Protective Effects of Bee Venom against Endotoxemia-Related Acute Kidney Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Procedures
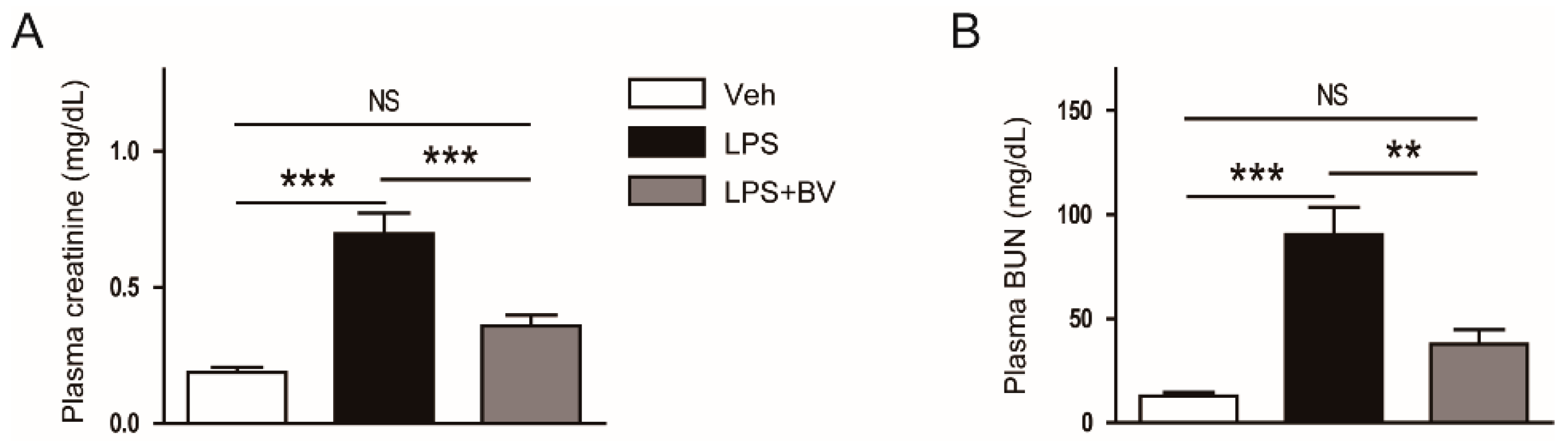
2.2. Assessment of Renal Function
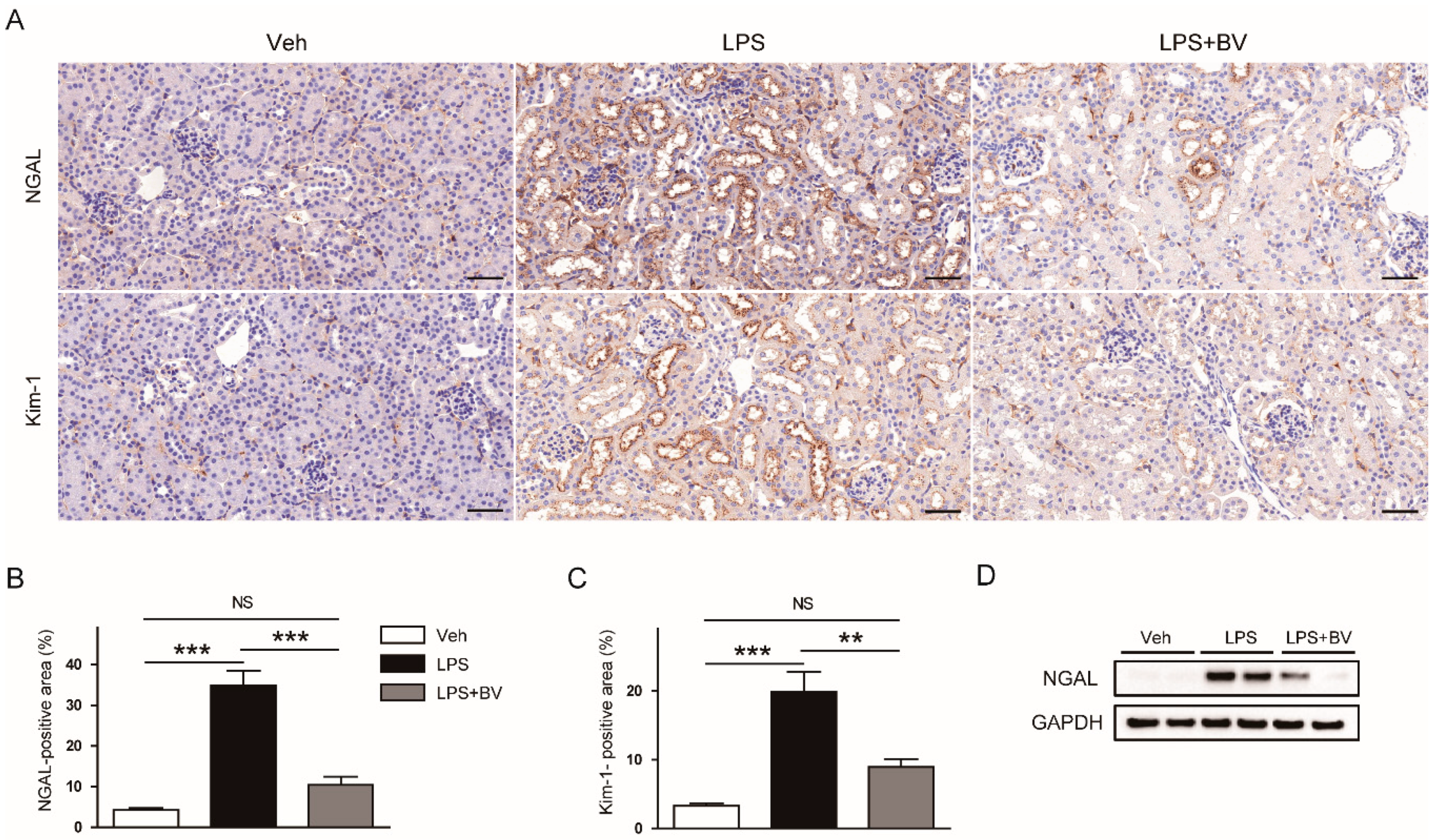
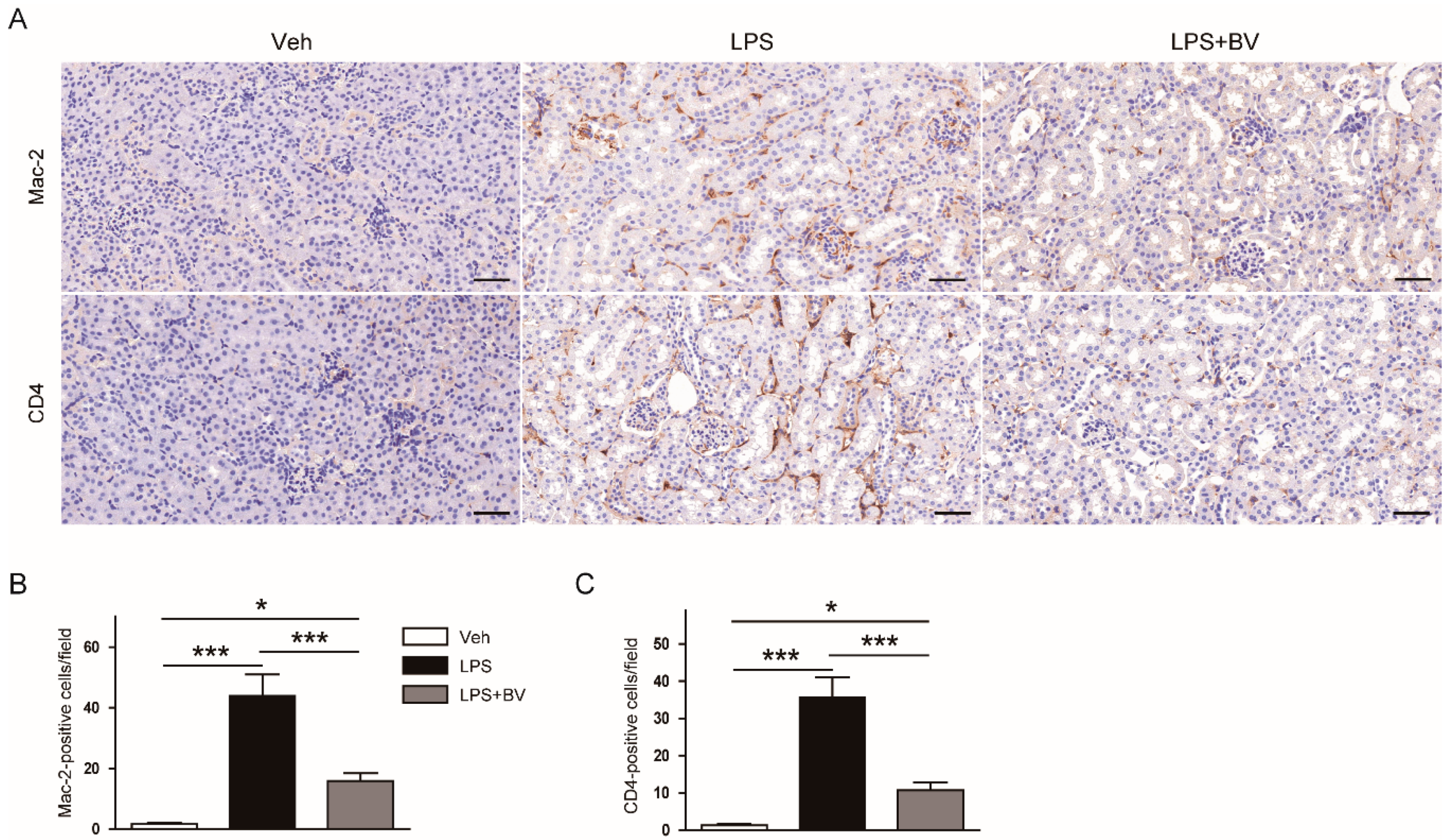
2.3. Histology, Immunohistochemistry (IHC), and Immunofluorescence
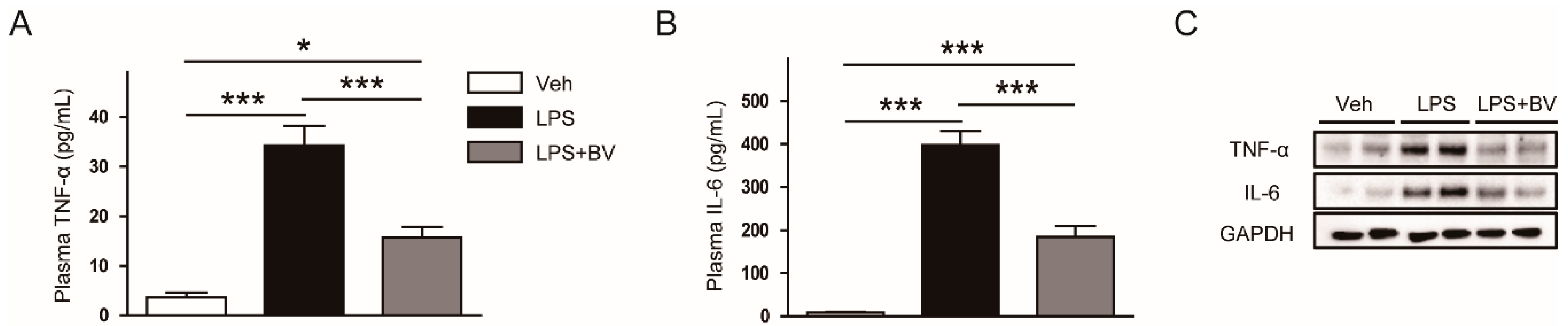
2.4. Mesurement of Plasma Cytokines
2.5. Western Blot Analysis
2.6. Measurement of Malondialdehyde (MDA)
2.7. TdT-Mediated dUTP Nick End Labeling (TUNEL) for Detection of Apoptotic Cells
2.8. Statistical Analysis
3. Results
3.1. Bee Venom Ameliorated LPS-Induced Kidney Damage
3.2. Bee Venom Suppressed LPS-Induced Inflammatory Responses
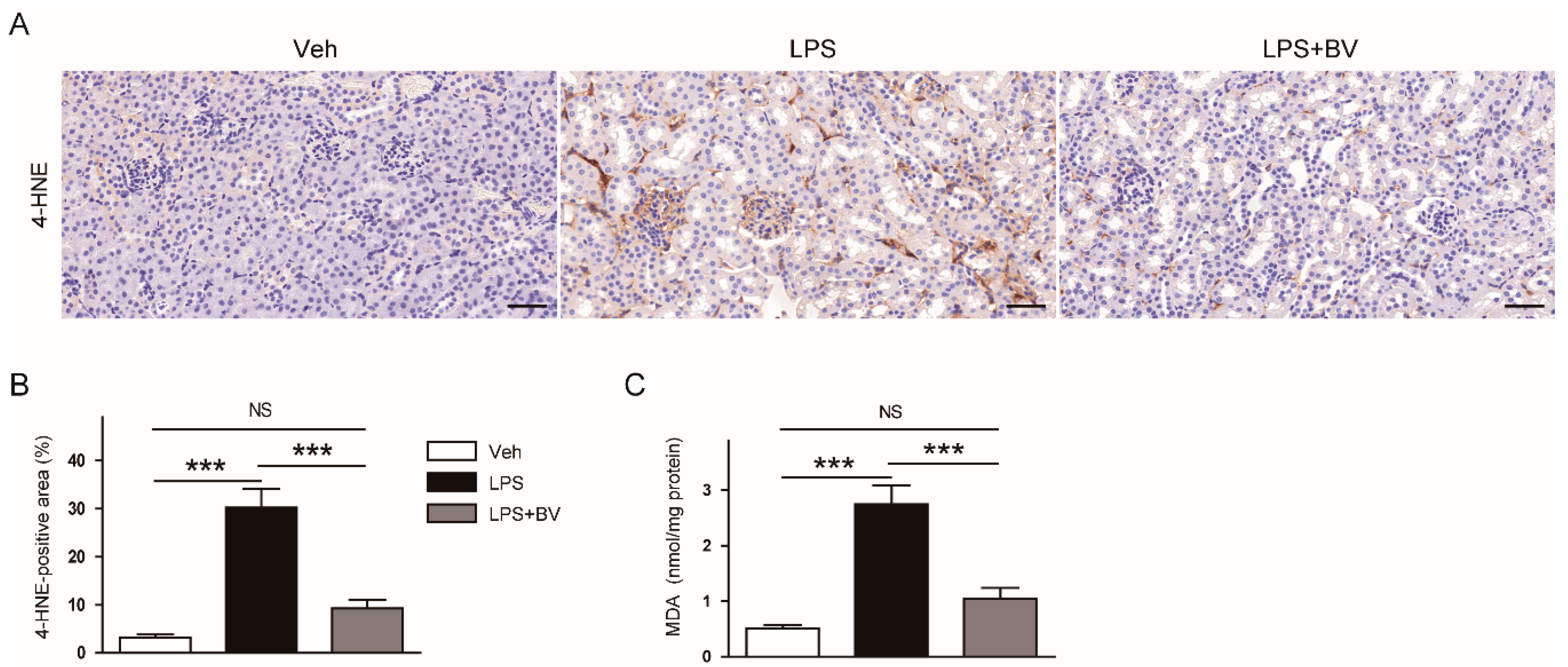
3.3. Bee Venom Attenuated LPS-Induced Oxidative Stress
3.4. Bee Venom Inhibited LPS-Induced Tubular Cell Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- James, M.T.; Bhatt, M.; Pannu, N.; Tonelli, M. Long-term outcomes of acute kidney injury and strategies for improved care. Nat. Rev. Nephrol. 2020, 16, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.J. A long journey for acute kidney injury biomarkers. Ren. Fail. 2020, 42, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms-Biomarkers-Interventions, and Future Perspectives. Oxid. Med. Cell. Longev. 2017, 2017, 6193694. [Google Scholar] [CrossRef]
