Stress Changes the Resting-State Cortical Flow of Information from Distributed to Frontally Directed Patterns
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Resting-State EEG
2.2.1. Acquisition
2.2.2. Preprocessing
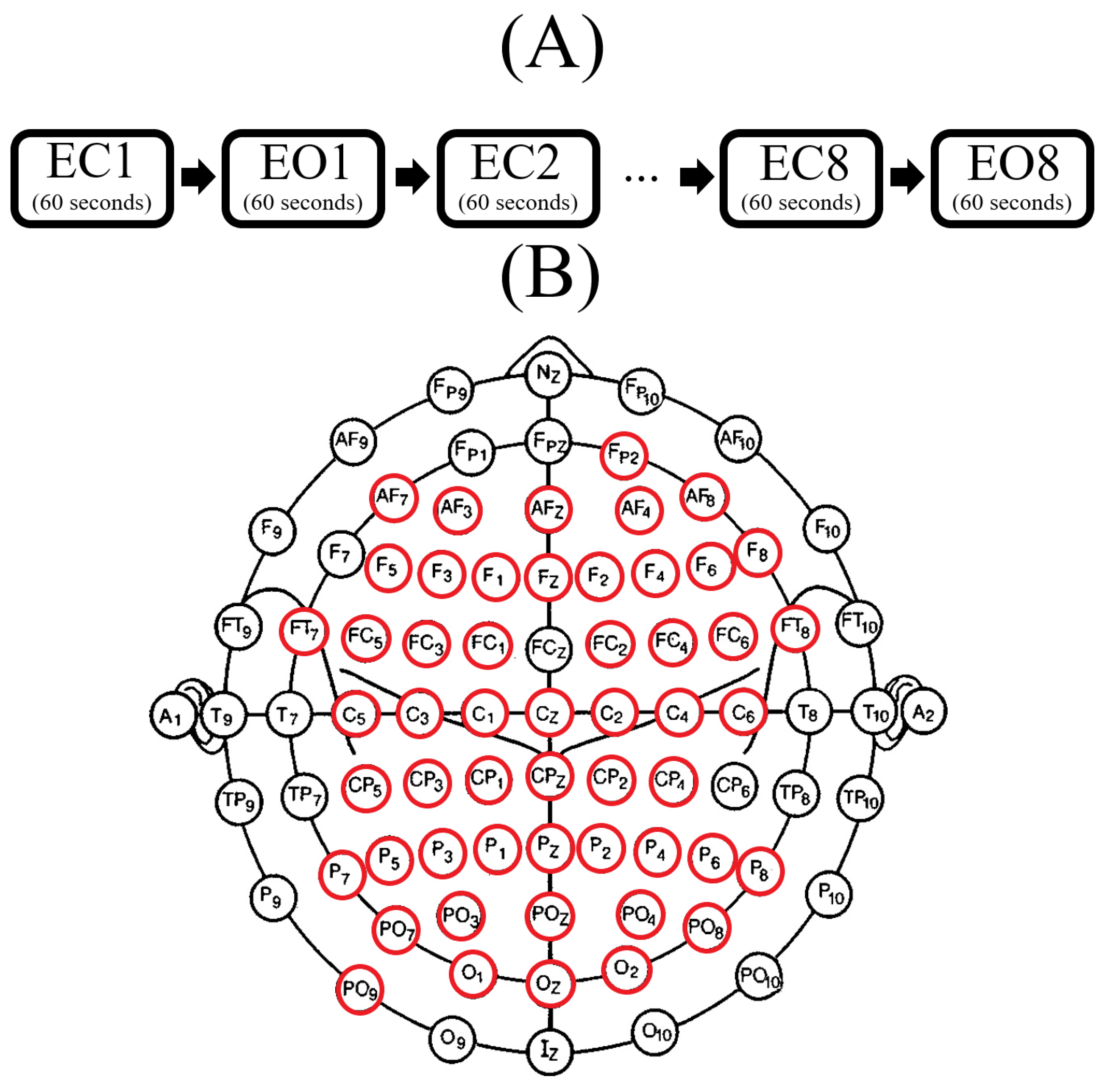
2.3. EEG Channels Inclusion
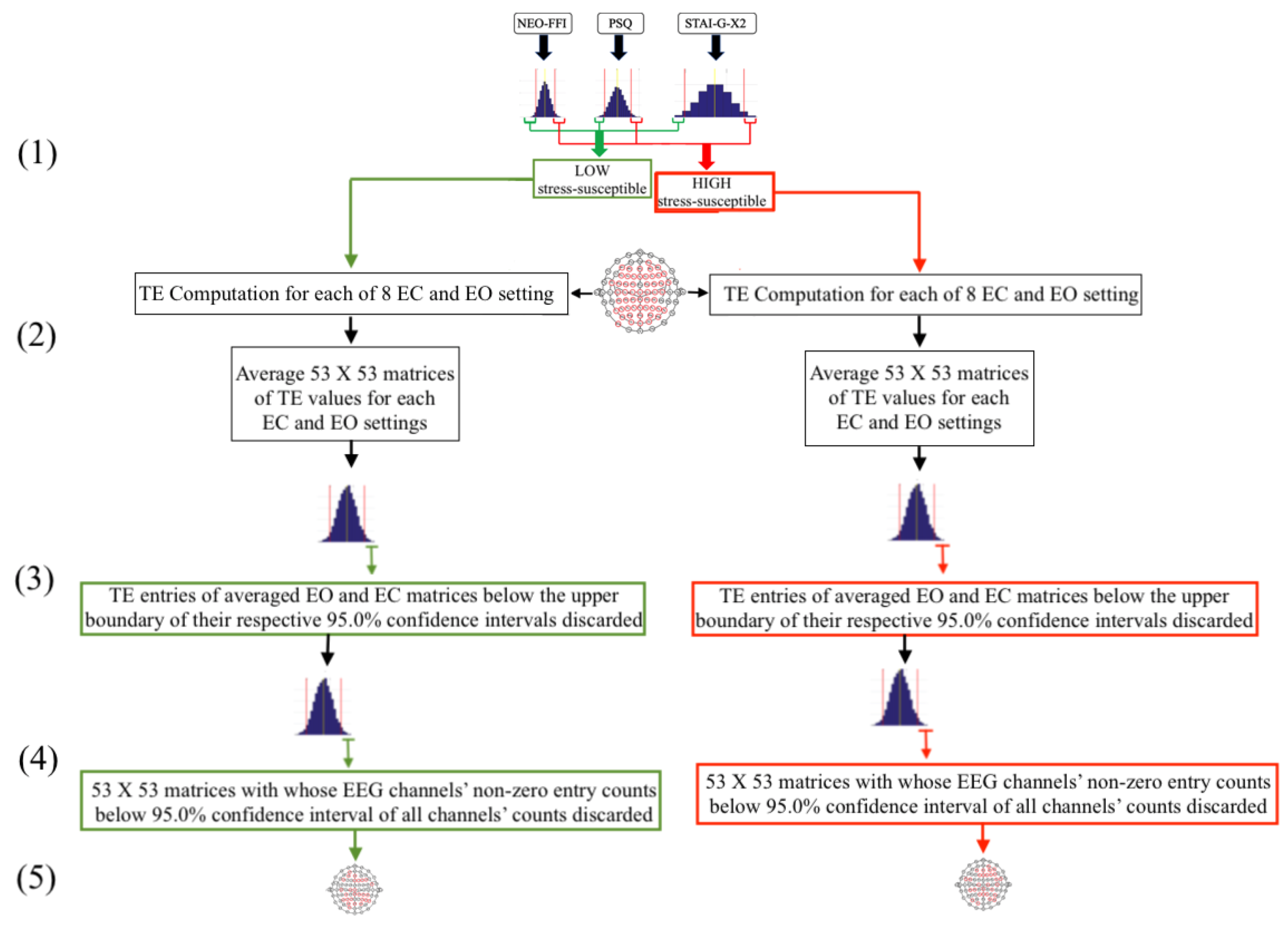
2.4. An Overview of the Participants’ Selection and EEG Inclusion Process
- As a first step (Figure 2(1)), the 95.0% confidence intervals of the participants’ responses to each of NEO-FFI, PSQ, and STAI-G-X2 were calculated separately. Subsequently, those individuals whose responses to all of these three questionnaires fell in lower/upper boundary of respective confidence interval of its related questionnaire (indicated by green/red lines, respectively) were used to form the LOW/HIGH stress-susceptible groups.
- In the second step (Figure 2(2)), pair-wise TE values for all available 53-EEG-channels (Figure 1B), per LOW and HIGH stress-susceptible participants, per EC and EO settings’ matrices (i.e., 8 matrices, per setting) were calculated. This resulted in 8 TE matrices of size 53 × 53 (i.e., paired EEG channels’ TE values), per EC and EO settings. These 8 TE matrices were then averaged, per EC and EO settings, per participant, thereby yielding one averaged 53 × 53 TE matrix, per EC and EO settings, per participant.
- Third step (Figure 2(3)) included computing the 95.0% confidence interval for TE values, per EC and EO settings. For this purpose, TE values from the averaged EC and EO TE matrices of all participants in each LOW and HIGH stress-susceptible groups were separately combined (i.e., 10 × 53 × 53 = 28090 TE values, per EC and EO settings). Next, the TE values, per participant, per setting, that were below the upper boundary of their respective group’s confidence interval (i.e., per EC and EO settings) were discarded (i.e., set to zero).
- In step four (Figure 2(4)), we first counted the number of non-zero entries in each row of the averaged TE matrices, per individual, per EC and EO settings. Next, we combined the individuals’ counts for LOW and HIGH stress-susceptible groups separately and computed the 95.0% confidence intervals for these counts (i.e., per EC and EO settings, per stress-susceptible groups). We then discarded those EEG channels whose number of non-zero TE entries were below the upper boundary of their related confidence interval, per individual, per stress-susceptible groups, and per EC and EO settings.
- In step five (Figure 2(5)) we separately found the union of EEG channels among individuals in each of LOW and HIGH stress-susceptible groups, per EC and EO settings (Figure A2). In the case of EC, this step resulted in 18 EEG channels that were common between all participants in HIGH stress-susceptible group. These channels were AFZ, AF4, F1, F6, FT7, CZ, C2, C6, CP5, CP3, CP1, CP4, P7, P5, P4, P6, PO3, POZ. Similarly, there were 18 EEG channels that were common among all participants in LOW stress-susceptible group. They were FP2, AFZ, F3, F5, FZ, CP1, CP3, CP5, CPZ, P7, P3, P4, P2, P6, PO3, POZ, PO4, O2. We used the union of these EEG channels (without repetition) for comparative analyses between HIGH and LOW stress-susceptible groups in EC setting (Figure A2A). On the other hand, we found 21 EEG channels in EO setting that survived these thresholding steps and that were common between all participants in HIGH stress-susceptible group. Those channels were AF3, AF4, C1, C2, C6, CP3, CP4, CP5, F1, F2, F6, FC5, FC6, FT7, FT8, Fp2, Fz, O1, P1, P6, PO4. The number of such EEG channels in LOW stress-susceptible group was 17 (AF3, AF8, AFZ, C2, CP3, CPZ, F2, F4, FC5, FC6, FT7, Fp2, Fz, P2, PO7, PO8, PZ). Similar to the case of EC, we used the union of these EEG channels for comparative analyses between HIGH and LOW stress-susceptible groups for EO setting (Figure A2B).
