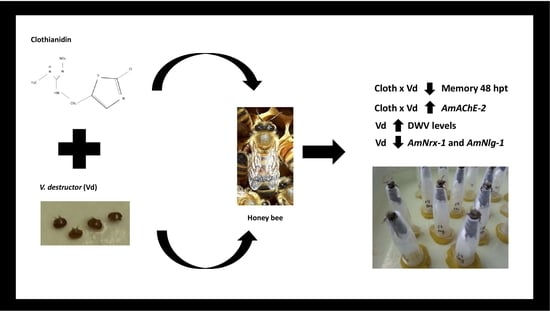
The Combined Effects of Varroa destructor Parasitism and Exposure to Neonicotinoids Affects Honey Bee (Apis mellifera L.) Memory and Gene Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Honey Bees and V. destructor Mites
2.2. Sublethal Doses of Clothianidin
2.3. Exposure to Clothianidin and/or V. destructor
2.4. Effect of Sublethal Doses of Clothianidin and/or V. destructor on Memory Retention
2.5. RNA Extraction and cDNA Synthesis
2.6. DWV Quantification
2.7. Quantitative Real Time (qRT-PCR) and Gene Expression Analysis
2.8. Statistical Analyses
3. Results
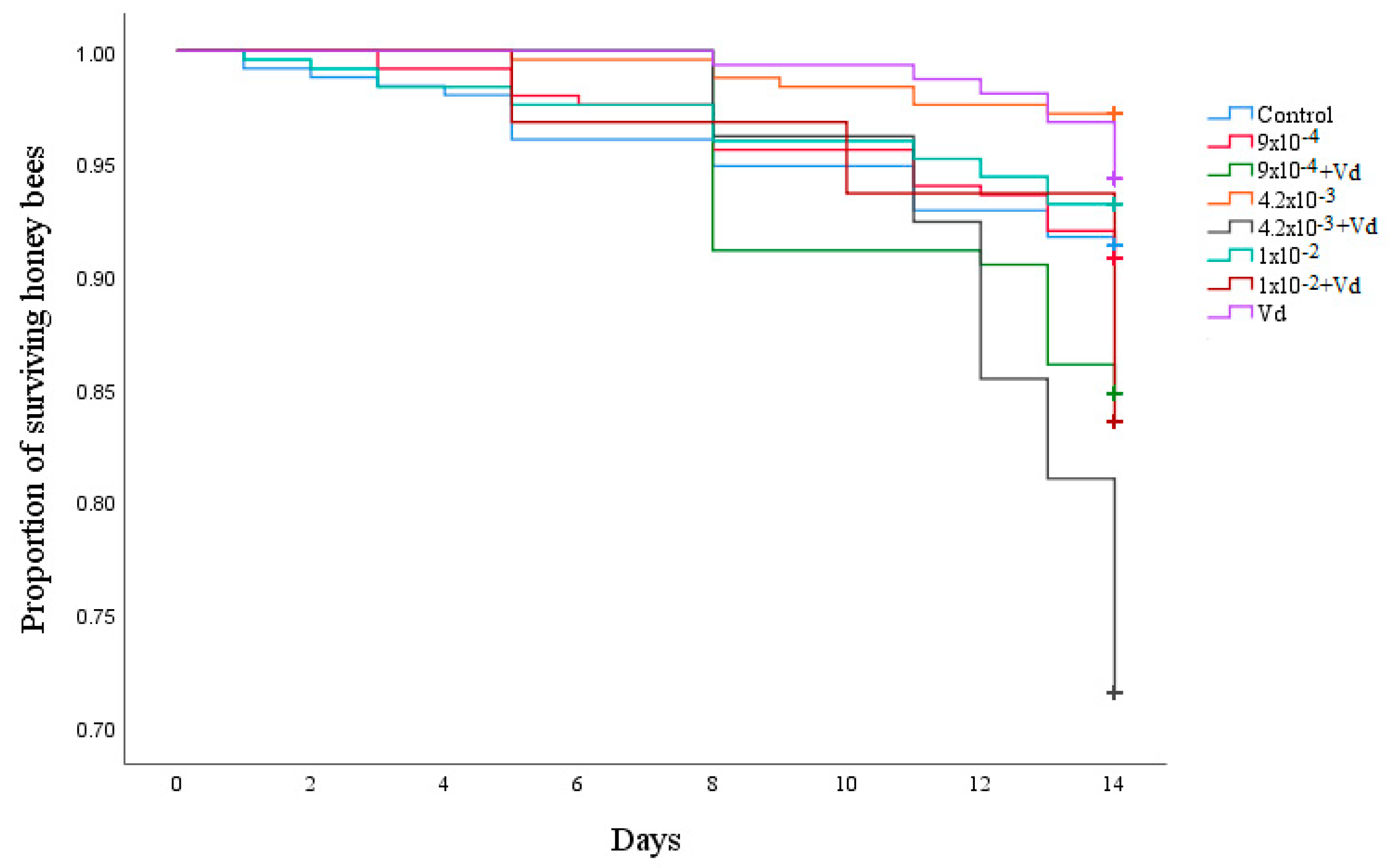
3.1. Survival and Sugar Consumption
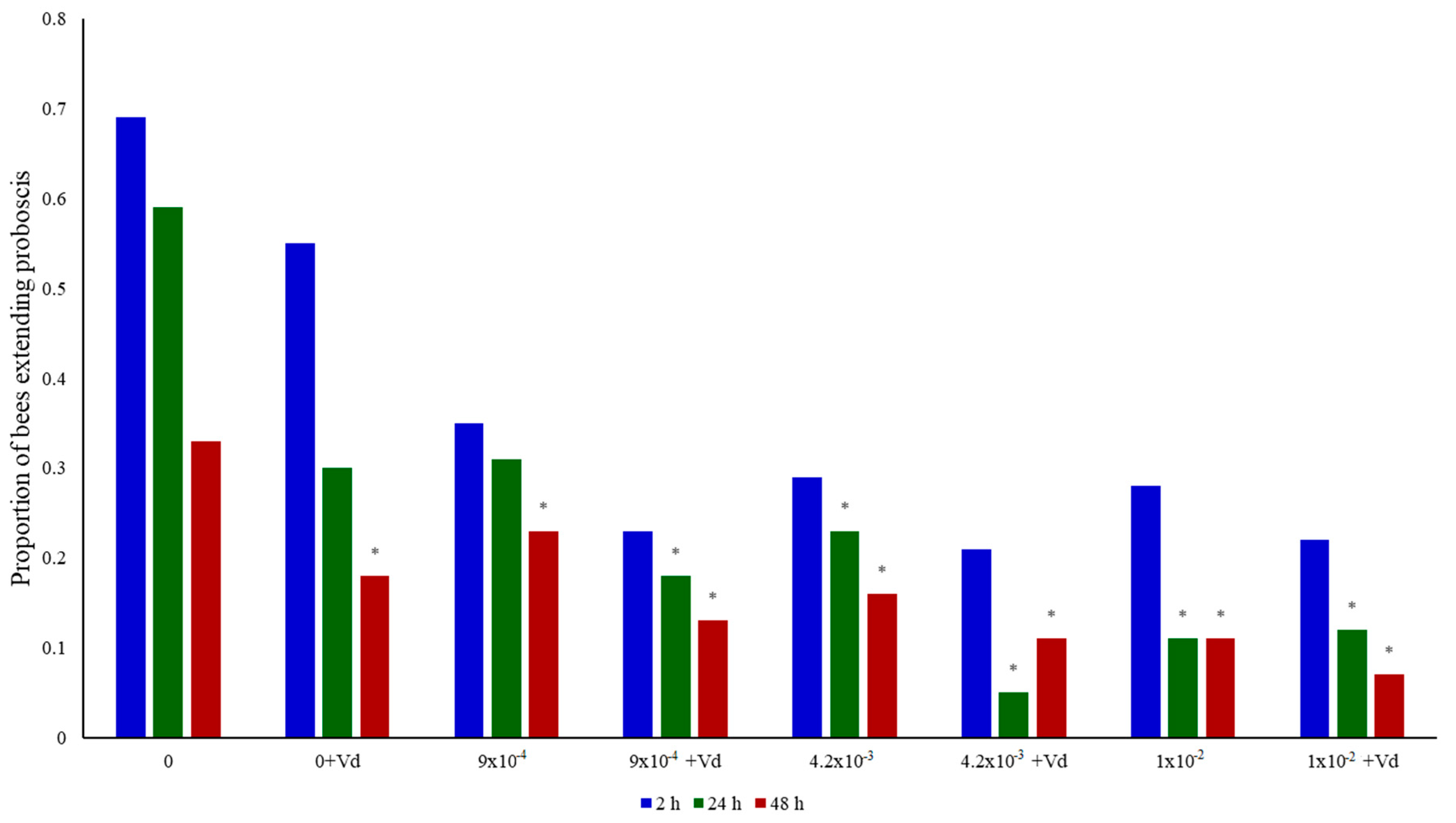
3.2. Memory Retention
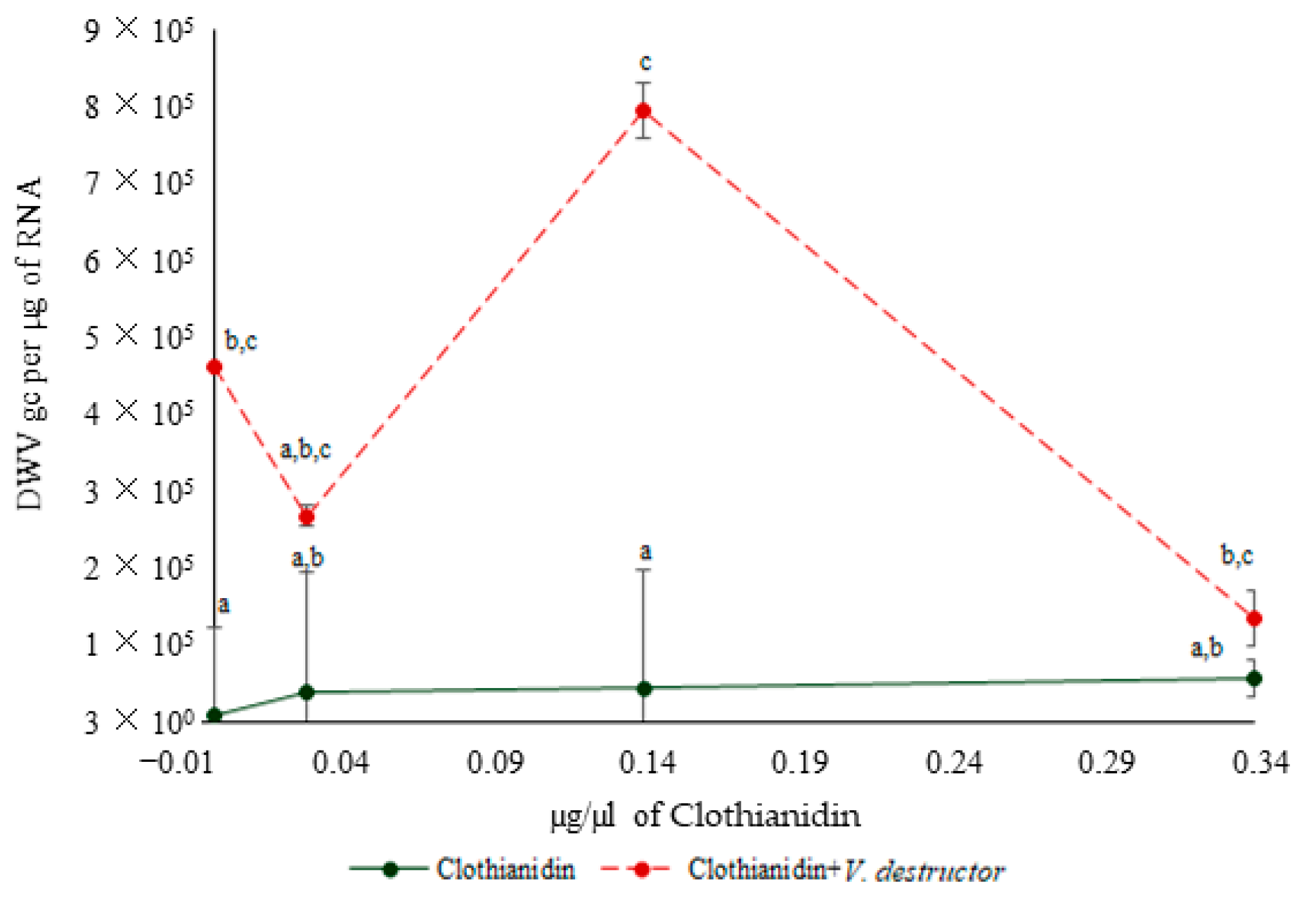
3.3. DWV Levels
3.4. Gene Expression in Bees Assessed for Memory Retention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Webb, B. Cognition in insects. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2715–2722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, J.L. The locale map of honey bees: Do insects have cognitive maps? Science 1986, 232, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Esch, H. Foraging Honey Bees: How Foragers Determine and Transmit Information About Feeding Site Locations; Springer Science and Business Media LLC: Berlin, Germany, 2011; pp. 53–64. [Google Scholar]
- Hopwood, J.; Vaughan, M.; Shepherd, M.; Biddinger, D.; Mader, E.; Black, S.H.; Mazzacano, C. Are Neonicotinoids Killing Bees? A Review of Research into the Effects of Neonicotinoid Insecticides on Bees, with Recommendations for Action; Xerces Society for Invertebrate Conservation: Portland, OR, USA, 2012. [Google Scholar]
- Belzunces, L.P.; Tchamitchian, S.; Brunet, J.-L. Neural effects of insecticides in the honey bee. Apidologie 2012, 43, 348–370. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, M.; Casida, J.E. Structure and diversity of insect nicotinic acetylcholine receptors. Pest Manag. Sci. 2001, 57, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.; Goulson, D. The environmental risks of neonicotinoid pesticides: A review of the evidence post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef]