- Koçkara, A.; Kayataş, M. Renal cell apoptosis and new treatment options in sepsis-induced acute kidney injury. Ren. Fail. 2013, 35, 291–294. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Ye, Y.; Wang, X.R.; Lin, L.T.; Xiao, L.Y.; Zhou, P.; Shi, G.X.; Liu, C.Z. Bee venom therapy: Potential mechanisms and therapeutic applications. Toxicon 2018, 148, 64–73. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef]
- Lee, W.R.; Pak, S.C.; Park, K.K. The protective effect of bee venom on fibrosis causing inflammatory diseases. Toxins 2015, 7, 4758–4772. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Hao, D.J.; Zhang, Q.; An, J.; Zhao, J.J.; Chen, B.; Zhang, L.L.; Yang, H. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother. Pharmacol. 2016, 78, 1113–1130. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, G.; Park, S.; Chung, H.S.; Lee, H.; Kim, J.Y.; Nam, S.; Kim, S.K.; Bae, H. Bee Venom Mitigates Cisplatin-Induced Nephrotoxicity by Regulating CD4(+)CD25(+)Foxp3(+) Regulatory T Cells in Mice. Evid. Based Complement. Altern. Med. 2013, 2013, 879845. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, K.H.; Lee, W.R.; Kim, J.Y.; Lee, S.J.; Pak, S.C.; Han, S.M.; Park, K.K. Anti-fibrotic effect of natural toxin bee venom on animal model of unilateral ureteral obstruction. Toxins 2015, 7, 1917–1928. [Google Scholar] [CrossRef]
- Khajevand-Khazaei, M.R.; Azimi, S.; Sedighnejad, L.; Salari, S.; Ghorbanpour, A.; Baluchnejadmojarad, T.; Mohseni-Moghaddam, P.; Khamse, S.; Roghani, M. S-allyl Cysteine Protects Against Lipopolysaccharide-Induced Acute Kidney Injury in the C57BL/6 Mouse Strain: Involvement of Oxidative Stress and Inflammation. Int. Immunopharmacol. 