A Lipidomic Analysis of Leaves of Esca-Affected Grapevine Suggests a Role for Galactolipids in the Defense Response and Appearance of Foliar Symptoms
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experimental Design
2.1.1. Grapevine Cultivar and Vineyard Characteristics
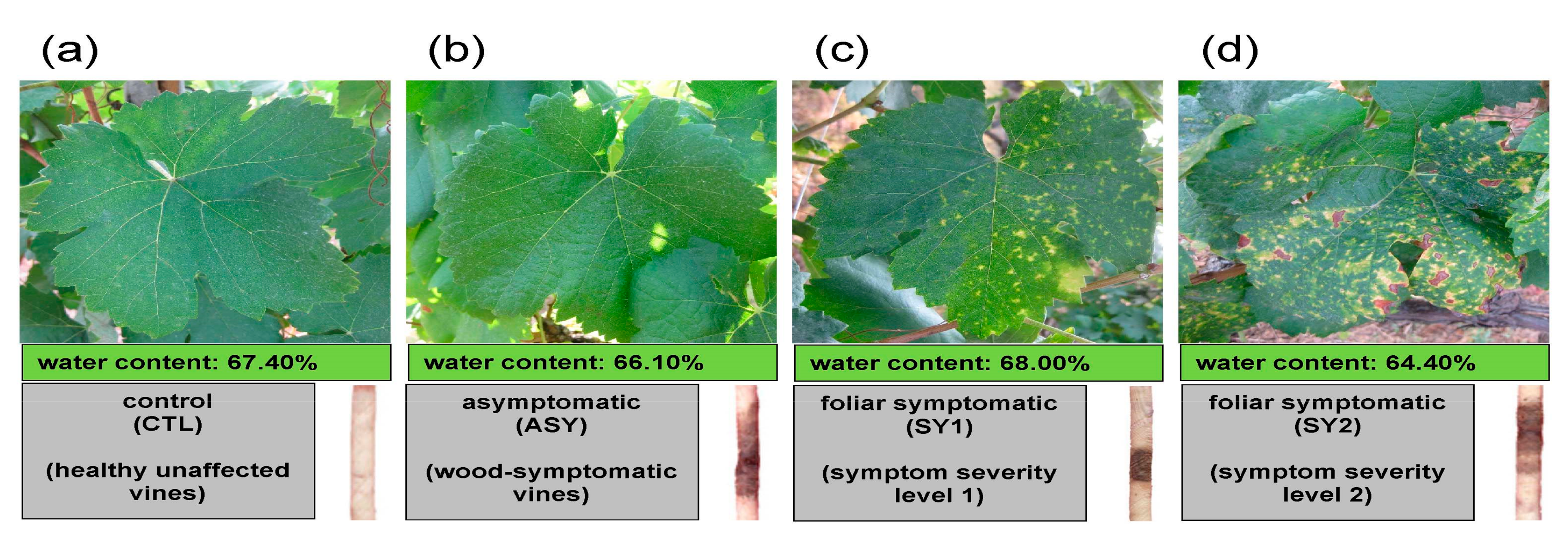
2.1.2. Internal and External Inspection of Vines
2.1.3. Collection and Processing of Leaf Samples
2.2. Lipidomic Analyses
2.2.1. Extraction of Compounds
2.2.2. Chromatographic Separations
2.2.3. Data Processing and Identification of Lipids
2.2.4. Statistical Calculations and Data Presentation
3. Results
3.1. Identification of Lipids Species, Hormones and Other Compounds of the Metabolism of Lipids in Grapevine Leaves
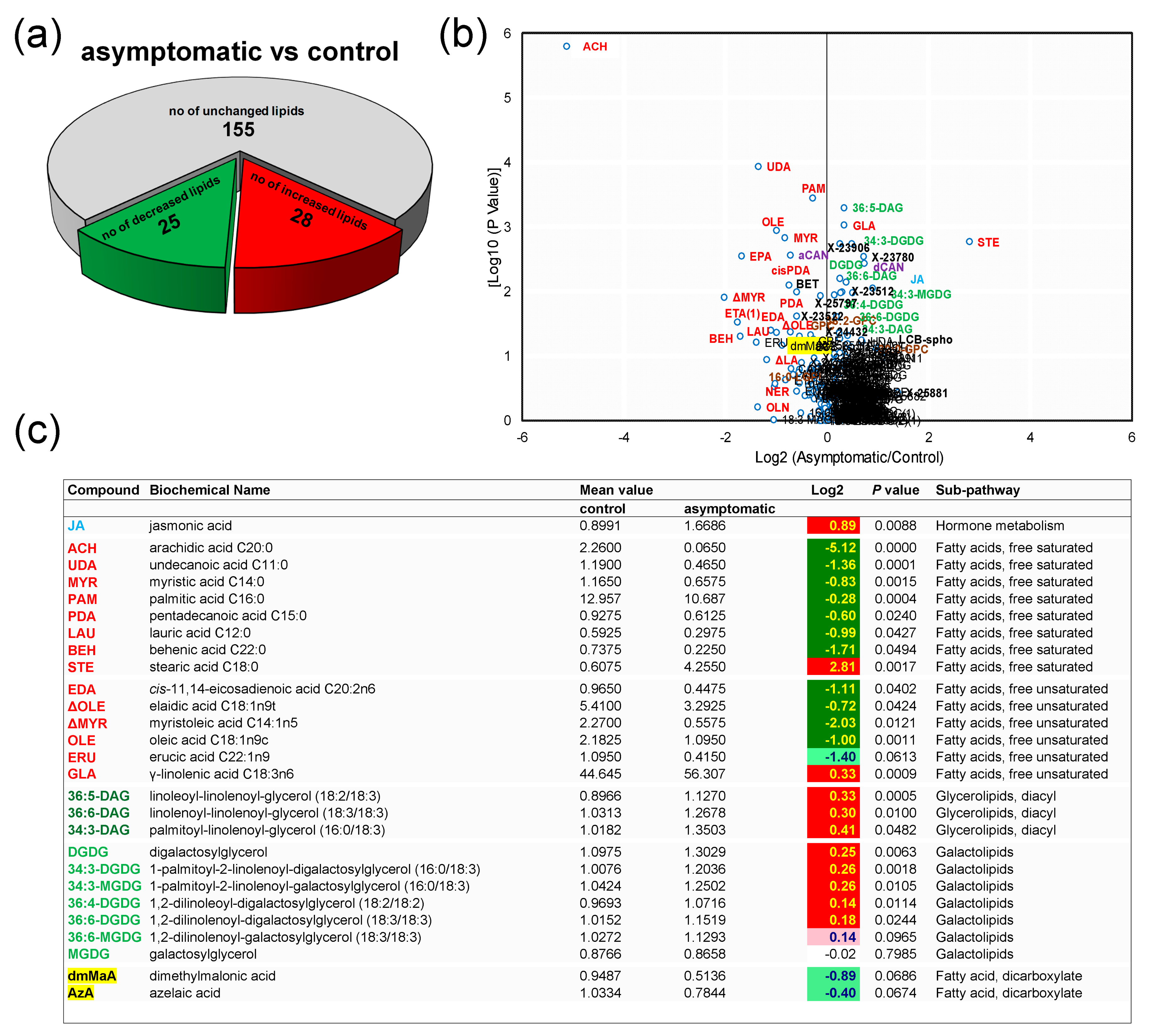
3.2. Control Samples versus Asymptomatic Samples
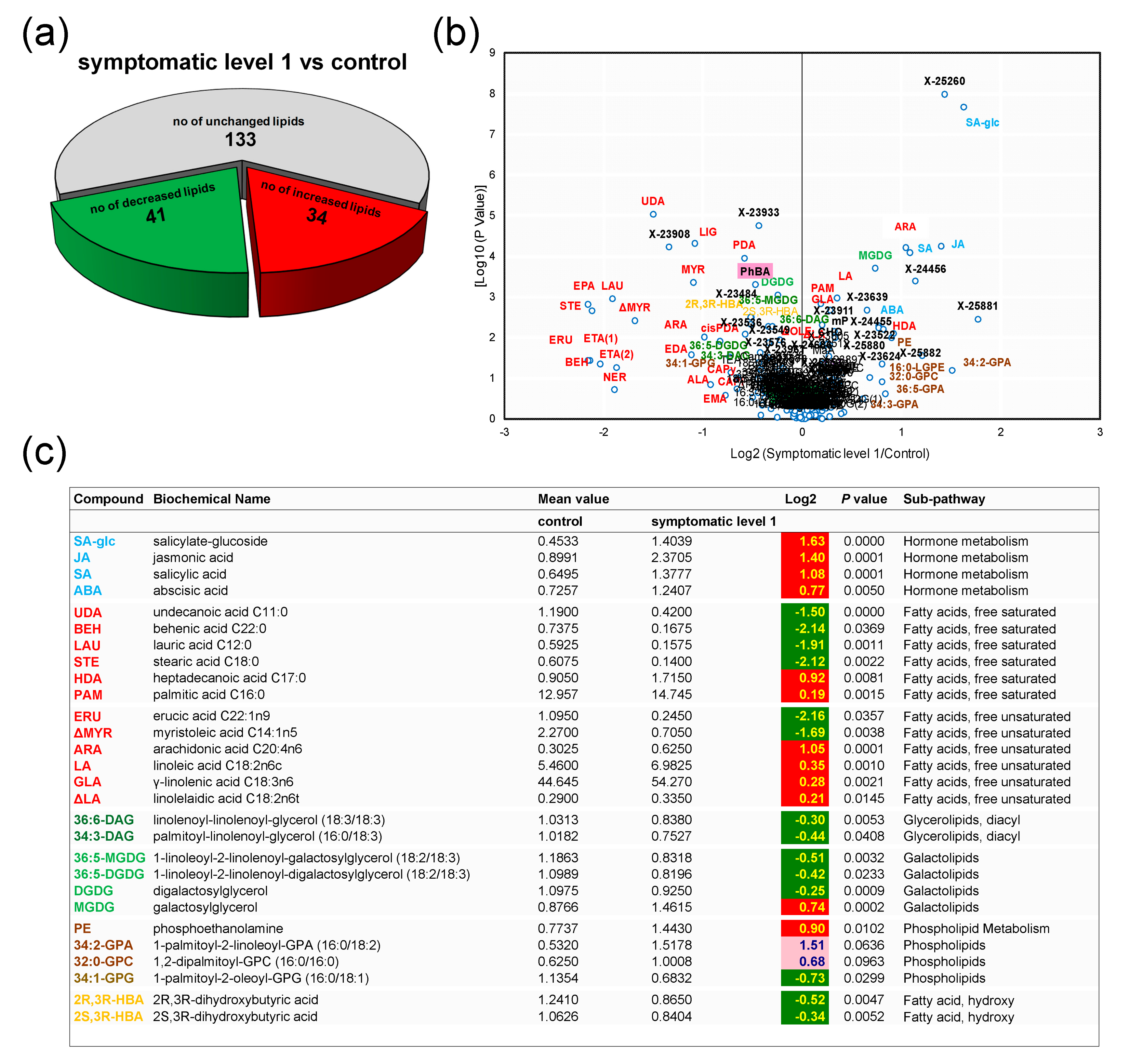
3.3. Control Samples versus Symptomatic Samples (Severity Level 1)
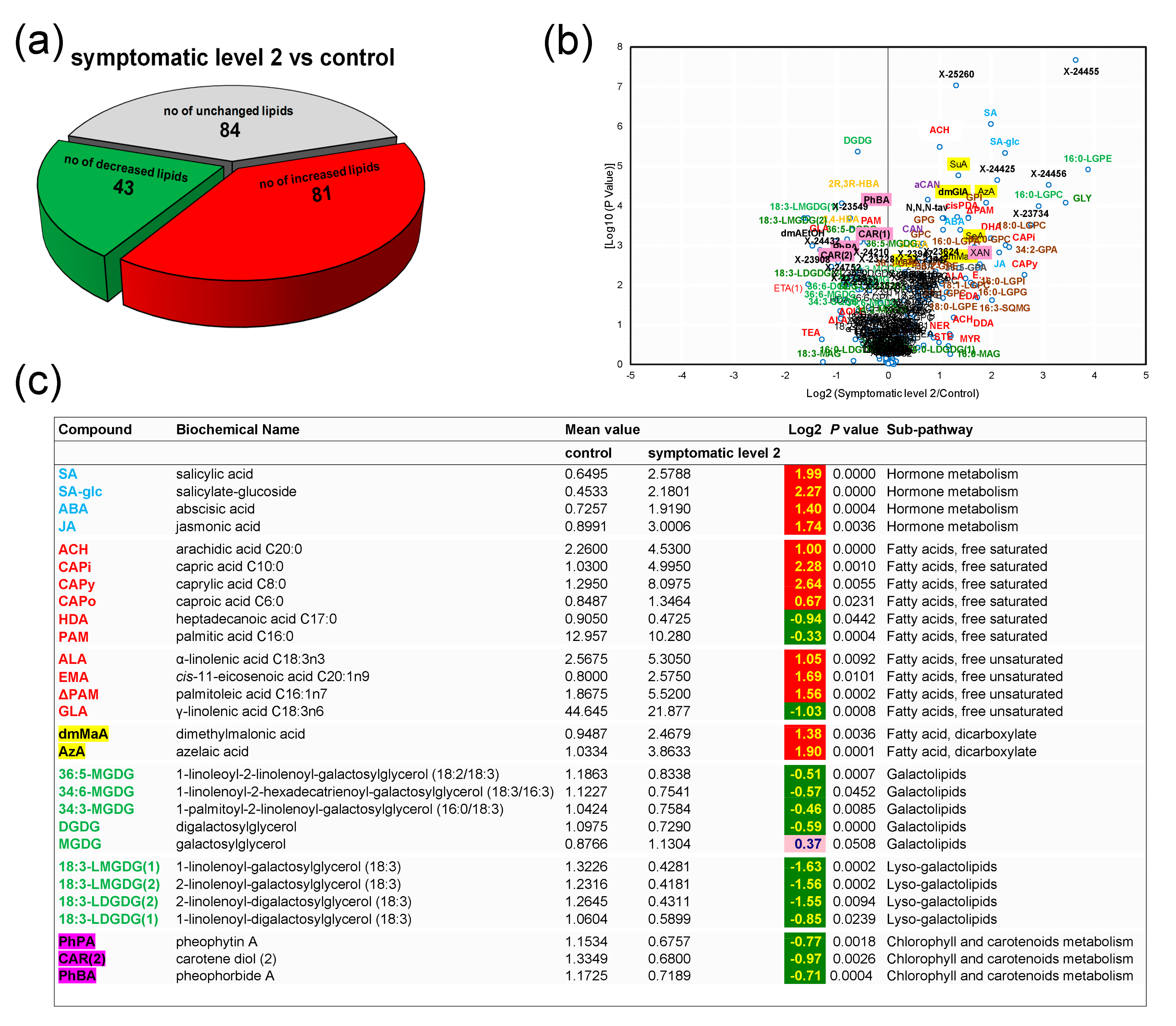
3.4. Control Samples versus Symptomatic Samples (Severity Level 2)
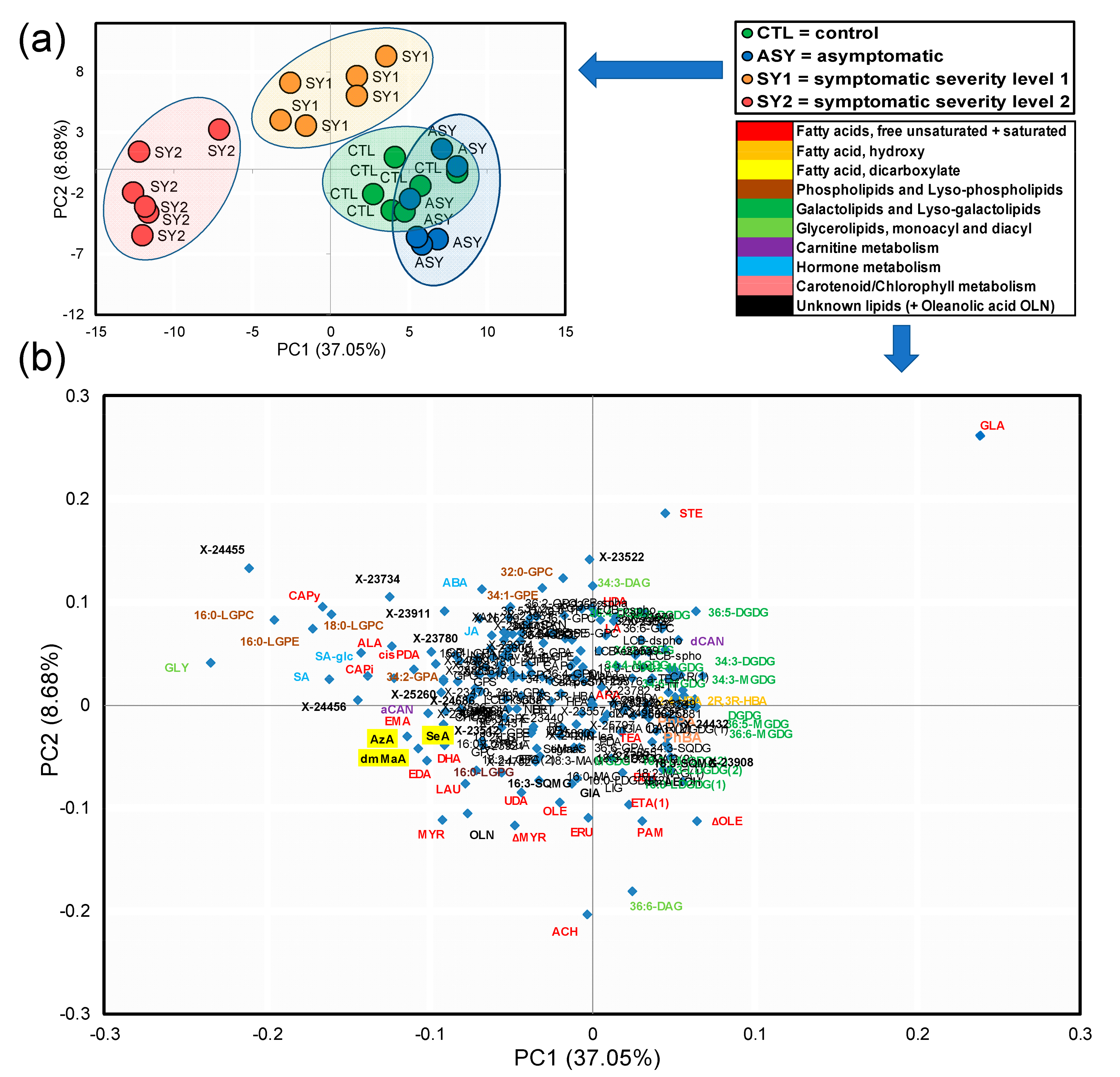
3.5. PCA Highlight of Holistic Differences among Experimental Groups
3.6. Identification of Top Lipid Candidate Markers
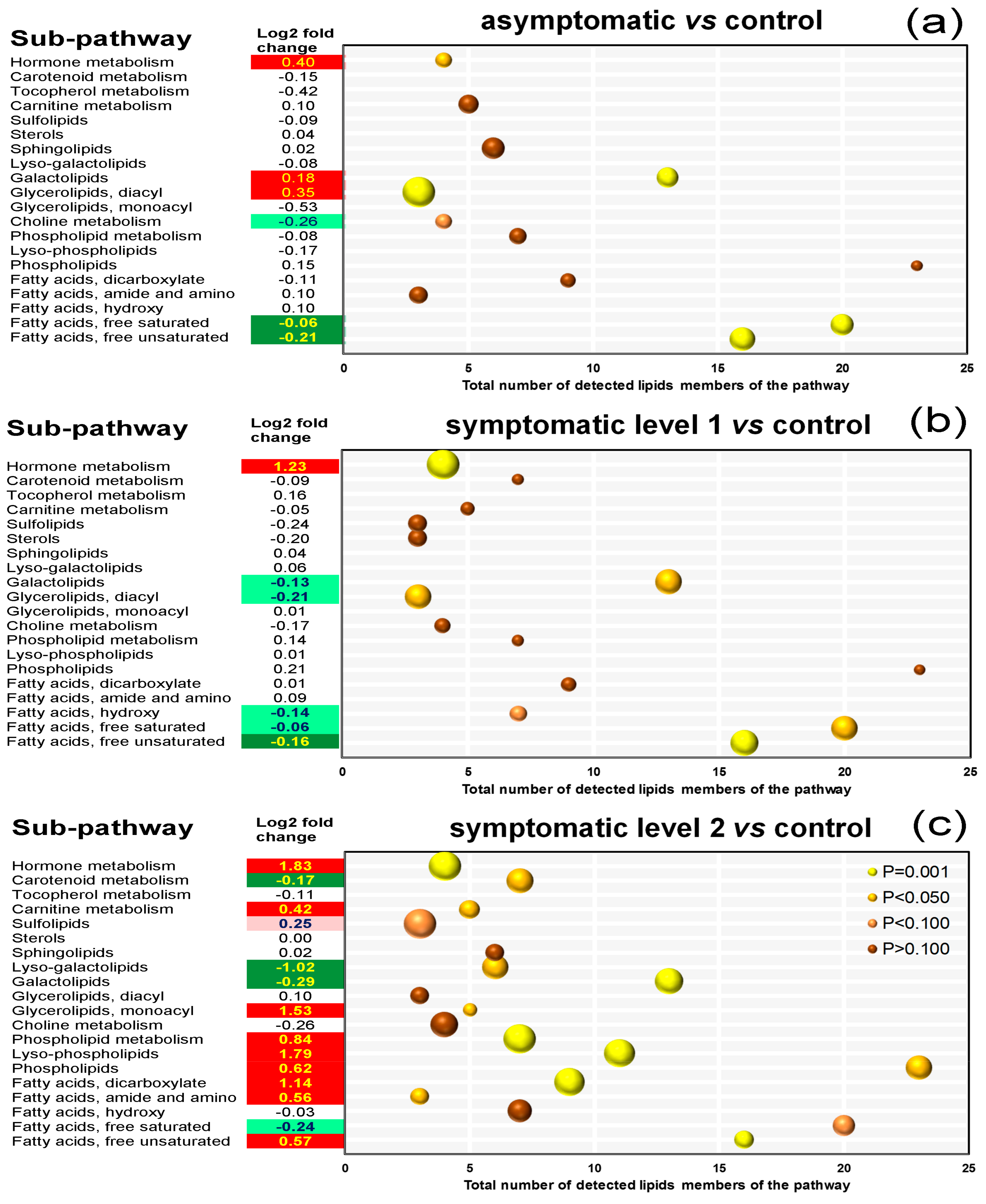
3.7. Pathway Enrichment Analysis of Differentially Accumulated Lipid Species
4. Discussion
4.1. Mobile Signals Are Generated in the Fungi-Infected Woods and Systemically Activate a Defense Response in Healthy Leaves
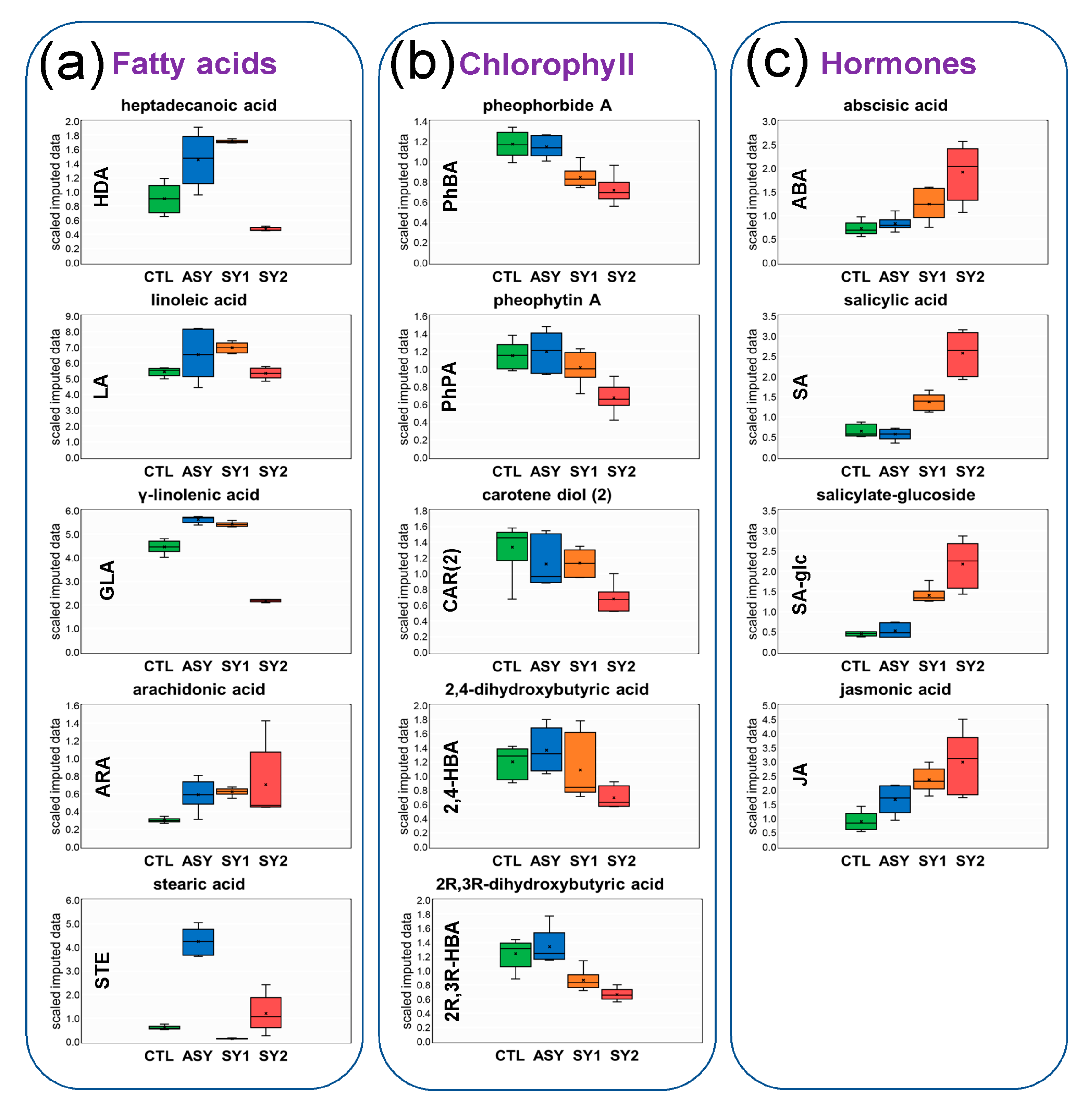
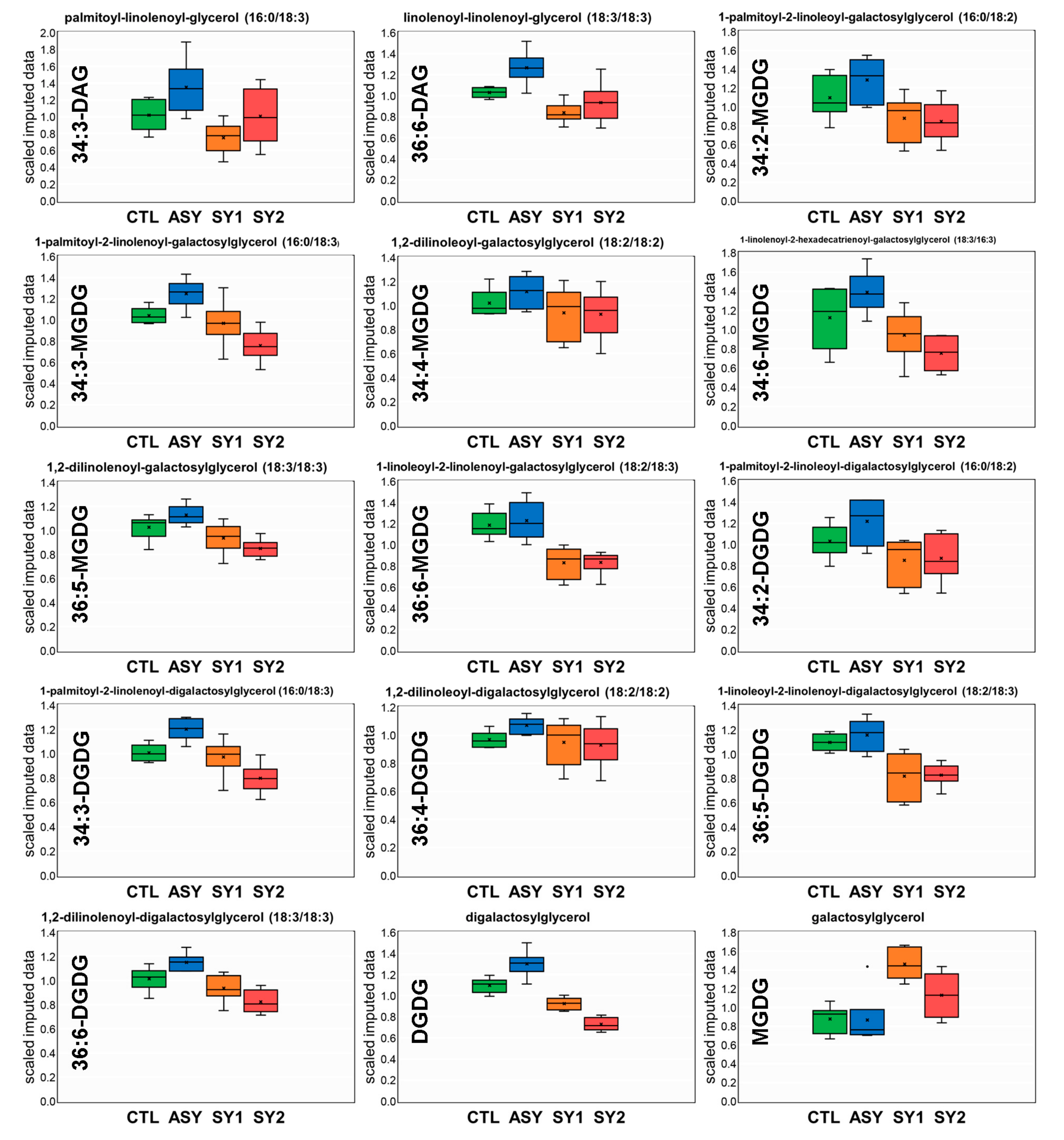
4.2. Except for Galactolipids and Prenol Lipids, There Is a Positive Association between Lipid Levels and Symptom Severity
4.3. Galactolipids Likely Play a Role in the Mechanism Underlying Inhibition of Symptom Formation
4.4. Accumulation of JA in All Stressed Leaves Suggests a Signaling Role in Grapevine Defense Response to ESCA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CTL | healthy leaves from healthy unaffected control vines |
| ASY | healthy leaves from foliar-asymptomatic and wood-symptomatic vines |
| SY1 | chlorotic leaves from foliar-symptomatic and wood-symptomatic vines |
| SY2 | spotted/scorched leaves from foliar-symptomatic and wood-symptomatic vines |
| FA | fatty acids |
| DGDG | digalactosyldiacylglycerol |
| MGDG | monogalactosyldiacylglycerol |
| MAPK | mitogen-activated protein kinase |
| NO | nitric oxide |
| ROS | reactive oxygen species |
| PA | phosphatidic acid |
| OLE | oleic acid |
| SA | salicylic acid |
| JA | jasmonic acid |
| ET | ethylene |