- Uneme, H. Chemistry of clothianidin and related compounds. J. Agric. Food Chem. 2011, 59, 2932–2937. [Google Scholar] [CrossRef]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; McField, M.; et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. 2014, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Tomizawa, M.; Casida, J.E. Molecular recognition of neonicotinoid insecticides: The determinants of life or death. Accounts Chem. Res. 2009, 42, 260–269. [Google Scholar] [CrossRef]
- Matsuda, K.; Buckingham, S.D.; Kleier, D.; Rauh, J.J.; Grauso, M.; Sattelle, D.B. Neonicotinoids: Insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2001, 22, 573–580. [Google Scholar] [CrossRef]
- Blacquière, T.; Smagghe, G.; Van Gestel, C.A.M.; Mommaerts, V. Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology 2012, 21, 973–992. [Google Scholar] [CrossRef] [Green Version]
- Le Conte, Y.; Ellis, M.; Ritter, W. Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie 2010, 41, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Guzman-Novoa, E.; Eccles, L.; Calvete, Y.; McGowan, J.; Kelly, P.G.; Correa-Benítez, A. Varroa destructor is the main culprit for the death and reduced populations of overwintered honey bee (Apis mellifera) colonies in Ontario, Canada. Apidologie 2010, 41, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Weinberg, K.P.; Madel, G. The Influence of the mite Varroa jacobsoni Oud. on the protein concentration and the haemolymph volume of the brood of worker bees and drones of the honey bee Apis mellifera L. Apidologie 1985, 16, 421–436. [Google Scholar] [CrossRef] [Green Version]
- Kralj, J.; Fuchs, S. Parasitic Varroa destructor mites influence flight duration and homing ability of infested Apis mellifera foragers. Apidologie 2006, 37, 577–587. [Google Scholar] [CrossRef] [Green Version]
- Navajas, M.; Migeon, A.; Alaux, C.; Martin-Magniette, M.; Robinson, G.E.; Evans, J.D.; Cros-Arteil, S.; Crauser, D.; Le Conte, Y. Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genom. 2008, 9, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzman-Novoa, E. Effect of Varroa destructor, wounding and varroa homogenate on gene expression in brood and adult honey bees. PLoS ONE 2017, 12, e0169669. [Google Scholar] [CrossRef] [Green Version]
