Abstract
Aside from two samples collected nearly 50 years ago, little is known about the microbial composition of wind tidal flats in the hypersaline Laguna Madre, Texas. These mats account for ~42% of the lagoon’s area. These microbial communities were sampled at four locations that historically had mats in the Laguna Madre, including Laguna Madre Field Station (LMFS), Nighthawk Bay (NH), and two locations in Kenedy Ranch (KRN and KRS). Amplicon sequencing of 16S genes determined the presence of 51 prokaryotic phyla dominated by Bacteroidota, Chloroflexi, Cyanobacteria, Desulfobacteria, Firmicutes, Halobacteria, and Proteobacteria. The microbial community structure of NH and KR is significantly different to LMFS, in which Bacteroidota and Proteobacteria were most abundant. Twenty-three cyanobacterial taxa were identified via genomic analysis, whereas 45 cyanobacterial taxa were identified using morphological analysis, containing large filamentous forms on the surface, and smaller, motile filamentous and coccoid forms in subsurface mat layers. Sample sites were dominated by species in Oscillatoriaceae (i.e., Lyngbya) and Coleofasciculaceae (i.e., Coleofasciculus). Most cyanobacterial sequences (~35%) could not be assigned to any established taxa at the family/genus level, given the limited knowledge of hypersaline cyanobacteria. A total of 73 cyanobacterial bioactive metabolites were identified using ultra performance liquid chromatography-Orbitrap MS analysis from these commu nities. Laguna Madre seems unique compared to other sabkhas in terms of its microbiology.
1. Introduction
Surface biotic microlayers are essential in many habitats for soil stabilization [,], building behavior of aquatic animals [], and surface aquatic biofilm formation []. Surface layers can be termed biofilms, biological crusts, or mats, depending on development stage [,]. Biological crusts have been extensively studied in desert and polar regions, and include fungal, bacterial, and algal constituents [,]. Although most common in coastal and hypersaline areas (Table 1), these mats can occur in freshwater hot springs [] and alkaline lakes [,].

Table 1.
Previous studies of microbial communities from hypersaline benthic communities.
The Laguna Madre is the largest hypersaline system in the world if the international border that splits the Laguna Madre into the US portion and Mexican Laguna Tamaulipas is ignored []. This ecosystem is renowned for its fishery, clear water, and extensive seagrass beds. The Laguna Madre is located on the Central Flyway and serves as the winter feeding grounds for many endangered avian species []. Over 40% of the Laguna’s area is covered by wind tidal algal flats, based on Bureau of Economic Geology mapping efforts []. A unique feature of the Laguna Madre is a constriction in the middle of its length by sand—this is a coastal sabkha unlike those found in the Persian Gulf and Mediterranean Seas. Laguna Madre water is occasionally pushed by wind over this shallow area, forming brines that are mixed into deeper lagoonal water [,,]. Little is known about the benthic microbial community of Laguna Madre occurring in these tidal flats [,]. In each case, a single algal sample was examined by a noted phycologist who identified the dominant taxa present. Collectively, the community was identified to be dominated by Lyngbya aestuarii and Microcoleus chthonoplastes (two cyanobacterial species) with occasional diatoms (e.g., Amphora normanii Rabenhorst, Amphora spp., Nitzschia spp.) present. Pulich and Rabalais [] conducted nitrogen fixation experiments seasonally, finding significant “new” N resulting from nitrogen fixation in the algal mats. Mats can consist of up to three centimeters’ depth with the attachment of sand and clays to the biological consortium []. During the past 80 years, considerable alterations have occurred within the Laguna []. These include: dredging of the intercoastal waterway that increased water depth to ~4 meters in the main channel (the average depth otherwise is <1 m), opening several passes through the barrier island, removing the sill between the Laguna and Corpus Christi Bay, as well as increased nutrient loading from development, including housing and agriculture [].
As is widely appreciated, taxonomic analysis of mat communities is nearly impossible using morphological criteria alone for cyanobacteria [,] and for bacteria (Table 1). This study used a polyphasic approach, including morphological, isolation/culturing, next-generation analyses, and chemical approaches, to describe the biodiversity within these complex biofilm/mat communities. There is an urgency to understand unique habitats such as these hypersaline mats, as sea-level rise and Anthropocene influences are likely to alter their habitat. The isolation and identification of these organisms may provide unique resources for natural product discovery.
2. Materials and Methods
2.1. Study Site
Sandflat algal mat samples for morphological, isolation/culturing, next-generation, and toxin analyses were collected from 4 different sample sites in the Laguna Madre during May 2018 (Figure 1). Each site contained an elevational gradient of wetting and were identified as: Kenedy Ranch (KRN and KRS; 2 transects about 1.3 km apart, near Tres Marias Island), Nighthawk Bay (NH; just north of Bird Island), and on Laguna Madre Field Station island (LMFS). Samples were collected along a gradient from wet (nearly submersed), an intermediate site with no visible water, and a dry site more shoreward in a sterile plastic container (18 cm × 32 cm × 5 cm deep) and immediately placed in a darkened cooler on ice packs until processed in the laboratory.
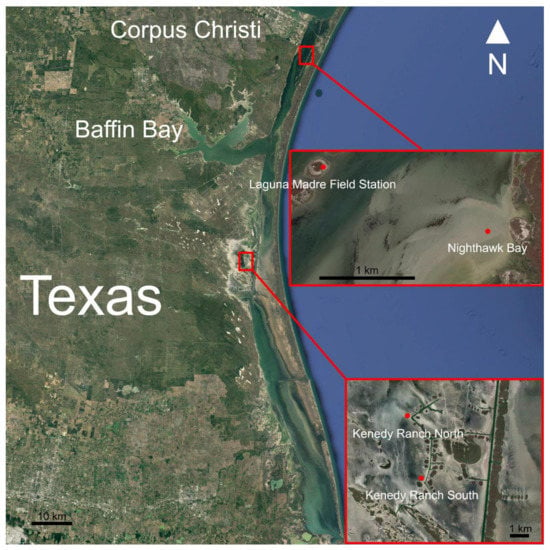
Figure 1.
Map of the Laguna Madre showing the sample sites.
2.2. Microscopy
Structure of the mats was captured using an Olympus SZ60 stereo microscope (Olympus, Tokyo, Japan) that was equipped with an AmScope 10 MP Microscope Digital Camera, MU1000 controlled with AmScope v3.7.1 software (AmScope, Irvine, CA, USA). Species composition of the cyanobacteria were examined using either a Wild M5 inverted microscope (Wild Herrburgg, Heerbrugg, Switzerland) or a Zeiss Axio Imager A1 Microscope (Carl Zeiss Inc., Hallbergmoos, Germany). Taxa were named according to the current nomenclature [,,,,]. Identified cyanobacterial taxa were classified as dominant (>51%), common (20%), and rare (5% or less) according to their occurrence on the surface of the mat. In some cases, identification occurred from enrichment cultures grown in f/100 media [] at salinity 47 ppt. Subsamples of each site were viewed alive and after being preserved using 4% formalin. For SEM examination, samples were dehydrated through an ethanol gradient (25%, 50%, and 75%) to a final concentration of 100% and then transferred to a coverslip. Samples were chemically dried using hexamethyldisilazane (cat. #379212; Sigma-Aldrich, St. Louis, MO, USA), then attached to an aluminum stub using carbon adhesive tape and coated with gold/palladium using a Denton Desk II sputter coating system (Denton Vacuum, LLC, Moorestown, NJ, USA). Samples were examined using a Hitachi 3400 N-II Scanning Electron Microscope (Tokyo, Japan). Photo plates were assembled in Microsoft PowerPoint (Microsoft Cooperation, Redmond, WA, USA) and Adobe Photoshop CC (Adobe System Inc., San Jose, CA, USA).