2.5. Analysis
2.5.1. Responses to Neuroticism (NEO-FFI), Worries (PSQ), Tension (PSQ), and STAI Trait Anxiety (STAI-G-X2)
2.5.2. Total TEs
2.5.3. Distributed TEs
2.5.4. TE Computation, Effect Sizes and Bonferroni Correction
3. Results
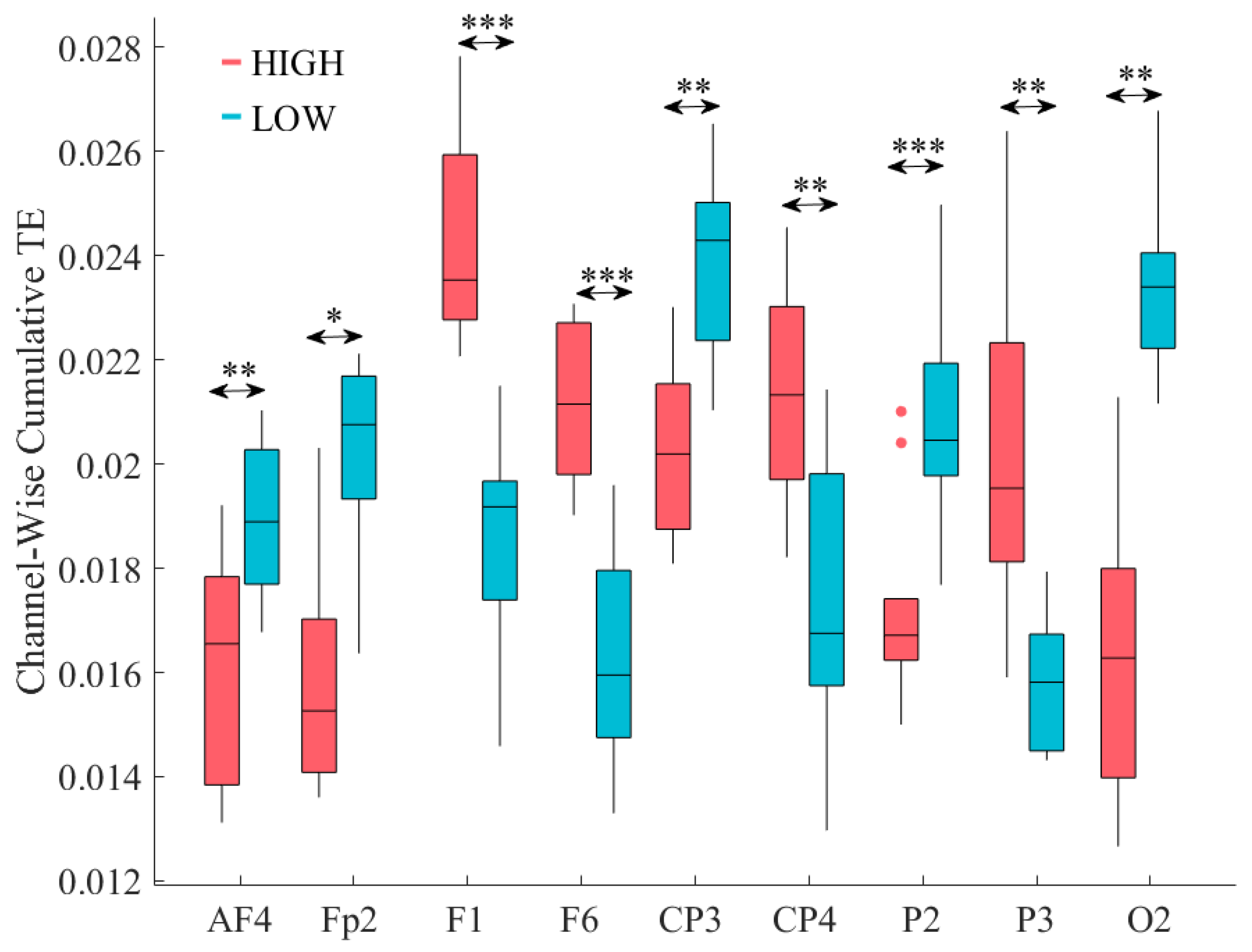
3.1. Total TEs
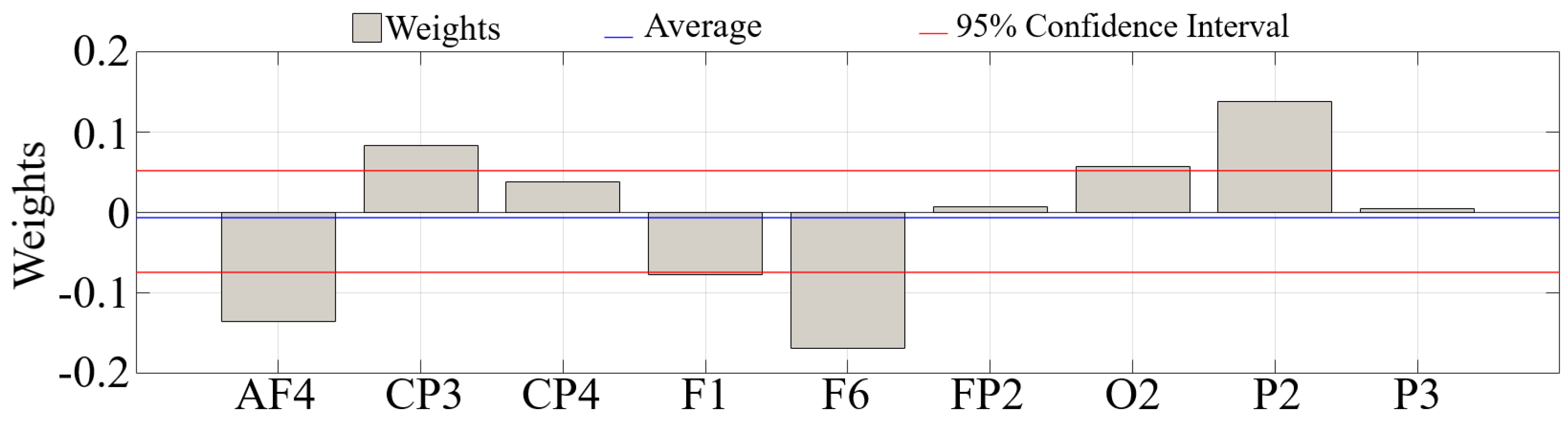
3.2. GLM Analysis of the Channels with Significantly Different Total TEs
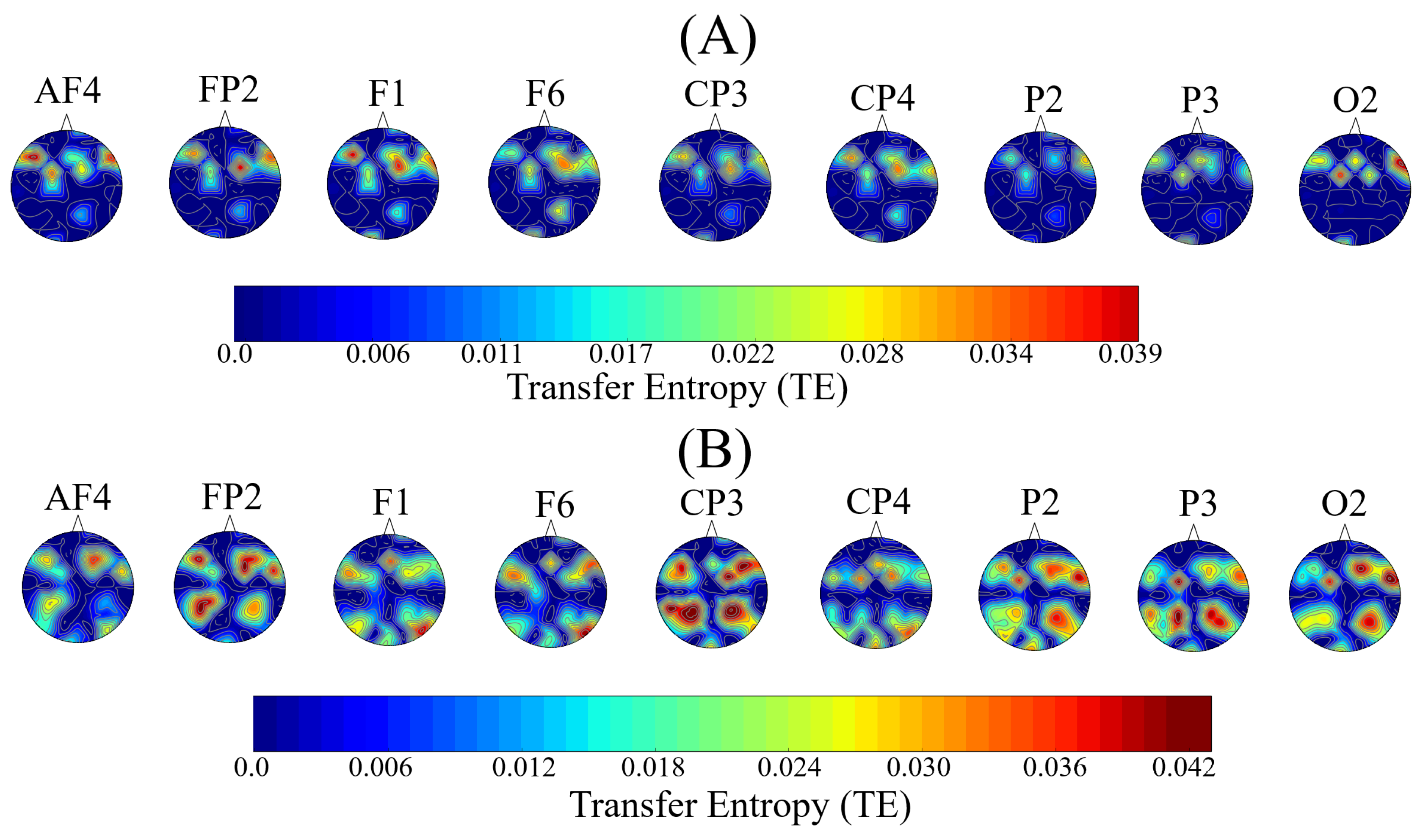
3.3. Distribution of the Information Transferred by the Channels with Significantly Different Total TEs
4. Discussion
5. Limitations and Future Direction
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Detailed Participants and EEG Selection Procedure
Appendix A.1. Determination of Participants’ Stress-Susceptibility
Appendix A.1.1. Participants’ Selection Based on Their Responses to NEO-FFI, PSQ, STAI-G-X2
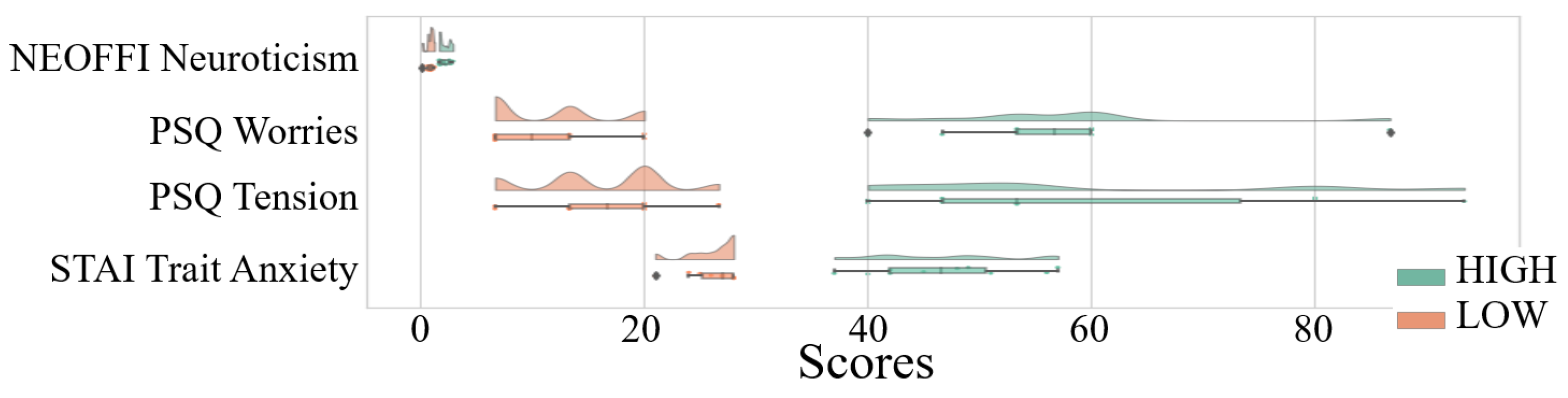
| Questionnaire | M | SD | CI95.0% | Minimum | Maximum |
|---|---|---|---|---|---|
| NEO-FFI Neuroticism | 1.53 | 0.54 | [1.44 1.63] | 0.17 | 2.92 |
| PSQ Worries | 29.18 | 16.49 | [26.39 32.30] | 6.67 | 86.67 |
| PSQ Tension | 31.48 | 17.8 | [28.52 34.81] | 6.67 | 93.33 |
| STAI Trait Anxiety | 31.48 | 17.80 | [28.58 34.75] | 6.67 | 93.33 |
Appendix A.2. Quantification of the Directed Transfer of Information between EEG Channels
Appendix A.2.1. Overview of Transfer Entropy (TE)
Appendix A.2.2. Participants’ TE Computation
Appendix A.2.3. Thresholding the Participants’ TEs
| Group | M | SD | Mdn | CI95.0% |
|---|---|---|---|---|
| HIGH | 0.0346 | 0.003 | 0.0349 | [0.0303 0.0386] |
| LOW | 0.0344 | 0.003 | 0.0341 | [0.0282 0.0392] |
| Group | M | SD | Mdn | CI95.0% |
|---|---|---|---|---|
| HIGH | 0.0349 | 0.002 | 0.0354 | [0.0297 0.0378] |
| LOW | 0.0349 | 0.003 | 0.0353 | [0.0312 0.0386] |
| Group | M | SD | CI95.0% |
|---|---|---|---|
| HIGH | 26.65 | 8.67 | [25.90 27.39] |
| LOW | 27.20 | 9.82 | [26.36 28.04] |
| Group | M | SD | CI95.0% |
|---|---|---|---|
| HIGHs | 27.49 | 10.12 | [26.61 28.36] |
| LOW | 27.23 | 9.30 | [26.45 28.01] |

Appendix B. Eyes-Open (EO) Setting
Appendix B.1. Total TEs
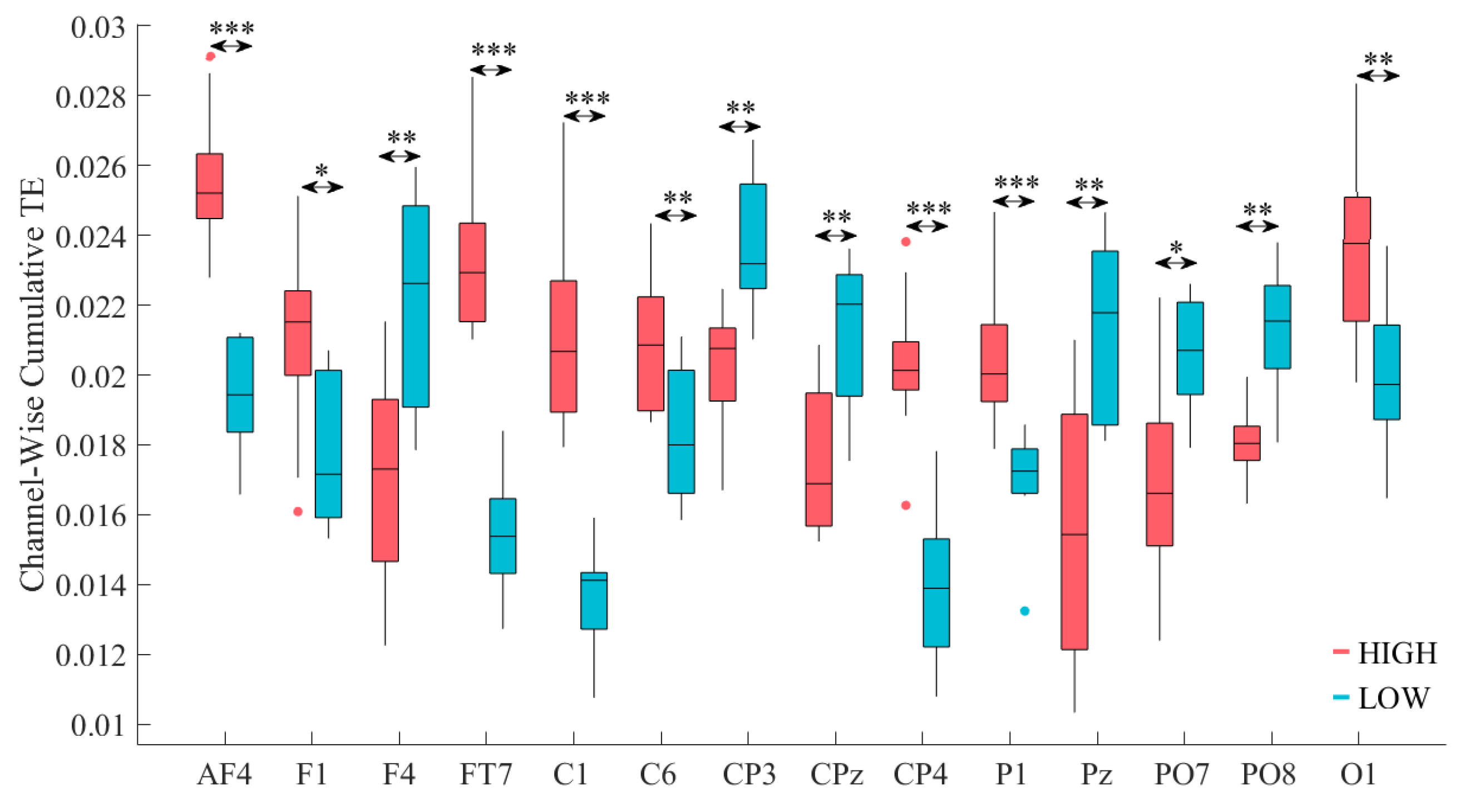
| Channel | p = | W(18) | r | ||||
|---|---|---|---|---|---|---|---|
| AF4 | 0.0002 | 3.74 | 0.84 | 0.0256 | 0.002 | 0.019 | 0.002 |
| F1 | 0.0110 | 2.46 | 0.55 | 0.021 | 0.003 | 0.018 | 0.002 |
| FT7 | 0.0002 | 3.74 | 0.84 | 0.023 | 0.002 | 0.015 | 0.002 |
| C1 | 0.0002 | 3.74 | 0.84 | 0.021 | 0.003 | 0.014 | 0.001 |
| C6 | 0.0073 | 2.68 | 0.60 | 0.021 | 0.002 | 0.018 | 0.002 |
| CP4 | 0.0002 | 3.67 | 0.82 | 0.020 | 0.002 | 0.013 | 0.002 |
| P1 | 0.0009 | 3.44 | 0.77 | 0.0204 | 0.002 | 0.017 | 0.001 |
| O1 | 0.0046 | 2.83 | 0.63 | 0.024 | 0.003 | 0.020 | 0.002 |
| Channel | p = | W(18) | r | ||||
|---|---|---|---|---|---|---|---|
| F4 | 0.0046 | −2.83 | 0.63 | 0.017 | 0.003 | 0.022 | 0.003 |
| CP3 | 0.0010 | −3.29 | 0.74 | 0.020 | 0.002 | 0.024 | 0.002 |
| CPz | 0.0028 | −2.99 | 0.67 | 0.017 | 0.002 | 0.021 | 0.002 |
| Pz | 0.0036 | −2.91 | 0.65 | 0.016 | 0.004 | 0.021 | 0.002 |
| PO7 | 0.0120 | −2.46 | 0.55 | 0.017 | 0.003 | 0.020 | 0.002 |
| PO8 | 0.0010 | −3.29 | 0.74 | 0.018 | 0.001 | 0.021 | 0.002 |
Appendix B.2. GLM Analysis of the Channels with Significantly Different Total TEs
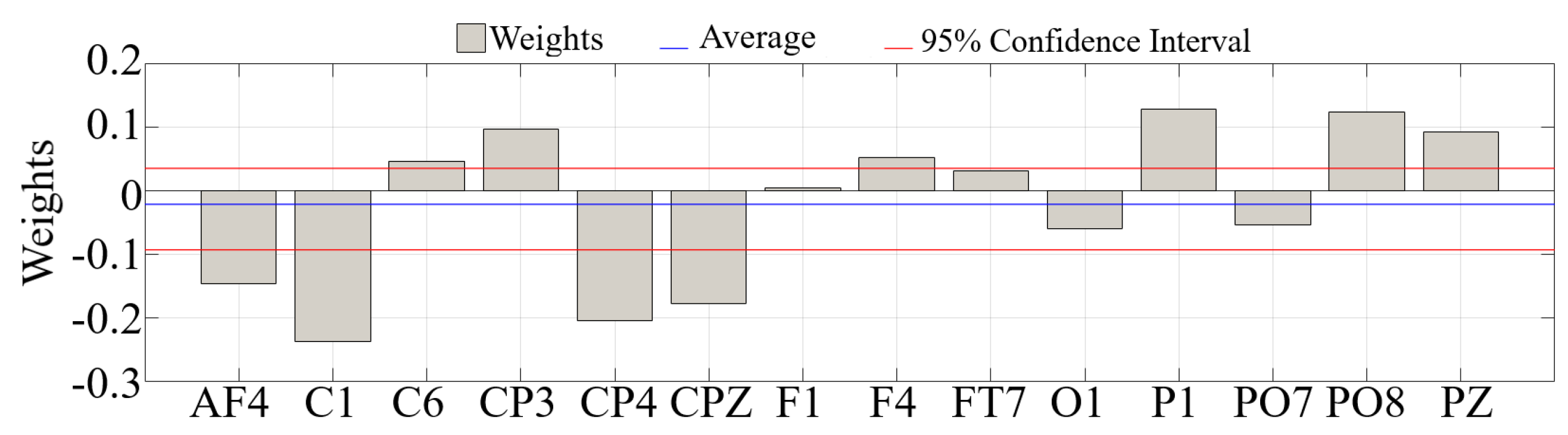
| Channel | p = | F | |
|---|---|---|---|
| AF4 | 0.8885 | 0.021 | 0.003 |
| F1 | 0.9962 | 0.000 | 0.000 |
| F4 | 0.9804 | 0.001 | 0.0001 |
| FT7 | 0.9872 | 0.000 | 0.000 |
| C1 | 0.8604 | 0.033 | 0.005 |
| C6 | 0.9524 | 0.004 | 0.0006 |
| CP3 | 0.9012 | 0.017 | 0.003 |
| CPZ | 0.9027 | 0.016 | 0.003 |
| CP4 | 0.8872 | 0.022 | 0.004 |
| P1 | 0.9037 | 0.016 | 0.003 |
| PZ | 0.9380 | 0.007 | 0.001 |
| PO7 | 0.9563 | 0.003 | 0.001 |
| PO8 | 0.8608 | 0.033 | 0.005 |
| O1 | 0.9597 | 0.003 | 0.0004 |
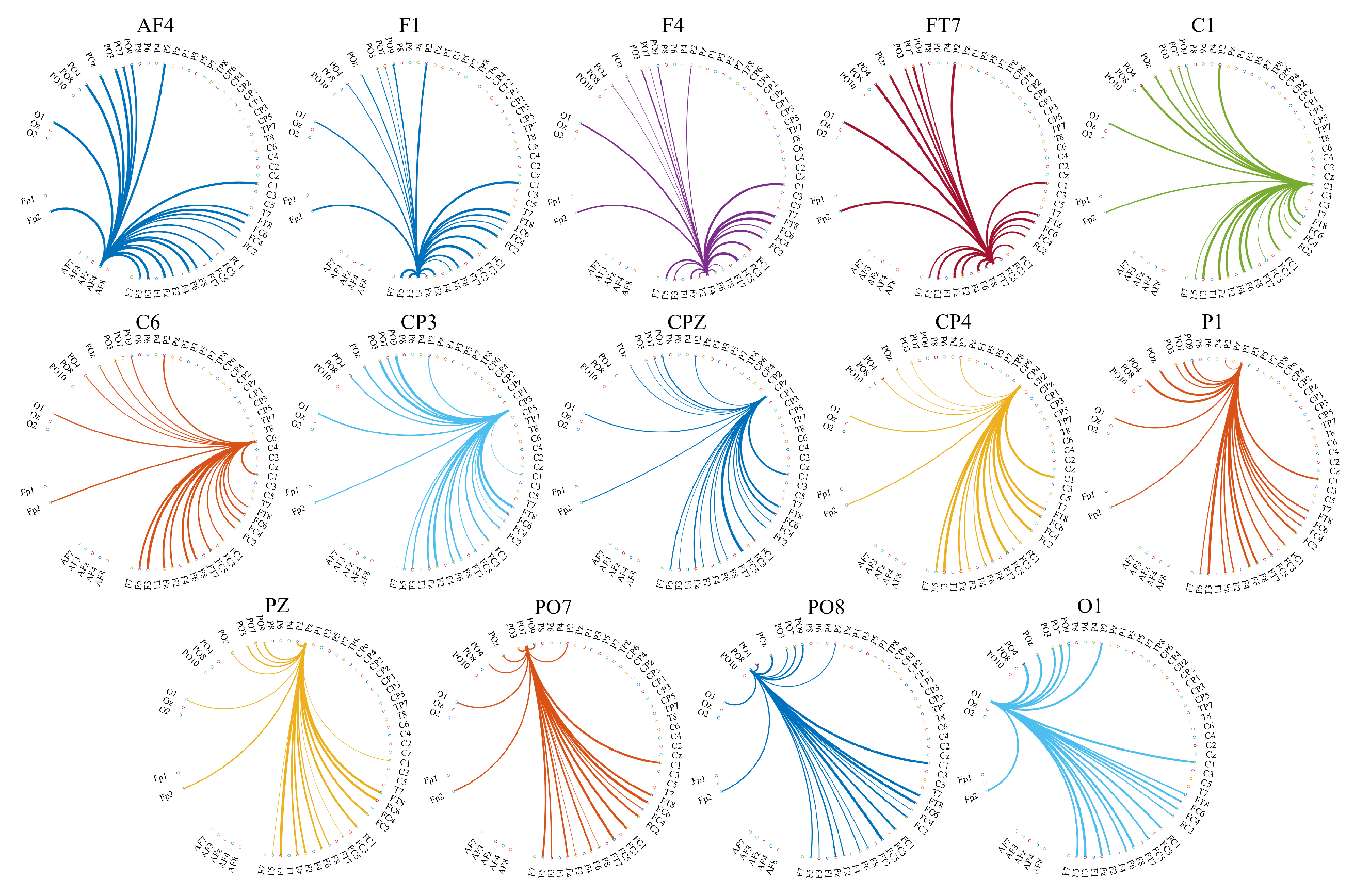
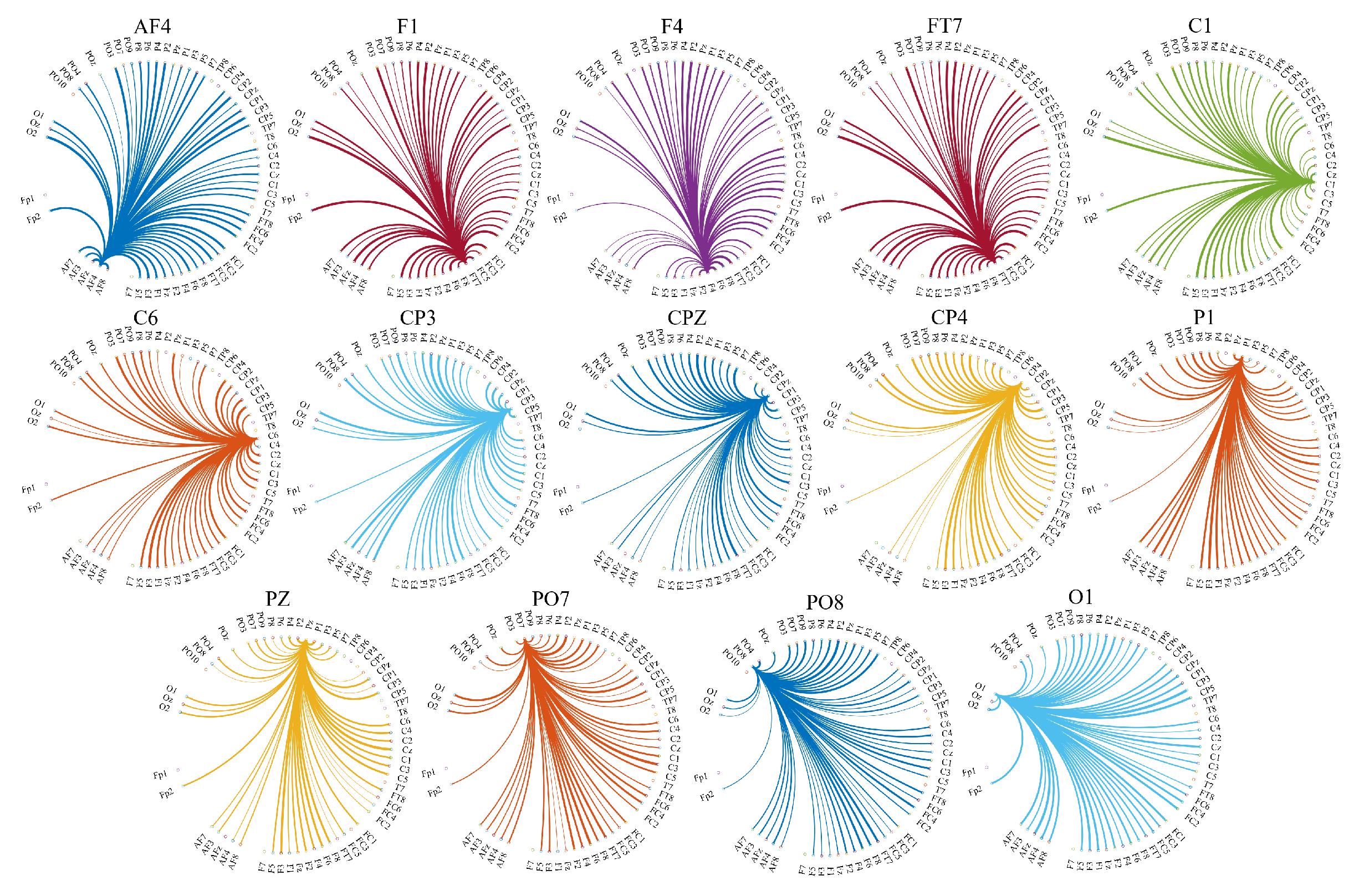
Appendix B.3. Distribution of the Information Transferred by the Channels with Significantly Different Total TEs
| Channel | p = | W(18) | r | ||||
|---|---|---|---|---|---|---|---|
| AF4 | 0.0000 | 4.13 | 0.13 | 0.026 | 0.017 | 0.019 | 0.019 |
| F1 | 0.0066 | 2.72 | 0.08 | 0.021 | 0.018 | 0.017 | 0.018 |
| FT7 | 0.0000 | 5.59 | 0.17 | 0.023 | 0.018 | 0.015 | 0.019 |
| C1 | 0.0000 | 5.70 | 0.175 | 0.021 | 0.018 | 0.014 | 0.018 |
| C6 | 0.0024 | 3.04 | 0.09 | 0.021 | 0.019 | 0.018 | 0.018 |
| CP4 | 0.0000 | 5.61 | 0.17 | 0.020 | 0.019 | 0.014 | 0.018 |
| P1 | 0.0029 | 2.98 | 0.091 | 0.020 | 0.018 | 0.017 | 0.018 |
| O1 | 0.004 | 2.85 | 0.089 | 0.024 | 0.018 | 0.020 | 0.018 |
| Channel | p = | W(18) | r | ||||
|---|---|---|---|---|---|---|---|
| F4 | 0.0000 | −4.24 | 0.13 | 0.017 | 0.018 | 0.022 | 0.018 |
| CP3 | 0.0027 | −3.00 | 0.09 | 0.020 | 0.019 | 0.024 | 0.018 |
