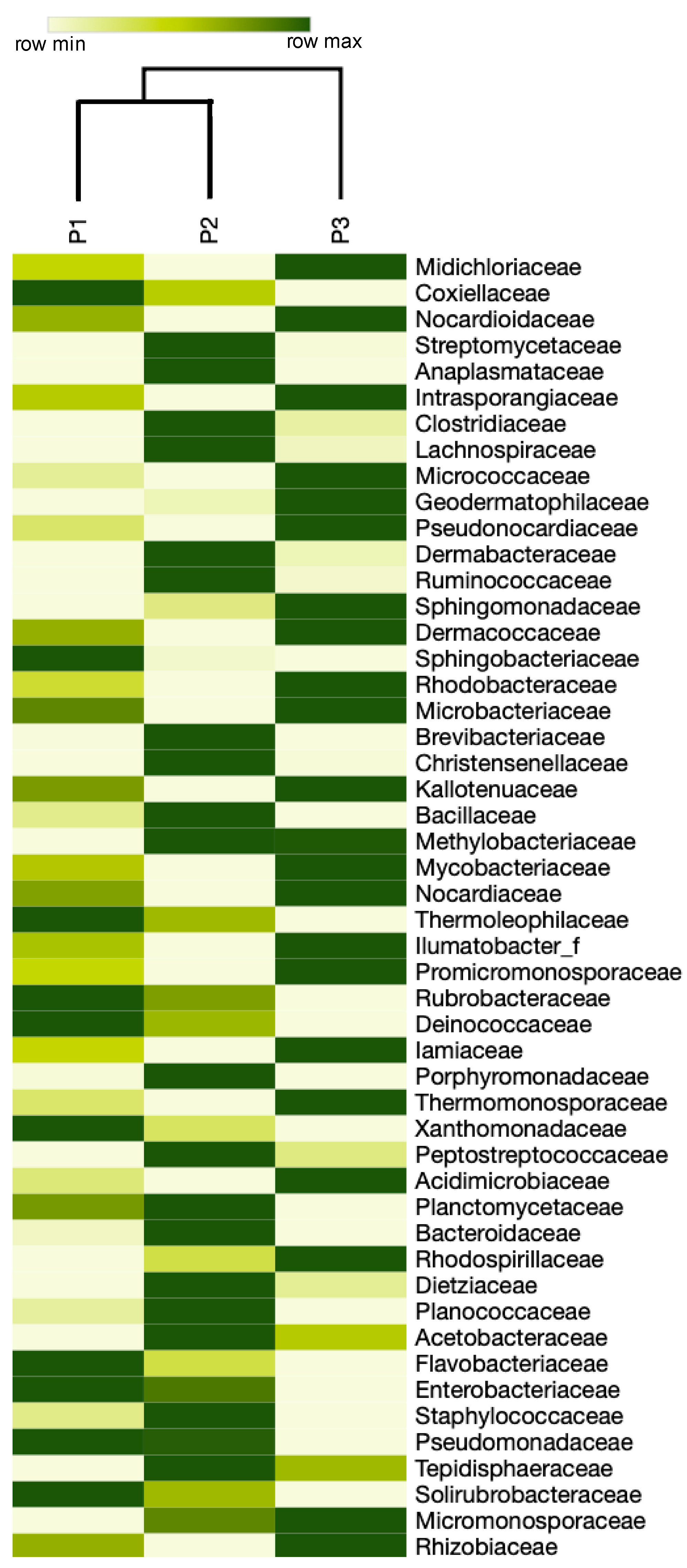
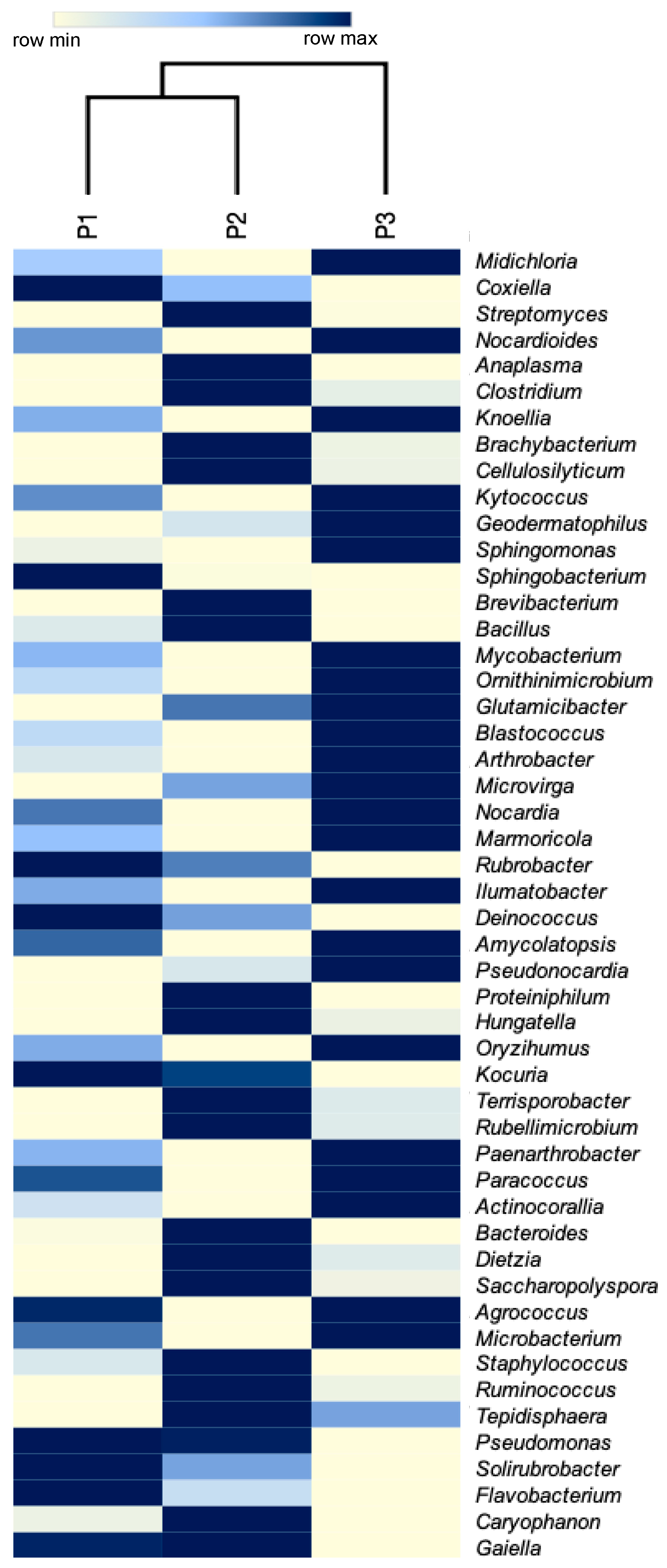
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manzano-Román, R.; Díaz-Martín, V.; de la Fuente, J.; Pérez-Sánchez, R. Soft ticks as pathogen vectors: Distribution, surveillance and control. In Parasitology; Manjur, M., Ed.; Intech: Rijeka, Croatia, 2012; pp. 125–162. [Google Scholar]
- Gupta, S.; Mortensen, M.S.; Schjørring, S.; Trivedi, U.; Vestergaard, G.; Stokholm, J.; Bisgaard, H.; Krogfelt, K.A.; Sørensen, S.J. Amplicon sequencing provides more accurate microbiome information in healthy children compared to culturing. Commun. Biol. 2019, 2, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollet, T.; Sprong, H.; Lejal, E.; Krawczyk, A.I.; Moutailler, S.; Cosson, J.-F.; Vayssier-Taussat, M.; Estrada-Peña, A. The scale affects our view on the identification and distribution of microbial communities in ticks. Parasite. Vector. 2020, 13, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The tick microbiome: Why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Vayssier-Taussat, M.; Albina, E.; Citti, C.; Cosson, J.F.; Jacques, M.-A.; Lebrun, M.-H.; Le Loir, Y.; Ogliastro, M.; Petit, M.-A.; Roumagnac, P. Shifting the paradigm from pathogens to pathobiome: New concepts in the light of meta-omics. Front. Cell. Infect. Microbiol. 2014, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Narasimhan, S.; Fikrig, E. Tick microbiome: The force within. Trends Parasitol. 2015, 31, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Lalzar, I.; Friedmann, Y.; Gottlieb, Y. Tissue tropism and vertical transmission of Coxiella in Rhipicephalus sanguineus and Rhipicephalus turanicus ticks. Environ. Microbiol. 2014, 16, 3657–3668. [Google Scholar] [CrossRef]
- Varela-Stokes, A.S.; Park, S.H.; Kim, S.; Ricke, S.C. Microbial communities in North American ixodid ticks of veterinary and medical importance. Front. Vet. Sci. 2017, 4, 179. [Google Scholar] [CrossRef] [Green Version]