- Koleoglu, G.; Goodwin, P.H.; Reyes-Quintana, M.; Hamiduzzaman, M.M.; Guzmán-Novoa, E. Varroa destructor parasitism reduces hemocyte concentrations and prophenol oxidase gene expression in bees from two populations. Parasitol. Res. 2018, 117, 1175–1183. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.P.; Higgins, J.A.; Feldlaufer, M.F. Quantitative real-time reverse transcription-PCR analysis of deformed wing virus infection in the honeybee (Apis mellifera L.). Appl. Environ. Microbiol. 2005, 71, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Emsen, B.; Guzman-Novoa, E.; Kelly, P.G. Honey production of honey bee (Hymenoptera: Apidae) colonies with high and low Varroa destructor (Acari: Varroidae) infestation rates in eastern Canada. Can. Èntomol. 2013, 146, 236–240. [Google Scholar] [CrossRef]
- Kralj, J.; Brockmann, A.; Fuchs, S.; Tautz, J. The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J. Comp. Physiol. A 2006, 193, 363–370. [Google Scholar] [CrossRef]
- Iqbal, J.; Mueller, U. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B Biol. Sci. 2007, 274, 1517–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, K. Classical conditioned response in the honey bee. J. Insect Physiol. 1961, 6, 168–179. [Google Scholar] [CrossRef]
- Bitterman, M.E.; Menzel, R.; Fietz, A.; Schäfer, S. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 1983, 97, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Decourtye, A.; Armengaud, C.; Renou, M.; Devillers, J.; Cluzeau, S.; Gauthier, M.; Pham-Delègue, M.-H. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pestic. Biochem. Physiol. 2004, 78, 83–92. [Google Scholar] [CrossRef]
- Dacher, M.; Lagarrigue, A.; Gauthier, M. Antennal tactile learning in the honeybee: Effect of nicotinic antagonists on memory dynamics. Neuroscience 2005, 130, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Aliouane, Y.; El Hassani, A.K.; Gary, V.; Armengaud, C.; Lambin, M.; Gauthier, M. Subchronic Exposure of honeybees to sublethal doses of pesticides: Effects on behavior. Environ. Toxicol. Chem. 2009, 28, 113. [Google Scholar] [CrossRef]