| CPZ | 0.0012 | −3.23 | 0.10 | 0.017 | 0.018 | 0.021 | 0.018 |
| PZ | 0.0000 | −4.13 | 0.13 | 0.016 | 0.018 | 0.021 | 0.018 |
| PO7 | 0.0053 | −2.79 | 0.09 | 0.017 | 0.018 | 0.020 | 0.018 |
| PO8 | 0.0041 | −2.87 | 0.09 | 0.018 | 0.018 | 0.021 | 0.018 |

Appendix C. Responses to Questionnaires
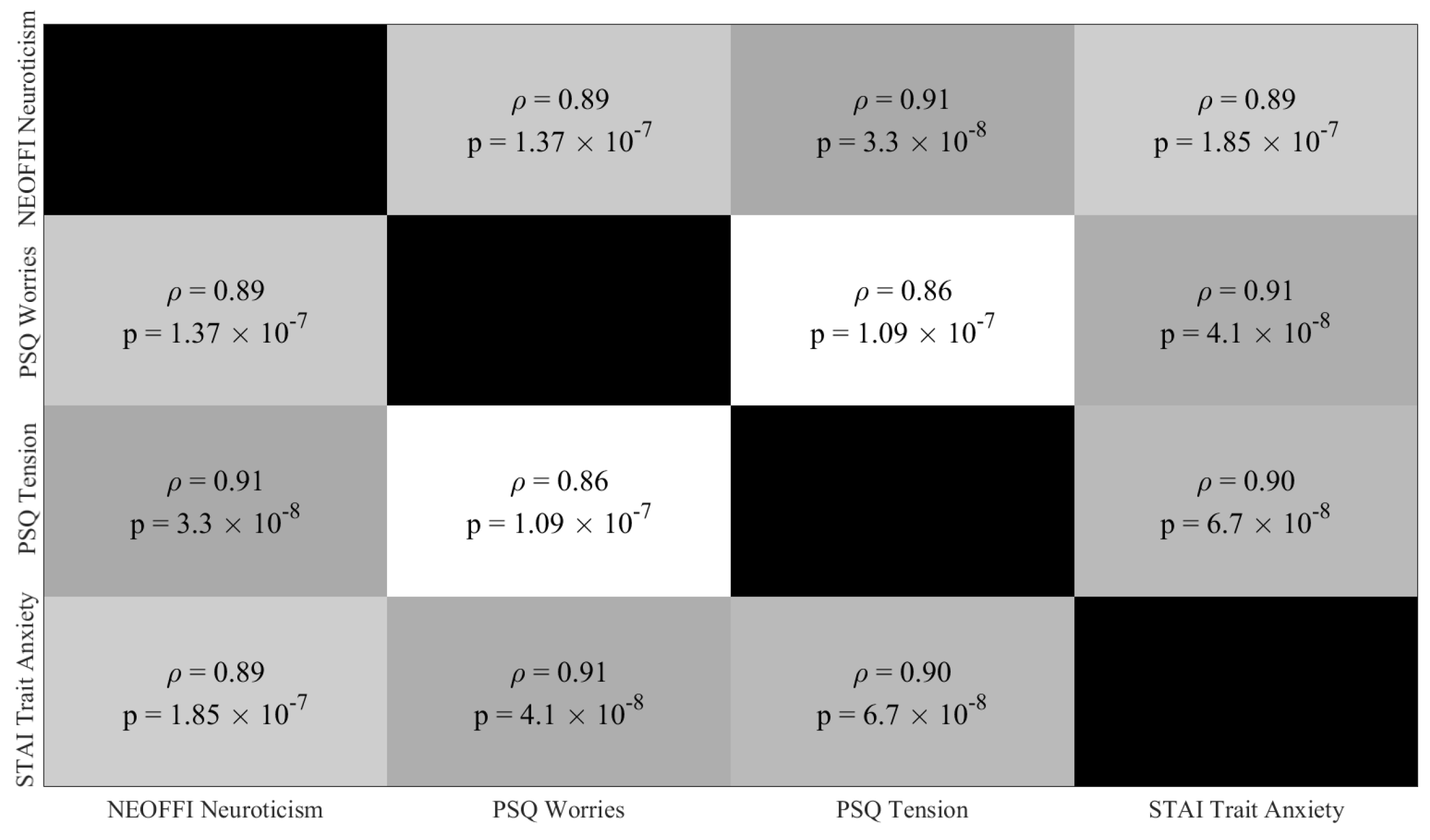
Appendix C.1. Paired Partial (Spearman) Correlations: All 122 Participants from Resting-State EEG Recordings

Appendix C.2. Paired Partial (Spearman) Correlations: All HIGH (26 Participants) and LOW (14 Participants) Stress-Susceptible Participants Prior to Discarding Younger Females and Older Females and Males
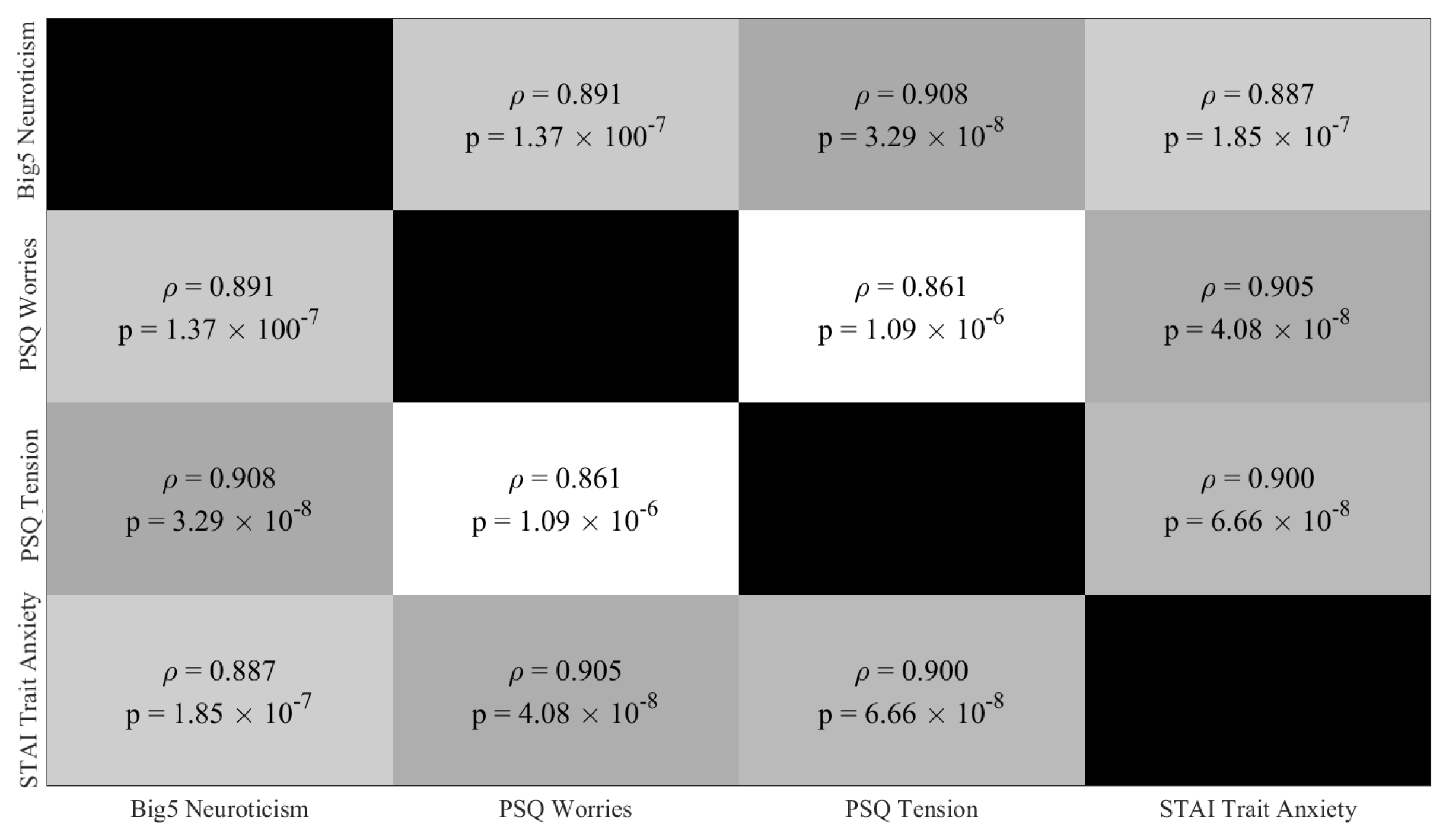
Appendix C.3. Paired Partial (Spearman) Correlations: Final HIGH (10 Participants) and LOW (10 Participants) Stress-Susceptible Participants
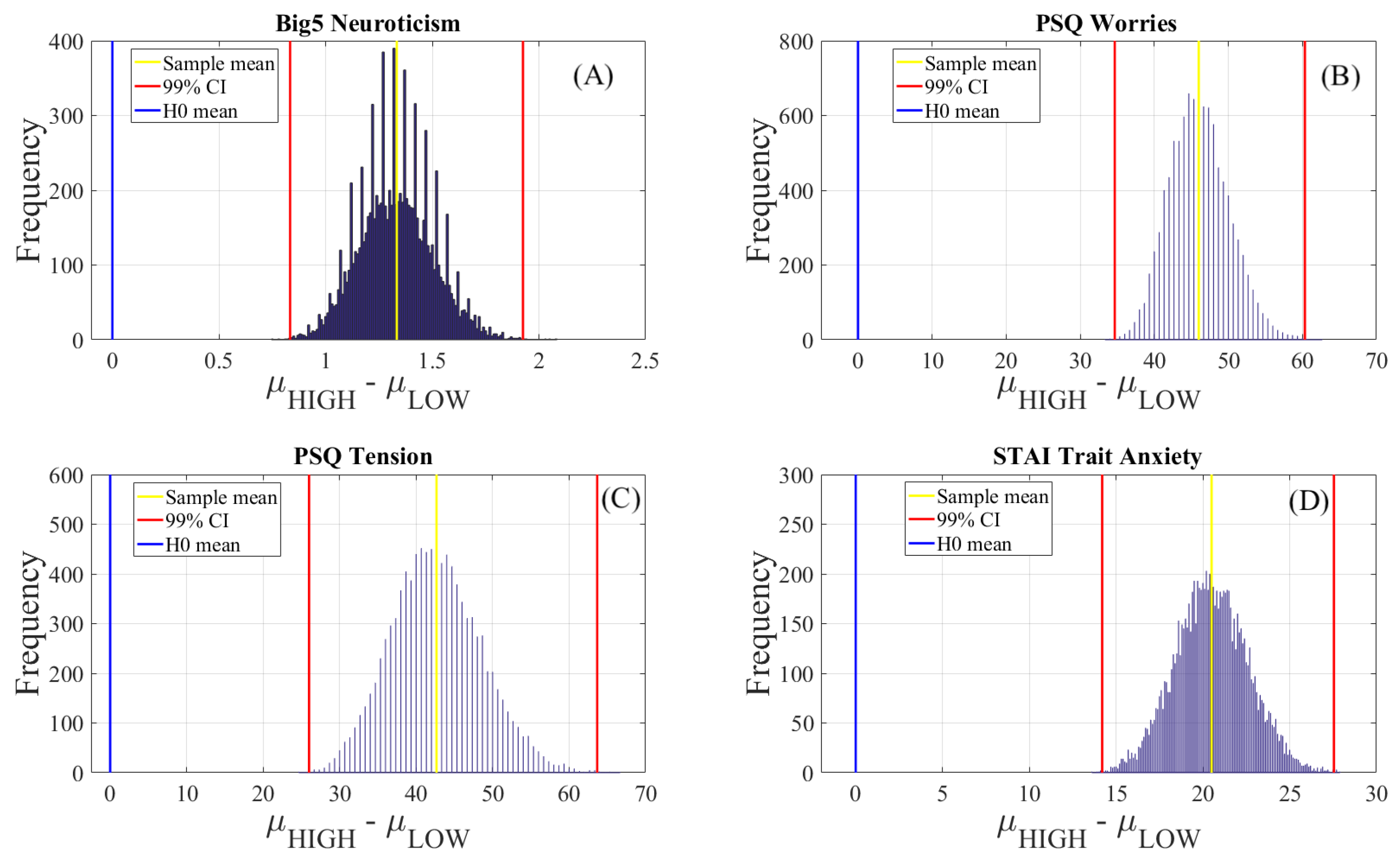
Appendix C.4. Within-Questionnaire Test of Significant Difference

| Questionnaire | M | SD | 99.0% CI |
|---|---|---|---|
| Neuroticism (Big5) | 1.33 | 0.17 | [0.82 1.91] |
| Worries (PSQ) | 45.99 | 4.02 | [35.00 61.00] |
| Tension (PSQ) | 42.62 | 6.02 | [26.00 63.67] |
| Trait Anxiety (STAI) | 20.51 | 2.13 | [13.95 28.00] |
Appendix D. Correlation between Participants’ Responses to Questionnaires and Their TEs
Appendix D.1. Eyes-Closed (EC) Setting
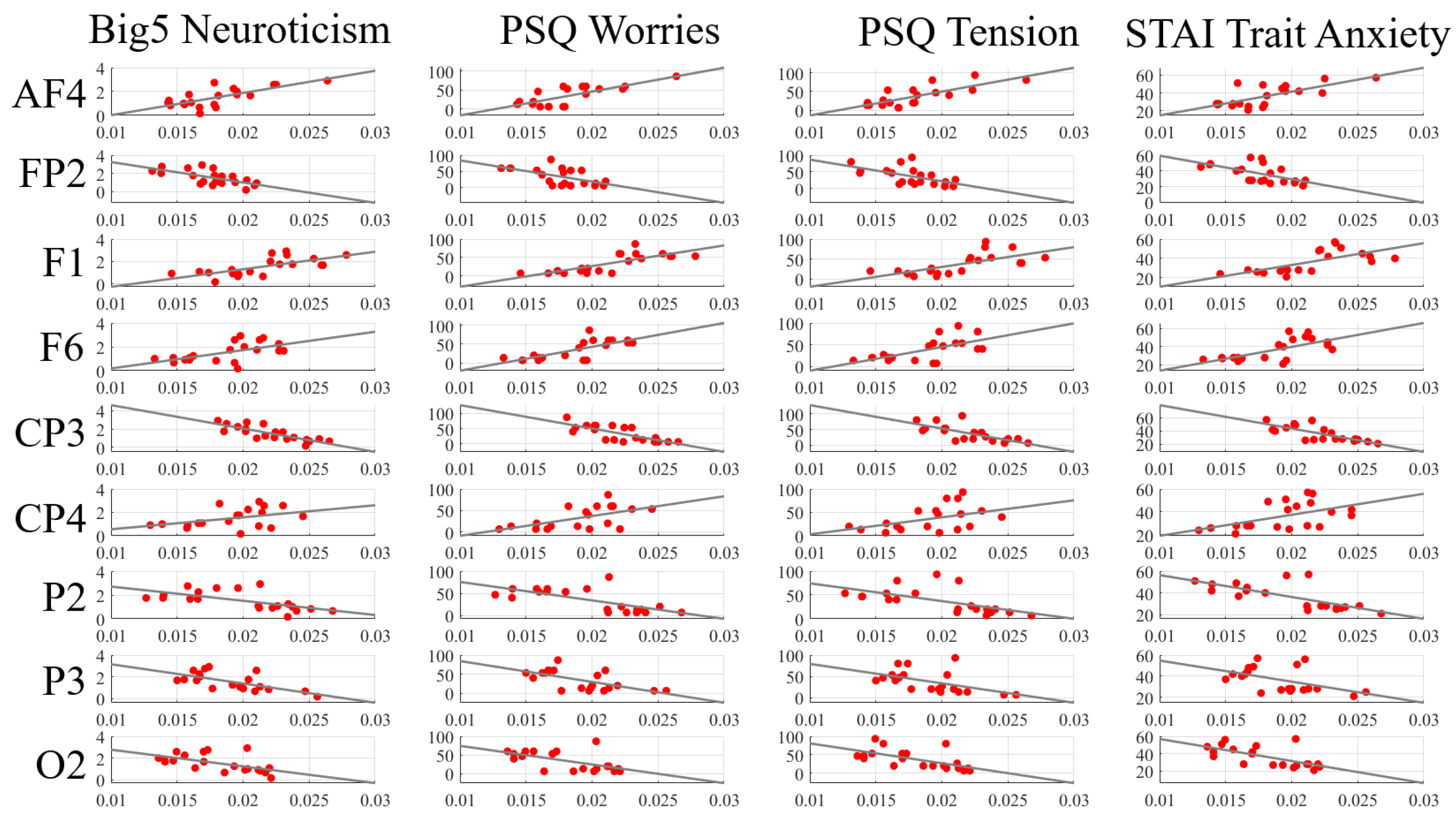
| Channels | Neuroticism (Big5) | Worries (PSQ) | Tension (PSQ) | Trait Anxiety (STAI) |
|---|---|---|---|---|
| AF4 | = 0.62 | = 0.64 | = 0.71 | = 0.59 |
| p = 0.0030 | p = 0.0026 | p = 0.0004 | p = 0.0059 | |
| FP2 | = −0.62 | = −0.56 | = −0.59 | = −0.62 |
| p = 0.0034 | p = 0.0097 | p = 0.0059 | p = 0.0039 | |
| F1 | = 0.68 | = 0.75 | = 0.72 | = 0.72 |
| p = 0.0010 | p = 0.0001 | p = 0.0003 | p = 0.0003 | |
| F6 | = 0.59 | = 0.73 | = 0.60 | = 0.65 |
| p = 0.0061 | p = 0.0002 | p = 0.0051 | p = 0.0021 | |
| CP3 | = −0.89 | = −0.74 | = −0.77 | = −0.76 |
| p = 1.2 ×10 | p = 0.0002 | p = 0.0001 | p = 0.0001 | |
| CP4 | = 0.38 | = 0.53 | = 0.46 | = 0.50 |
| p = 0.0971 | p = 0.0151 | p = 0.0411 | p = 0.0245 | |
| P2 | = −0.70 | = −0.65 | = −0.70 | = −0.72 |
| p = 0.0005 | p = 0.0021 | p = 0.0006 | p = 0.0003 | |
| P3 | = −0.63 | = −0.55 | = −0.58 | = −0.43 |
| p = 0.0031 | p = 0.0125 | p = 0.0076 | p = 0.0562 | |
| O2 | = −0.62 | = −0.53 | = −0.70 | = −0.62 |
| p = 0.0035 | p = 0.0161 | p = 0.0006 | p = 0.0033 |
Appendix D.2. Eyes-Open (EO) Setting
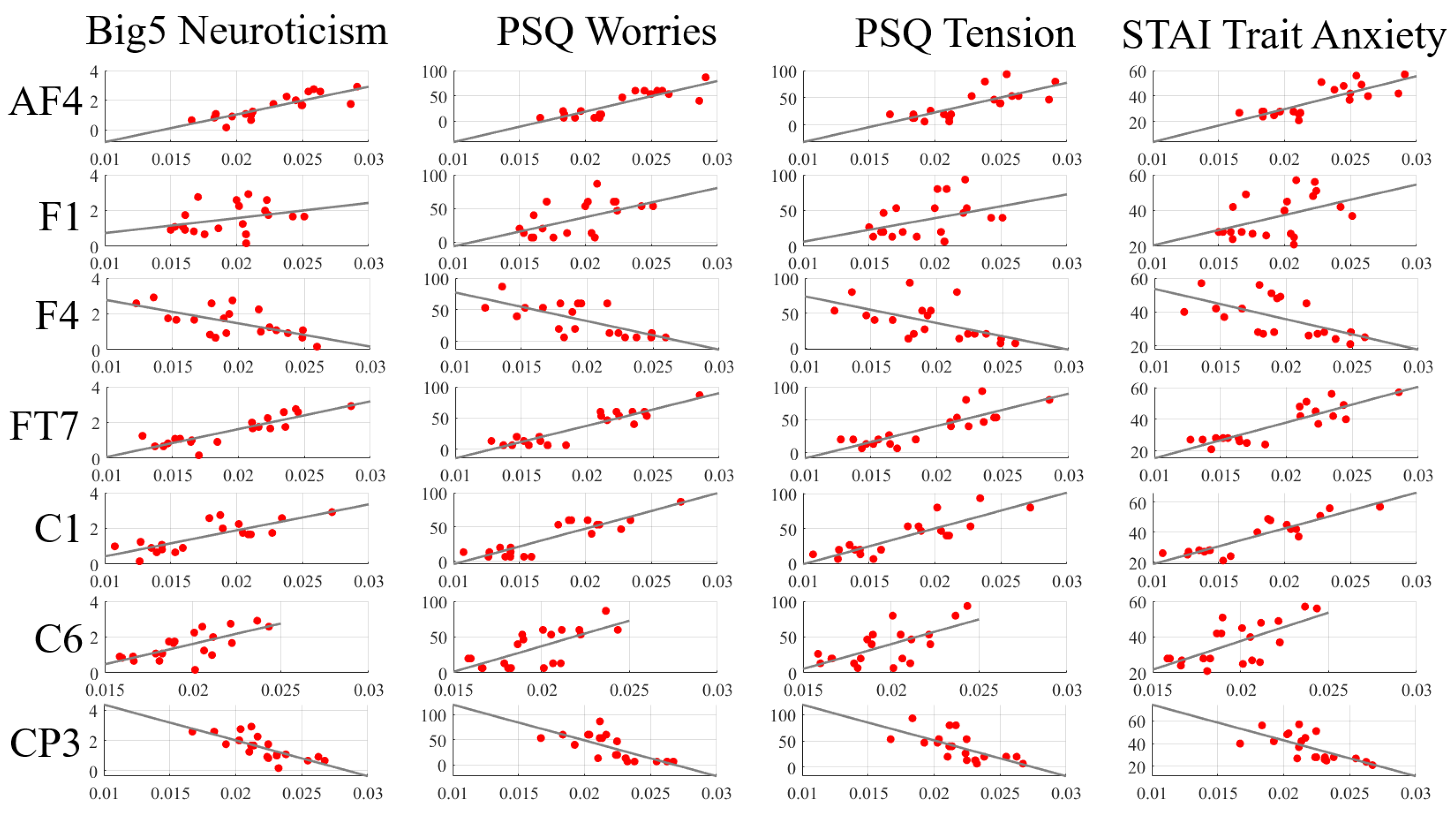
| Channels | Neuroticism (Big5) | Worries (PSQ) | Tension (PSQ) | Trait Anxiety (STAI) |
|---|---|---|---|---|
| AF4 | = 0.87 | = 0.78 | = 0.76 | = 0.74 |
| p = 7.37 × 10 | p = 4.21 × 10 | p = 0.0001 | p = 0.0002 | |
| F1 | = 0.33 | = 0.45 | = 0.35 | = 0.39 |
| p = 0.1552 | p = 0.0439 | p = 0.1336 | p = 0.0907 | |
| F4 | = −0.54 | = −0.62 | = −0.62 | = −0.61 |
| p = 0.0149 | p = 0.0035 | p = 0.0034 | p = 0.0044 | |
| FT7 | = 0.82 | = 0.77 | = 0.81 | = 0.75 |
| p = 9.32 × 10 | p = 6.25 × 10 | p = 1.85 × 10 | p = 0.0002 | |
| C1 | = 0.73 | = 0.75 | = 0.79 | = 0.81 |
| p = 0.0002 | p = 0.0001 | p = 4.07 × 10 | p = 0.0001 | |
| C6 | = 0.69 | = 0.65 | = 0.55 | = 0.55 |
| p = 0.0007 | p = 0.0019 | p= 0.0129 | p = 0.0123 | |
| CP3 | = −0.80 | = −0.79 | = −0.73 | = −0.71 |
| p = 0.0002 | p = 3.05 ×10 | p = 0.0002 | p = 0.0004 | |
| CPZ | = −0.65 | = −0.71 | = −0.72 | = −0.67 |
| p = 0.0020 | p = 0.0004 | p = 0.0003 | p = 0.0012 | |
| CP4 | = 0.69 | = 0.75 | = 0.64 | = 0.72 |
| p = 0.0008 | p = 0.0001 | p = 0.0024 | p = 0.0003 | |
| P1 | = 0.63 | = 0.65 | = 0.64293 | = 0.69 |
| p = 0.0030 | p = 0.0018 | p = 0.0022 | p = 0.0008 | |
| PZ | = −0.56 | = −0.53 | = −0.56 | = −0.56 |
| p = 0.0108 | p = 0.0176 | p = 0.0106 | p = 0.0097 | |
| PO7 | = −0.48 | = −0.53 | = −0.48 | = −0.54 |
| p = 0.0331 | p = 0.0173 | p = 0.0325 | p = 0.0133 | |
| PO8 | = −0.67 | = −0.76 | = −0.78 | = −0.72 |
| p = 0.0013 | p = 0.0001 | p = 0.0001 | p = 0.0003 | |
| O1 | = 0.65 | = 0.59 | = 0.59 | = 0.48 |
| p = 0.0019 | p = 0.0067 | p = 0.0062 | p = 0.0302 |
References
- Tozzi, L.; Staveland, B.; Holt-Gosselin, B.; Chesnut, M.; Chang, S.E.; Choi, D.; Shiner, M.L.; Wu, H.; Lerma-Usabiaga, G.; Sporns, O.; et al. The human connectome project for disordered emotional states: Protocol and rationale for a research domain criteria study of brain connectivity in young adult anxiety and depression. NeuroImage 2020, 214, 116715. [Google Scholar] [CrossRef] [PubMed]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; et al. Global burden of disease attributable to mental and substance use disorders: Findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Van Marle, H.J.; Hermans, E.J.; Qin, S.; Fernández, G. From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biol. Psychiatry 2009, 66, 649–655. [Google Scholar] [CrossRef]