| PCD | programmed cell death |
| ALA | α-linolenic acid |
| ARA | arachidonic acid |
| GTD | grapevine trunk disease |
| GLA | γ-linolenic acid |
| LA | linoleic acid |
| ESCA | Esca complex disease |
| PRs | pathogenesis-related proteins |
| MS: | mass spectrometry |
| GLSD | grapevine leaf stripe disease |
| UPLC | ultrahigh performance liquid chromatography |
| GC | gas chromatography |
| RI | retention index |
| PCA | principal components analysis |
| UFA | free unsaturated fatty acids |
| SFA | free saturated fatty acids |
| ACH | arachidic acid |
| STE | stearic acid |
| HDA | heptadecanoic acid |
| AzA | azelaic acid |
| ABA | abscisic acid |
| PhBA | pheophorbide A |
| 16:0-LGPE | 1-palmitoyl-sn-glycero-3-phosphoethanolamine |
| 2,4-HBA | 2,4-dihydroxybutyric acid |
| 2R,3R-HBA | 2R,3R-dihydroxybutyric acid |
| PhPA | pheophytin A |
| ΔOLE | elaidic acid |
| SAR | systemic acquired resistance |
| G3P | glycerol 3-phosphate |
References
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef]
- Bortolami, G.; Gambetta, A.G.; Delzon, S.; Lamarque, J.L.; Pouzoulet, J.; Badel, E.; Burlett, R.; Charrier, G.; Cochard, H.; Dayer, S.; et al. Exploring the hydraulic failure hypothesis of esca leaf symptom formation. Plant Physiol. 2019, 181, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Pouzoulet, J.; Pivovaroff, L.A.; Santiago, S.L.; Rolshausen, E.P. Can vessel dimension explain tolerance toward fungal vascular wilt diseases in woody plants? Lessons from Dutch elm disease and esca disease in grapevine. Front. Plant Sci. 2014, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.H.; Singhal, R.; Kachroo, A.; Kachroo, P. Fatty acid- and lipid-mediated signaling in plant defense. Ann. Rev. Phytopathol. 2017, 55, 505–536. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Compant, S.; Pierron, R.J.G.; Gorfer, M.; Jacques, A.; Thines, E.; Berger, H. Differing alterations of two esca associated fungi, Phaeoacremonium aleophilum and Phaeomoniella chlamydospora on transcriptomic level, to co- cultured Vitis vinifera L. calli. PLoS ONE 2016, 11, e0163344. [Google Scholar] [CrossRef] [PubMed]
- Escoriaza, G.; Lampasona, G.S.; Talquenca, G.S.; Piccoli, P. In vitro plants of Vitis vinifera respond to infection with the fungus Phaeoacremonium parasiticum by synthesizing the phytoalexin nerolidol. Plant Cell Tissue Org. Cult. 2019, 138, 459–466. [Google Scholar] [CrossRef]
