Lymphoid Tissue in Teleost Gills: Variations on a Theme
Abstract
1. Introduction
2. Materials and Methods
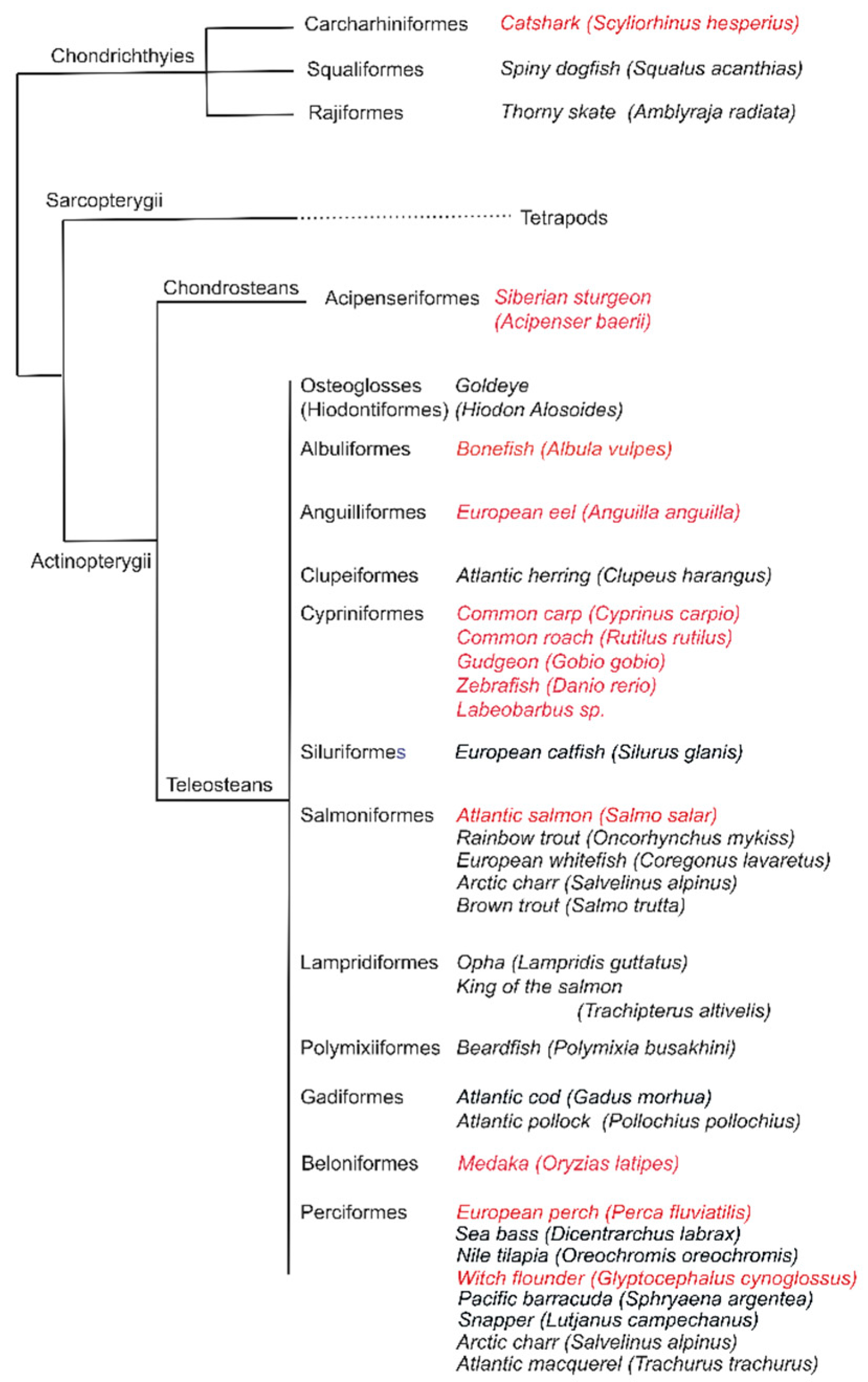
2.1. Species Investigated
2.2. Histochemistry
2.3. Confocal Imaging
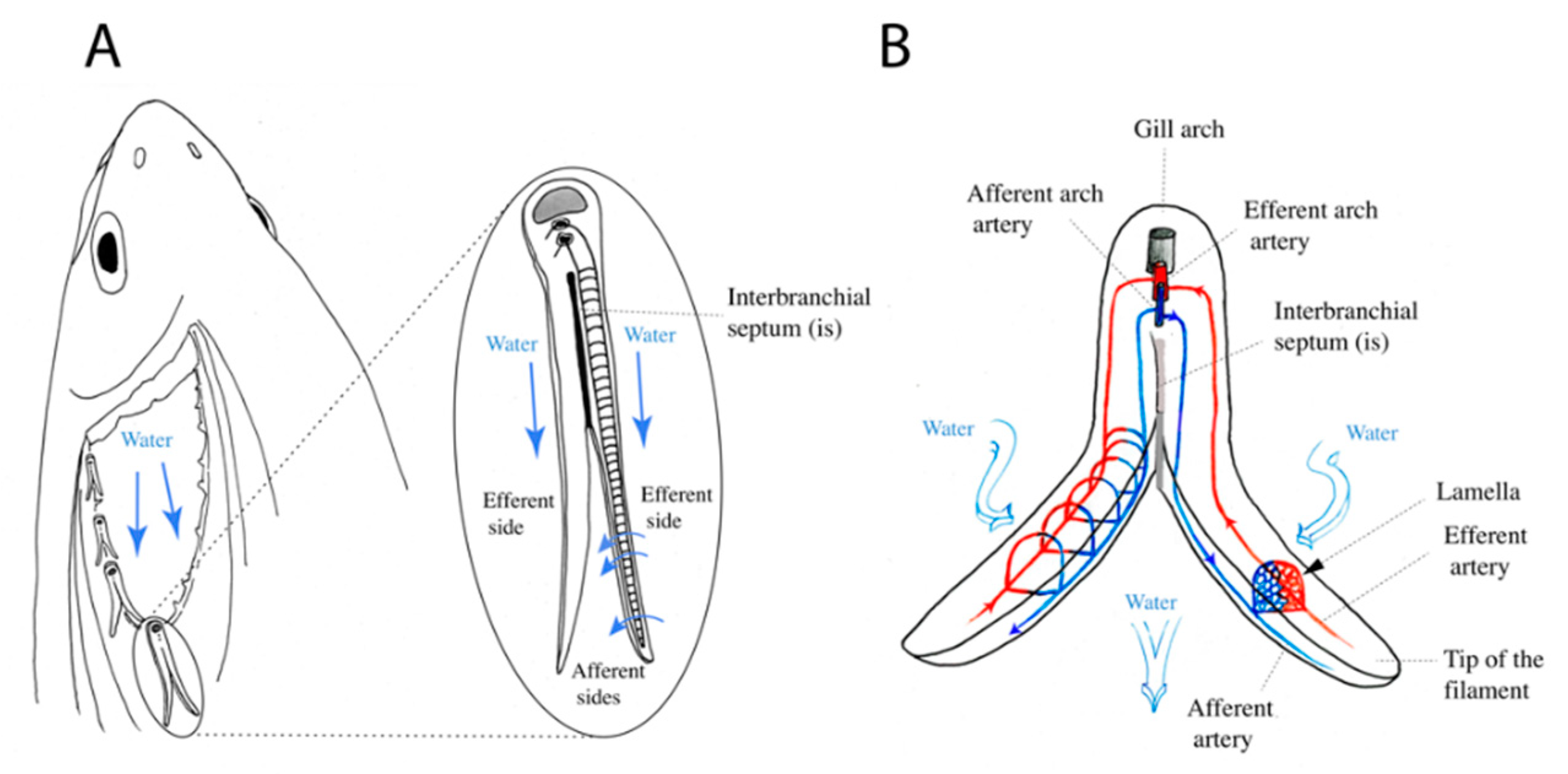
3. Results
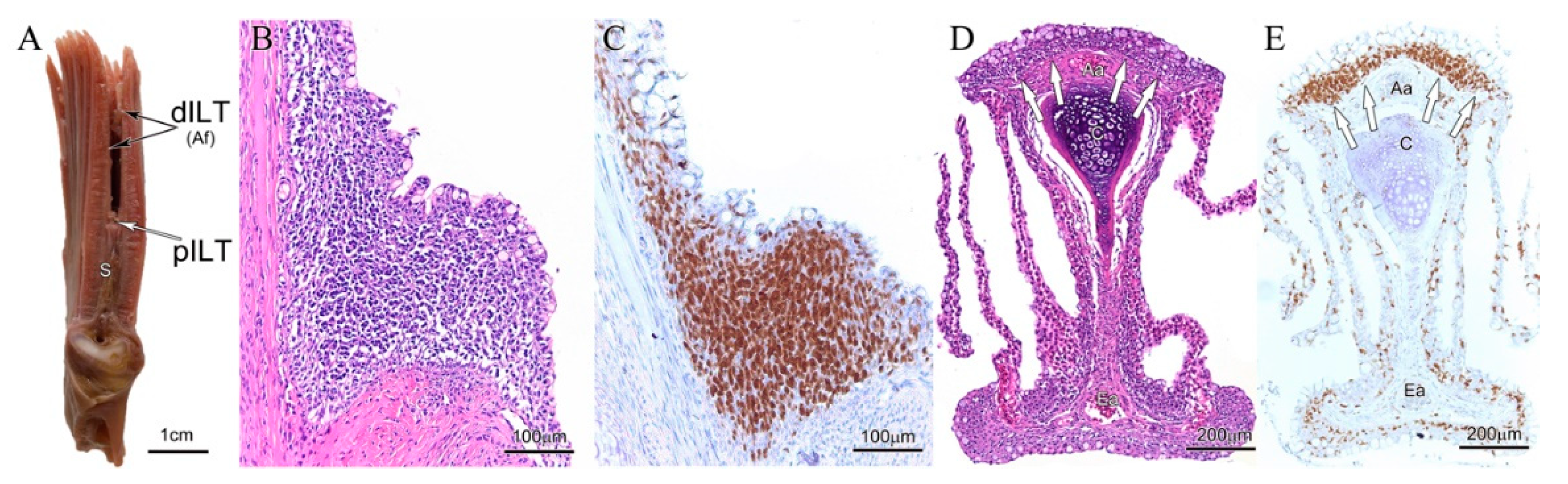
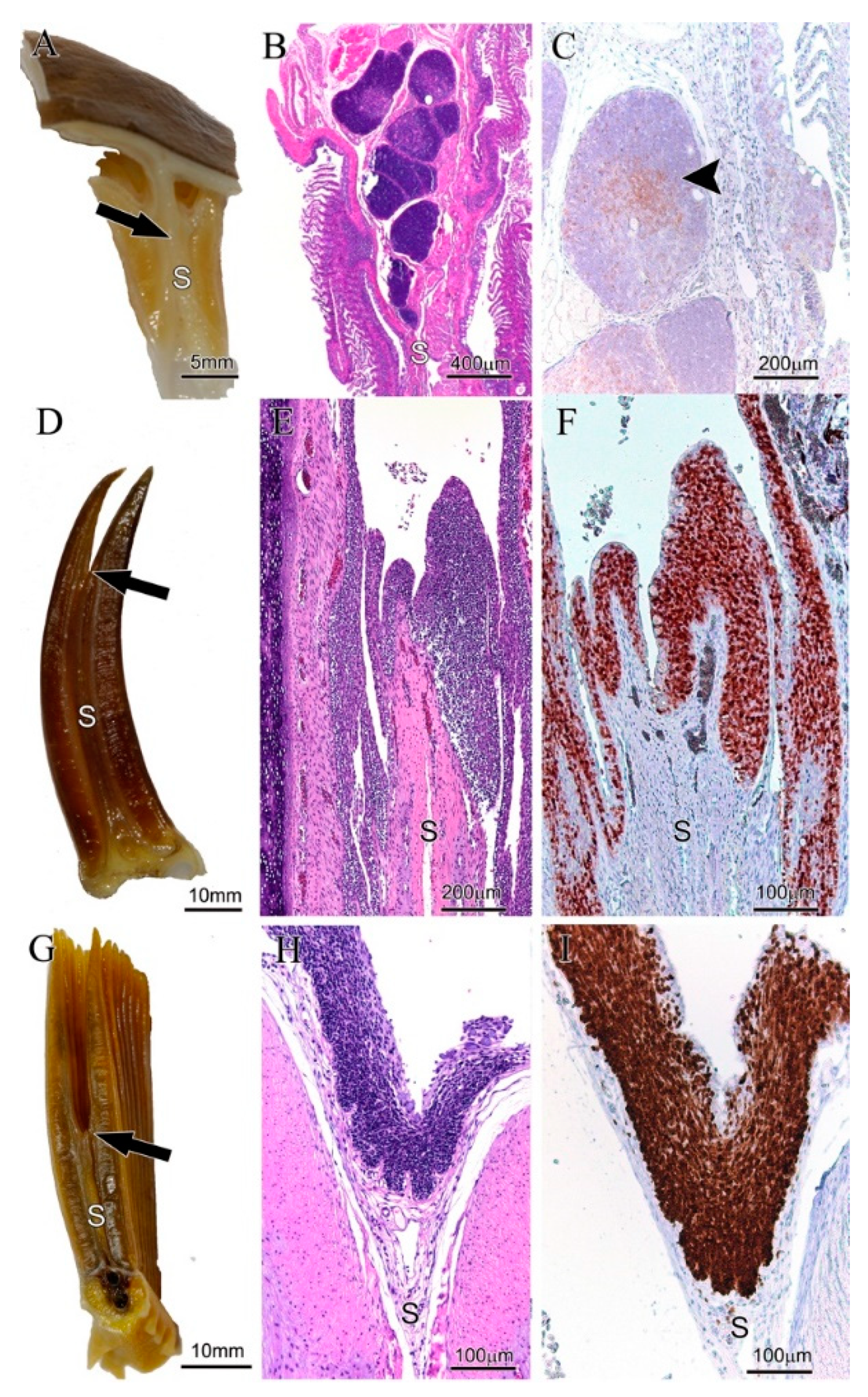
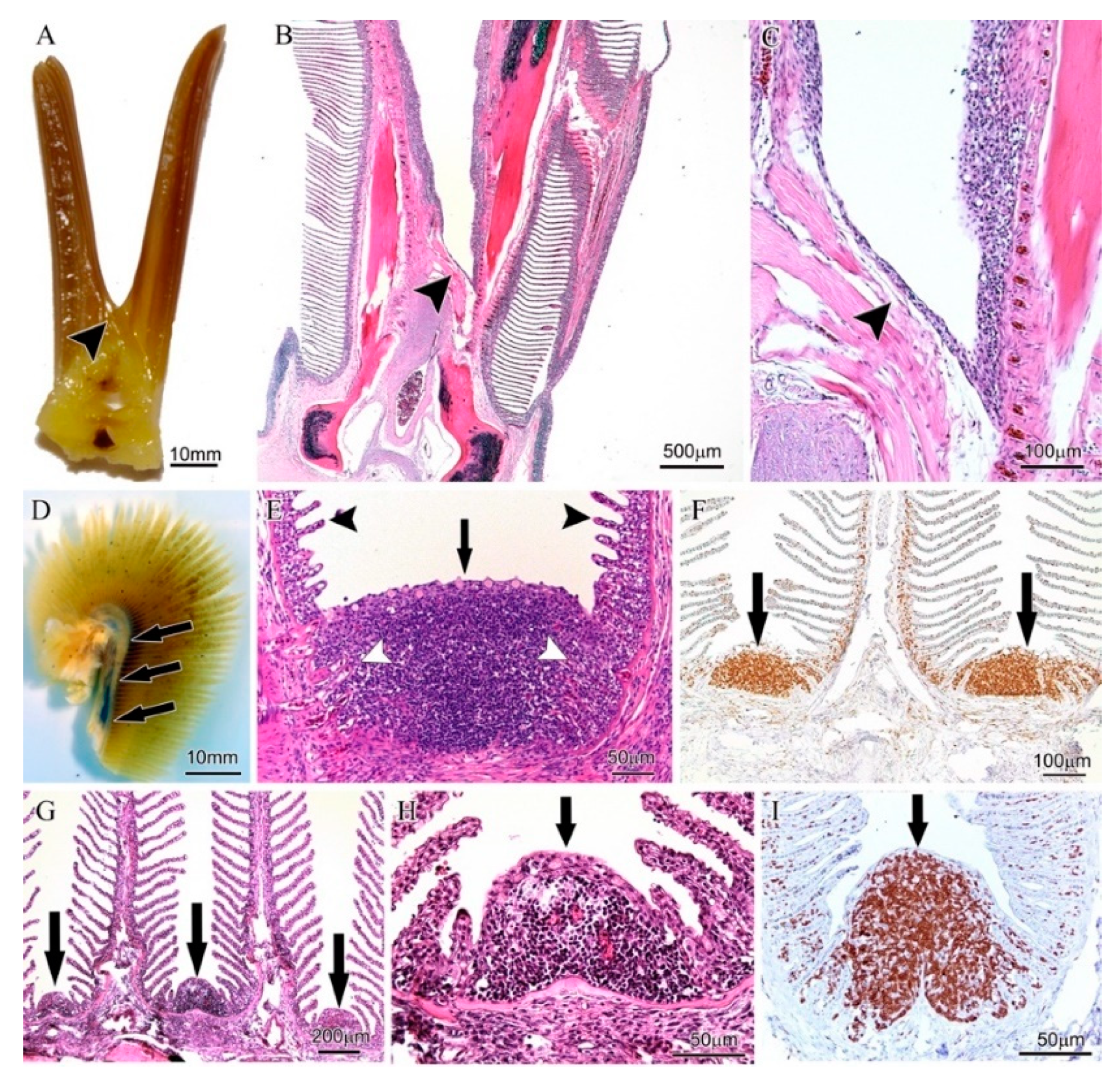
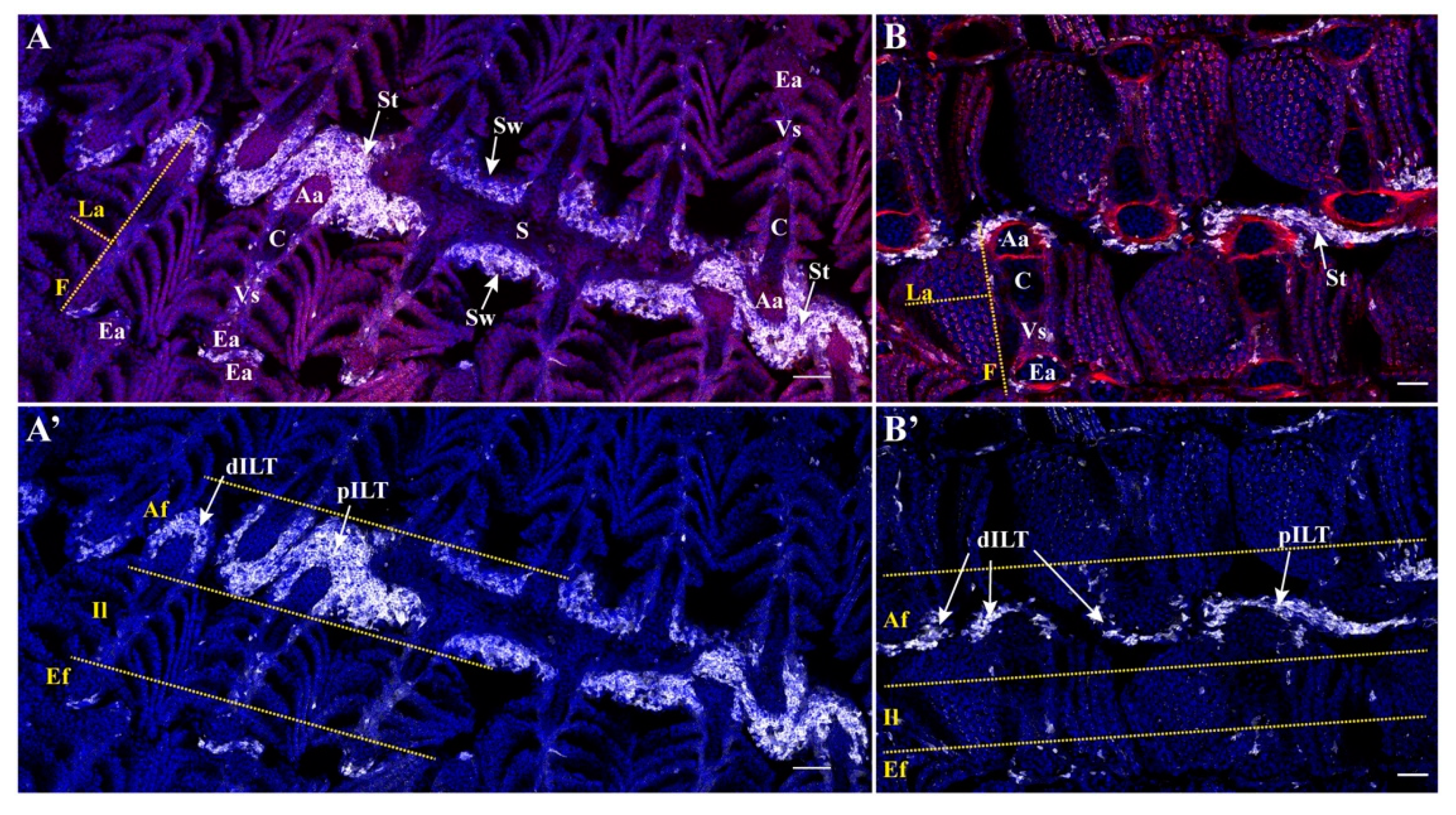
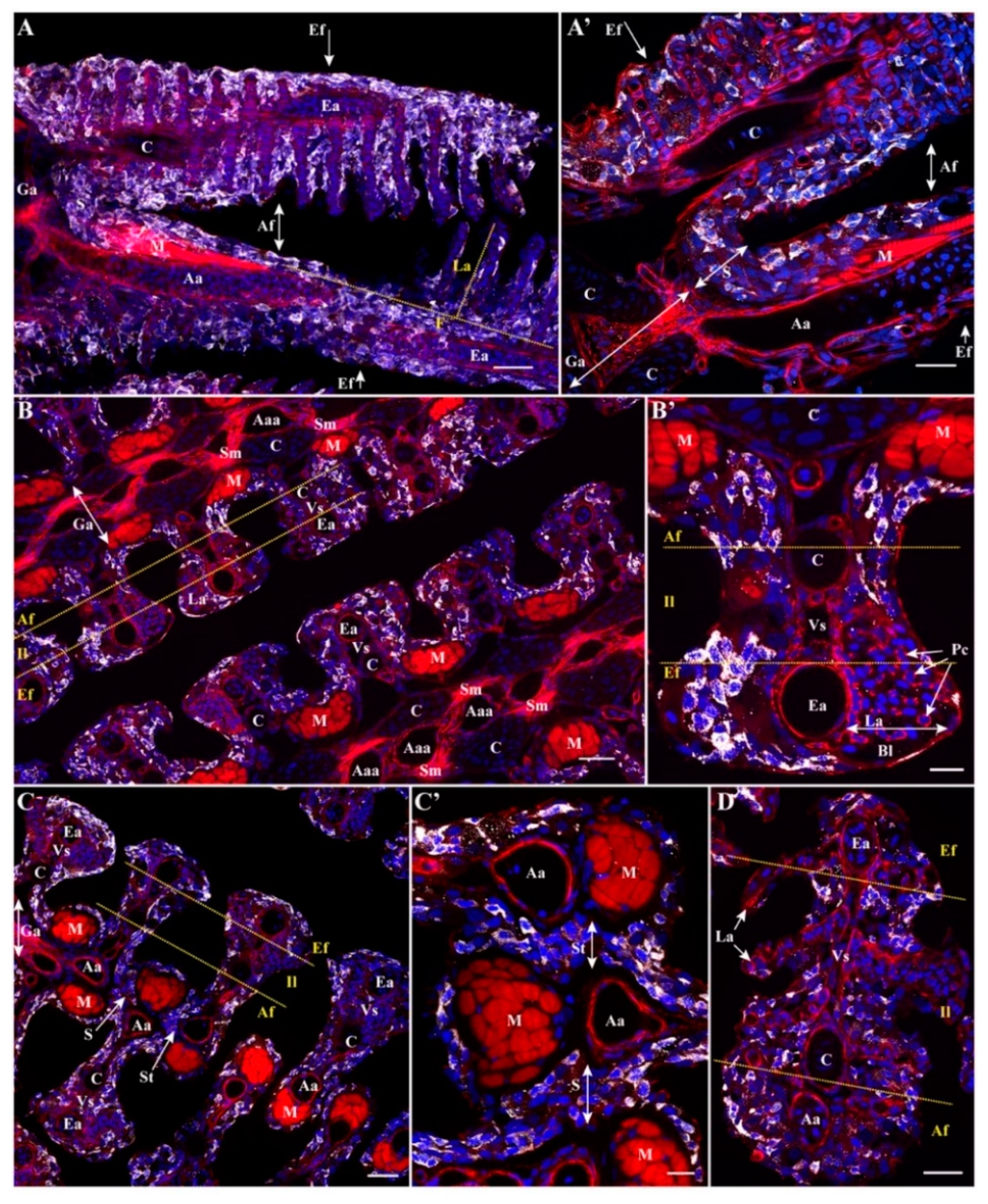
3.1. Variations of Interbranchial Lymphoid Tissue (ILT) across Representative Fish Orders
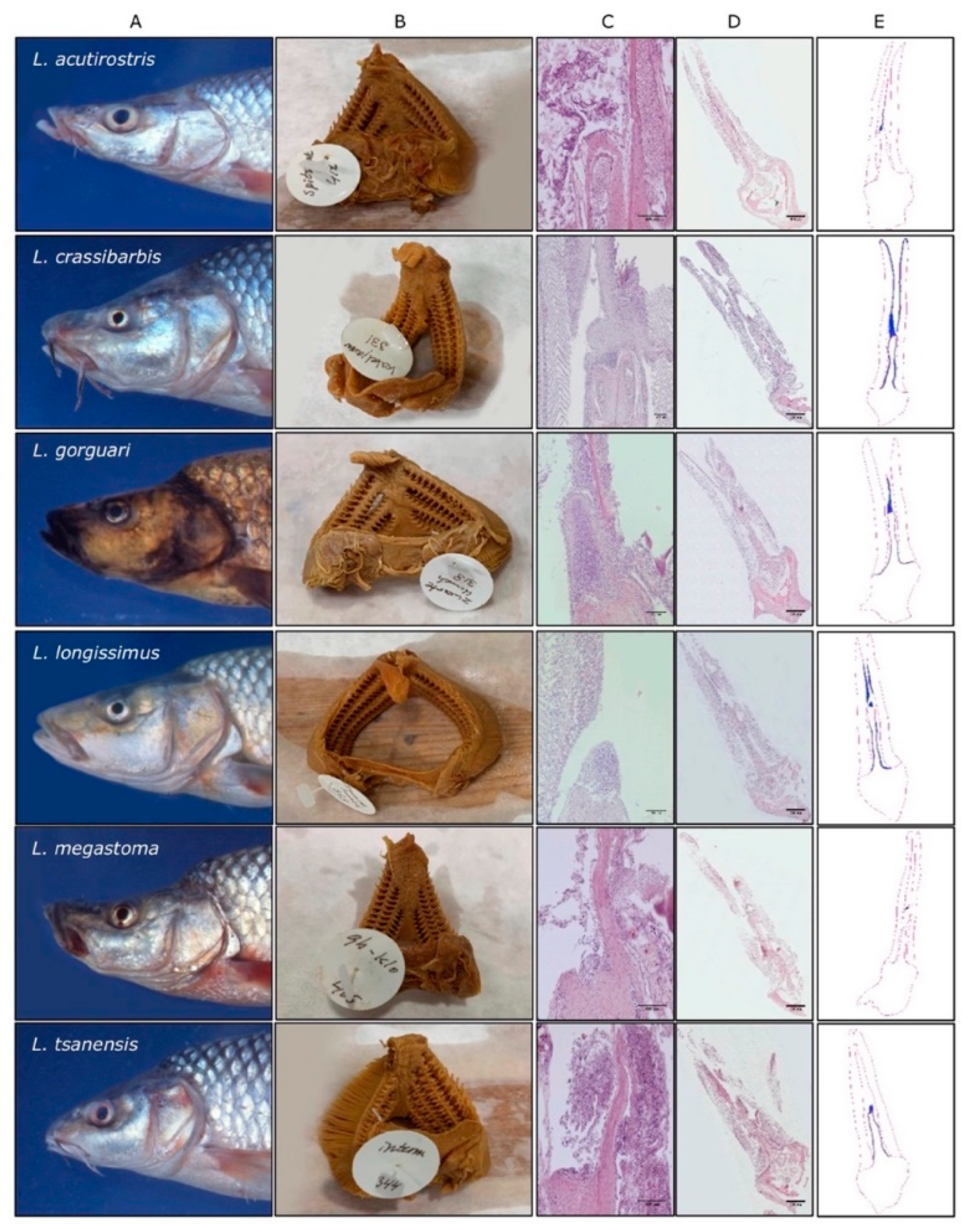
3.2. Body Size Variation Does Not Impose Drastic Restructuring of the ILT
3.3. Adaptation of Jaw Morphology to Different Feeding Modes Does Not Impose Drastic Restructuring the ILT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, J.S. Fishes of the World; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Laurent, P. Morphology and physiology of organs of aquatic respiration in vertebrates: The gill. J. Physiol. 1984, 79, 98–112. [Google Scholar]