- Panksepp, J. Affective Neuroscience: The Foundations of Human and Animal Emotions; Oxford University Press, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Cahill, L.; Prins, B.; Weber, M.; McGaugh, J.L. β-Adrenergic activation and memory for emotional events. Nature 1994, 371, 702–704. [Google Scholar] [CrossRef]
- Roozendaal, B.; McEwen, B.S.; Chattarji, S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009, 10, 423–433. [Google Scholar] [CrossRef]
- Joëls, M.; Pu, Z.; Wiegert, O.; Oitzl, M.S.; Krugers, H.J. Learning under stress: How does it work? Trends Cogn. Sci. 2006, 10, 152–158. [Google Scholar] [CrossRef]
- Qin, S.; Hermans, E.J.; van Marle, H.J.; Luo, J.; Fernández, G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol. Psychiatry 2009, 66, 25–32. [Google Scholar] [CrossRef]
- Miller, A.B.; Prinstein, M.J. Adolescent suicide as a failure of acute stress-response systems. Annu. Rev. Clin. Psychol. 2019, 15, 425–450. [Google Scholar] [CrossRef]
- Arnsten, A.F. Stress signalling pathways that impair prefrontal cortex structure and function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Bressler, S.L. Large-scale cortical networks and cognition. Brain Res. Rev. 1995, 20, 288–304. [Google Scholar] [CrossRef]
- Van Oort, J.; Tendolkar, I.; Hermans, E.J.; Mulders, P.C.; Beckmann, C.F.; Schene, A.H.; Fernández, G.; van Eijndhoven, P.F. How the brain connects in response to acute stress: A review at the human brain systems level. Neurosci. Biobehav. Rev. 2017, 83, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.J.; Henckens, M.J.; Joëls, M.; Fernández, G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014, 37, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.J.; Van Marle, H.J.; Ossewaarde, L.; Henckens, M.J.; Qin, S.; Van Kesteren, M.T.; Schoots, V.C.; Cousijn, H.; Rijpkema, M.; Oostenveld, R.; et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 2011, 334, 1151–1153. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Soares, J.M.; Marques, P.; Magalhaes, R.; Santos, N.C.; Sousa, N. The association between stress and mood across the adult lifespan on default mode network. Brain Struct. Funct. 2017, 222, 101–112. [Google Scholar] [CrossRef]
- Laird, A.R.; Eickhoff, S.B.; Li, K.; Robin, D.A.; Glahn, D.C.; Fox, P.T. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J. Neurosci. 2009, 29, 14496–14505. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Dedovic, K.; Khalili-Mahani, N.; Engert, V.; Pruessner, M.; Buss, C.; Renwick, R.; Dagher, A.; Meaney, M.J.; Lupien, S. Deactivation of the limbic system during acute psychosocial stress: Evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol. Psychiatry 2008, 63, 234–240. [Google Scholar] [CrossRef]
- Koric, L.; Volle, E.; Seassau, M.; Bernard, F.A.; Mancini, J.; Dubois, B.; Pelissolo, A.; Levy, R. How cognitive performance?induced stress can influence right VLPFC activation: An fMRI study in healthy subjects and in patients with social phobia. Hum. Brain Mapp. 2012, 33, 1973–1986. [Google Scholar] [CrossRef]
- Albert, K.; Pruessner, J.; Newhouse, P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology 2015, 59, 14–24. [Google Scholar] [CrossRef]
- Beckmann, C.F.; DeLuca, M.; Devlin, J.T.; Smith, S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef]
- van Marle, H.J.; Hermans, E.J.; Qin, S.; Fernández, G. Enhanced resting-state connectivity of amygdala in the immediate aftermath of acute psychological stress. NeuroImage 2010, 1, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Veer, I.M.; Oei, N.Y.; Spinhoven, P.; van Buchem, M.A.; Elzinga, B.M.; Rombouts, S.A. Beyond acute social stress: Increased functional connectivity between amygdala and cortical midline structures. NeuroImage 2011, 57, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Vaisvaser, S.; Lin, T.; Admon, R.; Podlipsky, I.; Greenman, Y.; Stern, N.; Fruchter, E.; Wald, I.; Pine, D.S.; Tarrasch, R.; et al. Neural traces of stress: Cortisol related sustained enhancement of amygdala-hippocampal functional connectivity. Front. Hum. Neurosci. 2013, 7, 313. [Google Scholar] [CrossRef]
- Viard, A.; Mutlu, J.; Chanraud, S.; Guenolé, F.; Egler, P.J.; Gérardin, P.; Baleyte, J.M.; Dayan, J.; Eustache, F.; Guillery-Girard, B. Altered default mode network connectivity in adolescents with post-traumatic stress disorder. NeuroImage Clin. 2019, 22, 101731. [Google Scholar] [CrossRef]
- Zhang, W.; Hashemi, M.M.; Kaldewaij, R.; Koch, S.B.; Beckmann, C.; Klumpers, F.; Roelofs, K. Acute stress alters the ’default’ brain processing. NeuroImage 2019, 189, 870–877. [Google Scholar] [CrossRef]
