Hydralazine Sensitizes to the Antifibrotic Effect of 5-Aza-2′-deoxycytidine in Hepatic Stellate Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Treatment of Cells with 5-Aza-dC and/or HDZ
2.3. WST-1 Assay
2.4. Semi-Quantitative Real-Time Reverse Transcription PCR (RT–PCR)
2.5. Statistical Analysis
3. Results
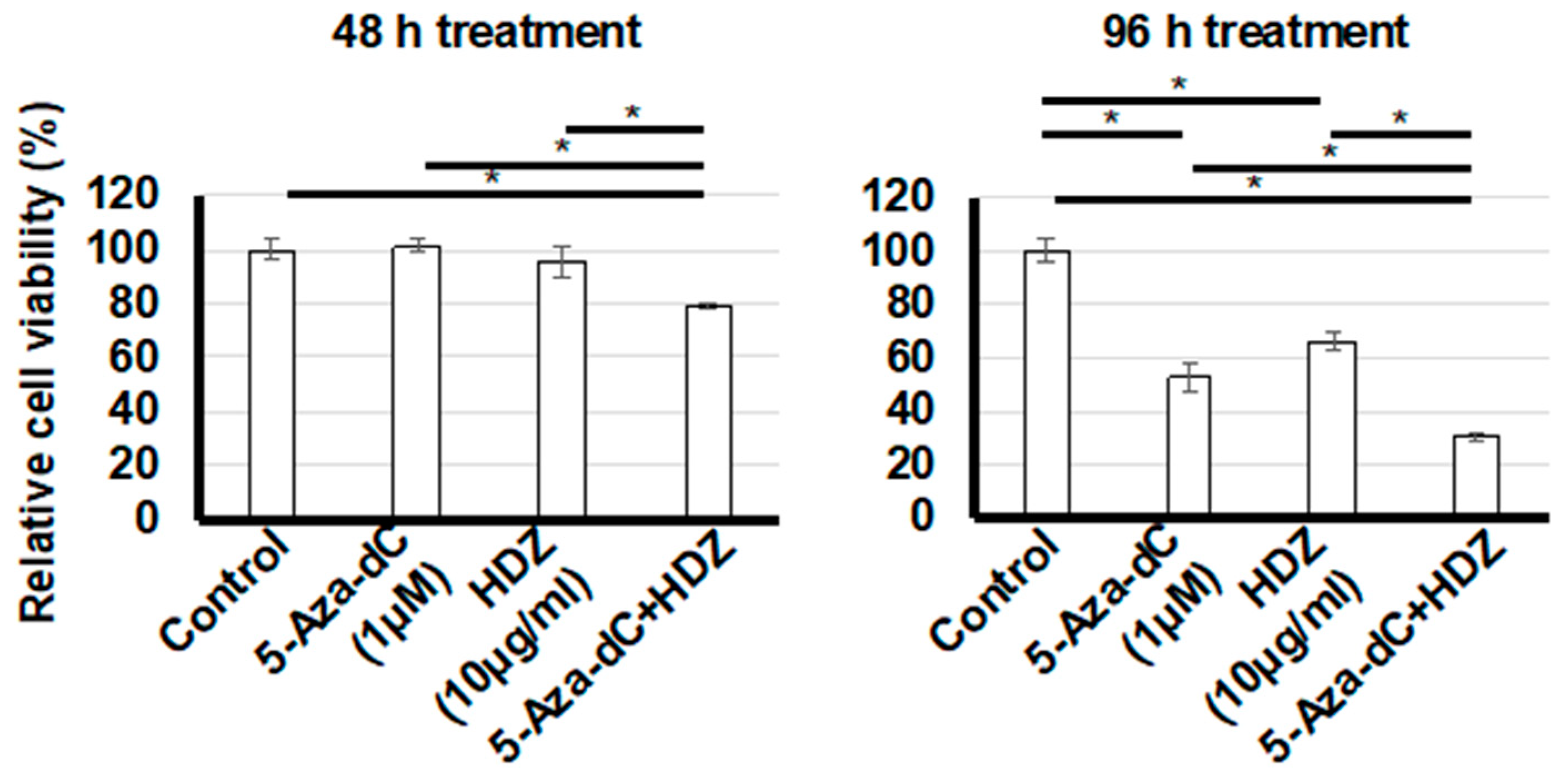
3.1. Toxicity of HDZ and 5-Aza-dC in HSC-T6 cells
3.2. Effect of Combined Treatment with 5-Aza-dC and HDZ on Cell Proliferation
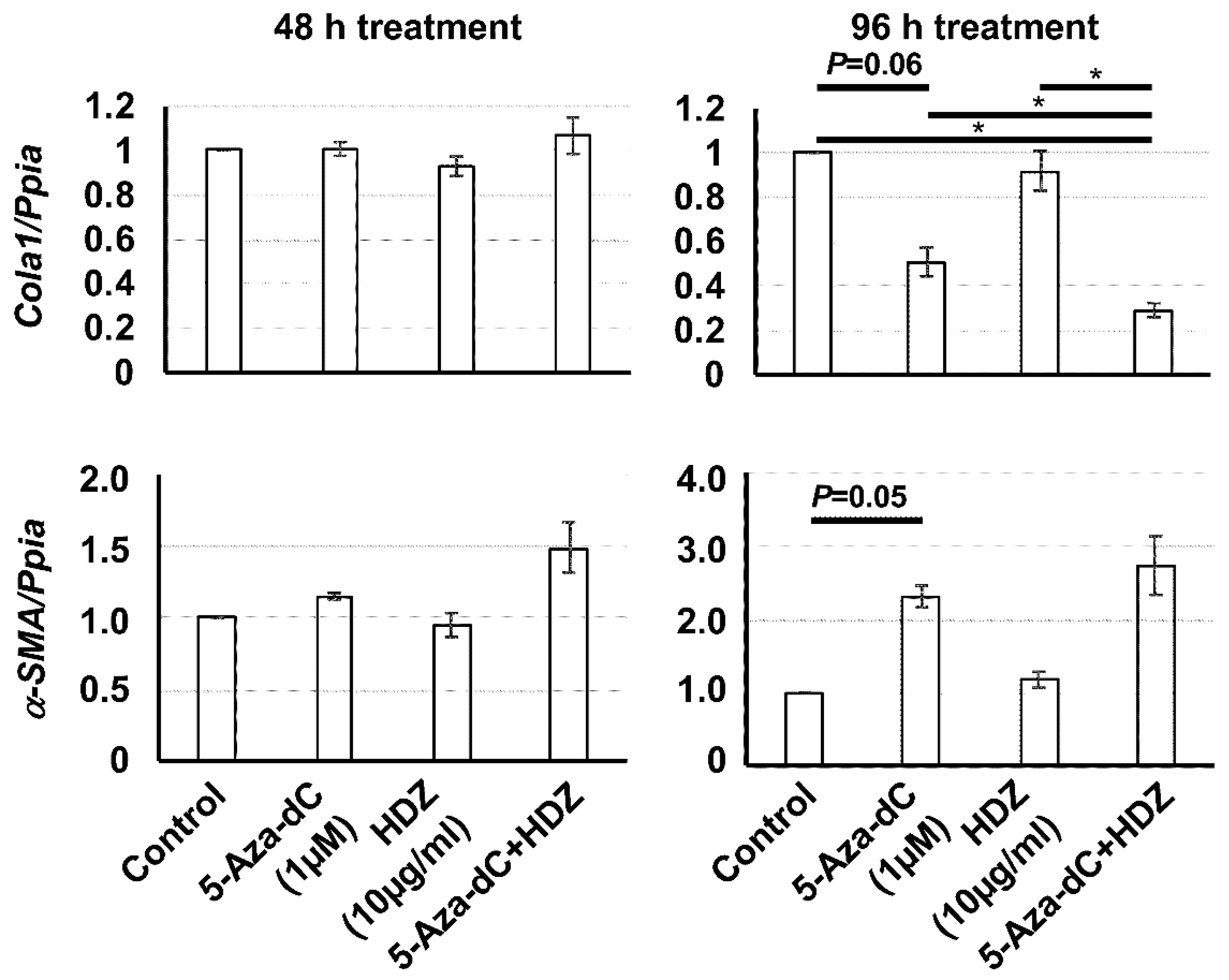
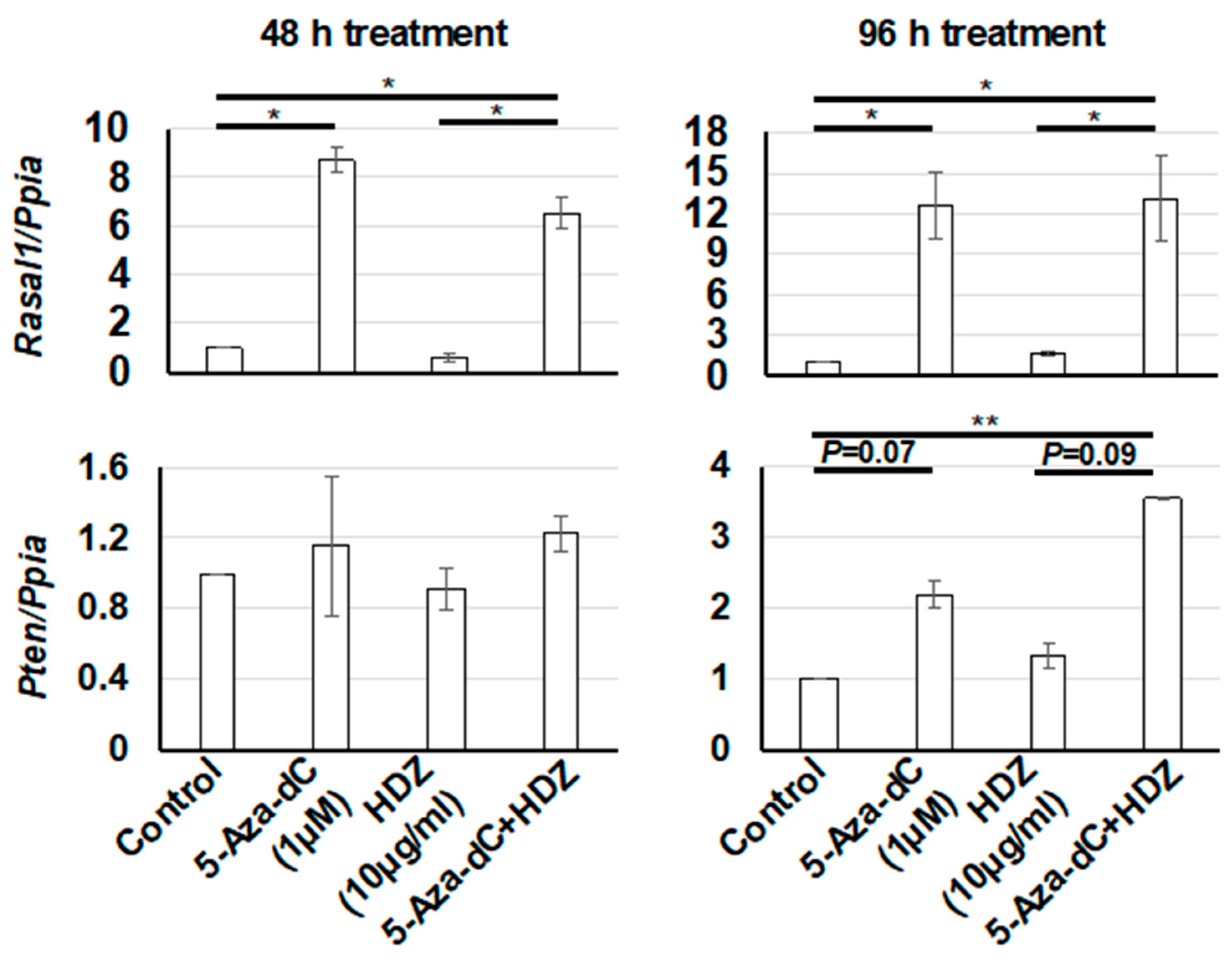
3.3. Effect of the Combined Treatment with 5-Aza-dC and HDZ on Cola1, α-SMA, Rasal1, and Pten mRNA Expression in HSC-T6 Cells
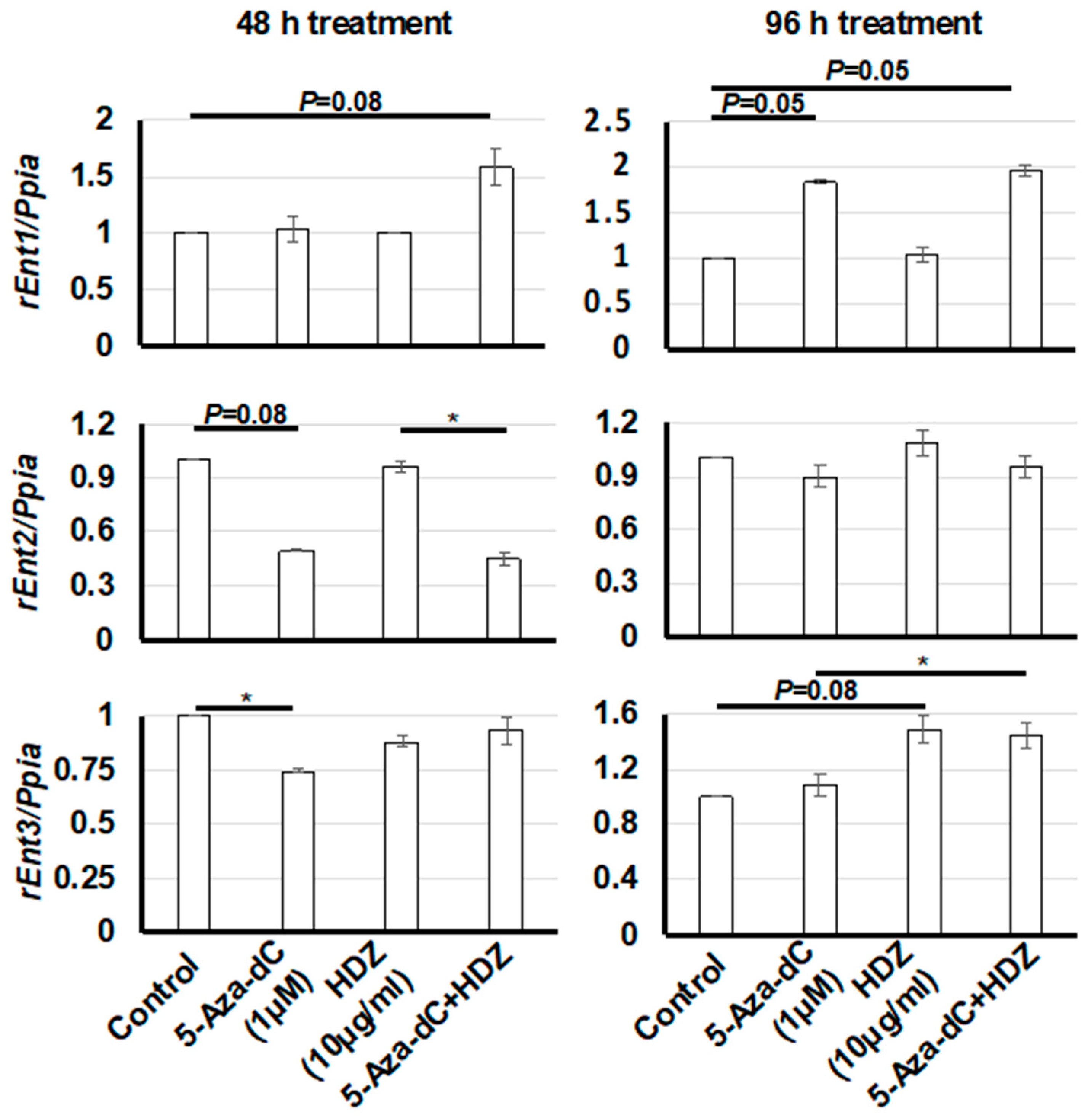