2.3. Molecular Methods and Sequencing
Total DNA was extracted using a DNeasy PowerSoil Kit (QIAGEN, Hilden, Germany). The V4 region of the 16S rRNA gene was amplified using a HotStarTaq Plus Master Mix Kit (QIAGEN) and the improved 515f and 806r primer set with barcoded forward primers []. Amplification conditions were 94 °C for 3 minutes followed by 28 cycles of 30 seconds at 94 °C, 40 seconds at 54 °C, and 72 °C for one minute. Final elongation occurred at 72 °C for 5 minutes. Amplification was checked for success visually using gel electrophoresis, and samples were pooled in equal proportions based on their molecular weight and DNA concentrations. Pooled PCR products were then purified using calibrated Ampure XP beads (Beckman Coulter, Indianapolis, IN, USA), and the resulting pooled library was sequenced on an Illumina MiSeq instrument using paired-end chemistry (2 × 150 bp) at Molecular Research LP (Shallowater, TX, USA). Raw sequence reads were processed using a combination of QIIME v1.9 and QIIME2 version 2020.2 []. Barcodes were extracted from the paired-end reads using the ‘extract_barcodes.py’ tool within QIIME v1.9, and then imported into QIIME2 for the remainder of the analysis. Sequence reads were demultiplexed and then denoised using DADA2, using trim lengths of 242 bp and 233 bp on the forward and reverse read, respectively. In addition, DADA2 improves sequences quality by removing chimeric sequences, and merges paired-end reads. Principal co-ordinate analysis of weighted UniFrac values was also carried out using the q2-diversity plugin. Taxonomy was assigned using a Naïve Bayes classifier trained on the SILVA release 138 operational taxonomic units (OTUs) database at 99% criterion, where sequences had been trimmed to include only the 250 bases from the 16S region that were sequenced. Taxonomic bar plots were generated using ggplot2 package (ver. 3.3.0) and biodiversity was analyzed using Bray Curtis dissimilarity index calculated from the vegan package (ver. 2.5.6) in RStudio program (R ver. 4.0.0) to visualize taxonomic differences between samples.
2.4. Mat Cyanotoxin Analysis
Presence of toxins was determined using an ultra performance liquid chromatography (UPLC) Thermo Scientific Orbitrap Fusion Tribrid mass spectrometer (ThermoScientific Waltham, MA, USA). A library of known cyanobacterial metabolites was used for confirmation of retention time []. Toxins were extracted after a 4-hour period using acidified acetonitrile [] at 4 °C, then clarified using 0.2 µm filters prior to analysis. Samples were loaded onto a C18 column (3 × 100 mm, 3 µm particle size, Phenomenex Corp., Torrance, CA, USA), with formic-acid-added ultrapure water and acetonitrile as mobile phases. As standards for most toxins were not available, toxin concentration was not determined. Toxin confirmation was achieved through the alignment of the retention time, the difference in predicted and detected masses (<3 ppm), the peak abundance (>104 unit), and the isotope patterns.
3. Results
3.1. Microscopic Based Taxonomic Structure of the Mat
Mats from all locations were composed of layered organic and inorganic strata largely occupied by cyanobacteria, with infrequent pennate diatoms, euglenoids, and flagellate algae present in the upper 1 cm. Cyanobacteria formed a thin surface layer 0.1–0.5 mm with inorganic material associated with the sheath of at least some taxa. During collections, the only animals found were dipteran flies in mats and annelids occasionally in the submerged mats at LMFS and NB.
Mats from submersed and dry regions contained different algal communities (Figure 2A–F and Figure 3A–AB). Submerged mats were less developed in terms of surface coverage and were less thick (Figure 2A,B). Based on light microscopy, the species richness of cyanobacteria in the microbial mats from the Laguna Madre was high, with 45 species/unique morphotypes identified (Table 2). Of these, 27 taxa were filamentous forms (62% of the flora) from seven different orders. Synechococcales, Spirulinales, Oscillatoriales, Nostocales were dominant numerically. Other taxa were identified from the Pleurocapsales, Chroococcales, and rarely Chroococcidiopsidales. Species richness of the individual samples was ~10 species always with at least one dominant taxon. Coleofasiculus spp. accounted for ~70% of the taxa present in the submerged sites (Figure 3E), with “Lyngbya-like” filaments commonly found. (Figure 2C, 3N,O). Collectively these taxa accounted for ca. 95% of the taxa on the sediment surface but were rarely found buried within the mat. Coleofasciculus chthonoplastes found in the Laguna Madre (Figure 3E) was similar to the type species C. chthonoplastes WW7 isolated from the wind flats of the Baltic Sea, Germany []. Morphologically the “Lyngbya” sp. from the Laguna Madre was similar to Lyngbya aestuarii Liebman ex Gomont, except our material lacked the calyptra found in this taxon (Figure 3O). A brown sheath covering Lyngbya was evident (Figure 3N). Species of several cyanobacteria genera (i.e., Geitlerinema, Oscillatoria, Pseudanabaena, and Spirulina) were commonly found in the microbial mats from the Laguna Madre (Figure 3Q,V,X). The taxon Leibleinia subtilis (Figure 3M) was rarely encountered, growing coiled on “Lyngbya” sp. A newly described taxon, Perforafilum tunnelli, with a tapered end and boring ability, was rarely found in the mats (Figure 3R). Most coccoid cyanobacteria were rarely found in the Laguna Madre microbial mat, except Aphanocapsa, Bacularia, and Synechococcus (Figure 3B,AA). Unique multicelluar-colonial Chroococcus, Cyanosarcina, Eucapsis, Gloeocapsa, and Gloeocapsosis were identified in the Laguna Madre mats with rare abundance (Figure 3D,G–J). A rare pleurocapsalean taxon Pleurocapsa sp. (Figure 3T) was found on shell fragments of the bivalve Anomalocardia auberiana. Pleurocapsa sp. and the rarely encountered coccoid species Chroococcus cf. pulcherrimus (Figure 3F) were enveloped by a brown sheath. A poorly know taxon, Johannesbaptistia sp., was found intermixed with other cyanobacteria in the mat (Figure 3K). This taxon’s morphometric measurements and morphology differed from values reported for the type species, Johannesbaptistia pellucida. Cyanobacterial genera known from the literature to be bioactive metabolite producers were present, including Lyngbya, Nodosilinea, Oscillatoria, Perforafilum, Phormidium, Pleurocapsa, Pseudanabaena, Schizothrix, Spirulina, and Synechococcus (Figure 3N–T,V–X,AA).