- Van Oort, J.; Kohn, N.; Vrijsen, J.N.; Collard, R.; Duyser, F.A.; Brolsma, S.C.A.; Fernández, G.; Schene, A.H.; Tendolkar, I.; van Eijndhoven, P.F. Absence of default mode downregulation in response to a mild psychological stressor marks stress-vulnerability across diverse psychiatric disorders. NeuroImage: Clin. 2020, 25, 102176. [Google Scholar] [CrossRef]
- Wutz, A.; Loonis, R.; Roy, J.E.; Donoghue, J.A.; Miller, E.K. Different levels of category abstraction by different dynamics in different prefrontal areas. Neuron 2018, 97, 716–726. [Google Scholar] [CrossRef]
- Reid, A.T.; Headley, D.B.; Mill, R.D.; Sanchez-Romero, R.; Uddin, L.Q.; Marinazzo, D.; Lurie, D.J.; Valdés-Sosa, P.A.; Hanson, S.J.; Biswal, B.B.; et al. Advancing functional connectivity research from association to causation. Nat. Neurosci. 2019, 22, 1751–1760. [Google Scholar] [CrossRef]
- Horwitz, B. The elusive concept of brain connectivity. Neuroimage 2003, 19, 466–470. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 3–36. [Google Scholar] [CrossRef] [PubMed]
- Stam, C.J. Nonlinear dynamical analysis of EEG and MEG: Review of an emerging field. Clin. Neurophysiol. 2005, 116, 2266–2301. [Google Scholar] [CrossRef] [PubMed]
- Pereda, E.; Quiroga, R.Q.; Bhattacharya, J. Nonlinear multivariate analysis of neurophysiological signals. Prog. Neurobiol. 2005, 77, 1–37. [Google Scholar] [CrossRef]
- Fagerholm, E.D.; Lorenz, R.; Scott, G.; Dinov, M.; Hellyer, P.J.; Mirzaei, N.; Leeson, C.; Carmichael, D.W.; Sharp, D.J.; Shew, W.L.; et al. Cascades and cognitive state: Focused attention incurs subcritical dynamics. J. Neurosci. 2015, 35, 4626–4634. [Google Scholar] [CrossRef] [PubMed]
- Shew, W.L.; Plenz, D. The functional benefits of criticality in the cortex. Neuroscientist 2013, 19, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Arieli, A.; Sterkin, A.; Grinvald, A.; Aertsen, A.D. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science 1996, 273, 1868–1871. [Google Scholar] [CrossRef] [PubMed]
- McDonough, I.M.; Nashiro, K. Network complexity as a measure of information processing across resting-state networks: Evidence from the Human Connectome Project. Front. Hum. Neurosci. 2014, 8, 409. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.R.; Vakorin, V.; Kovacevic, N.; Wang, H.; Diaconescu, A.; Protzner, A.B. Spatiotemporal dependency of age-related changes in brain signal variability. Cereb. Cortex 2013, 24, 1806–1817. [Google Scholar] [CrossRef]
- Yang, A.C.; Huang, C.C.; Yeh, H.L.; Liu, M.E.; Hong, C.J.; Tu, P.C.; Chen, J.F.; Huang, N.E.; Peng, C.K.; Lin, C.P.; et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: A multiscale entropy analysis. Neurobiol. Aging 2013, 34, 428–438. [Google Scholar] [CrossRef]
- Pearson, J. The human imagination: The cognitive neuroscience of visual mental imagery. Nat. Rev. Neurosci. 2019, 273, 1868–1871. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.; Moran, R.; Seth, A.K. Analysing connectivity with Granger causality and dynamic causal modelling. Curr. Opin. Neurobiol. 2013, 23, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.K.; Barrett, A.B.; Barnett, L. Granger causality analysis in neuroscience and neuroimaging. J. Neurosci. 2015, 35, 3293–3297. [Google Scholar] [CrossRef] [PubMed]
- Wilber, A.A.; Skelin, I.; Wu, W.; McNaughton, B.L. Laminar organization of encoding and memory reactivation in the parietal cortex. Neuron 2017, 95, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T. Measuring information transfer. Phys. Rev. Lett. 2000, 85, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Lungarella, M.; Sporns, O. Mapping information flow in sensorimotor networks. PLoS Comput. Biol. 2006, 2, 1301–1312. [Google Scholar] [CrossRef]
- Kaiser, A.; Schreiber, T. Information transfer in continuous processes. Physica 2002, 166, 43–62. [Google Scholar] [CrossRef]
- Barnett, L.; Barrett, A.B.; Seth, A.K. Granger causality and transfer entropy are equivalent for Gaussian variables. Phys. Rev. Lett. 2009, 103, 238701. [Google Scholar] [CrossRef]
- Barnett, L.; Bossomaier, T. Transfer entropy as a log-likelihood ratio. Phys. Rev. Lett. 2013, 109, 0138105. [Google Scholar] [CrossRef]
- Granger, C.W.J. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 1969, 37, 424–438. [Google Scholar] [CrossRef]
- Geweke, J. Measures of conditional linear dependence and feedback between time series. J. Am. Stat. Assoc. 1984, 79, 907–915. [Google Scholar] [CrossRef]
- Babayan, A.; Erbey, M.; Kumral, D.; Reinelt, J.D.; Reiter, A.M.; Röbbig, J.; Schaare, H.L.; Uhlig, M.; Anwander, A.; Bazin, P.L.; et al. A mind-brain-body dataset of MRI, EEG, cognition, emotion, and peripheral physiology in young and old adults. Sci. Data 2019, 6, 180308. [Google Scholar] [CrossRef]
- Goldberg, L.R. An alternative “description of personality”: The big-five factor structure. J. Personal. Soc. Psychol. 1990, 59, 1216–1229. [Google Scholar] [CrossRef]
