1. Introduction
Plants are constantly exposed to environmental stresses responsible for physiological changes and oxidative stress. Water deficit stress is the major stressor and presents great potential to adversely affect crop development [
1,
2,
3,
4].
Sometimes, even under water deficit conditions, it is necessary to apply a fungicide. It occurs because most growers have been using the application time following one predetermined calendar schedule, according to the critical development stage of the plant, the first application, or even the residual period of the products [
5]. This can lead plants to combined stress factors if the fungicide is a xenobiotic, which causes alterations in plant metabolism [
6].
A large amount of research has shown plant responses to isolated stresses, which is incompatible with the reality of field conditions [
7], but recent studies have confirmed that plants are exposed to a combination of stresses [
7,
8], therefore demanding findings in this sense. Thus, although plant performance in a water-restricted environment is already consolidated, still needs to elucidate plant to multiple stresses [
9,
10], such as water deficit stress and fungicide application.
In recent years, efforts have been made to understand plant responses to combined stresses [
8,
11]. A combined stress situation is when there is simultaneous exposure of the plant to different stress conditions, and means a new state of stress, and therefore requires new mechanisms and different pathways of defense response [
7,
10,
11,
12].
One situation of multiple stress factors that has been receiving attention in studies with is simultaneous water deficit and high temperature, which are almost dependent as it usually occurs at the same time in the field [
13]. In these conditions, soybean was affected with greater intensity when water stress was imposed at higher temperatures [
14]. The response is not a simple additive of every single stress, but a synergistic result of the combination [
15]. In addition to these studies, soybean crop under water deficit stress is also evaluated recently in combination with UV-B radiation [
16], cadmium [
17], and heavy metal [
18].
Antioxidant agents, also called biostimulants, are products used to minimize stress damage when plants are exposed to multiple stressors [
19,
20,