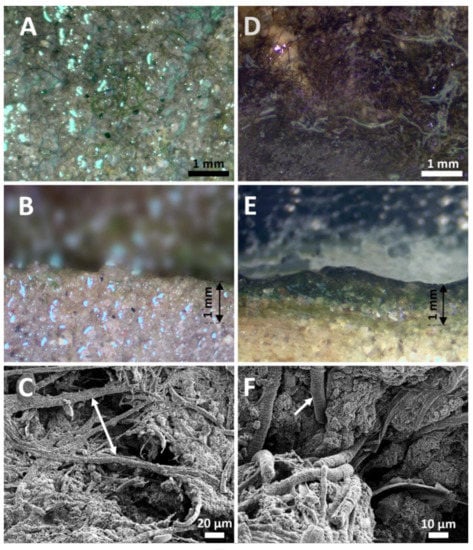
Figure 2.
Light photographs and SEM images of submerged (A,B,C) and dry (D,E,F) mats. (A) Surface of a submerged, poorly developed mat with multitrichal filaments of the dominant species Coleofasciculus chthonoplastes. Note limited cyanobacterial coverage. (B) Cross section of the submerged mat with barely seen surface layer of cyanobacteria. Some filament occurred within the mat between the sand grains, at depths of ~0.2 mm due to active locomotion of C. chthonoplastes. (C) SEM image of the submerged mat with threads of C. chthonoplastes embracing mineral’s particles. (D) Surface of a dry, well developed mat with abundant filaments of the “Lyngbya” sp. covering almost 95% of area. (E) Cross section of a dry mat with visible colonies of “Lyngbya” sp. on the surface and blue-green layer underneath with other filamentous taxa. Note that the mat has pigmentation/coloration all the way down to 1 mm compared to thinner mats in submerged mat. (F) SEM picture of a dry mat with large rounded filaments of “Lyngbya” sp. having short discoid cells.

Figure 3.
Representative morphologies of cyanobacterial taxa identified using light microscopy. (A) Anagnostidinema sp., a filamentous cyanobacterium with rounded end. (B) Aphanocapsa sp., a coccoid form with thick mucilage covering the colonies. (C) Aphanothece sp., a coccoid cyanobacterium with thin layer of mucilage. (D) Chroococcus sp., a coccoid form with thick mucilage covering each cell. (E) Coleofasciculus chthonoplastes (Thuret ex Gomont) M. Siegesmund et al., a filamentous form with tapered end and thick sheath covering the trichomes. (F) Cyanocohniella sp., a thermal cyanobacterium that was originally isolated from thermal springs. (G) Cyanosarcina sp., a unicellular-colonial, usually 2–16-multicelled cyanobacterium. (H) Eucapsis prescottii (Drouet et Daily) Komárek et Hindák, a unicellular-colonial, irregular, and diffluent mucilage-forming cyanobacterium. (I) Gloeocapsa sp., a unicellular-colonial cyanobacterium in a form of irregular aggregations with brown sheath. (J) Gloeocapsopsis sp., a less spherical unicellular-colonial cyanobacterium with brown sheath. (K) Johannesbaptistia pellucida (Dickie) W. R. Taylor et Drouet., a unicellular-pseudofilamentous taxon, that possessed a gelatinous sheath. (L) Kamptonema sp., an unsheathed cyanobacterium with rounded ends. (M) Leibleinia sp., a thin sheath-covered filamentous cyanobacterium that tangled in old stages. (N) Lyngbya sp., a filamentous cyanobacterium with brown sheath. (O) Lyngbya sp. with a thickened outer cell wall at the end cell. (P) Nodosilinea sp., a filamentous cyanobacterium with nitrogen-fixing potentials. (Q) Oscillatoria nigro-viridis Thwaites ex Gomont, a motile filamentous cyanobacterium. (R) Perforafilum tunnelli, a tapered-ended, sometimes hooked-ended, filamentous cyanobacterium with boring ability. (S) Phormidium sp., a filamentous cyanobacterium with tight sheath. (T) Pleurocapsa sp., a cyanobacterium that is unicellular-pseudofilamentous and crust-like, composed of irregular groups of cells. (U) Porphyrosiphon sp., a tapered-ended filamentous cyanobacterium with a firm and thick sheath. (V) Pseudanabaena sp., a filamentous cyanobacterium. (W) Schizothrix sp., a filamentous colonial cyanobacterium. (X) Spirulina tenerrima Kützing ex Gomont., a filamentous screw-like cyanobacterium. (Y) Stenomitos sp., a rounded-ended filamentous cyanobacterium. (Z) Symplocastrum sp., a filamentous cyanobacterium. (AA) Synechococcus sp., a unicellular-colonial, oval-shaped cyanobacterium without mucilage. (AB) Xenococcus sp., a unicellular-colonial cyanobacterium containing mucilaginous sheath. Scale bar in each figure represents 10 µm.

Table 2.
List of the cyanobacterial species found in the sand flat mats in Laguna Madre. Med-medium; R-rare; C-common; A-abundant.
3.2. Molecular and Phylogenetic Analysis
The complete OTU table is summarized in the supplemental material (Table S1). According to principal coordinate ordinations (Figure S1), microbial communities of KRS and KRN sample sites (all conditions) tended to group together; however, that trend was not significant. The dry and medium sites of NH grouped with KR sample sites, supporting the idea that dry sites within LMFS and NH are taxonomically similar to wet sites in KR. The microbial community from the sand flats of the Laguna Madre were dominated by members of the phyla Proteobacteria and Bacteroidota, which collectively accounted for 20–60% of OTU relative abundance (Figure 4). Bray–Curtis dissimilarity index showed that the LMFS-dry site had a unique microbial composition dominated by Firmicutes and Proteobacteria, whereas the microbial compositions of the other three dry sites were similar. All sites in KR shared similar microbial composition at the phyla level to the two NH sites, i.e., NH-medium and NH-dry. KRN-wet sites were dominated by Chloroflexi, followed by Proteobacteria and Bacteroidota. According to MiSeq analysis, 50 microbial phyla were identified from KR mats. Members of the phylum Cyanobacteria represented less than 15% of the total relative abundance of the microbial community from all sample sites and all conditions (Figure 4); the KRS-medium wetness site had the largest Cyanobacteria abundance, which was double the next closest site (KRN-dry).
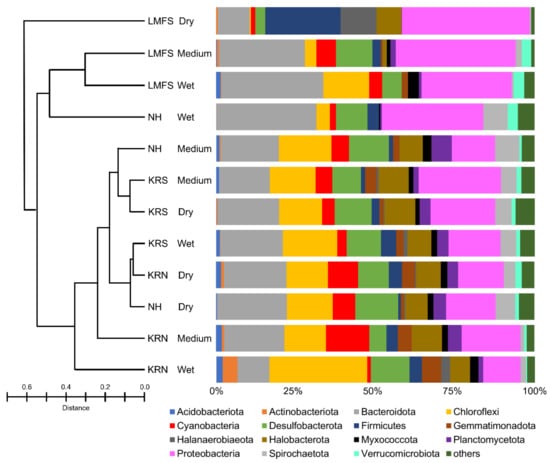
Figure 4.