- Wilson, J.M.; Laurent, P. Fish gill morphology: Inside out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Grevellec, A.; Tucker, A.S. The pharyngeal pouches and clefts: Development, evolution, structure and derivatives. Semin. Cell Dev. Biol. 2010, 21, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Hughes, G.M. General anatomy of the gills. Fish Physiol. 1984, 10, 1–72. [Google Scholar]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Berillis, P.; Mente, E.; Nikouli, E.; Makridis, P.; Grundvig, H.; Bergheim, A.; Gausen, M. Improving aeration for efficient oxygenation in sea bass sea cages. Blood, brain and gill histology. Open Life Sci. 2016, 11, 270–279. [Google Scholar] [CrossRef]
- Olson, K.R. Chapter 21-Respiratory system. In The Laboratory Fish; Ostrander, G.K., Ed.; Academic Press: London, UK, 2000; pp. 357–367. [Google Scholar] [CrossRef]
- Bergh, O.; Borsheim, K.Y.; Bratbak, G.; Heldal, M. High abundance of viruses found in aquatic environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Haugarvoll, E.; Bjerkas, I.; Nowak, B.F.; Hordvik, I.; Koppang, E.O. Identification and characterization of a novel intraepithelial lymphoid tissue in the gills of Atlantic salmon. J. Anat. 2008, 213, 202–209. [Google Scholar] [CrossRef]