- Tison, L.; Holtz, S.; Adeoye, A.; Kalkan, Ö.; Irmisch, N.S.; Lehmann, N.; Menzel, R. Effects of sublethal doses of thiacloprid and its formulation Calypso® on the learning and memory performance of honey bees. J. Exp. Biol. 2017, 220, 3695–3705. [Google Scholar] [CrossRef] [Green Version]
- Tison, L.; Rößner, A.; Gerschewski, S.; Menzel, R. The neonicotinoid clothianidin impairs memory processing in honey bees. Ecotoxicol. Environ. Saf. 2019, 180, 139–145. [Google Scholar] [CrossRef]
- Demares, F.; Pirk, C.W.; Nicolson, S.; Human, H. Neonicotinoids decrease sucrose responsiveness of honey bees at first contact. J. Insect Physiol. 2018, 108, 25–30. [Google Scholar] [CrossRef]
- Bartling, M.T.; Vilcinskas, A.; Lee, K.-Z. Sub-lethal doses of clothianidin inhibit the conditioning and biosensory abilities of the western honeybee Apis mellifera. Insects 2019, 10, 340. [Google Scholar] [CrossRef] [Green Version]
- Mustard, J.A.; Gott, A.; Scott, J.; Chavarria, N.L.; Wright, G.A. Honeybees fail to discriminate floral scents in a complex learning task after consuming a neonicotinoid pesticide. J. Exp. Biol. 2020, 223, jeb217174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, M.; Grünewald, B. Neurotransmitter Systems in the Honey Bee Brain: Functions in Learning and Memory; Springer Science and Business Media LLC: Berlin, Germany, 2011; pp. 155–169. [Google Scholar]
- Gauthier, M.; Belzunces, L.P.; Zaoujal, A.; Colin, M.E.; Richard, D. Modulatory effect of learning and memory on honey bee brain acetylcholinesterase activity. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1992, 103, 91–95. [Google Scholar] [CrossRef]
- Gauthier, M.; Dacher, M.; Thany, S.H.; Niggebrugge, C.; Deglise, P.; Kljucevic, P.; Armengaud, C.; Grunewald, B. Involvement of α-bungarotoxin-sensitive nicotinic receptors in long-term memory formation in the honeybee (Apis mellifera). Neurobiol. Learn. Mem. 2006, 86, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Silman, I.; Sussman, J.L. Acetylcholinesterase: How is structure related to function? Chem. Interactions 2008, 175, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.; Xie, W.; Boulianne, G.L. Neurexins and neuroligins: Recent insights from invertebrates. Mol. Neurobiol. 