- Thapa, S.; Zhang, Y.; Allen, M.S. Bacterial microbiomes of Ixodes scapularis ticks collected from Massachusetts and Texas, USA. BMC Microbiol. 2019, 19, 138. [Google Scholar] [CrossRef] [Green Version]
- Madison-Antenucci, S.; Kramer, L.D.; Gebhardt, L.L.; Kauffman, E. Emerging Tick-Borne Diseases. Clin. Microbiol. Rev. 2020, 33, 33. [Google Scholar] [CrossRef]
- Nava, S.; Venzal, J.M.; Acuña, D.G.; Martins, T.F.; Guglielmone, A.A. Ticks of the Southern Cone of America: Diagnosis, Distribution, and Hosts with Taxonomy, Ecology and Sanitary Importance; Academic Press: Cambridge, MA, USA, 2017; pp. 269–321. [Google Scholar] [CrossRef]
- Oliver, J.H., Jr. Biology and systematics of ticks (Acari: Ixodida). Annu. Rev. Ecol. Evol. Syst. 1989, 20, 397–430. [Google Scholar] [CrossRef]
- Donaldson, T.G.; de León, A.A.P.; Li, A.I.; Castro-Arellano, I.; Wozniak, E.; Boyle, W.K.; Hargrove, R.; Wilder, H.K.; Kim, H.J.; Teel, P.D. Assessment of the geographic distribution of Ornithodoros turicata (Argasidae): Climate variation and host diversity. PLOS Negl. Trop. Dis. 2016, 10, e0004383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milstrey, E.G. Bionomics and Ecology of Ornithodoros (P.) Turicata Americanus (Marx) (Ixodoidea: Argasidae) and Other Commensal Invertebrates Present in the Burrows of the Gopher Tortoise, Gopherus Polyphemus Daudin; University of Florida: Gainesville, FL, USA, 1987. [Google Scholar]
- Paredes-León, R.; García-Prieto, L.; Guzmán-Cornejo, C.; León-Regagnon, V.; Pérez, T.M. Metazoan parasites of Mexican amphibians and reptiles. Zootaxa 2008, 1904, 1–166. [Google Scholar] [CrossRef]
- SEMARNAT (Secretaria de Medio Ambiente y Recursos Naturales). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación 2010, 12, 1–180. [Google Scholar]