- Aas, I.B.; Austbø, L.; Falk, K.; Hordvik, I.; Koppang, E.O. The interbranchial lymphoid tissue likely contributes to immune tolerance and defense in the gills of Atlantic salmon. Dev. Comp. Immunol. 2017, 76, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Aas, I.B.; Austbø, L.; Konig, M.; Syed, M.; Falk, K.; Hordvik, I.; Koppang, E.O. Transcriptional characterization of the T cell population within the Salmonid interbranchial lymphoid tissue. J. Immunol. 2014, 193, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Dalum, A.S.; Austbø, L.; Bjørgen, H.; Skjodt, K.; Hordvik, I.; Hansen, T.; Fjelldal, P.G.; Press, C.M.; Griffiths, D.J.; Koppang, E.O. The interbranchial lymphoid tissue of Atlantic salmon (Salmo salar L) extends as a diffuse mucosal lymphoid tissue throughout the trailing edge of the gill filament. J. Morphol. 2015, 276, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Dalum, A.S.; Griffiths, D.J.; Valen, E.C.; Amthor, K.S.; Austbø, L.; Koppang, E.O.; Press, C.M.; Kvellestad, A. Morphological and functional development of the interbranchial lymphoid tissue (ILT) in Atlantic salmon (Salmo salar L). Fish Shellfish Immun. 