- Laureano, G.; Figueiredo, J.; Cavaco, A.R.; Duarte, B.; Caçador, I.; Malhó, R.; Silva, S.M.; Matos, A.R.; Figueiredo, A. The interplay between membrane lipids and phospholipase A family member in grapevine resistance against Plasmopara viticola. Sci. Rep. 2018, 8, 14538. [Google Scholar] [CrossRef]
- Yacoub, A.; Gerbore, J.; Magnin, N.; Chambon, P.; Dufour, M.-C.; Corio-Costet, M.-F.; Guyoneaud, R.; Rey, P. Ability of Pythium oligandrum strains to protect Vitis vinifera L., by inducing plant resistance against Phaeomoniella chlamydospora, a pathogen involved in Esca, a grapevine trunk disease. Biol. Control 2016, 92, 7–16. [Google Scholar] [CrossRef]
- Figueiredo, A.; Monteiro, F.; Sebastiana, M. First clues on a jasmonic acid role in grapevine resistance against the biotrophic fungus Plasmopara viticola. Eur. J. Plant Pathol. 2015, 142, 645–652. [Google Scholar] [CrossRef]
- Mandal, M.K.; Chandra-Shekara, A.C.; Jeong, R.D.; Yu, K.; Zhu, S.; Chanda, B.; Navarre, D.; Kachroo, A.; Kachroo, P. Oleic acid–dependent modulation of NITRIC OXIDE ASSOCIATED1 protein levels regulates nitric oxide–mediated defense signaling in Arabidopsis. Plant Cell 2012, 24, 1654–1674. [Google Scholar] [CrossRef]
- Repka, V.; Čarná, M.; Pavlovkin, J. Methyl jasmonate-induced cell death in grapevine requires both lipoxygenase activity and functional octadecanoid biosynthetic pathway. Biologia 2013, 68, 896–903. [Google Scholar] [CrossRef]
- Gao, Q.-M.; Yu, K.; Xia, Y.; Shine, M.B.; Wang, C.; Navarre, D.; Kachroo, A.; Kachroo, P. Mono- and Digalactosyldiacylglycerol lipids function nonredundantly to regulate systemic acquired resistance in plants. Cell Rep. 2014, 9, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Pecher, P.; Eschen-Lippold, L.; Herklotz, S.; Kuhle, K.; Naumann, K.; Bethke, G.; Uhrig, J.; Weyhe, M.; Scheel, D.; Lee, J. The Arabidopsis thaliana mitogen-activated protein kinases MPK3 and MPK6 target a subclass of ‘‘VQ-motif’’-containing proteins to regulate immune responses. New Phytol. 2014, 203, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.A.; Kalisch, B.; Hölzl, G.; Schulze, S.; Thiele, J.; Melzer, M.; Rostond, L.R.; Benning, C.; Dörmann, P. Synthesis and transfer of galactolipids in the chloroplast envelope membranes of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2016, 113, 10714–10719. [Google Scholar] [CrossRef]
- Li, T.; Cofer, T.; Engelberth, M.; Engelberth, J. Defense priming and jasmonates: A role for free fatty acids in insect elicitor-induced long distance signaling. Plants 2016, 5, 5. [Google Scholar] [CrossRef]
- Tenenboim, H.; Burgos, A.; Willmitzer, L.; Brotman, Y. Using lipidomics for expanding the knowledge on lipid metabolism in plants. Biochimie 2016, 130, 91–96. [Google Scholar] [CrossRef]