Bar plot showing the relative abundance (%) of amplified sequences of bacterial and archaeal phyla. Abundances were normalized to the total number of sequences, and taxonomic groups that were outside of the scope of research were grouped under the others category. The 15 most abundant orders across all samples are displayed in the legend. Bray–Curtis dissimilarity index is presented in the left panel.
Eighteen Bacteriodota families were identified in the Laguna Madre microbial community. Balneolaceae, Cyclobacteriaceae, Rhodothermaceae, and Saprospiraceae were the four most abundant families in the KR microbial mats, accounting for 45%~95% (mean = 72%) of the Bacteriodota OTUs (Figure S2). Species of Rhodothermaceae were less abundant in LMFS sites compared to the three other locations. Amoebophilaceae and Marinilaniliaceae were only found in LMFS and NH sites, whereas Salinivirgaceae was only present in NH sites. The Chloroflexi were dominated by unnamed species, with four families identified including the Anaerolineaceae, Ardenticatenaceae, Caldilineaceae, and Chloroflexaceae (Figure S3). The KRN-wet site was the only one that contained all four Chloroflexi families. Sixteen Desulfobacterota families were identified from the Laguna Madre. Four families were commonly present in all sites, including Bradymonadaceae, Desulfocapsaceae, Desulfococcaceae, and Desulfosarcinaceae (Figure S4). Another family, the Desulfovibrionaceae, was found in all sites but LMFS-wet; further, the abundance of this particular family in three LMFS sites was lower than other sites. Species of Desulfurivibtionaceae and Geothermobacteraceae were only found at the KRN sites. According to Bray–Curtis indices, it is clear that the composition of Desulfobacterota in LMFS and NH was different than KR sites. Firmicutes accounted for 25% of microbial abundance at LMFS-dry (Figure S5), whereas at LMFS-wet they were not present. Fifteen families in Firmicutes were identified, with Bacillaceae found in all sites but NH-dry. Fusibacteraceae were only found in LMFS and NH sites. Lachnospiraceae were only found at the NH-wet site. Thermotaleaceae were only found in KRN sites, i.e., KRN-medium and -dry locations. Five Halobacterota families were identified in the Laguna Madre mats, with Haloferacaceae and Halomicrobiaceae being the dominant families (Figure S6). KRN-dry and KRS-wet had all five families present, whereas both LMFS and NH wet sites had no occurrence of Halobacterota. Halobacteriaceae and Halococcaceae were only found in KR sites. A total of 58 proteobacterial families were found in the Laguna Madre mats (Table S1). Chromatiaceae and Rhodobacteraceae were commonly found in all sites, accounting for 10% to 50% of the OTUs, averaging 28% (Figure S7). Fodinicurvataceae had high abundance at all sites in NH and KR, other than the three sites in LMFS.
Species in the cyanobacterial families Coleofasiculaceae and Oscillatoriaceae were the most abundant in the mats, accounting for 25–85% (average 46%) of the cyanobacteria OTUs (Figure 5). The dry site at LMFS was the only site that did not contain species of Oscillatoriaceae. Unlike the Oscillatoriaceae, species in the Coleofasiculaceae were mostly found in dry areas. Coleofasiculaceae were either not found or present in low OUT abundance (<20%) in samples from all four wet sites across the Laguna Madre, i.e., KRN-wet, NH-wet, LMFS-medium and -wet. Leptolyngbyaceae were found in 10 sites, but not at KRN-wet and LFMS-dry. Microcoleaceae were only found in rare abundance at the other three low moisture sites, including NH-medium, KRN-medium, and KRS-dry. Chroococcaceae and Spirulinaceae were only found in LFMS and NH, whereas Merismopediaceae were only found in KR sites.
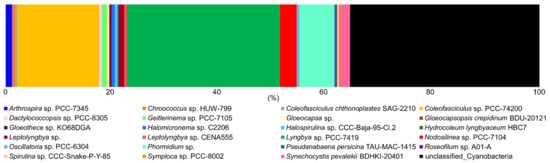
Figure 5.
Bar plot showing the relative abundance (%) of amplified sequences of total cyanobacterial species in mid and upper Laguna Madre. Abundances were normalized to the total number of sequences.
Total species richness of cyanobacteria, using the SILVA automatic classification, was 23 known taxa (Figure 6) within nine families (Table S1). Nine of the 23 taxa were not identified by light microscopy including species in the genera Arthrospira, Dactylococcopsis, Gloeothece, Halospirulina, Hydrocoleum, Leptolyngbya, Nodosilinea, Roseofilum, and Symploca. According to OTU analysis, Lyngby sp. PCC-7419 (30% of the total), Coleofasciculus sp. PCC-7420 (20%), and Phormidium sp. (8%) were the most abundant taxa in the mats (Figure 6). Overall, ~35% of OTUs were not matched to known species in the SILVA database. The medium and dry sites of KRN had the highest species richness of Lyngbya (Figure S8), supporting our idea that “Lyngbya” spp. are best suited for growth/survival in these dry environments and that multiple cryptic species are present (Table 2). All sites in LMFS, as well as wet and medium sites of NH, did not contain any “Lyngbya” spp. sequences. Coleofasciculus was most abundant in LMFS-dry, which differs from our microscopic data, where Coleofasciculus spp. were dominant only in the wet sites at LMFS and NH. Generally, filamentous and coccoid cyanobacteria co-occurred in the sand flat mats of the Laguna Madre, including hypersaline and thermo-tolerant forms, e.g., Halospirulina and Cyanocohniella.
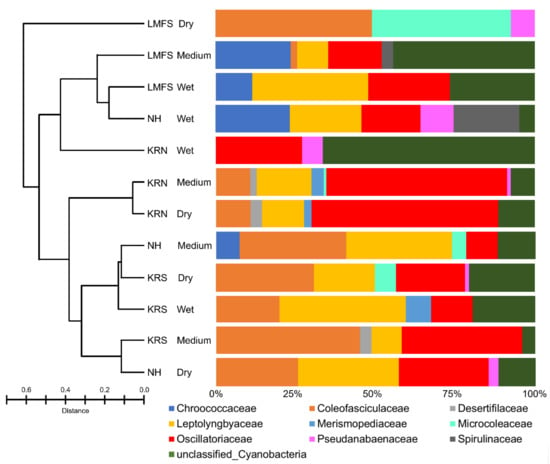
Figure 6.
Bar plot showing the relative abundance of cyanobacterial amplified sequences separated by regions and conditions. Abundances were normalized to the total number of sequences. Bray–Curtis dissimilarity index is shown on the left.