2016, 58, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.; Miyazawa, H.; Nakayama, Y.; Ikari, Y.; Kondo, H.; Yamaguchi, T.; Sano, M.; Fischer, U. A Novel antigen-sampling cell in the teleost gill epithelium with the potential for direct antigen presentation in mucosal tissue. Front Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Koppang, E.O.; Fischer, U.; Moore, L.; Tranulis, M.A.; Dijkstra, J.M.; Kollner, B.; Aune, L.; Jirillo, E.; Hordvik, I. Salmonid T cells assemble in the thymus, spleen and in novel interbranchial lymphoid tissue. J. Anat. 2010, 217, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Resseguier, J.; Delaune, E.; Coolen, A.L.; Levraud, J.P.; Boudinot, P.; Le Guellec, D.; Verrier, B. Specific and efficient uptake of surfactant-free poly (lactic acid) nanovaccine vehicles by mucosal dendritic cells in adult zebrafish after bath immersion. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef]
- Xu, Z.; Takizawa, F.; Parra, D.; Gomez, D.; Jorgensen, L.V.; LaPatra, S.E.; Sunyer, J.O. Mucosal immunoglobulins at respiratory surfaces mark an ancient association that predates the emergence of tetrapods. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Salinas, I. The mucosal immune system of teleost fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef]