- Liu, M.Y.; Burgos, A.; Ma, L.; Zhang, Q.; Tang, D.; Ruan, J. Lipidomics analysis unravels the effect of nitrogen fertilization on lipid metabolism in tea plant (Camellia sinensis L.). BMC Plant Biol. 2017, 17, 165. [Google Scholar] [CrossRef]
- Moradi, P.; Mahdavi, A.; Khoshkam, M.; Iriti, M. Lipidomics unravels the role of leaf lipids in thyme plant response to drought stress. Int. J. Mol. Sci. 2017, 18, 2067. [Google Scholar] [CrossRef]
- Arita, K.; Honma, T.; Suzuki, S. Comprehensive and comparative lipidome analysis of Vitis vinifera L. cv. Pinot Noir and Japanese indigenous V. vinifera L. cv. Koshu grape berries. PLoS ONE 2017, 12, e0186952. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Huang, B. Lipidomic reprogramming associated with drought stress priming-enhanced heat tolerance in tall fescue (Festuca arundinacea). Plant Cell Environ. 2019, 42, 947–958. [Google Scholar] [CrossRef]
- Yu, C.-W.; Lin, Y.-T.; Li, H.-M. Increased ratio of galactolipid MGDG:DGDG induces jasmonic acid overproduction and changes chloroplast shape. New Phytol. 2020, 1–9. [Google Scholar] [CrossRef]
- Okazaki, Y.; Kamide, Y.; Hirai, Y.M.; Saito, K. Plant lipidomics based on hydrophilic interaction chromatography coupled to ion trap time-of-flight mass spectrometry. Metabolomics 2013, 9, S121–S131. [Google Scholar] [CrossRef] [PubMed]
- Drissner, D.; Kunze, G.; Callewaert, N.; Gehrig, P.; Tamasloukht, M.; Boller, T.; Felix, G.; Amrhein, N.; Bucher, M. Lyso-phosphatidylcholine is a signal in the arbuscular mycorrhizal symbiosis. Science 2007, 318, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Negrel, L.; Halter, D.; Wiedemann-Merdinoglu, S.; Rustenholz, C.; Merdinoglu, D.; Hugueney, P.; Baltenweck, R. Identification of lipid markers of Plasmopara viticola infection in grapevine using a non-targeted metabolomic approach. Front Plant Sci. 2018, 9, 360. [Google Scholar] [CrossRef]
- Koussa, T.; Cherrad, M.; Zaoui, D.; Broquedis, M. Composition and content of fatty acids in Vitis vinifera. var. cabernet sauvignon leaves infected with the eutypiosis fungus, Eutypa lata. J. Int. Sci. Vigne Vin 1998, 32, 11–16. [Google Scholar] [CrossRef]
- Lecomte, P.; Diarra, B.; Carbonneau, A.; Rey, P.; Chevrier, C. Esca of grapevine and training practices in France: Results of a 10-year survey. Phytopathol. Mediterr. 2018, 57, 472–487. [Google Scholar] [CrossRef]