- Kiester, A.; Palomo-Ramos, R.; Ríos-Arana, J.; Goode, E. Gopherus flavomarginatus. The IUCN Red List of Threatened Species 2018: E.T9402A112660985. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T9402A112660985.en (accessed on 21 August 2020).
- Dziadzio, M.C.; Smith, L.L. Vertebrate use of gopher tortoise burrows and aprons. Southeast. Nat. 2016, 15, 586–594. [Google Scholar] [CrossRef]
- FASS. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; f Animal Science Journal: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- García, E. Modificaciones al Sistema de Clasificación Climática de Köppen; Universidad Nacional Autónoma de México: Ciudad de México, Mexico, 2004; 246p. [Google Scholar]
- Cornet, A. Principales caractéristiques climatiques. In Estudio Integrado de Los Recursos Vegetación, Suelo Y Agua en la Reserva de la Biósfera de Mapimí; Montaña, C., Ed.; Instituto de Ecología, A.C.: Veracruz, México, 1988; pp. 45–76. [Google Scholar]
- Rzedowski, J. Vegetación de México, 1st ed.; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad: Ciudad de México, México, 2006; p. 504.
- Barnard, S.M.; Durden, L.A. A Veterinary Guide to the Parasites of Reptiles; Krieger: Melbourne, FL, USA, 2000; Volume 2, 288p. [Google Scholar]
- Morafka, D.J. A historical biogeography of the Chihuahuan herpetofauna. In A Biogeographical Analysis of the Chihuahuan Desert through Its Herpetofauna; Springer: Dodlerk, The Netherlands, 1977; pp. 159–215. [Google Scholar]
- Couper, L.; Swei, A. Tick microbiome characterization by next-generation 16S rRNA amplicon sequencing. JoVE 2018, 138, e58239. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic. Acids. Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Illumina. 16S Metagenomic Sequencing Library Preparation, Preparing 16S Ribosomal RNA Gene Amplicons for the Illumina MiSeq System. Available online: https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf (accessed on 21 August 2020).