- Bjørgen, H.; Løken, O.M.; Aas, I.B.; Fjelldal, P.G.; Hansen, T.; Austbø, L.; Koppang, E.O. Visualization of CCL19-like transcripts in the ILT, thymus and head kidney of Atlantic salmon (Salmo salar L.). Fish Shellfish Immunol. 2019, 93, 763–765. [Google Scholar] [CrossRef]
- Nagelkerke, L.A.J.; Sibbing, F.A.; van den Boogaart, J.G.M.; Lammens, E.H.R.R.; Osse, J.W.M. The barbs (Barbus spp.) of lake Tana: A forgotten species flock? Environ. Biol. Fish 1994, 39, 1–22. [Google Scholar] [CrossRef]
- Nagelkerke, L.; Sibbing, F. A revision of the Large Barbs (Barbus spp., Cyprinidae, Teleostei) of Lake Tana, Ethiopia, with a Description of Seven New Species. The Barbs of Lake Tana, Ethiopia: Morphological Diversity and Its Implications for Taxonomy, Trophic Resource Partitioning and Fisheries. Ph.D. Thesis, Agricultural University Wageningen, Wageningen, The Netherlands, 1997; pp. 105–170. [Google Scholar]
- Helfman, G.; Collette, B.B.; Facey, D.E.; Bowen, B.W. The Diversity of Fishes: Biology, Evolution, and Ecology; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Piazzon, M.C.; Savelkoul, H.F.J.; Pietretti, D.; Wiegertjes, G.F.; Forlenza, M. Carp Il10 has anti-inflammatory activities on phagocytes, promotes proliferation of memory T cells, and regulates B cell differentiation and antibody secretion. J. Immunol. 