- Antonielli, L.; Compant, S.; Strauss, J.; Sessitsch, A.; Berger, H. Draft genome sequence of Phaeomoniella chlamydospora Strain RR-HG1, a Grapevine Trunk Disease (Esca)-related member of the Ascomycota. Genome Anounc. 2014, 2, 2013–2014. [Google Scholar] [CrossRef]
- Sofia, J.; Trovão, J.; Portugal, A.; Paiva de Carvalho, H.; Mesquita, N.; Nascimento, T.; Rego, C.; Gonçalves, M. Molecular and phenotypic characterisation of Phaeomoniella chlamydospora isolates from the demarcated wine region of Dão (Portugal). Phytopathol. Mediterr. 2015, 54, 403–413. [Google Scholar] [CrossRef]
- Sofia, J.; Mota, M.; Gonçalves, T.M.; Rego, C. Response of four Portuguese grapevine cultivars to infection by Phaeomoniella chlamydospora. Phytopathol. Mediterr. 2018, 57, 506–518. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Rolshausen, P.; Cantu, D. Draft genome sequence of the ascomycete Phaeoacremonium aleophilum Strain UCR-PA7, a causal agent of the esca disease. Genome Anounc. 2013, 1, 3–4. [Google Scholar] [CrossRef]
- Del Frari, G.; Cabral, A.; Nascimento, T.; Boavida Ferreira, R.; Oliveira, H. Epicoccum layuense a potential biological control agent of esca-associated fungi in grapevine. PLoS ONE 14, e0213273. [CrossRef] [PubMed]
- Del Frari, G.; Gobbi, A.; Aggerbeck, M.R.; Oliveira, H.; Hansen, L.H.; Ferreira, R.B. Characterization of the wood mycobiome of Vitis vinifera in a vineyard affected by esca. Spatial distribution of fungal communities and their putative relation with leaf symptoms. Front. Plant Sci. 10, 910. [CrossRef] [PubMed]
- Magnin-Robert, M.; Spagnolo, A.; Boulanger, A.; Joyeux, C.; Clément, C.; Abou-Mansour, E.; Fontaine, F. Changes in plant metabolism and accumulation of fungal metabolites in response to Esca proper and apoplexy expression in the whole grapevine. Phytopathology 2016, 106, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Calzarano, F.; Osti, F.; D’agostino, V.; Pepe, A.; Della Pelle, F.; De rosso, M.; Flamini, R.; Di Marco, S. Levels of phytoalexins in vine leaves with different degrees of grapevine leaf stripe disease symptoms (Esca complex of diseases). Phytopathol. Mediterr. 2017, 56, 494–501. [Google Scholar] [CrossRef]
- Magnin-Robert, M.; Adrian, M.; Trouvelot, S.; Spagnolo, A.; Jacquens, L.; Letousey, P.; Rabenoelina, F.; Harir, M.; Roullier-Gall, C.; Clément, C.; et al. Alterations in grapevine leaf metabolism occur prior to esca apoplexy appearance. Mol. Plant-Microbe Interact. 2017, 30, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Marques, C.A.; Cortez, I. Exhibition of local but not systemic induced phenolic defenses in Vitis vinifera L. affected by brown wood streaking, grapevine leaf stripe, and apoplexy (Esca complex). Plants 2019, 8, 412. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on fungal phytotoxins and their role in grapevine trunk diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef]