- Levenstein, S.; Prantera, C.; Varvo, V.; Scribano, M.L.; Berto, E.; Luzi, C.; Andreoli, A. Development of the Perceived Stress Questionnaire: A new tool for psychosomatic research. J. Psychosom. Res. 1993, 37, 19–32. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Luschene, R.E. Manual for the State-Trait Anxiety Inventory. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Mountain View, CA, USA, 1970. [Google Scholar]
- Chand, G.B.; Habes, M.; Dolui, S.; Detre, J.A.; Wolk, D.A.; Davatzikos, C. Estimating regional cerebral blood flow using resting-state functional MRI via machine learning. J. Neurosci. Methods 2020, 331, 108528. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, X.; Hildebrandt, A.; Zhou, C. Individual cortical entropy profile: Test-retest reliability, predictive power for cognitive ability, and neuroanatomical foundation. Cereb. Cortex Commun. 2020, 1, tgaa015. [Google Scholar] [CrossRef]
- Ieong, H.F.H.; Gao, F.; Yuan, Z. Machine learning: Assessing neurovascular signals in the prefrontal cortex with non-invasive bimodal electro-optical neuroimaging in opiate addiction. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Imani, M.; Braga-Neto, U. Gene regulatory network state estimation from arbitrary correlated measurements. EURASIP J. Adv. Signal Process. 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011, 6, 180308. [Google Scholar]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef]
- Schaworonkow, N.; Nikulin, V.V. Spatial neuronal synchronization and the waveform of oscillations: Implications for EEG and MEG. PLoS Comput. Biol. 2019, 15, e1007055. [Google Scholar] [CrossRef] [PubMed]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Mardia, K.; Kent, J.; Bibby, J. Multivariate analysis. In Probability and Mathematical Statistics; Academic Press Inc.: New York, NY, USA, 1979. [Google Scholar]
- Friston, K.; Holmes, A.; Worsley, K.; Poline, J.-B.; Frith, C.; Frackowiak, R. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1995, 2, 189–210. [Google Scholar] [CrossRef]
- Lizier, J.T. JIDT: An information-theoretic toolkit for studying the dynamics of complex systems. Front. Robot. AI 2014, 1, 11. [Google Scholar] [CrossRef]
- Rosenthal, R.; DiMatteo, M.R. Meta-analysis: Recent developments n quantitative methods for literature reviews. Annu. Rev. Psychol. 2001, 52, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R. Parametric measures of effect size. In The Handbook of Research Synthesis; Russell Sage Foundation: New York, NY, USA, 1994; pp. 231–244. [Google Scholar]
- Tomczak, M.; Tomczak, E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 2014, 1, 19–25. [Google Scholar]
- Vacha-Haase, T.; Thompson, B. How to estimate and interpret various effect sizes. J. Couns. Psychol. 2004, 51, 473–481. [Google Scholar] [CrossRef]
- Seo, D.; Jia, Z.; Lacadie, C.M.; Tsou, K.A.; Bergquist, K.; Sinha, R. Sex differences in neural responses to stress and alcohol context cues. Hum. Brain Mapp. 2011, 32, 1998–2013. [Google Scholar] [CrossRef]
- Steyer, R.; Schwenkmezger, P.; Notz, P.; Eid, M. Der Mehrdimensionale Befindlichkeitsfragebogen; Hogrefe: Göttingen, Germany, 1997. [Google Scholar]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. Year Cogn. Neurosci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R. The brain’s default network and its adaptive role in internal mentation. Neurosci. 2012, 18, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Damasio, A.; Carvalho, G.B. The nature of feelings: Evolutionary and neurobiological origins. Nat. Rev. Neurosci. 2013, 340, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kober, H.; Barrett, L.F.; Joseph, J.; Bliss-Moreau, E.; Lindquist, K.; Wager, T.D. Functional grouping and cortical/subcortical interactions in emotion: A meta-analysis of neuroimaging studies. Neuroimage 2008, 42, 998–1031. [Google Scholar] [CrossRef]
- Lindquist, K.A.; Satpute, A.B.; Wager, T.D.; Weber, J.; Barrett, L.F. The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cereb. Cortex 2015, 26, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.; Tellegen, A. Toward a consensual structure of mood. Psychol. Bull. 1985, 98, 219–235. [Google Scholar] [CrossRef]
- Zhang, W.; Llera, A.; Hashemi, M.M.; Kaldewaij, R.; Koch, S.B.; Beckmann, C.F.; Klumpers, F.; Roelofs, K. Discriminating stress from rest based on resting-state connectivity of the human brain: A supervised machine learning study. Hum. Brain Mapp. 2020, 36, 12083–12094. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Barch, D.M.; Price, J.L.; Rundle, M.M.; Vaishnavi, S.N.; Snyder, A.Z.; Mintun, M.A.; Wang, S.; Coalson, R.S.; Raichle, M.E. The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. USA 2009, 106, 1942–1947. [Google Scholar] [CrossRef]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef]