21]. Any protective effects of these compounds against biotic and abiotic stresses would be associated with reductions in reactive oxygen species (ROS) and antioxidant system activation and increases in the production of phenolic compounds [
20]. Thus, these products may act as osmoregulators, signaling molecules, and stomatal aperture modulators [
22].
We hypothesize that soybean plants exposed to combined water deficit and fungicide application will have their metabolism more impaired than plants exposed to one stress type, or without stress. However, it is expected that stress damages will be minimized by the antioxidant agent application. Thus, the objective of this study is to evaluate the soybean plant metabolism under water deficit stress and the application of fungicide and antioxidant agents.
4. Discussion
Studies have shown that there is a relationship between decreasing water potential with water deficit conditions [
39,
40,
41]. Literature uses leaf water potential values of −1.2 to −1.5 MPa as critical and indicative of water deficit stress for corn plants, which has a C4 metabolism and is, therefore, more resistant to water deficit [
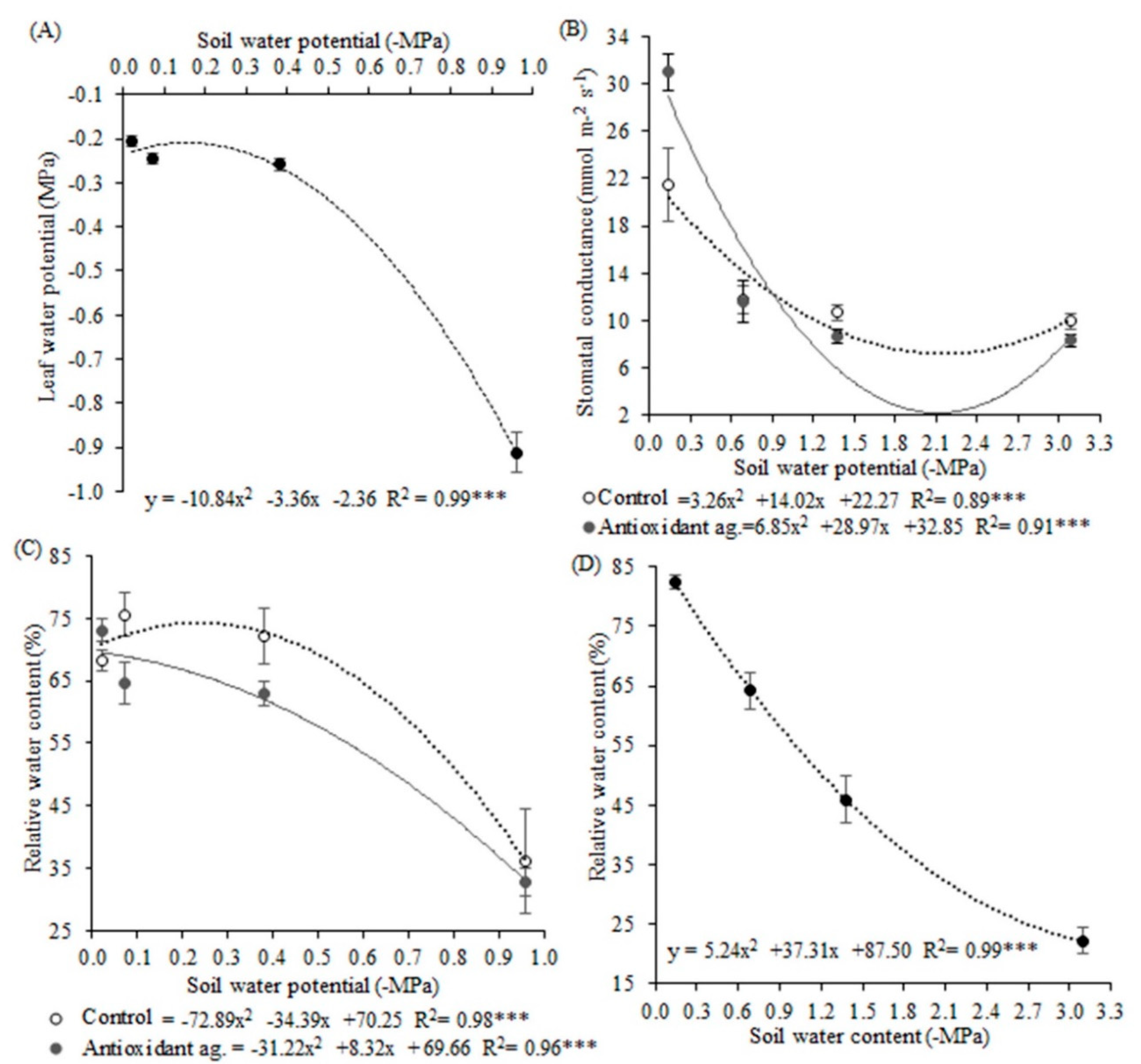
42]. In this work, which was developed in a controlled environment, values of −0.9 MPa indicated more severe deficit water stress for soybean plants. This value may be suggested as a stress indicator for the soybean crop.
Water deficit and concurrent xenobiotic application comprised a new stress situation, in agreement with [
11], not simply based on adding the isolated effects of deficit water to the isolated effects of xenobiotic. Other studies had shown positive results when stresses were combined, compared to isolated stresses [
43,
44,
45,
46]. This shows the complexity involved in different stress conditions, isolated or in combinations.
Plants exposed to deficit water stress had reduced their water status, leaf water potential, RWC, and stomatal conductance. These physiological changes are commonly observed and corroborate with [
47]. The decrease of the stomatal conductance reduced the gas exchanges, which may have contributed to the decrease in CO
2 absorption, which also favored the increase in the leaf temperature of these plants. Under low soil water availability, plants closed their stomata to prevent water loss by transpiration, maintain cell turgor, and regulate CO
2 absorption [