2011, 44, 426–440. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Reinhard, J.; Oakeshott, J.; Russell, R.; Srinivasan, M.V.; Claudianos, C. Sensory regulation of neuroligins and neurexin I in the honeybee brain. PLoS ONE 2010, 5, e9133. [Google Scholar] [CrossRef] [Green Version]
- Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. Interaction of field realistic doses of clothianidin and Varroa destructor parasitism on adult honey bee (Apis mellifera L.) health and neural gene expression, and antagonistic effects on differentially expressed genes. PLoS ONE 2020, 15, e0229030. [Google Scholar] [CrossRef] [Green Version]
- Arechavaleta-Velasco, M.E.; Guzman-Novoa, E. Relative effect of four characteristics that restrain the population growth of the mite Varroa destructor in honey bee (Apis mellifera) colonies. Apidologie 2001, 32, 157–174. [Google Scholar] [CrossRef] [Green Version]
- United States Environmental Protection Agency (USEPA). EFED Risk Assessment for the Seed Treatment of Clothianidin 600FS on Corn and Canola. 2003. Available online: https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-044309_20-Feb-03_a.pdf (accessed on 7 June 2020).
- United States Environmental Protection Agency (USEPA). Clothianidin Registration of Prosper T400 Seed Treatment on Mustard Seed (oilseed and condiments) and Poncho/Votivo Seed Treatment on Cotton. 2010. Available online: https://www.beyondpesticides.org/assets/media/documents/pollinators/clothianidinepamemo110210.pdf (accessed on 7 June 2020).
- Free, J.B.; Spencer-Booth, Y. Observations on the temperature regulation and food consumption of honeybees (Apis mellifera). J. Exp. Biol. 1958, 35, 930–937. [Google Scholar]
- Decourtye, A.; Lacassie, É.; Pham-Delègue, M.-H. Learning performances of honeybees (Apis mellifera L) are differentially affected by imidacloprid according to the season. Pest Manag. Sci. 2003, 59, 269–278. [Google Scholar] [CrossRef]