- Illumina. Nextera XT DNA Library Prep Kit Reference Guide. Available online: https://support.illumina.com/content/dam/illumina-support/documents/documentation/chemistry_documentation/samplepreps_nextera/nextera-xt/nextera-xt-library-prep-reference-guide-15031942-05.pdf (accessed on 21 August 2020).
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morpheus. Available online: https://software.broadinstitute.org/morpheus/ (accessed on 21 August 2020).
- Budachetri, K.; Gaillard, D.; Williams, J.; Mukherjee, N.; Karim, S. A snapshot of the microbiome of Amblyomma tuberculatum ticks infesting the gopher tortoise, an endangered species. Ticks Tick-Borne Dis. 2016, 7, 1225–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzatti, G.; Lopetuso, L.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. Biomed Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, P.; Qiu, Z.; Zhang, T.; Li, Y.; Wang, W.; Li, M.; Yu, Z.; Liu, J. Microbial diversity in the tick Argas japonicus (Acari: Argasidae) with a focus on Rickettsia pathogens. Med. Vet. Entomol. 2019, 33, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haitham, E.; Faisal, A.; Naser, A.A. A glimpse of the bacteriome of Hyalomma dromedarii ticks infesting camels reveals human Helicobacter pylori pathogen. J. Infect. Dev. Ctries. 2019, 13, 1001–1012. [Google Scholar]
- Zhang, Y.-K.; Yu, Z.-J.; Wang, D.; Bronislava, V.; Branislav, P.; Liu, J.-Z. The bacterial microbiome of field-collected Dermacentor marginatus and Dermacentor reticulatus from Slovakia. Parasites Vectors 2019, 12, 325. [Google Scholar] [CrossRef]
- Portillo, A.; Palomar, A.M.; de Toro, M.; Santibáñez, S.; Santibáñez, P.; Oteo, J.A. Exploring the bacteriome in anthropophilic ticks: To investigate the vectors for diagnosis. PLoS ONE 2019, 14, e0213384. [Google Scholar] [CrossRef] [Green Version]
- Ahantarig, A.; Trinachartvanit, V.; Baimai, V.; Grubhoffer, L. Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol. 2013, 58, 419–428. [Google Scholar] [CrossRef]
- Haine, E.R. Symbiont-mediated protection. Proc. R. Soc. B-Biol. Sci. 2008, 275, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Walker, T.; Johnson, P.; Moreira, L.; Iturbe-Ormaetxe, I.; Frentiu, F.; McMeniman, C.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Williams-Newkirk, A.J.; Rowe, L.A.; Mixson-Hayden, T.R.; Dasch, G.A. Presence, genetic variability, and potential significance of “Candidatus Midichloria mitochondrii” in the lone star tick Amblyomma americanum. Exp. Appl. Acarol. 2012, 58, 291–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Liu, M.-X.; Ren, Q.-Y.; Chen, Z.; Tian, Z.-C.; Hao, J.-W.; Wu, F.; Liu, X.-C.; Luo, J.-X.; Yin, H. Micropathogen community analysis in Hyalomma rufipes via high-throughput sequencing of small RNAs. Front. Cell. Infect. Microbiol. 2017, 7, 374. [Google Scholar] [CrossRef] [PubMed]
- Beninati, T.; Lo, N.; Sacchi, L.; Genchi, C.; Noda, H.; Bandi, C. A novel alpha-Proteobacterium resides in the mitochondria of ovarian cells of the tick Ixodes ricinus. Appl. Environ. Microbiol. 2004, 70, 2596–2602. [Google Scholar] [CrossRef] [Green Version]
- Lo, N.; Beninati, T.; Sassera, D.; Bouman, E.A.; Santagati, S.; Gern, L.; Sambri, V.; Masuzawa, T.; Gray, J.S.; Jaenson, T.G.; et al. Widespread distribution and high prevalence of an alpha-proteobacterial symbiont in the tick Ixodes ricinus. Environ. Microbiol. 2006, 8, 1280–1287. [Google Scholar] [CrossRef] [Green Version]