2015, 194, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Mitra, S.; Wyse, C.; Alnabulsi, A.; Zou, J.; Weerdenburg, E.M.; van der Sar, A.M.; Wang, D.; Secombes, C.J.; Bird, S. First demonstration of antigen induced cytokine expression by CD4-1+ lymphocytes in a poikilotherm: Studies in zebrafish (Danio rerio). PLoS ONE 2015, 10, e0126378. [Google Scholar] [CrossRef] [PubMed]
- Magadan, S.; Sunyer, O.J.; Boudinot, P. Unique features of fish immune repertoires: Particularities of adaptive immunity within the largest group of vertebrates. In Pathogen-Host Interactions: Antigenic Variation v. Somatic Adaptations; Hsu, E., Du Pasquier, L., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 235–264. [Google Scholar] [CrossRef]
- Grant, P.R.; Grant, B.R. How and Why Species Multiply: The Radiation of Darwin’s Finches; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Aguirre, W.E.; Bell, M.A. Twenty years of body shape evolution in a threespine stickleback population adapting to a lake environment. Biol. J. Linn. Soc. 2012, 105, 817–831. [Google Scholar] [CrossRef]
- Van Rijssel, J.C.; Hoogwater, E.S.; Kishe-Machumu, M.A.; van Reenen, E.; Spits, K.V.; van der Stelt, R.C.; Wanink, J.H.; Witte, F. Fast adaptive responses in the oral jaw of lake Victoria cichlids. Evolution 2015, 69, 179–189. [Google Scholar] [CrossRef]
- Fu, C.; Wilson, J.M.; Rombough, P.J.; Brauner, C.J. Ions first: Na+ uptake shifts from the skin to the gills before O-2 uptake in developing rainbow trout, Oncorhynchus mykiss. Proc. R. Soc. B Biol. Sci. 2010, 277, 1553–1560. [Google Scholar] [CrossRef]
- Pabst, R. Plasticity and heterogeneity of lymphoid organs what are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunol. Lett. 2007, 112, 1–8. [Google Scholar] [CrossRef]
- Gebert, A.; Pabst, R. M cells at locations outside the gut. Semin. Immunol. 1999, 11, 165–170. [Google Scholar] [CrossRef]
- Iwata, M.; Sato, A. Morphological and immunohistochemical studies of the lungs and bronchus-associated lymphoid-tissue in a rat model of chronic pulmonary infection with pseudomonas-aeruginosa. Infect. Immun. 1991, 59, 1514–1520. [Google Scholar] [CrossRef]