- Ouadi, L.; Bruez, E.; Bastien, S.; Vallance, J.; Lecomte, P.; Domec, J.-C.; Rey, P. Ecophysiological impacts of Esca, a devastating grapevine trunk disease, on Vitis vinifera L. PLoS ONE 2019, 14, e0222586. [Google Scholar] [CrossRef]
- Reis, P.; Pierron, R.; Larignon, P.; Lecomte, P.; Abou-Mansour, E.; Farine, S.; Bertsch, C.; Jacques, A.; Trotel-Aziz, P.; Rego, C.; et al. Vitis methods to understand and develop strategies for diagnosis and sustainable control of grapevine trunk diseases. Phytopathology 2019, 109, 916–931. [Google Scholar] [CrossRef]
- Calzarano, F.; Di Marco, S. Further evidence that calcium, magnesium and seaweed mixtures reduce grapevine leaf stripe symptoms and increase grape yields. Phytopathol. Mediterr. 2018, 57, 459–471. [Google Scholar] [CrossRef]
- Roblin, G.; Luini, E.; Fleurat-Lessard, P.; Larignon, P.; Berjeaud, J.-M. Towards a preventive and/or curative treatment of esca in grapevine trunk disease: General basis in the elaboration of treatments to control plant pathogen attacks. Crop Prot. 2019, 116, 156–169. [Google Scholar] [CrossRef]
- Goufo, P.; Fontem, D.A.; Ngnokam, D. Evaluation of plant extracts for tomato late blight control in Cameroon. N. Z. J. Crop Hortic. Sci. 2010, 38, 171–176. [Google Scholar] [CrossRef]
- Goufo, P.; Teugwa Mofor, C.; Fontem, D.A.; Ngnokam, D. High efficacy of extracts of Cameroon plants against tomato late blight disease. Agron. Sustain. Dev. 2008, 28, 567–573. [Google Scholar] [CrossRef]
- Moret, F.; Lemaître-Guillier, C.; Grosjean, C.; Clément, G.; Coelho, C.; Negrel, J.; Jacquens, L.; Morvan, G.; Mouille, G.; Trouvelot, S.; et al. Clone-dependent expression of esca disease revealed by leaf metabolite analysis. Front. Plant Sci. 2019, 9, 1960. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference list of phenolic compounds (including stilbenes) in grapevine (Vitis vinifera L.) roots, woods, canes, stems, and leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Tarazona, P.; Feussner, K.; Feussner, I. An enhanced plant lipidomics method based on multiplexed liquid chromatography–mass spectrometry reveals additional insights into cold- and drought-induced membrane remodeling. Plant J. 2015, 84, 621–633. [Google Scholar] [CrossRef]