3.3. Cyanobacterial Bioactive Metabolite Analysis
A total of 57 toxins were identified from the mat samples (Table 3). The most abundant class of toxins found are the aeruginosins (14), with 10 unique microcystins, 7 microcycloamides, and 6 cyanopeptolins identified. One lynbelloside and two aeruginosins had high area counts (exceeding 10,000). Cyanobacterial genera that are reported in the literature to produce six of 57 bioactive metabolites were identified in this study, whereas the cyanobacterial family reported as the original source of 28% metabolites were identified. About 50% of the metabolites present were originally isolated from Microcystis in the literature, of which eight associated genera (e.g., Aphanothece and Chroococcus) were identified using morphological and genomic analyses. Four bioactive metabolites found in this study were originally isolated from marine sponge Theonella sp. and seahare Dolabella sp., indicating the source should be their dietary cyanobacteria.

Table 3.
HPLC Orbitrap results for Laguna Madre wind-tidal flat screening for all known cyanobacterial bioactive metabolites, organized as in Huang and Zimba (2019). Listed compounds had peak exceeding 10,000 area counts. Names followed by an asterisk were area counts exceeding 100,000, with a double asterisk indicating mass difference equals 0 ppm. Occurrence (Occr) refers to how many samples (of 9 total) contained each toxin. Literature of metabolite origins was summarized in Huang and Zimba (2019). Occr—bioactive metabolite occurrence; Cy—Cyanobacteria; Y—yes; N—no; N/A—not applicable.
4. Discussion
Climate change is affecting numerous ecosystems worldwide, including widespread alteration of tidal flats []. Mats positioned between the ocean and coastlines are impacted by land hardening, population growth/nutrification, as well as sea level rise. It is estimated that 16% of these areas have been destroyed in the past 32 years [,]. Changes of centimeters in elevation (sea-level rise) inundate mats more frequently, thereby allowing less hardy species to proliferate. Unique habitats such as the Laguna Madre are susceptible to changing environmental conditions, because of the selective unique conditions that helped organisms adapt to this unique sabkha. Alterations of salinity (from levels up to 100 practical salinity unit to near seawater), increased wetting frequency of the mats due to channelization of the Intercoastal Waterway and opening more passes to the Gulf of Mexico, and alteration/development of land collectively threaten to alter these mats in terms of species composition and biomass present []. Increases in water level or inundation frequency will affect mat composition, as these new disturbances (in the form of salinity decreases) have been shown to alter composition of saline pond communities of San Salvador Island, Bahamas []. Increased biodiversity of cyanobacteria was associated with the near four-fold decrease in salinity after hurricane passage. Climate changes may alter species composition or physiological activity of the microbial consortia. Exopolysaccharide secretion are typically required for initiation of adhesion of bacteria [,] and cyanobacteria [] to the inorganic soil/sediments. This adhesive layer is critical for mat stability during desiccation periods, to develop more diverse composition [,], and for differential metabolite formation in pathogenic bacteria []. The dominant taxon in the Laguna Madre, Lyngbya, can produce copious amounts of extracellular polymeric substances [] and so changes in environmental conditions may threaten its current architecture, functionality, and ecosystem role.
Mat dominance by bacteria, particularly Proteobacteria and Bacteroidetes, have been observed in other hypersaline communities (Table 1). Vogt et al. [] reported up to 60% dominance of Bacteroidetes in the hypersaline microbial communities of the Gulf of Oman, with similar results from the surface of the microbial mats from the hypersaline Guerrero Negro, Baja California, Mexico [,]. Both Proteobacteria and Bacteroidetes possess diverse nitrogen metabolism and sulfur reduction [,,,,]. Other than Bacteroidetes and Proteobacteria, species in Chloroflexi, Desulfobacteria, Firmicutes, and archaea-Halobacterota were also commonly present in the Laguna Madre mats. Chloroflexi have been reported from thermal environments and are known for their anaerobic decomposing ability [,], whereas Halobacterota are well-known for their occurrence in hypersaline environments and utilization of cellulose and chitin as nutrient sources [,]. Unlike other hypersaline sites, Laguna Madre has abundant oil and natural gas deposits. The drilling process of harvesting these natural sources may have resulted in the richness of sulfur-containing substances, which support the success of species of Desulfobacteria. Alternatively, the seasonal deposition of seagrass wrack provides an anaerobic habitat suitable for growth of these taxa. The occurrence of Firmicutes was ~25% at LMFS-dry while it was almost absent from other sampled sites. This particular site was nearly always (~90% of time) exposed to the air, allowing the community to be largely affected by the low-moisture conditions and solar radiation. The ultra-structure of Firmicutes, preventing damage from solar radiation [] and the loss of intracellular moisture [], may explain the abundance of this phylum at LMFS-dry compared to other phyla. Further, the presence of a rare phylum, Planctomycetota, suggests the potential for anaerobic ammonium oxidation to occur in the Laguna Madre microbial mats []. The microbial composition revealed by next-generation sequencing suggested the Laguna Madre mats are unique compared to other locations.
Taxonomic cyanobacterial composition of Laguna Madre mats was similar to other tropic/sub-tropical hypersaline systems (Table 2) with a total of 55 taxa found using either light microscopy or Next-generation 16S rRNA analyses. Of these, 13 taxa were found using both approaches, with the dominance of Lyngbya (~30% overall) and Coleofasiculus (~18%). High abundance of Coleofasciculus spp. at the medium and dry sites in the Laguna Madre based on SILVA automatic classification data suggested the preference of this genus regarding wetness (Figure S8). This occurrence of Coleofasciculus is opposite to the finding in Vogt et al. [], which reported Coleofasciculus prefer lower tidal sites (high wetness conditions). Both morphological observation and SILVA automatic classification revealed several species of Coleofasciculus, but none of these analyses could resolve species level taxonomy. Unlike Coleofasciculus, Lyngbya was rarely found in NH and LMFS sites (except NH-dry), but was commonly occupied in the samples from KR, especially KRN-medium and -dry sites, as Lyngbya were present in the medium to dry sample sites from other studies []. This distribution could be a result of (1) the favorable conditions, and (2) the structure of Lyngbya. Both NH and LMFS sites were exposed to the direct current in the Laguna Madre, whereas two KR locations were at a divergence from the main Laguna Madre stream (Figure 1). Compared to KRS, which was along the shore of KR, KRN was at an angle that could decelerate the water flow. With the significant dominance of Lyngbya at KRN, it is likely this particular taxon favors a slow/static environment. An alternative explanation is that heavily sheathed “Lyngbya” spp. had poor extraction of DNA. Cyanobacterial taxa such as Cyanocohniella and Oscillatoria have been identified in other hypersaline and thermal environments [,] and were present in this study (Figure 3F,Q). Many of the taxa do not fit within described taxa for either the dominant (Lyngbya and Coleofasiculus, for example) or rarer taxa (Perforafilum tunnelli) as seen in other analysis of benthic saline cyanobacteria [,]. We feel this is a result of limited analysis of benthic hypersaline communities, as recent taxonomic revisions have resulted in several new genera and species such as Dapis being separated from Lyngbya [], and Toxifilum, a cryptic taxon from the hypersaline Corpus Christi Bay, serving to establish a new family (the Laspinaceae) []. According to Vogt et al. [], coccoid cyanobacteria such as Euhalothece sp. and species in Chroococcales had dominated the upper tidal site (high dryness conditions). There was no general trend in the occurrence of coccoid cyanobacteria in this study due to the dominance of filamentous cyanobacteria in all sites.