- Dosenbach, N.U.; Visscher, K.M.; Palmer, E.D.; Miezin, F.M.; Wenger, K.K.; Kang, H.C.; Burgund, E.D.; Grimes, A.L.; Schlaggar, B.L.; Petersen, S.E. A core system for the implementation of task sets. Neuron 2006, 50, 799–812. [Google Scholar] [CrossRef]
- Van Snellenberg, J.X.; Wager, T.D. Cognitive and motivational functions of the human prefrontal cortex. In Luria’s Legacy in the 21st Century; Goldberg, E., Bougakov, D., Eds.; Oxford University Press: Oxford, UK, 2009; pp. 30–61. [Google Scholar]
- Yamashita, M.; Yoshihara, Y.; Hashimoto, R.; Yahata, N.; Ichikawa, N.; Sakai, Y. A prediction model of working memory across health and psychiatric disease using whole-brain functional connectivity. ELife 2018, 7, e38844. [Google Scholar] [CrossRef]
- Avery, E.W.; Yoo, K.; Rosenberg, M.D.; Greene, A.S.; Gao, S.; Na, D.L.; Scheinost, D.; Constable, T.R.; Chun, M.M. Distributed Patterns of Functional Connectivity Predict Working Memory Performance in Novel Healthy and Memory-impaired Individuals. J. Cogn. Neurosci. 2019, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Northoff, G. Is emotion regulation self-regulation? Trends Cogn. Sci. 2006, 9, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, E.; McKinnon, M.C.; Levine, B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia 2006, 44, 2189–2208. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, K.A.; Wager, T.D.; Kober, H.; Bliss-Moreau, E.; Barrett, L.F. The brain basis of emotion: A meta-analytic review. Behav. Brain Sci. 2012, 35, 121–143. [Google Scholar] [CrossRef] [PubMed]
- Forbes, C.E.; Grafman, J. The role of the human prefrontal cortex in social cognition and moral judgment. Annu. Rev. Neurosci. 2010, 33, 299–324. [Google Scholar] [CrossRef]
- Krain, A.L.; Wilson, A.M.; Arbuckle, R.; Castellanos, F.X.; Milham, M.P. Distinct neural mechanisms of risk and ambiguity: A meta-analysis of decisionmaking. Neuroimage 2006, 32, 477–484. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.; Laird, A.R.; Bullmore, E. N-Back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Arias, J.A.; Williams, C.; Raghvani, R.; Aghajani, M.; Baez, S.; Belzung, C.; Booij, L.; Busatto, G.; Chiarella, J.; Fu, C.H.; et al. The Neuroscience of Sadness: A Multidisciplinary Synthesis and Collaborative Review for the Human Affectome Project. Neurosci. Biobehav. Rev. 2020, 111, 199–228. [Google Scholar] [CrossRef]
- Cohen, J.R.; D’Esposito, M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 2016, 36, 12083–12094. [Google Scholar] [CrossRef]
- Gazzaniga, M.S.; Ivry, R.B.; Mangun, G.R. Cognitive Neuroscience. The Biology of the Mind, 5th ed.; W. W. Norton and Company: New York, NY, USA, 2019. [Google Scholar]
- Kyeong, S.; Kim, J.; Kim, J.; Kim, E.J.; Kim, H.E.; Kim, J.J. Differences in the modulation of functional connectivity by self-talk tasks between people with low and high life satisfaction. NeuroImage 2020, 217, 116929. [Google Scholar] [CrossRef]
- Powers, J.P.; Graner, J.L.; LaBar, K.S. Multivariate Patterns of Posterior Cortical Activity Differentiate Forms of Emotional Distancing. Cereb. Cortex 2020, 30, 2766–2776. [Google Scholar] [CrossRef] [PubMed]
- Liston, C.; McEwen, B.S.; Casey, B.J. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. USA 2009, 106, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.; Wang, M.J.; Paspalas, C.D. Neuromodulation of thought: Flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 2012, 76, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, E.V.; Rosenberg, M.D.; Seo, D.; Constable, R.T.; Sinha, R. Hippocampal seed connectome-based modeling predicts the feeling of stress. Nat. Commun. 2020, 11, 2650. [Google Scholar] [CrossRef]
- Lighthall, N.R.; Sakaki, M.; Vasunilashorn, S.; Nga, L.; Somayajula, S.; Chen, E.Y.; Samii, N.; Mather, M. Gender differences in reward-related decision processing under stress. Soc. Cogn. Affect. Neurosci. 2012, 74, 476–484. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Peng, C.K.; Lipsitz, L.A. What is physiologic complexity and how does it change with aging and disease? Neurobiol. Aging 2002, 23, 23–26. [Google Scholar] [CrossRef]
- Sleimen-Malkoun, R.; Temprado, J.J.; Hong, S.L. Aging induced loss of complexity and dedifferentiation: Consequences for coordination dynamics within and between brain, muscular and behavioral levels. Front. Aging Neurosci. 2014, 6, 140. [Google Scholar] [CrossRef]
- Takahashi, T.; Cho, R.Y.; Murata, T.; Mizuno, T.; Kikuchi, M.; Mizukami, K.; Kosaka, H.; Takahashi, K.; Wada, Y. Age-related variation in EEG complexity to photic stimulation: A multiscale entropy analysis. Clin. Neurophysiol. 2009, 120, 476–483. [Google Scholar] [CrossRef]