- Mariconti, M.; Epis, S.; Gaibani, P.; Valle, C.D.; Sassera, D.; Tomao, P.; Fabbi, M.; Castelli, F.; Marone, P.; Sambri, V. Humans parasitized by the hard tick Ixodes ricinus are seropositive to Midichloria mitochondrii: Is Midichloria a novel pathogen, or just a marker of tick bite? Pathog. Glob. Health 2012, 106, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Moran, N.A.; McCutcheon, J.P.; Nakabachi, A. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 2008, 42, 165–190. [Google Scholar] [CrossRef] [Green Version]
- Duron, O.; Noël, V.; Mccoy, K.D.; Bonazzi, M.; Sidi-Boumedine, K.; Morel, O.; Vavre, F.; Zenner, L.; Jourdain, E.; Durand, P. The recent evolution of a maternally-inherited endosymbiont of ticks led to the emergence of the Q fever pathogen, Coxiella burnetii. PLoS Pathog. 2015, 11, e1004892. [Google Scholar] [CrossRef] [Green Version]
- Trinachartvanit, W.; Maneewong, S.; Kaenkan, W.; Usananan, P.; Baimai, V.; Ahantarig, A. Coxiella-like bacteria in fowl ticks from Thailand. Parasites Vectors 2018, 11, 670. [Google Scholar] [CrossRef]
- Balashov, Y.S.; Daiter, A.B. Blood-Feeding Artropods and Rickettsiae; Herald Russian Academy of Sciences; Nauka: Leningrad, Russia, 1973; 251p. [Google Scholar]
- Noda, H.; Munderloh, U.G.; Kurtti, T.J. Endosymbionts of ticks and their relationship to Wolbachia spp. and tick-borne pathogens of humans and animals. Appl. Environ. Microbiol. 1997, 63, 3926–3932. [Google Scholar] [CrossRef] [Green Version]
- Mediannikov, O.; Fenollar, F.; Socolovschi, C.; Diatta, G.; Bassene, H.; Molez, J.-F.; Sokhna, C.; Trape, J.-F.; Raoult, D. Coxiella burnetii in humans and ticks in rural Senegal. PLOS Negl. Trop. Dis. 2010, 4, e654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldin, C.; Melenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J.-L.; Maurin, M.; Raoult, D. From Q fever to Coxiella burnetii infection: A paradigm change. Clin. Microbiol. Rev. 2017, 30, 115–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duron, O.; Sidi-Boumedine, K.; Rousset, E.; Moutailler, S.; Jourdain, E. The importance of ticks in Q fever transmission: What has (and has not) been demonstrated? Trends Parasitol. 2015, 31, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Sprong, H.; Tijsse-Klasen, E.; Langelaar, M.; De Bruin, A.; Fonville, M.; Gassner, F.; Takken, W.; Van Wieren, S.; Nijhof, A.; Jongejan, F.; et al. Prevalence of Coxiella burnetii in ticks after a large outbreak of Q fever. Zoonoses Public Health 2012, 59, 69–75. [Google Scholar] [CrossRef]
- Hornok, S.; Szőke, K.; Meli, M.L.; Sándor, A.D.; Görföl, T.; Estók, P.; Wang, Y.; Tu, V.T.; Kováts, D.; Boldogh, S.A.; et al. Molecular detection of vector-borne bacteria in bat ticks (Acari: Ixodidae, Argasidae) from eight countries of the Old and New Worlds. Parasites Vectors 2019, 12, 50. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Sánchez, S.; Hernández-Jarguín, A.; Torina, A.; Fernández de Mera, I.G.; Blanda, V.; Caracappa, S.; Gortazar, C.; de la Fuente, J. Characterization of the bacterial microbiota in wild-caught Ixodes ventalloi. Ticks Tick Borne Dis. 2019, 10, 336–343. [Google Scholar] [CrossRef]
- Jacobson, E.R.; Berry, K.H.; Wellehan, J.F., Jr.; Origgi, F.; Childress, A.L.; Braun, J.; Schrenzel, M.; Yee, J.; Rideout, B. Serologic and molecular evidence for Testudinid herpesvirus 2 infection in wild Agassiz’s desert tortoises, Gopherus agassizii. J. Wildl. Dis. 2012, 48, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Guthrie, A.L.; White, C.L.; Brown, M.B.; deMaar, T.W. Detection of Mycoplasma agassizii in the Texas Tortoise (Gopherus berlandieri). J. Wildl. Dis. 2013, 49, 704–708. [Google Scholar] [CrossRef]