46,
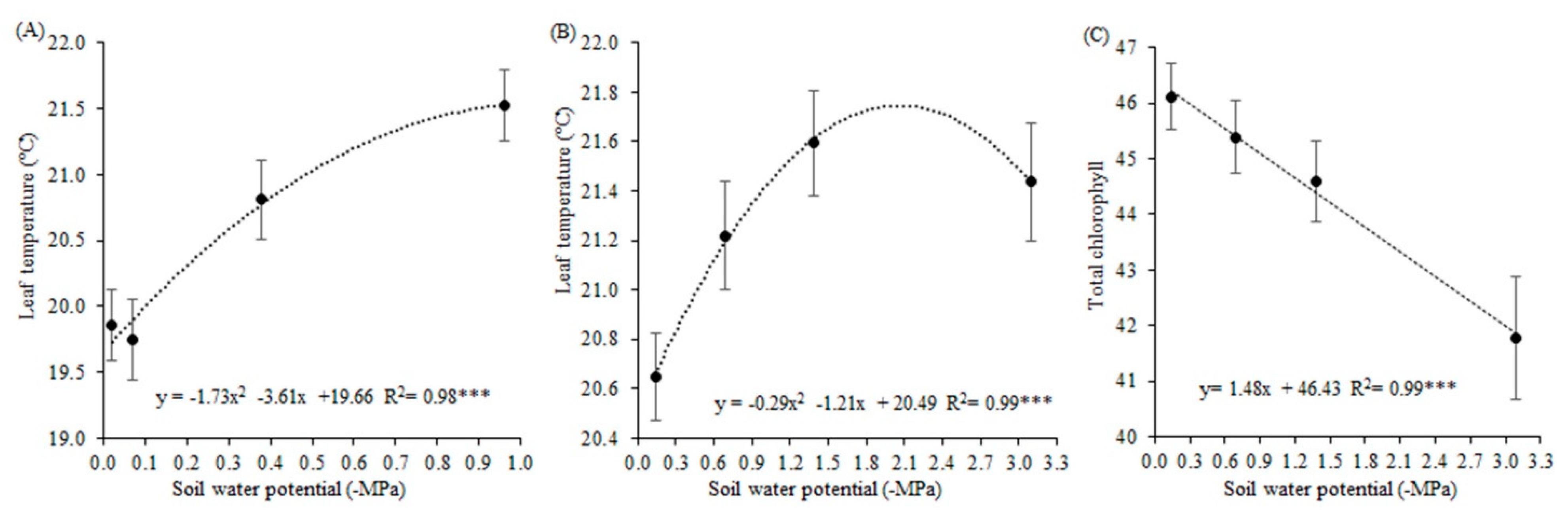
48]. According to these authors, the heat and water that would be removed from the leaves by evaporative cooling stayed in the cells, increasing leaf temperature.
This increasing temperature may have contributed to the degradation of photosynthetic pigments, or even alteration in the biosynthesis of these pigments because they were reduced. According to [
49], these cited factors are considered the main cause of pigment reduction in stress situations. Reduced chlorophyll content also may be due to lower photosynthesis of plants, and oxidative stress. [
50] also observed reductions in the photosynthetic activity of soybean plants when under soil water potential of −0.026 MPa. Photosynthetic pigments were also reduced by xenobiotic application, which can cause less photon absorption by these molecules and consequently diminish the plant’s ability to absorb light.
Quantum yield was also affected by water deficit, and the plants under this stress for eight days (−3.09 MPa) had a quantum yield of less than 0.75 mmol CO
2 mmol photons
−1, which is considered a critical limit. Below this limit, plants may suffer damage to PSII [
51]. The reduction in the quantum yield of PSII results from the decrease in the efficiency with which the PSII reaction centers capture the energy [
52]. Chlorophyll fluorescence is an evaluation of plant stress, then the increased chlorophyll fluorescence in xenobiotic treated plants indicated changes in photosynthetic activity [
53], an indication of greater photoinhibition and consequent physical damage to PSII.
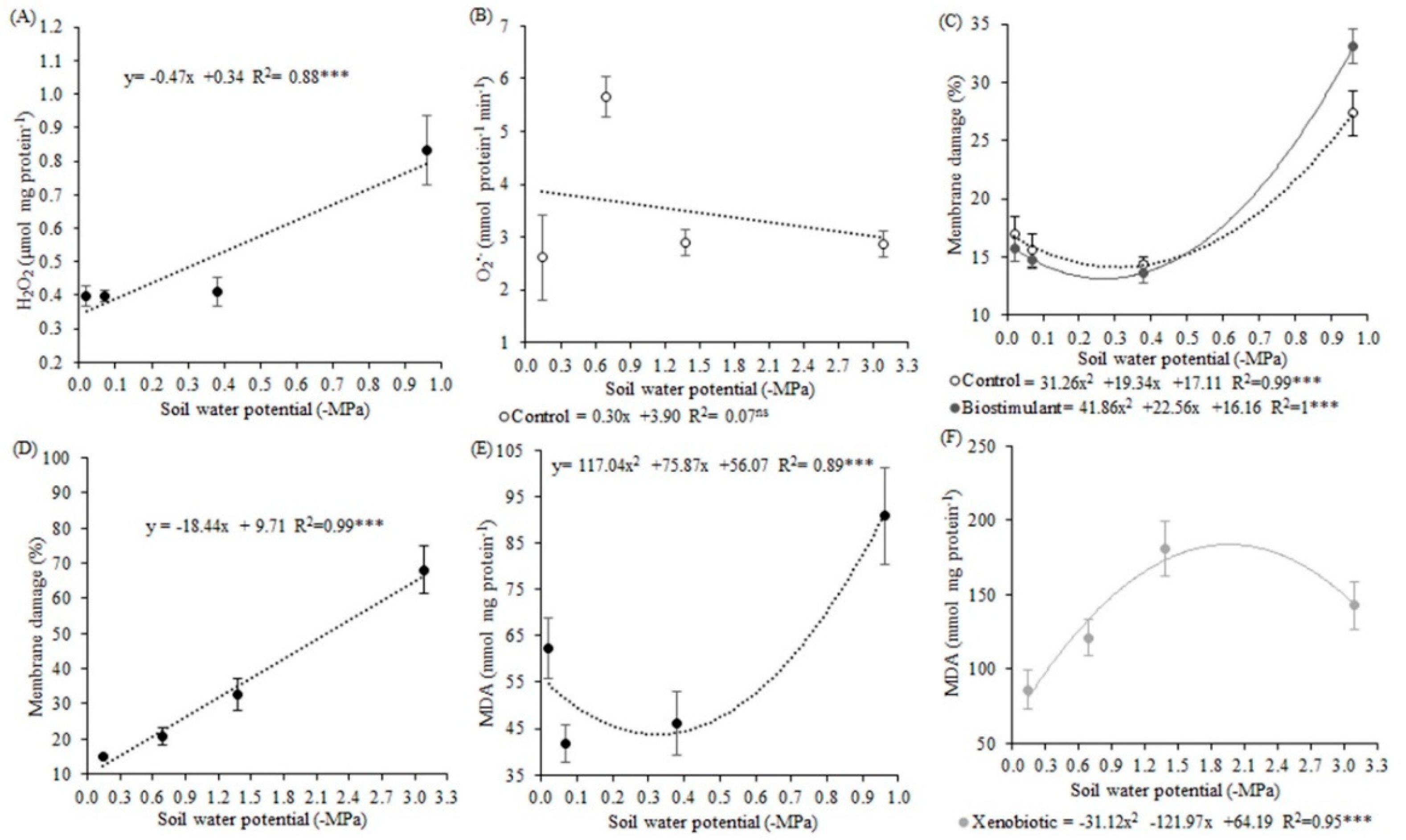
Increased ROS production, mainly H
2O
2, was observed in plants under water deficit, a result that is already well known in the literature [
54,
55,
56,
57]. This species accumulation may be due to reductions in liquid photosynthesis, triggered by the CO
2 limited entry, due to the stomatal closure [
54]. Moreover, thermal stress due to deficit causes disturbances in electron flow and destabilization of the energy reactions, which leads to ROS accumulation [
48]. Increased production of these molecules in cells can cause an imbalance in the antioxidant system, which may result in the generation of oxidative stress [
58].
The proportion of this increase may vary in each situation, depending on the stress intensity and duration, plant development stage, genetics, and even the evaluated tissue [