Cyanobacterial genera that have been reported as bioactive metabolite producers were present in the Laguna Madre microbial mats, including Nodosilinea, Lyngbya, Oscillatoria, Perforafilum, Phormidium, Pleurocapsa, Pseudanabaena, Schizothrix, Spirulina, and Synechococcus (Table 3) []. Given the widespread production of cyanobacterial bioactive metabolites (Table 3), it is tempting to suggest that these metabolites may serve as a deterrent for growth of animals and microbes, but it is also likely that the lack of water for animal survival may better explain the absence of grazing organisms. Identified bioactive compounds that had high peak abundance (>106) have documented protein phosphatase inhibition and cytotoxic activity (Table 3) []. These mats appear to be a rich, diverse source of numerous poorly known toxins []. Our culturing and analysis approach, allowing the systematic assignment of toxins, e.g., Perforafilum tunnelli, produces seven of the 57 known toxins found in the Laguna Madre mats []. This assignment of species and toxicity is essential for understanding the impacts of toxin production and potential utility of these compounds as done for dolastatins and other cyanobacterial bioactive compounds [].
5. Conclusions
In summary, the hypersaline mats found in the Laguna Madre potentially appear to differ from other systems based on dominance of multiple species, cryptic undescribed species, and different environmental drivers. The fate of these mats depends on the rate of sea level rise, and ability of these mats to migrate to high elevations as encroached by seawater. Future work will include seasonal analysis of these mats using these combined approaches, coupled with production and nitrogen fixation, to better understand the ecosystem services provided by these unique organisms.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-7737/9/8/183/s1. Table S1: OTU table, Figure S1: Principal coordinate ordinations relating the variation in microbial community composition between all 12 communities, Figure S2: Bar plot showing the relative abundance of Bacteroidota amplified sequences separated by regions and conditions, Figure S3: Bar plot showing the relative abundance of Chloroflexi amplified sequences separated by regions and conditions, Figure S4: Bar plot showing the relative abundance of Desulfobacterota amplified sequences separated by regions and conditions, Figure S5: Bar plot showing the relative abundance of Firmicutes amplified sequences separated by regions and conditions, Figure S6: Bar plot showing the relative abundance of Halobacterota amplified sequences separated by regions and conditions, Figure S7: Bar plot showing the relative abundance of Proteobacteria amplified sequences separated by regions and conditions, Figure S8: Bar plot showing the relative abundance of cyanobacterial species amplified sequences separated by regions and conditions.
Author Contributions
Conceptualization, I.-S.H., J.W.T., H.A. and P.V.Z.; Data curation, I.-S.H., and L.J.P.; Formal analysis, I.-S.H., L.J.P., L.B., E.W.L. and P.V.Z.; Facquisition, P.V.Z.; Investigation, I.-S.H., J.W.T., H.A. and P.V.Z.; Methodology, I.-S.H., J.W.T., H.A. and P.V.Z.; Project administration, I.-S.H., and P.V.Z.; Software, I.-S.H.; Supervision, P.V.Z.; Validation, I.-S.H.; Visualization, I.-S.H.; Writing—Original draft, I.-S.H. and P.V.Z.; Writing—Review and editing, I.-S.H., L.J.P. and P.V.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors appreciate funding from the Division of Research and Innovation, TAMUCC, that defrayed genomic analysis costs. We thank Sergei Shalygin for photography and his diverse knowledge of cyanobacteria taxonomy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Belnap, J. The world at your feet: Desert biological soil crusts. Front. Ecol. Environ. 2003, 1, 181–189. [Google Scholar] [CrossRef]
- Williams, A.; Kane, D.A.; Ewing, P.M.; Atwood, L.W.; Jilling, A.; Li, M.; Lou, Y.; Davis, A.S.; Grandy, A.S.; Huerd, S.C.; et al. Soil functional zone management: A vehicle for enhancing production and soil ecosystem services in row-crop agroecosystems. Front. Plant Sci. 2016, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D. The functional significance of selection of particles by aquatic animals during building behavior. In The Biology of Particles in Aquatic Systems, 2nd ed.; Wooten, R.S., Ed.; Lewis: Boca Raton, FL, USA, 1994; pp. 289–312. [Google Scholar]
- Allison, D.G.; Sutherland, L.W. Role of exopolysaccharides in adhesion of freshwater bacteria. J. Gen. Microbiol. 1987, 133, 1319–1327. [Google Scholar] [CrossRef][Green Version]
- Evans, R.D.; Johansen, J.R. Microbiotic crusts and ecosystem processes. Crit. Rev. Plant Sci. 1999, 18, 183–225. [Google Scholar] [CrossRef]
- Osorio-Santos, K.; Pietrasiak, N.; Bohunická, M.; Miscoe, L.H.; Kováčik, L.; Martin, M.P.; Johansen, J.R. Seven new species of Oculatella (Pseudanabaenales, Cyanobacteria): Taxonomically recognizing cryptic diversification. Eur. J. Phycol. 2014, 49, 450–470. [Google Scholar] [CrossRef]
- Castenholz, R.W. Composition of hot spring microbial mats: A summary. In Microbial Mats Stromatolites; Cohen, Y., Castenholz, R.W., Halvorson, H.O., Alan, R., Eds.; Liss Publ.: New York, NY, USA, 1984; pp. 101–119. [Google Scholar]
- Oremland, R.S.; Des Marms, D.J. Distribution, abundance and carbon isotopic composition of gaseous hydrocarbons in Big Soda Lake, Nevada an alkaline, meromictic lake. Geochim. Cosmochim. Ac. 1983, 47, 2107–2114. [Google Scholar] [CrossRef]
- Gerasimenko, L.M.; Mityushina, L.L.; Namsaraev, B.B. Microcoleus mats from alkaliphilic and halophilic communities. Microbiology 2002, 72, 71–79. [Google Scholar] [CrossRef]
- Tunnell, J.W.J.; Judd, F.W. The Laguna Madre of Texas and Tamaulipas; Texas A&M University Press: College Station, TX, USA, 2002; pp. 1–346. [Google Scholar]
- Gallardo, J.C.; Macias, V.; Velarde, E. Birds (Vertebrate: Aves) of the Gulf of Mexico. In Gulf of Mexico Origin, Water, and Biota. Biodiversity; Felder, D.L., Camp, D.K., Eds.; Texas A&M University: College Station, TX, USA, 2009; pp. 1321–1342. [Google Scholar]
- Amdurer, M.; Land, L.S. Geochemistry, hydrology, and mineralogy of the sand bulge area, Laguna Madre Flats, south Texas. J. Sediment. Petrol. 1982, 52, 703–716. [Google Scholar]