- Morfin, N.; Goodwin, P.H.; Hunt, G.J.; Guzman-Novoa, E. Effects of sublethal doses of clothianidin and/or V. destructor on honey bee (Apis mellifera) self-grooming behavior and associated gene expression. Sci. Rep. 2019, 9, 5196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenas, A.; Farina, W.M. Age and rearing environment interact in the retention of early olfactory memories in honeybees. J. Comp. Physiol. A 2008, 194, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Felsenberg, J.; Gehring, K.B.; Antemann, V.; Eisenhardt, D. Behavioural pharmacology in classical conditioning of the proboscis extension response in honeybees (Apis mellifera). J. Vis. Exp. 2011, e2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.-C.; Chang, H.-C.; Wu, W.-Y.; Chen, Y.-W. Impaired olfactory associative behavior of honeybee workers due to contamination of imidacloprid in the larval stage. PLoS ONE 2012, 7, e49472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhardt, D. Molecular mechanisms underlying formation of long-term reward memories and extinction memories in the honeybee (Apis mellifera). Learn. Mem. 2014, 21, 534–542. [Google Scholar] [CrossRef] [Green Version]
- Hammer, M. The neural basis of associative reward learning in honeybees. Trends Neurosci. 1997, 20, 245–252. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.D. Beepath: An ordered quantitative-PCR array for exploring honey bee immunity and disease. J. Invertebr. Pathol. 2006, 93, 135–139. [Google Scholar] [CrossRef]
- Thompson, G.J.; Yockey, H.; Lim, J.; Oldroyd, B.P. Experimental manipulation of ovary activation and gene expression in honey bee (Apis mellifera) queens and workers: Testing hypotheses of reproductive regulation. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2007, 307, 600–610. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR Data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [Green Version]
- Morfin, N.; Given, K.; Evans, M.; Guzman-Novoa, E.; Hunt, G.J. Grooming behavior and gene expression of the Indiana “mite-biter” honey bee stock. Apidologie 2019, 51, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆ CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- The R Project for Statistical Computing. Available online: http://www.r-project.org/ (accessed on 13 February 2012).