- Tumanov, S.; Zubenko, Y.; Greven, M.; Greenwood, D.R.; Shmanai, V.; Villas-Boas, S.G. Comprehensive lipidome profiling of Sauvignon blanc grape juice. Food Chem. 2015, 180, 249–256. [Google Scholar] [CrossRef]
- Goufo, P.; Moutinho-Pereira, J.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; Antonio, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) Metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, 586. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Guo, J.; Du, B.; He, G.; Zhang, Y.; Chen, R.; Li, J. Lipid profiles reveal different responses to brown planthopper infestation for pest susceptible and resistant rice plants. Metabolomics 2018, 14, 120. [Google Scholar] [CrossRef]
- Goufo, P.; Isabel, C. Lipidome of leaves of esca-affected grapevine. Mendeley Data 2020. [Google Scholar] [CrossRef]
- Goufo, P.; Ferreira, L.M.M.; Carranca, C.; Rosa, E.A.S.; Trindade, H. Effect of elevated carbon dioxide concentration on rice quality: Proximate composition, dietary fibres and free sugars. Cereal Chem. 2014, 91, 293–299. [Google Scholar] [CrossRef]
- Goufo, P.; Falco, V.; Brites, C.; Wessel, D.F.; Kratz, S.; Rosa, E.A.S.; Carranca, C.; Trindade, H. Effect of elevated carbon dioxide concentration on rice quality: Nutritive value, color, milling, cooking and eating qualities. Cereal Chem. 2014, 91, 513–521. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Van der Gheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
| LIPID MAPS Class 1 | Sub-Pathway 2 | KEGG Map 2 | Detection Platform 3 | No of Species |
|---|---|---|---|---|
| Fatty acyls | Fatty acids, free saturated | 01212 | LC/MS Neg, GC/MS | 16 |
| Fatty acyls | Fatty acids, free unsaturated | 01040 | GC/MS | 20 |
| Fatty acyls | Fatty acids, hydroxy | 00073 | LC/MS Polar | 7 |
| Fatty acyls | Fatty acids, amide and amino | 00061 | LC/MS Pos Early, LC/MS Pos Late | 3 |
| Fatty acyls | Fatty acids, dicarboxylate | 00071 | LC/MS Polar | 9 |
| NA | Carnitine metabolism | 00260 | LC/MS Pos Early | 5 |
| NA | Hormone metabolism | 04075 | LC/MS Neg, LC/MS Polar | 4 |
| Glycerolipids | Glycerolipids, monoacyl | 00561 | LC/MS Neg | 5 |
| Glycerolipids | Glycerolipids, diacyl | 00561 | LC/MS Pos Late | 3 |
| Glycerophospholipids | Galactolipids | 00564 | LC/MS Pos Late | 13 |
| Glycerophospholipids | Lyso-galactolipids | 00564 | LC/MS Pos Late, LC/MS Neg | 6 |
| Glycerophospholipids | Sulfolipids | 00564 | LC/MS Neg | 3 |
| Glycerophospholipids | Phospholipids | 00564 | LC/MS Pos Late | 23 |
| Glycerophospholipids | Lyso-phospholipids | 00564 | LC/MS Pos Late | 11 |