- Lipsitz, L.A.; Goldberger, A.L. Loss of ’complexity’ and aging: Potential applications of fractals and chaos theory to senescence. J. Am. Med Assoc. (JAMA) 1992, 267, 1806–1809. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Beckmann, C.F.; Arigita, E.S.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Rombouts, S.A.R.B. Reduced resting-state brain activity in the "default network" in normal aging. Cereb. Cortex 2008, 18, 1856–1864. [Google Scholar] [CrossRef]
- MacDuffie, K.E.; Knodt, A.R.; Radtke, S.R.; Strauman, T.J.; Hariri, A.R. Self-rated amygdala activity: An auto-biological index of affective distress. Personal. Neurosci. 2019, 2, e1. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Guo, C.; Luk, W.; Weston, S. Accelerating transfer entropy computation. In Proceedings of the 2014 International Conference on Field-Programmable Technology (FPT), Shanghai, China, 10–12 December 2014; pp. 60–67. [Google Scholar]
- Li, J.; Liang, C.; Zhu, X.; Sun, X.; Wu, D. Risk contagion in Chinese banking industry: A transfer entropy-based analysis. Entropy 2013, 15, 5549–5564. [Google Scholar] [CrossRef]
- Kanning, U.P. NEO-Fünf-Faktoren-Inventar nach Costa und McCrae (NEO-FFI). Z. Für Arbeits-Und Organ. A O 2009, 53, 194–198. [Google Scholar] [CrossRef]
- Costa, P.T.; McCrae, R.R. Revised NEO Personality Inventory (NEO PI-R) and NEO Five Factor Inventory (NEO-FFI) Professional Manual; Psychological Assessment Resources Inc.: Lutz, FL, USA, 1992. [Google Scholar]
- Fliege, H.; Rose, M.; Arck, P.; Levenstein, S.; Klapp, B.F. Validierung des Perceived Stress Questionnaire (PSQ) an einer deutschen Stichprobe. Diagnostica 2001, 47, 142–152. [Google Scholar] [CrossRef]
- Laux, L.; Glanzmann, P.; Schaffner, P.; Spielberger, C.D. Das State-Trait-Angstinventar; Beltz Test GmbH: Göttingen, Germany, 1981. [Google Scholar]
- Dotson, V.M.; McClintock, S.M.; Verhaeghen, P. Depression and Cognitive Control across the Lifespan: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2020, 2. [Google Scholar] [CrossRef]
- Cover, T.; Thomas, J. Elements of Information Theory, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]


| Channel | p = | W(18) | r | ||||
|---|---|---|---|---|---|---|---|
| AF4 | 0.0010 | 3.29 | 0.74 | 0.020 | 0.003 | 0.016 | 0.001 |
| F1 | 0.0002 | 3.74 | 0.84 | 0.024 | 0.002 | 0.019 | 0.002 |
| F6 | 0.0004 | 3.52 | 0.79 | 0.021 | 0.001 | 0.016 | 0.002 |
| CP4 | 0.0091 | 2.61 | 0.58 | 0.021 | 0.002 | 0.017 | 0.003 |
| Channel | p = | W(18) | r | ||||
|---|---|---|---|---|---|---|---|
| FP2 | 0.0100 | −2.47 | 0.55 | 0.016 | 0.002 | 0.0190 | 0.002 |
| CP3 | 0.0013 | −3.21 | 0.72 | 0.020 | 0.002 | 0.024 | 0.002 |
| P2 | 0.0003 | −3.59 | 0.80 | 0.016 | 0.003 | 0.023 | 0.002 |
| P3 | 0.0036 | −2.91 | 0.65 | 0.017 | 0.002 | 0.021 | 0.002 |
| O2 | 0.0013 | −3.21 | 0.72 | 0.016 | 0.0021 | 0.020 | 0.002 |
| Channel | p = | F | |
|---|---|---|---|
| AF4 | 0.6734 | 0.19 | 0.02 |
| FP2 | 0.9813 | F = 0.001 | 0.0001 |
| F1 | 0.8818 | F = 0.02 | 0.002 |
| F6 | 0.6864 | 0.17 | 0.014 |
| CP3 | 0.8292 | 0.05 | 0.004 |
| CP4 | 0.9290 | 0.008 | 0.0007 |
| P2 | 0.8000 | 0.07 | 0.006 |
| P3 | 0.9893 | F = 0.0002 | 0.00002 |
| O2 | 0.8915 | 0.020 | 0.002 |
| Channel | p = | W(1058) | r | ||||
|---|---|---|---|---|---|---|---|
| AF4 | 0.0003 | 3.61 | 0.11 | 0.020 | 0.018 | 0.016 | 0.018 |
| F1 | 0.000001 | 4.50 | 0.15 | 0.024 | 0.018 | 0.019 | 0.018 |
| F6 | 0.00003 | 4.18 | 0.13 | 0.021 | 0.018 | 0.016 | 0.018 |
| CP4 | 0.00003 | 4.20 | 0.13 | 0.021 | 0.018 | 0.017 | 0.018 |
| Channel | p = | W(1058) | r | ||||
|---|---|---|---|---|---|---|---|
| FP2 | 0.0052 | −2.79 | 0.09 | 0.016 | 0.018 | 0.019 | 0.019 |
| CP3 | 0.0002 | −3.71 | 0.11 | 0.020 | 0.019 | 0.024 | 0.018 |
| P2 | 0.0000 | 5.46 | 0.17 | 0.016 | 0.018 | 0.023 | 0.018 |
| P3 | 0.0014 | −3.19 | 0.10 | 0.017 | 0.019 | 0.021 | 0.018 |
| O2 | 0.0009 | −3.33 | 0.10 | 0.016 | 0.019 | 0.020 | 0.019 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keshmiri, S. Stress Changes the Resting-State Cortical Flow of Information from Distributed to Frontally Directed Patterns. Biology 2020, 9, 236. https://doi.org/10.3390/biology9080236
Keshmiri S. Stress Changes the Resting-State Cortical Flow of Information from Distributed to Frontally Directed Patterns. Biology. 2020; 9(8):236. https://doi.org/10.3390/biology9080236
Chicago/Turabian StyleKeshmiri, Soheil. 2020. "Stress Changes the Resting-State Cortical Flow of Information from Distributed to Frontally Directed Patterns" Biology 9, no. 8: 236. https://doi.org/10.3390/biology9080236
APA StyleKeshmiri, S. (2020). Stress Changes the Resting-State Cortical Flow of Information from Distributed to Frontally Directed Patterns. Biology, 9(8), 236. https://doi.org/10.3390/biology9080236