- IBM Corp. IBM SPSS Statistics for Windows, Version 25.0; IBM Corp: Armonk, NY, USA, 2017. [Google Scholar]
- Abbo, P.M.; Kawasaki, J.K.; Hamilton, M.; Cook, S.C.; Degrandi-Hoffman, G.; Li, W.F.; Liu, J.; Chen, Y.P. Effects of imidacloprid and Varroa destructor on survival and health of European honey bees, Apis mellifera. Insect Sci. 2016, 24, 467–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosi, S.; Nieh, J.C.; Sgolastra, F.; Cabbri, R.; Medrzycki, P. Neonicotinoid pesticides and nutritional stress synergistically reduce survival in honey bees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straub, L.; Williams, G.R.; Vidondo, B.; Khongphinitbunjong, K.; Retschnig, G.; Schneeberger, A.; Chantawannakul, P.; Dietemann, V.; Neumann, P. Neonicotinoids and ectoparasitic mites synergistically impact honeybees. Sci. Rep. 2019, 9, 8159. [Google Scholar] [CrossRef] [PubMed]
- Siede, R.; Meixner, M.D.; Almanza, M.T.; Schöning, R.; Maus, C.; Büchler, R. A long-term field study on the effects of dietary exposure of clothianidin to varroosis-weakened honey bee colonies. Ecotoxicology 2018, 27, 772–783. [Google Scholar] [CrossRef] [Green Version]
- Doublet, V.; Labarussias, M.; De Miranda, J.R.; Moritz, R.F.A.; Paxton, R.J. Bees under stress: Sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environ. Microbiol. 2014, 17, 969–983. [Google Scholar] [CrossRef]
- Guideline on test methods for evaluating the side-effects of plant protection products on honeybees. EPPO Bull. 1992, 22, 203–215. [CrossRef]
- Dukas, R. Evolutionary biology of insect learning. Annu. Rev. Èntomol. 2008, 53, 145–160. [Google Scholar] [CrossRef] [Green Version]
- Andrione, M.; Vallortigara, G.; Antolini, R.; Haase, A. Neonicotinoid-induced impairment of odour coding in the honeybee. Sci. Rep. 2016, 6, 38110. [Google Scholar] [CrossRef] [Green Version]
- Alkassab, A.T.; Kirchner, W.H. Impacts of chronic sublethal exposure to clothianidin on winter honeybees. Ecotoxicology 2016, 25, 1000–1010. [Google Scholar] [CrossRef] [PubMed]
- Piiroinen, S.; Goulson, D. Chronic neonicotinoid pesticide exposure and parasite stress differentially affects learning in honeybees and bumblebees. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.P.; Huang, Z.Y. Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie 2010, 41, 364–374. [Google Scholar] [CrossRef] [Green Version]
- McMenamin, A.J.; Genersch, E. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef]
- Annoscia, D.; Brown, S.P.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Frizzera, D.; Zanni, V.; Galbraith, D.A.; Caprio, E.; Grozinger, C.M.; et al. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, S.; Ochoa, R.; Bauchan, G.R.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [Green Version]
- Zanni, V.; Degirmenci, L.; Annoscia, D.; Scheiner, R.; Nazzi, F. The reduced brood nursing by mite-infested honey bees depends on their accelerated behavioral maturation. J. Insect Physiol. 2018, 109, 47–54. [Google Scholar] [CrossRef]