- Fisk, H.N. Padre Island and the Laguna Madre flats, coastal South Texas. Louisiana State University, 2nd Coastal Geography Conference 1959, 6–9, 103–151. [Google Scholar]
- Sorensen, L.O.; Conover, J.T. Algal mat communities of Lyngbya confervoides (Agardh) Gomont. Publ. Inst. Mar. Sci. Univ. Texas 1962, 8, 61–74. [Google Scholar]
- Pulich, W.; Rabalais, S. Primary production potential of blue-green algal mats on southern Texas tidal flats. Southwest. Nat. 1986, 31, 39–47. [Google Scholar] [CrossRef]
- Withers, K. Wind-tidal flats. In The Laguna Madre of Texas and Tamaulipas; Tunnell, J.W., Jr., Judd, F.W., Eds.; Texas A&M Press: College Station, TX, USA, 2002; pp. 114–126. [Google Scholar]
- Kirkwood, A.E.; Buchheim, J.A.; Buchheim, M.A.; Henley, W.J. Cyanobacterial diversity and halotolerance in a variable hypersaline environment. Microb. Ecol. 2008, 55, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Bernt Sørensen, K.; Canfield, D.E.; Oren, A. Salinity responses of benthic microbial communities in a solar saltern (Eilat, Israel). Appl. Environ. Microbiol. 2004, 70, 1608–1616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, J.; Yang, H.; Zhao, M.; Zhanga, X. Spatial distribution patterns of benthic microbial communities along the Pearl Estuary, China. Syst. Appl. Microbiol. 2014, 37, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Yoshida, T.; Nishimura, M.; Ohwada, K. Characterization of depth-related population variation in microbial communities of a coastal marine sediment using 16S rDNA-based approaches and quinone profiling. Environ. Microbiol. 2000, 2, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.B.; Revsbech, N.P.; Cohen, Y. Photosynthesis and structure of benthic microbial mats: microelectrode and SEM studies of four cyanobacterial communities. Limnol. Oceanogr. 1983, 28, 1075–1093. [Google Scholar] [CrossRef]
- John, J.; Hay, M.; Paton, J. Cyanobacteria in benthic microbial communities in coastal salt lakes in Western Australia. Algol. Stud. 2009, 130, 125–135. [Google Scholar] [CrossRef]
- Vogt, J.C.; Albach, D.C.; Palinska, K.A. Cyanobacteria of the Wadden Sea: Seasonality and sediment influence on community composition. Hydrobiologia 2018, 811, 103–117. [Google Scholar] [CrossRef]
- Echnique-Subiabre, I.; Villeneuve, A.; Golubic, S.; Turquet, J.; Humbert, J.-F.; Gugger, M. Influence of local and global environmental parameters on the composition of cyanobacterial mats in a tropical lagoon. Microb. Ecol. 2015, 69, 234–244. [Google Scholar] [CrossRef]
- Ramos, V.M.C.; Castelo-Branco, R.; Leão, P.N.; Martins, J.; Carvalhal-Gomes, S.; Sobrinho da Silva, F.; Mendonça Filho, J.G.; Vasconcelos, V.M. Cyanobacterial diversity in microbial mats from the hypersaline lagoon system of Araruama, Brazil: An in-depth polyphasic study. Front. Microbiol. 2017, 8, 1233. [Google Scholar] [CrossRef]
- Bauer, K.; Diez, B.; Seppala, S.; Borg, A.J.; Bergman, B. Variability in diazotrophy and cyanobacterial diversity in a tropical intertidal lagoon. FEMS Microbiol. Ecol. 2008, 63, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Omoregie, E.; Crumbliss, L.L.; Bebout, B.M.; Zehr, J.P. Determination of nitrogen-fixing phylotypes in Lyngbya sp. and Microcoleus chthonoplastes cyanobacterial mats from Geurro Negro, Baja California, Mexico. Appl. Environ. Microb. 2004, 70, 2119–2128. [Google Scholar] [CrossRef]
- Harris, J.K.; Caporaso, J.G.; Walker, J.J.; Spear, J.R.; Gold, N.J.; Robertson, C.E.; Hugenholtz, P.; Goodrich, J.; McDonald, D.; Knights, D.; et al. Phylogenetic stratigraphy in the Guerrero Negro hypersaline microbial mat. ISME J. 2013, 7, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, H.; Fillinger, L.; Stal, L.S. Coastal microbial mat diversity along a natural salinity gradient. PLoS ONE 2013, 8, e63166. [Google Scholar] [CrossRef]
- Jungblut, A.D.; Hawes, I.; Mountfort, D.; Hitzfeld, B.; Dietrich, D.R.; Burns, B.P.; Neilan, B.A. Diversity within cyanobacterial mat communities in variable salinity meltwater ponds of McMurdo Ice Shelf, Antarctica. Environ. Microbiol. 2005, 7, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.D.J.; McDonald, I.R.; Herbold, C.W.; Lee, C.K.; Cary, C.S. Benthic microbial communities of coastal terrestrial and ice shelf Antarctic meltwater ponds. Front. Microbiol. 2015, 6, 485. [Google Scholar] [CrossRef]
- Zoppini, A.; Amalfitano, S.; Fazi, S.; Puddu, A. Dynamics of a benthic microbial community in a riverine environment subject to hydrological fluctuations (Mulargia River, Italy). Hydrobiologia 2010, 215, 37–51. [Google Scholar] [CrossRef]
- Fourçans, A.; García de Oteyza, T.; Wieland, A.; Solé, A.; Diestra, E.; van Bleijswijk, J.; Grimalt, J.O.; Kühl, M.; Esteve, I.; Muyzer, G.; et al. Characterization of functional bacterial groups in a hypersaline microbial mat community (Salins-de-Giraud, Camargue, France). FEMS Microbiol. Ecol. 2004, 51, 55–70. [Google Scholar] [CrossRef]
- Yannarell, A.; Steppe, T.F.; Paerl, H.W. Genetic variance in the composition of two functional group (diazotrophs and cyanobacteria) from a hypersaline microbial mat. Appl. Environ. Microbiol. 2006, 72, 1207–1217. [Google Scholar] [CrossRef]
- Komárek, J. Subwasserflora von Mitteleuropa, Bd. 19/1: Cyanprokaryota. Teil/Part 1: Chrococcales; Gustav Fisher: Jena, Germany, 1999. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Subwasserflora von Mitteleuropa, Bd. 19/2: Cyanprokaryota. Tiel/ Part 2: Oscillatoriales; Springer: Heidelburg, Germany, 2007. [Google Scholar]
- Hindak, F. Colour Atlas of Cyanophytes; VEDA Publ.: Bratislavia, Slovak, 2008. [Google Scholar]
- Komárek, J. Subwasserflora von Mitteleuropa, Bd. 19/3: Cyanprokaryota. Teil/Part 3: Heterocystous genera; Springer: Heidelburg, Germany, 2013. [Google Scholar]
- Komárek, J.; Kaštovský, J.; Mares, J.; Joansen, J.R. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera), using a polyphasic approach. Preslia 2014, 86, 295–335. [Google Scholar]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, A.; Gilbert, J.A.; Jannson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; Apprill, A.; et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems 2016, 1, e9–e15. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Huttley, G.A.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 86, 139–209. [Google Scholar]