2019, 69, 19–26. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Su, J.; Liu, Z.; Fang, S.; Li, L.; Deng, J.; Fan, G. Toddalolactone Protects Lipopolysaccharide-Induced Sepsis and Attenuates Lipopolysaccharide-Induced Inflammatory Response by Modulating HMGB1-NF-κB Translocation. Front. Pharmacol. 2020, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; An, H.J.; Kim, J.Y.; Kim, W.H.; Gwon, M.G.; Kim, H.J.; Han, S.M.; Park, I.; Park, S.C.; Leem, J.; et al. Bee venom attenuates Porphyromonas gingivalis and RANKL-induced bone resorption with osteoclastogenic differentiation. Food Chem. Toxicol. 2019, 129, 344–353. [Google Scholar] [CrossRef]
- Gu, H.; Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Han, S.M.; Leem, J.; Park, K.K. Therapeutic effects of bee venom on experimental atopic dermatitis. Mol. Med. Rep. 2018, 18, 3711–3718. [Google Scholar] [CrossRef]
- Kim, J.W.; Jo, J.; Kim, J.Y.; Choe, M.; Leem, J.; Park, J.H. Melatonin Attenuates Cisplatin-Induced Acute Kidney Injury through Dual Suppression of Apoptosis and Necroptosis. Biology 2019, 8, 64. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, J.; Kim, K.; An, H.J.; Gwon, M.G.; Gu, H.; Kim, H.J.; Yang, A.Y.; Kim, S.W.; Jeon, E.J.; et al. Pharmacological Activation of Sirt1 Ameliorates Cisplatin-Induced Acute Kidney Injury by Suppressing Apoptosis, Oxidative Stress, and Inflammation in Mice. Antioxidants 2019, 8, 322. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, J.H.; Kim, K.; Jo, J.; Leem, J.; Park, K.K. Pharmacological Inhibition of Caspase-1 Ameliorates Cisplatin-Induced Nephrotoxicity through Suppression of Apoptosis, Oxidative Stress, and Inflammation in Mice. Mediat. Inflamm. 2018, 2018, 6571676. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Cheng, W.N.; Bae, H.; Lee, K.W.; Han, S.M.; Petriello, M.C.; Lee, H.G.; Seo, H.G.; Han, S.G. Bee Venom Decreases LPS-Induced Inflammatory Responses in Bovine Mammary Epithelial Cells. J. Microbiol. Biotechnol. 2017, 27, 1827–1836. [Google Scholar] [CrossRef]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Park, J.B.; Sung, W.J.; Kwon, Y.C.; Park, K.D.; Han, S.M.; et al. Bee Venom Inhibits Porphyromonas gingivalis Lipopolysaccharides-Induced Pro-Inflammatory Cytokines through Suppression of NF-κB and AP-1 Signaling Pathways. Molecules 2016, 21, 1508. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, S.J.; Park, J.H.; Kim, K.H.; Chang, Y.C.; Park, Y.Y.; Lee, K.G.; Han, S.M.; Yeo, J.H.; Pak, S.C.; et al. Bee venom reduces atherosclerotic lesion formation via anti-inflammatory mechanism. Am. J. Chin. Med. 2010, 38, 1077–1092. [Google Scholar] [CrossRef]
- Li, H.; Chen, W.; Chen, Y.; Zhou, Q.; Xiao, P.; Tang, R.; Xue, J. Neferine Attenuates Acute Kidney Injury by Inhibiting NF-κB Signaling and Upregulating Klotho Expression. Front. Pharmacol. 2019, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.M.; Ravuri, H.G.; Pradhan, R.K.; Narra, S.; Kumar, J.M.; Kuncha, M.; Kanjilal, S.; Sistla, R. Ferulic acid protects lipopolysaccharide-induced acute kidney injury by suppressing inflammatory events and upregulating antioxidant defenses in Balb/c mice. Biomed. Pharmacother. 