| NA | Phospholipid metabolism | 00564 | LC/MS Pos Early, LC/MS Polar | 7 |
| NA | Choline metabolism | 00564 | LC/MS Pos Early | 4 |
| Sphingolipids | Sphingolipids | 00600 | LC/MS Pos Late | 6 |
| Sterol lipids | Sterols | 00100 | LC/MS Pos Late | 3 |
| Prenol lipids | Tocopherol metabolism | 00130 | LC/MS Pos Late | 3 |
| NA | Carotenoid/Chlorophyll metabol | 00860/00906 | LC/MS Pos Late, LC/MS Neg | 7 |
| Unknown lipids | NOT APPLICABLE (NA) | NA | LC/MS Neg, LC/MS Pos Early | 50 |
| Unknown Lipid | Detection Platform | Retention Indice (RI) | Molecular Ion | ASY/CTL | SY1/CTL | SY2/CTL |
|---|---|---|---|---|---|---|
| X-24686 | LC/MS Pos Early | 1876 | 224.1126 | −0.05 | 0.25 | 0.62 |
| X-25260 | LC/MS Neg | 1465 | 315.0721 | −0.07 | 1.43 | 1.32 |
| X-24455 | LC/MS Pos Early | 2641 | 237.0867 | 0.20 | 0.82 | 3.64 |
| X-24456 | LC/MS Pos Early | 2538 | 237.0866 | 0.17 | 1.14 | 3.11 |
| X-24425 | LC/MS Pos Early | 2378 | 130.0974 | −0.20 | −0.19 | 2.12 |
| X-23734 | LC/MS Pos Early | 881 | 174.0758 | 0.42 | 0.39 | 2.77 |
| X-23512 | LC/MS Neg | 1306 | 267.1084 | 0.37 | 0.27 | 0.29 |
| X-23522 | LC/MS Neg | 1417 | 267.1085 | 0.50 | 0.77 | 0.41 |
| X-23780 | LC/MS Pos Early | 2190 | 144.1017 | 0.72 | 0.04 | −0.18 |
| X-23911 | LC/MS Neg | 800 | 283.1037 | 0.25 | 0.20 | −0.14 |
| X-25880 | LC/MS Pos Early | 2208 | 222.0761 | −0.12 | 0.33 | 0.17 |
| X-24432 | LC/MS Pos Early | 618 | 210.0606 | −0.55 | 0.07 | −1.32 |
| X-23908 | LC/MS Neg | 1237 | 188.0929 | −0.12 | −1.34 | −1.11 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goufo, P.; Cortez, I. A Lipidomic Analysis of Leaves of Esca-Affected Grapevine Suggests a Role for Galactolipids in the Defense Response and Appearance of Foliar Symptoms. Biology 2020, 9, 268. https://doi.org/10.3390/biology9090268
Goufo P, Cortez I. A Lipidomic Analysis of Leaves of Esca-Affected Grapevine Suggests a Role for Galactolipids in the Defense Response and Appearance of Foliar Symptoms. Biology. 2020; 9(9):268. https://doi.org/10.3390/biology9090268
Chicago/Turabian StyleGoufo, Piebiep, and Isabel Cortez. 2020. "A Lipidomic Analysis of Leaves of Esca-Affected Grapevine Suggests a Role for Galactolipids in the Defense Response and Appearance of Foliar Symptoms" Biology 9, no. 9: 268. https://doi.org/10.3390/biology9090268
APA StyleGoufo, P., & Cortez, I. (2020). A Lipidomic Analysis of Leaves of Esca-Affected Grapevine Suggests a Role for Galactolipids in the Defense Response and Appearance of Foliar Symptoms. Biology, 9(9), 268. https://doi.org/10.3390/biology9090268