59]. Thus, in this work, the highest concentration of H
2O
2 was not observed for the lowest soil water potential (−3.09 MPa, in the second experiment), except for plants submitted to −0.96 MPa. A possible reason for this is that ROS, such as H
2O
2, are considered as signaling molecules of stress despite being toxic molecules at a certain concentration in cells [
60]. Thus, alterations in plant metabolism, such as increased production of H
2O
2 molecules, were triggered under water deficit and may have signaled this stressful situation, and thus the plant induced defense mechanisms.
The different behavior of the observed ROS species can be due to the anticipated production of O
2•− at lower soil water potential (−0.69 MPa). This is because O
2•− is usually the first ROS generated, being produced by oxygen reduction during electron transport along the electron transport chain [
61]. In the O
2•− dismutation, there is a reduction of two electrons of oxygen, which is a determinant factor in H
2O
2 and hydroxyl radical formation [
61]. Hydroxyl radical is the most toxic and reactive molecule formed by the Fenton reaction, which occurs in the presence of transition metals, like copper and iron [
41,
60,
61].
We observed that a combined water deficit and fungicide application increased H
2O
2 production by 25%, which is very considerable. Changes in H
2O
2 levels in cells can act as indicators of the membrane structural integrity of stress-exposed plants [
62] because membranes are often the first target of abiotic stresses [
9]. In this sense, the greatest membrane damage observed in plants of the lower soil water potentials may have been due to higher ROS production. According to [
63] under stress conditions, ROS production increase results in electrolyte leakage, which leads to MD increases.
The MDA content is another indication of membrane damage that was increased in plants under water deficit. MDA is the last product of lipid peroxidation that occurs in the membranes [
64]. The effect of increasing leaf temperature caused by lower water availability increased membrane fluidity, which is related to the rupture and peroxidation of the lipids constituent of these structures, corroborating [
9]. This damage was potentialized by xenobiotic application in the second experiment.