- Gofton, A.W.; Doggett, S.; Ratchford, A.; Oskam, C.L.; Paparini, A.; Ryan, U.; Irwin, P. Bacterial profiling reveals novel “Ca. Neoehrlichia”, Ehrlichia, and Anaplasma species in Australian human-biting ticks. PLoS ONE 2015, 10, e0145449. [Google Scholar] [CrossRef] [Green Version]
- Atif, F.A. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol. Res. 2015, 114, 3941–3957. [Google Scholar] [CrossRef]
- Cabezas-Cruz, A.; Gallois, M.; Fontugne, M.; Allain, E.; Denoual, M.; Moutailler, S.; Devillers, E.; Zientara, S.; Memmi, M.; Chauvin, A. Epidemiology and genetic diversity of Anaplasma ovis in goats in Corsica, France. Parasites Vectors 2019, 12, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schouls, L.M.; Van De Pol, I.; Rijpkema, S.G.; Schot, C.S. Detection and Identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J. Clin. Microbiol. 1999, 37, 2215–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawahara, M.; Rikihisa, Y.; Isogai, E.; Takahashi, M.; Misumi, H.; Suto, C.; Shibata, S.; Zhang, C.; Tsuji, M. Ultrastructure and phylogenetic analysis of “Candidatus Neoehrlichia mikurensis” in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 2004, 54, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Beck, R.; Oteo, J.A.; Pfeffer, M.; Sprong, H. Neoehrlichiosis: An emerging tick-borne zoonosis caused by Candidatus Neoehrlichia mikurensis. Exp. Appl. Acarol. 2016, 68, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Kim, C.-M.; Kim, D.-M.; Yoon, N.-R.; Jha, B.; Park, J.W.; Chung, J.K. First detection and identification of Candidatus Neoehrlichia mikurensis in South Korea. PLoS ONE 2018, 13, e0209685. [Google Scholar] [CrossRef] [PubMed]
- Yabsley, M.J.; Murphy, S.M.; Luttrell, M.P.; Wilcox, B.R.; Howerth, E.W.; Munderloh, U.G. Characterization of “Candidatus Neoehrlichia lotoris” (family Anaplasmataceae) from raccoons (Procyon lotor). Int. J. Syst. Evol. Microbiol. 2008, 58, 2794. [Google Scholar] [CrossRef]
- Gofton, A.W.; Oskam, C.L.; Lo, N.; Beninati, T.; Wei, H.; McCarl, V.; Murray, D.C.; Paparini, A.; Greay, T.L.; Holmes, A.J. Inhibition of the endosymbiont “Candidatus Midichloria mitochondrii” during 16S rRNA gene profiling reveals potential pathogens in Ixodes ticks from Australia. Parasites Vectors 2015, 8, 345. [Google Scholar] [CrossRef]
- Davis, G.E. A Relapsing Fever Spirochete, Borrelia mazzottii (sp. nov.), from Ornithodoros talaje from Mexico. Am. J. Hyg. 1956, 63, 13–17. [Google Scholar] [CrossRef]
- Krishnavajhala, A.; Armstrong, B.A.; Lopez, J.E. Vector competence of geographical populations of Ornithodoros turicata for the tick-borne relapsing fever spirochete Borrelia turicatae. Appl. Environ. Microbiol. 2018, 84, e01505–e01518. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Li, A.Y.; Teel, P.D.; de León, A.A.P.; Seshu, J.; Liu, J. Biological and physiological characterization of in vitro blood feeding in nymph and adult stages of Ornithodoros turicata (Acari: Argasidae). J. Insect. Physiol. 2015, 75, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Sethi, M. Poikilotherms as reservoirs of Q-fever (Coxiella burnetii) in Uttar Pradesh. J. Wildl. Dis. 1979, 15, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Majláthová, V.; Majláth, I.; Hromada, M.; Tryjanowski, P.; Bona, M.; Antczak, M.; Víchová, B.; Dzimko, Š.; Mihalca, A.; Peťko, B. The role of the sand lizard (Lacerta agilis) in the transmission cycle of Borrelia burgdorferi sensu lato. Int. J. Med. Microbiol. 2008, 298, 161–167. [Google Scholar] [CrossRef]