2018, 100, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.H.; An, H.J.; Kim, J.Y.; Gwon, M.G.; Gu, H.; Jeon, M.; Sung, W.J.; Han, S.M.; Pak, S.C.; Kim, M.K.; et al. Beneficial effects of melittin on ovalbumin-induced atopic dermatitis in mouse. Sci. Rep. 2017, 7, 17679. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Kim, J.Y.; Kim, W.H.; Gwon, M.G.; Gu, H.M.; Jeon, M.J.; Han, S.M.; Pak, S.C.; Lee, C.K.; Park, I.S.; et al. Therapeutic effects of bee venom and its major component, melittin, on atopic dermatitis in vivo and in vitro. Br. J. Pharmacol. 2018, 175, 4310–4324. [Google Scholar] [CrossRef]
- Hanafi, M.Y.; Zaher, E.L.M.; El-Adely, S.E.M.; Sakr, A.; Ghobashi, A.H.M.; Hemly, M.H.; Kazem, A.H.; Kamel, M.A. The therapeutic effects of bee venom on some metabolic and antioxidant parameters associated with HFD-induced non-alcoholic fatty liver in rats. Exp. Ther. Med. 2018, 15, 5091–5099. [Google Scholar] [CrossRef]
- Khalil, W.K.; Assaf, N.; ElShebiney, S.A.; Salem, N.A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochem. Int. 2015, 80, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H. Alkannin protects human renal proximal tubular epithelial cells from LPS-induced inflammatory injury by regulation of microRNA-210. Biomed. Pharmacother. 2018, 108, 1679–1685. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, B.; Gan, H. CIDEC Is Involved in LPS-Induced Inflammation and Apoptosis in Renal Tubular Epithelial Cells. Inflammation 2018, 41, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Wang, Y.; Minto, A.W.; Quigg, R.J.; Cunningham, P.N. Acute renal failure in endotoxemia is dependent on caspase activation. J. Am. Soc. Nephrol. 2004, 15, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kum, Y.S.; Park, Y.Y.; Park, J.H.; Kim, S.J.; Lee, W.R.; Lee, K.G.; Han, S.M.; Park, K.K. The protective effect of bee venom against ethanol-induced hepatic injury via regulation of the mitochondria-related apoptotic pathway. Basic Clin. Pharmacol. Toxicol. 2010, 107, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, K.H.; Kim, S.J.; Lee, W.R.; Lee, K.G.; Park, K.K. Bee venom protects hepatocytes from tumor necrosis factor-alpha and actinomycin D. Arch. Pharm. Res. 2010, 33, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Lee, K.W.; Choi, S.M.; Yang, E.J. Bee Venom Protects against Rotenone-Induced Cell Death in NSC34 Motor Neuron Cells. Toxins 2015, 7, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Hozzein, W.N.; Badr, G.; Badr, B.M.; Allam, A.; Ghamdi, A.A.; Al-Wadaan, M.A.; Al-Waili, N.S. Bee venom improves diabetic wound healing by protecting functional macrophages from apoptosis and enhancing Nrf2, Ang-1 and Tie-2 signaling. Mol. Immunol. 2018, 103, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.S.; An, H.J.; Cheon, S.Y.; Kwon, K.R.; Lee, K.H. Bee venom suppresses testosterone-induced benign prostatic hyperplasia by regulating the inflammatory response and apoptosis. Exp. Biol. Med. (Maywood) 2015, 240, 1656–1663. [Google Scholar] [CrossRef]