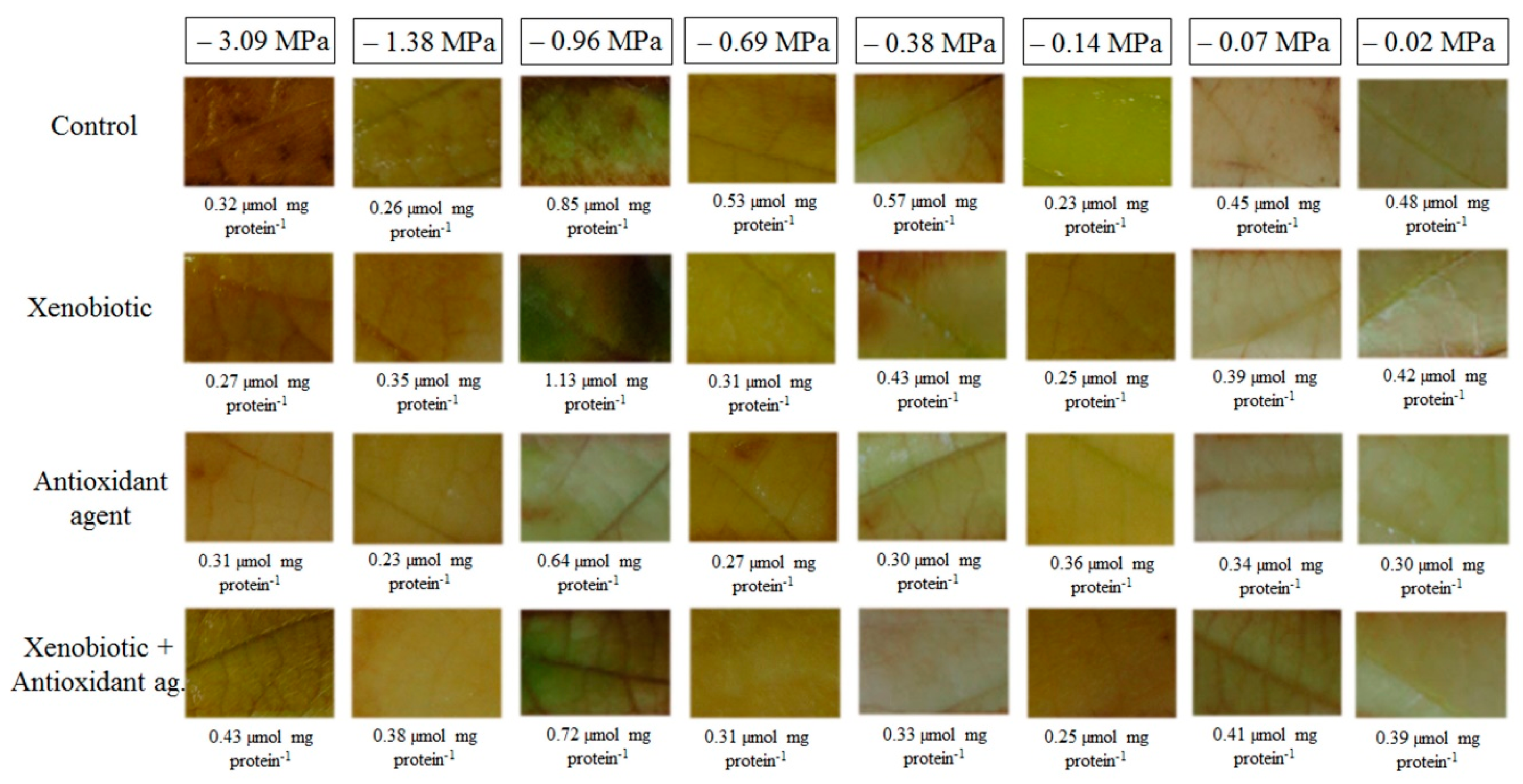
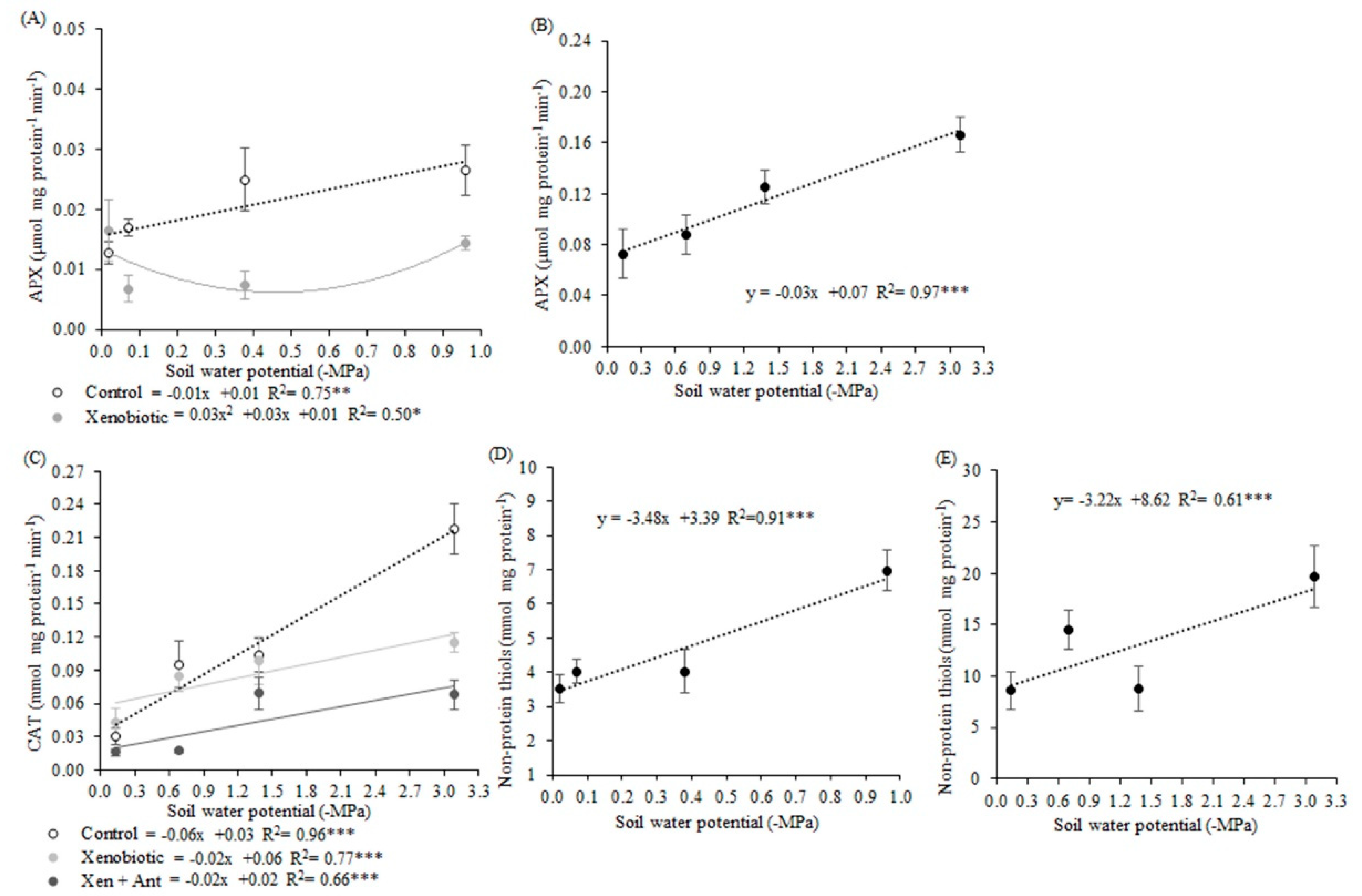
As H2O2 increased in combined stresses, the MDA content also increased. Because higher MDA contents represent oxidative damages that may be caused by ROS, fungicide application in plants submitted to very low soil water potentials may lead to the generation of oxidative stress. This H2O2 content estimated at 0.83 mmol mg protein−1 min−1 at the potential −0.96 MPa, may, therefore, be suggested as a borderline. This value could be considered to separate what would be a basal level of H2O2, which is essential and positive for the plant, from a level that could be considered critical and capable of causing damage, and which was therefore signaled and neutralized by antioxidant machinery.