- Peter, T.F.; Burridge, M.J.; Mahan, S.M. Competence of the african tortoise tick, Amblyomma marmoreum (acari: Ixodidae), as a vector of the agent of heartwater (Cowdria ruminantium). J. Parasitol. 2000, 86, 438–441. [Google Scholar] [PubMed]
- Široký, P.; Kamler, M.; Modrý, D. Long-term occurrence of Hemolivia cf. mauritanica (Apicomplexa: Adeleina: Haemogregarinidae) in captive Testudo marginata (Reptilia: Testudinidae): Evidence for cyclic merogony? J. Parasitol. 2004, 90, 1391–1393. [Google Scholar] [CrossRef]
- Råberg, L.; Sim, D.; Read, A.F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007, 318, 812–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, D.S.; Ayres, J.S. Two ways to survive infection: What resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 2008, 8, 889–895. [Google Scholar] [CrossRef]
- Medzhitov, R.; Schneider, D.S.; Soares, M.P. Disease tolerance as a defense strategy. Science 2012, 335, 936–941. [Google Scholar] [CrossRef] [Green Version]

| Pool | Total | Assembled | Discarded | ChS | QS | BS | OTUs |
|---|---|---|---|---|---|---|---|
| P1 | 424,827 | 278,006 | 146,821 | 1580 | 276,426 | 272,339 | 56,998 |
| P2 | 457,353 | 202,476 | 254,877 | 1633 | 200,843 | 195,622 | 41,791 |
| P3 | 159,929 | 109,211 | 50,718 | 278 | 108,616 | 104,347 | 34,318 |
| Mean | 347,370 | 196,564 | 150,805 | 1164 | 195,295 | 190,769 | 44,369 |
| Bacterial Species | Bacterial Species | Bacterial Species |
|---|---|---|
| Actinocorallia libanotica | Kribbia dieselivorans | Phycicoccus jejuensis |
| Actinophytocola timorensis | Kytococcus aerolatus | Phytomonospora endophytica |
| Aeromicrobium panaciterrae | Kytococcus schroeteri | Piscicoccus intestinalis |
| Agrococcus terreus | Kytococcus sedentarius | Planosporangium thailandense |
| Anaerocella delicata | Lactobacillus sakei | Propionibacterium acnés |
| Anaplasma marginale | Lactococcus lactis | Rubellimicrobium mesophilum |
| Anaplasma ovis | Lentzea kentuckyensis | Saccharopolyspora hirsuta |
| Anaplasma phagocytophilum | Leucobacter celer | Salmonella entérica |
| Anseongella ginsenosidimutans | Leucobacter chromiireducens | Segetibacter koreensis |
| Arthrobacter halodurans | Leucobacter tardus | Seinonella peptonophila |
| Bacillus cecembensis | Leucobacter zeae | Serinicoccus profundi |
| Bacillus halosaccharovorans | Lysobacter dokdonensis | Sinomonas mesophila |
| Bacillus niacini | Magnospira bakii | Skermanella aerolata |
| Bauldia litoralis | Marmoricola bigeumensis | Skermanella stibiiresistens |
| Blautia wexlerae | Marmoricola pocheonensis | Smaragdicoccus niigatensis |
| Brachybacterium ginsengisoli | Marmoricola terrae | Sphingoaurantiacus polygranulatus |
| Brachybacterium phenoliresistens | Massilia brevitalea | Sphingobacterium changzhouense |
| Brachybacterium squillarum | Methylobacterium oxalidis | Sphingobacterium hotanense |
| Brachybacterium zhongshanense | Micromonospora taraxaci | Sphingobacterium mucilaginosum |
| Cellulosilyticum lentocellum | Midichloria mitochondrii | Sphingobacterium siyangense |
| Citricoccus yambaruensis | Mobilicoccus pelagius | Sphingobacterium suaedae |
| Clavibacter michiganensis | Mycobacterium rutilum | Stackebrandtia cavernae |
| Clostridium butyricum | Nocardia brevicatena | Staphylococcus cohnii |
| Clostridium chartatabidum | Nocardia puris | Staphylococcus hominis |
| Clostridium máximum | Nocardioides aestuarii | Staphylococcus saprophyticus |
| Coxiella burnetii | Nocardioides agariphilus | Staphylococcus sciuri |