- Shah, K.S.; Evans, E.C.; Pizzorno, M.C. Localization of deformed wing virus (DWV) in the brains of the honeybee, Apis mellifera Linnaeus. Virol. J. 2009, 6, 182. [Google Scholar] [CrossRef] [Green Version]
- Yue, C.; Genersch, E. RT-PCR analysis of Deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). J. Gen. Virol. 2005, 86, 3419–3424. [Google Scholar] [CrossRef]
- Tentcheva, D.; Gauthier, L.; Bagny, L.; Fievet, J.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Comparative analysis of deformed wing virus (DWV) RNA in Apis mellifera and Varroa destructor. Apidologie 2005, 37, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A Virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Prisco, G.; Zhang, X.; Pennacchio, F.; Caprio, E.; Li, J.; Evans, J.D.; DeGrandi-Hoffman, G.; Hamilton, M.C.; Chen, Y.P. Dynamics of persistent and acute deformed wing virus infections in honey bees, Apis mellifera. Viruses 2011, 3, 2425–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Èntomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demas, G.E.; Adamo, S.A.; French, S.S. Neuroendocrine-immune crosstalk in vertebrates and invertebrates: Implications for host defence. Funct. Ecol. 2011, 25, 29–39. [Google Scholar] [CrossRef]
- Tan, J.; Galligan, J.J.; Hollingworth, R.M. Agonist actions of neonicotinoids on nicotinic acetylcholine receptors expressed by cockroach neurons. Neuro Toxicol. 2007, 28, 829–842. [Google Scholar] [CrossRef]

| Treatment | Mean Consumption of Sugar Syrup (± S.E.) |
|---|---|
| 0 ng/µL | 29.41 ± 0.84 µL |
| 9 × 10−4 ng/µL | 28.65 ± 0.85 µL |
| 4.2 × 10−3 ng/µL | 28.53 ± 0.55 µL |
| 1 × 10−2 ng/µL | 28.85 ± 0.90 µL |
| 0 ng/µL + V. destructor | 27.64 ± 0.44 µL |
| 9 × 10−4 ng/µL + V. destructor | 27.80 ± 0.55 µL |
| 4.2 × 10−3 ng/µL + V. destructor | 27.89 ± 0.36 µL |
| 1 × 10−2 ng/µL + V. destructor | 29.97 ± 0.63 µL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morfin, N.; Goodwin, P.H.; Guzman-Novoa, E. The Combined Effects of Varroa destructor Parasitism and Exposure to Neonicotinoids Affects Honey Bee (Apis mellifera L.) Memory and Gene Expression. Biology 2020, 9, 237. https://doi.org/10.3390/biology9090237
Morfin N, Goodwin PH, Guzman-Novoa E. The Combined Effects of Varroa destructor Parasitism and Exposure to Neonicotinoids Affects Honey Bee (Apis mellifera L.) Memory and Gene Expression. Biology. 2020; 9(9):237. https://doi.org/10.3390/biology9090237
Chicago/Turabian StyleMorfin, Nuria, Paul H. Goodwin, and Ernesto Guzman-Novoa. 2020. "The Combined Effects of Varroa destructor Parasitism and Exposure to Neonicotinoids Affects Honey Bee (Apis mellifera L.) Memory and Gene Expression" Biology 9, no. 9: 237. https://doi.org/10.3390/biology9090237
APA StyleMorfin, N., Goodwin, P. H., & Guzman-Novoa, E. (2020). The Combined Effects of Varroa destructor Parasitism and Exposure to Neonicotinoids Affects Honey Bee (Apis mellifera L.) Memory and Gene Expression. Biology, 9(9), 237. https://doi.org/10.3390/biology9090237