Antioxidant agent application resulted in the lowest ROS production, and this stress mitigation by this biostimulant would be due to its composition. It contains folcysteine, which is formed by folic acid and cysteine. The sulfur element needs to be reduced to sulfate and cysteine to be assimilated by the plant. There is a need for electron donation among the steps for this reduction, and glutathione is one of the possible donors [
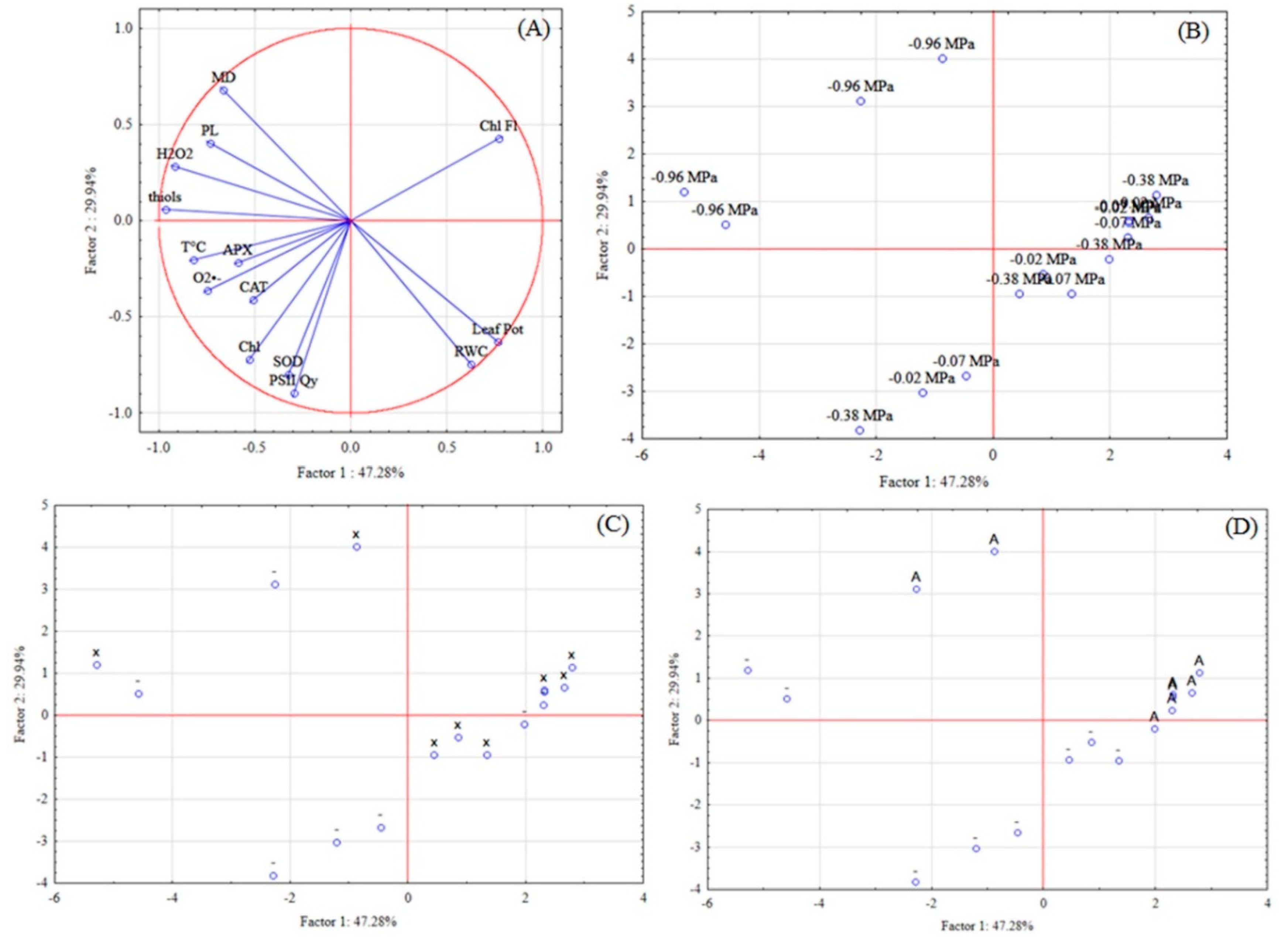
48]. Thus, glutathione, an important antioxidant, is related to cysteine and can act in the stress attenuation when the antioxidant agent is applied. According to the projection of the variables and cases, in PCA, glutathione can be suggested as the main compound of the product as a stress mitigator.
Thermal stress due to water deficit may impair plant metabolism because of its effect on protein and enzyme stability [
48]. Activation of defense mechanisms such as antioxidant enzymes, like induction of APX activity and non-protein thiols, predominantly glutathione (GSH), was observed with increases in temperature (low soil water potentials). These defense mechanisms are a result of increased respiratory activity. GSH represent the non-enzymatic antioxidant compounds and is formed by the enzyme glutathione reductase, which uses NADPH to reduce GSH-oxidized glutathione that acts by directly reducing ROS [
65].