| Cronobacter dublinensis | Nocardioides albertanoniae | Staphylococcus succinus |
| Dermabacter vaginalis | Nocardioides daedukensis | Stenotrophobacter namibiensis |
| Eubacterium eligens | Nocardioides glacieisoli | Streptomyces bacillaris |
| Flavobacterium anatoliense | Nocardioides islandensis | Streptomyces baliensis |
| Geodermatophilus arenarius | Nocardioides kongjuensis | Streptomyces barkulensis |
| Geodermatophilus nigrescens | Nocardioides lianchengensis | Streptomyces cacaoi |
| Geodermatophilus obscurus | Nocardioides luteus | Streptomyces drozdowiczii |
| Geodermatophilus saharensis | Nocardioides mesophilus | Streptomyces fenghuangensis |
| Geodermatophilus soli | Nocardioides pyridinolyticus | Streptomyces glaucosporus |
| Glutamicibacter creatinolyticus | Nocardioides tritolerans | Streptomyces mangrovi |
| Glutamicibacter nicotianae | Ornithinimicrobium humiphilum | Streptomyces murinus |
| Helcobacillus massiliensis | Ornithinimicrobium kibberense | Streptomyces thermoviolaceus |
| Janibacter alkaliphilus | Ornithinimicrobium murale | Syntrophomonas curvata |
| Janibacter corallicola | Ornithinimicrobium pekingense | Syntrophomonas wolfei |
| Kallotenue papyrolyticum | Ornithinimicrobium tianjinense | Taibaiella chishuiensis |
| Kibdelosporangium aridum | Paenarthrobacter nitroguajacolicus | Terracoccus luteus |
| Kineococcus radiotolerans | Paraburkholderia caledonica | Tetrasphaera elongata |
| Kineosphaera limosa | Paracoccus saliphilus | Tetrasphaera remsis |
| Knoellia aerolata | Paracoccus sphaerophysae | Tetrasphaera vanveenii |
| Knoellia sinensis | Pasteurella testudinis | Thermobaculum terrenum |
| Kocuria flava | Phycicoccus endophyticus |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barraza-Guerrero, S.I.; Meza-Herrera, C.A.; García-De la Peña, C.; González-Álvarez, V.H.; Vaca-Paniagua, F.; Díaz-Velásquez, C.E.; Sánchez-Tortosa, F.; Ávila-Rodríguez, V.; Valenzuela-Núñez, L.M.; Herrera-Salazar, J.C. General Microbiota of the Soft Tick Ornithodoros turicata Parasitizing the Bolson Tortoise (Gopherus flavomarginatus) in the Mapimi Biosphere Reserve, Mexico. Biology 2020, 9, 275. https://doi.org/10.3390/biology9090275
Barraza-Guerrero SI, Meza-Herrera CA, García-De la Peña C, González-Álvarez VH, Vaca-Paniagua F, Díaz-Velásquez CE, Sánchez-Tortosa F, Ávila-Rodríguez V, Valenzuela-Núñez LM, Herrera-Salazar JC. General Microbiota of the Soft Tick Ornithodoros turicata Parasitizing the Bolson Tortoise (Gopherus flavomarginatus) in the Mapimi Biosphere Reserve, Mexico. Biology. 2020; 9(9):275. https://doi.org/10.3390/biology9090275
Chicago/Turabian StyleBarraza-Guerrero, Sergio I., César A. Meza-Herrera, Cristina García-De la Peña, Vicente H. González-Álvarez, Felipe Vaca-Paniagua, Clara E. Díaz-Velásquez, Francisco Sánchez-Tortosa, Verónica Ávila-Rodríguez, Luis M. Valenzuela-Núñez, and Juan C. Herrera-Salazar. 2020. "General Microbiota of the Soft Tick Ornithodoros turicata Parasitizing the Bolson Tortoise (Gopherus flavomarginatus) in the Mapimi Biosphere Reserve, Mexico" Biology 9, no. 9: 275. https://doi.org/10.3390/biology9090275
APA StyleBarraza-Guerrero, S. I., Meza-Herrera, C. A., García-De la Peña, C., González-Álvarez, V. H., Vaca-Paniagua, F., Díaz-Velásquez, C. E., Sánchez-Tortosa, F., Ávila-Rodríguez, V., Valenzuela-Núñez, L. M., & Herrera-Salazar, J. C. (2020). General Microbiota of the Soft Tick Ornithodoros turicata Parasitizing the Bolson Tortoise (Gopherus flavomarginatus) in the Mapimi Biosphere Reserve, Mexico. Biology, 9(9), 275. https://doi.org/10.3390/biology9090275