- Zolfagharian, H.; Mohajeri, M.; Babaie, M. Bee Venom (Apis Mellifera) an Effective Potential Alternative to Gentamicin for Specific Bacteria Strains: Bee Venom an Effective Potential for Bacteria. J. Pharmacopunct. 2016, 19, 225–230. [Google Scholar] [CrossRef]
- Al-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Pharmacological synergism of bee venom and melittin with antibiotics and plant secondary metabolites against multi-drug resistant microbial pathogens. Phytomedicine 2015, 22, 245–255. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Lee, S.-J.; Maeng, Y.-I.; Leem, J.; Park, K.-K. Protective Effects of Bee Venom against Endotoxemia-Related Acute Kidney Injury in Mice. Biology 2020, 9, 154. https://doi.org/10.3390/biology9070154
Kim J-Y, Lee S-J, Maeng Y-I, Leem J, Park K-K. Protective Effects of Bee Venom against Endotoxemia-Related Acute Kidney Injury in Mice. Biology. 2020; 9(7):154. https://doi.org/10.3390/biology9070154
Chicago/Turabian StyleKim, Jung-Yeon, Sun-Jae Lee, Young-In Maeng, Jaechan Leem, and Kwan-Kyu Park. 2020. "Protective Effects of Bee Venom against Endotoxemia-Related Acute Kidney Injury in Mice" Biology 9, no. 7: 154. https://doi.org/10.3390/biology9070154
APA StyleKim, J.-Y., Lee, S.-J., Maeng, Y.-I., Leem, J., & Park, K.-K. (2020). Protective Effects of Bee Venom against Endotoxemia-Related Acute Kidney Injury in Mice. Biology, 9(7), 154. https://doi.org/10.3390/biology9070154