- Cesta, M.F. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol. Pathol. 2006, 34, 599–608. [Google Scholar] [CrossRef]
- Olson, K.R. Respiratory system. In The Laboratory Fish; Elsevier: Amsterdam, The Netherlands, 2000; pp. 357–367. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rességuier, J.; Dalum, A.S.; Du Pasquier, L.; Zhang, Y.; Koppang, E.O.; Boudinot, P.; Wiegertjes, G.F. Lymphoid Tissue in Teleost Gills: Variations on a Theme. Biology 2020, 9, 127. https://doi.org/10.3390/biology9060127
Rességuier J, Dalum AS, Du Pasquier L, Zhang Y, Koppang EO, Boudinot P, Wiegertjes GF. Lymphoid Tissue in Teleost Gills: Variations on a Theme. Biology. 2020; 9(6):127. https://doi.org/10.3390/biology9060127
Chicago/Turabian StyleRességuier, Julien, Alf S. Dalum, Louis Du Pasquier, Yaqing Zhang, Erling Olaf Koppang, Pierre Boudinot, and Geert F. Wiegertjes. 2020. "Lymphoid Tissue in Teleost Gills: Variations on a Theme" Biology 9, no. 6: 127. https://doi.org/10.3390/biology9060127
APA StyleRességuier, J., Dalum, A. S., Du Pasquier, L., Zhang, Y., Koppang, E. O., Boudinot, P., & Wiegertjes, G. F. (2020). Lymphoid Tissue in Teleost Gills: Variations on a Theme. Biology, 9(6), 127. https://doi.org/10.3390/biology9060127