- Zimba, P.V.; Huang, I.H.; Foley, J.E.; Linton, E.W. Identification of a new to science cyanobacterium, Toxifilum mysidocida gen. nov. and sp. nov. (Cyanobacteria, cyanophyceae). J. Phycol. 2017, 53, 188–197. [Google Scholar] [CrossRef]
- Siegesmund, M.A.; Johansen, J.R.; Karsten, U.; Friedl, T. Coleofasciculus gen. nov. (cyanobacteria): Morphological and molecular criteria for revision of the genus Microcoleus Gomont. J. Phycol. 2008, 44, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.J.; Phinn, S.R.; DeWitt, M.; Ferrari, R.; Johnston, R.; Lyons, M.R.; Clinton, C.; Thau, D.; Fuller, R.A. The global distribution and trajectory of tidal flats. Nature 2019, 565, 222–225. [Google Scholar] [CrossRef]
- Preisner, E.C.; Fichot, F.B.; Norman, R.S. Microbial mat composition and functional sensitivity to environmental disturbnace. Front. Microbiol. 2016, 7, 1632. [Google Scholar] [CrossRef]
- Blanco, Y.; Rivas, L.A.; Gonzalez-Toril, E.; Ruiz-Bermejo, M.; Morenzo-Paz, M.; Parro, V.; Palacin, A.; Aquilera, A.; Puente-Sanchez, F. Environmental parameters, and not phylogeny, determine the composition of extracellular polymeric substances in microbial mats from extreme environments. Sci. Total Environ. 2019, 650, 384–393. [Google Scholar] [CrossRef]
- Callejas, C.; Azziz, G.; Souza, E.M.; Gill, P.R.; Batista, S. Prokaroytic diversity in four microbial mats of the Fildes Peninsula, King George Island, maritime Antarctica. Polar Biol. 2018, 41, 935–943. [Google Scholar] [CrossRef]
- Engene, N.; Tronholm, A.; Paul, V.J. Uncovering cryptic diversity of Lyngbya: The new tropical marine cyanobacterial genus Dapis (Oscillatoriales). J. Phycol. 2018, 54, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, P.; Meletzus, D.; Green, A.; He, L.; Kennedy, C. Regulation of nitrogen fixation by ammonium in diazotrophic species of proteobacteria. Soil Biol. Biochem. 1997, 5-6, 831–841. [Google Scholar] [CrossRef]
- Perlova, O.; Ureta, A.; Meletzus, D.; Nordlund, S. Sensing of n-status in Gluconacetobacter diazotrophicus: Biochemistry and genetics of nitrogen fixation and assimilation. Symbiosis 2003, 35, 73–84. [Google Scholar]
- Campbell, B.J.; Summers Engel, A.; Porter, M.L.; Takai, K. The versatile ε-proteobacteria: Key players in sulphidic habitats. Nat. Rev. Microbiol. 2006, 4, 458–468. [Google Scholar] [CrossRef]
- Gregersen, L.H.; Bryant, D.A.; Frigaard, N. Mechanisms and evolution of oxidative sulfur metabolism in green sulfur bacteria. Front. Microbiol. 2011, 2, 116. [Google Scholar] [CrossRef]
- Inoue, J.; Oshima, K.; Suda, W.; Sakamoto, M.; Iino, T.; Noda, S.; Hongoh, Y.; Hattori, M.; Ohkuma, M. Distribution and evolution of nitrogen fixation genes in the phylum Bacteroidetes. Microbes Environ. 2015, 30, 44–50. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Yamada, T.; Hanada, S.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermophila gen. nov., sp. nov. and Caldilinea aerophila gen. nov., sp. nov., novel filamentous thermophiles that represent a previously uncultured lineage of the domain Bacteria at the subphylum level. Int. J. Syst. Evol. Microbiol. 2003, 53, 1843–1851. [Google Scholar] [CrossRef]
- Yamada, T.; Imachi, H.; Ohashi, A.; Harada, H.; Hanada, S.; Kamagata, Y.; Sekiguchi, Y. Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., strictly anaerobic, filamentous bacteria of the phylum Chloroflexi isolated from methanogenic propionate-degrading consortia. Int. J. Syst. Evol. Microbiol. 2007, 57, 2299–2306. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Toshchakov, S.V.; Kolganova, T.V.; Kublanov, I.V. Halo (natrono) archaea isolated from hypersaline lakes utilize cellulose and chitin as growth substrates. Front. Microbiol. 2015, 6, 942. [Google Scholar] [CrossRef]
- Quadri, I.; Hassani, I.I.; l’Haridon, S.; Chalopin, M.; Hacène, H.; Jebbar, M. Characterization and antimicrobial potential of extremely halophilic archaea isolated from hypersaline environments of the Algerian Sahara. Microbiol. Res. 2016, 186–187, 119–131. [Google Scholar] [CrossRef]
- Rao, S.; Chan, O.W.; Lacap-Bugler, D.C.; Pointing, S.B. Radiation-tolerant bacteria isolated from high altitude soil in Tibet. Indian J. Microbiol. 2016, 56, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Karaoz, U.; Couradeau, E.; da Rocha, U.M.; Lim, H.; Northen, T.; Garcia-Pichel, F.; Brodie, E.L. Large blooms of Bacillales (Firmicutes) underlie the response to wetting of cyanobacterial biocrusts at various stages of maturity. mBio 2018, 9, e01366-e16. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.S.M.; Sliekers, O.; Kuypers, M.; Dalsgaard, T.; van Niftrik, L.; Cirpus, I.; van de Pas-Schoonen, K.; Lavik, G.; Thamdrup, B.; Le Paslier, D.; et al. Anaerobic ammonium oxidation by marine and freshwater planctomycete-like bacteria. Appl. Microbiol. Biotechnol. 2003, 63, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Kaštovský, J.; Berrendero Gómez, E.; Hladil, J.; Johansen, J.R. Cyanocohniella calida gen. et sp. nov. (Cyanobacteria: Aphanizomenonaceae) a new cyanobacterium from the thermal springs from Karlovy Vary, Czech Republic. Phytotaxa 2014, 18, 3. [Google Scholar] [CrossRef]
- Caroppo, C.; Turicchia, S.; Margheri, M.C. Phytoplankton assemblages in coastal waters of the northern Ionian Sea (eastern Mediterranean), with special reference to cyanobacteria. J. Mar. Biolog. Assoc. U.K. 2006, 86, 927–937. [Google Scholar] [CrossRef]
- Engene, N.; Rottacker, E.C.; Kastovsky, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komárek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bioactive secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62, 1171–1178. [Google Scholar] [CrossRef]
- Zimba, P.V.; Shalygin, S.; Huang, I.S.; Momčilović, M.; Abdulla, H. A new boring toxin producer-Perforafilum tunnelli gen. & sp. nov. (Oscillatoriales, Cyanobacteria) isolated from Laguna Madre sand-tidal flats, Texas, USA. Phycologia 2020. submitted for publication. [Google Scholar]
- Quiblier, C.; Wood, S.; Echenique-Subiabre, I.; Heath, M.; Villeneuve, A.; Humbert, J.-F. A review of current knowledge on toxic benthic freshwater cyanobacteria-ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar]
- Tidgewell, J.; Clark, B.R.; Gerwick, W.H. 2.06-The Natural Products Chemistry of Cyanobacteria. In Comprehensive Natural Products; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).