Defense antioxidant mechanisms are considered important adaptive strategies to minimize oxidative damage and were activated to combat the ROS generated by stress situations [
60,
61,
66]. Thus, higher thiol contents that were found in the lower soil water potentials showed the increased antioxidant activity to protect the plant from water deficit. Incremental CAT activity was observed in lower soil water potentials, with a linear response similar to H
2O
2 production, because this enzyme acts on the H
2O
2 defense route [
67].
As observed for CAT, the activity of APX was also increased in deficit water stress for the control plants. APX is considered an active defense line in the neutralization of excessive production and possible negative effects of H
2O
2 [
67]. According to [
10], enzymes such as CAT and glutathione peroxidase, for example, are induced in water deficit conditions. Other defense routes can be triggered for combined stresses, such as glutathione reductase (GR) and glutathione-S-transferase.
Antioxidant agent application reduced SOD, CAT, and APX activities in the different soil water potentials. Another hypothesis to be considered is that the mitigation of oxidative stress, visualized by the lower ROS production in plants treated with the antioxidant agent, may have resulted due to the presence of folcysteine on its composition. Cysteine residues can react with O
2•− and with H
2O
2, with constant reaction rates varying by 7 × 105 and 1-2, respectively, for these two species [
60,
68,
69,
70].
ROS species interact with several signaling pathways, and signals are transmitted through proteins, with cysteine being the target of these molecules [
68]. Cysteine may be in several states of oxidation because it contains the sulfur atom with many electrons, causing cysteine residues to be important modification sites [
71]. Signal transduction sensors can be unraveled by these modified proteins that contain cysteine [
72]. The cysteine, as well as glutamate, glycine, and phenylalanine, are considered signaling amino acids, which in small doses can increase the activity of antioxidant enzymes [
73].
Thus, the antioxidant agent may have been essential for stress signaling by the ROS, which may have contributed to an increase in the activity of the antioxidant machinery and a reduction in the production and harmful effects of ROS. However, studies such as that of [
74] only recognize H
2O
2 as a secondary messenger, considering O
2•− only as a precursor of H
2O
2 and not a direct participant in signaling. It could also be supposed by the multivariate analysis that the defense mechanisms, like the catalase enzyme and the glutathione (thiols) can be activated by the antioxidant agent, which minimized the damage. The ROS may be the result of combined stresses, such as ROS or as signaling molecules that allow the early perception of stress.
Biostimulants, like the antioxidant agent used in this study, ameliorate the uptake and efficiency of nutrients and also increase the tolerance to abiotic stress [
19,
75]. This tolerance was probably obtained from modifications in antioxidant properties, such as enzyme activities [
76]. Biostimulants improve the antioxidant defense capacity of plants, which helps in stress tolerance. However, it is noticeable that there is a need for studies that indicate which mechanisms are responsible for this, a fact also observed by [
77].
Although folcysteine is not the only component of the antioxidant agent used in this study, it is the one with the highest amount. The interactions between the components of this product are complex and potentiate the folcysteine action. There is no way to indicate which component is responsible for the observed effects but to state that the effect comes from this formulation, which contributes to the mobility of folcysteine, in the case of nitrogen and phosphorus [
48]. According to the same authors, zinc, magnesium, and potassium acting in the activation of the enzymes contributes to the antioxidant system being metal enzymatic cofactors [
48]. In addition, potassium also regulates osmotic potential, and iron and manganese are known for redox reactions and electron transfer [
48]. Therefore, the sum of each isolated effect led to the potentiation of the effects and resulted in the ability to mitigate the effects of water deficit and xenobiotic stresses.
It is important to highlight that results, according to the literature that already exists, that plants under stress closure stomata, reducing gas exchanges and consequently photosynthesis. These alterations lead to mitochondrial respiration changes and the generation of ROS in photosynthetic electron transport [
60]. These toxic molecules can cause chemical damage to DNA, protein damage, impaired cellular effects, and gene expression changes.
With the antioxidant agent application, some of the antioxidant defenses were activated, neutralizing the toxic effects of ROS, and maintaining a basal level able to signal the stress, and preventing harmful effects, and also modulating cell expansion throughout the stiffness and relaxation of the cell wall [
67]. Amino acids, nutrients, hormones, and humic acids containing in the biostimulant composition probably triggered metabolic alterations in the plants, that quite changed the oxidation responses to stress, but maybe had not yet reached the physiological level.