3.4. Effect of HDZ on the mRNA Expression of Three Rat Equilibrative Nucleoside Transporter Genes (rEnt1, rEnt2, and rEnt3) in HSC-T6 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Wallace, M.C.; Friedman, S.L. Pathobiology of liver fibrosis: A translational success story. Gut 2015, 64, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin. Liver Dis. 2001, 21, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Yoshiji, H.; Kuriyama, S.; Yoshii, J.; Ikenaka, Y.; Noguchi, R.; Nakatani, T.; Tsujinoue, H.; Fukui, H. Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats. Hepatology 2001, 34, 745–750. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Massey, V.; Cabezas, J.; Bataller, R. Epigenetics in Liver Fibrosis. Semin. Liver Dis. 2017, 37, 219–230. [Google Scholar] [CrossRef]
- El Taghdouini, A.; van Grunsven, L.A. Epigenetic regulation of hepatic stellate cell activation and liver fibrosis. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 1397–1408. [Google Scholar] [CrossRef]
- Asada, K.; Aihara, Y.; Takaya, H.; Noguchi, R.; Namisaki, T.; Moriya, K.; Uejima, M.; Kitade, M.; Mashitani, T.; Takeda, K.; et al. DNA methylation of angiotensin II receptor gene in nonalcoholic steatohepatitis-related liver fibrosis. World J. Hepatol. 2016, 8, 1194–1199. [Google Scholar] [CrossRef]
- Mann, D.A. Epigenetics in liver disease. Hepatology 2014, 60, 1418–1425. [Google Scholar] [CrossRef]
- Liu, X.; Brenner, D.A. Liver: DNA methylation controls liver fibrogenesis. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 126–128. [Google Scholar] [CrossRef]
- Chuang, J.C.; Yoo, C.B.; Kwan, J.M.; Li, T.W.; Liang, G.; Yang, A.S.; Jones, P.A. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2’-deoxycytidine. Mol. Cancer Ther. 2005, 4, 1515–1520. [Google Scholar] [CrossRef]
- Gnyszka, A.; Jastrzebski, Z.; Flis, S. DNA methyltransferase inhibitors and their emerging role in epigenetic therapy of cancer. Anticancer Res. 2013, 33, 2989–2996. [Google Scholar] [PubMed]
- Mani, E.; Medina, L.A.; Isaac-Olive, K.; Duenas-Gonzalez, A. Radiosensitization of cervical cancer cells with epigenetic drugs hydralazine and valproate. Eur. J. Gynaecol. Oncol. 2014, 35, 140–142. [Google Scholar] [PubMed]
- Candelaria, M.; Burgos, S.; Ponce, M.; Espinoza, R.; Duenas-Gonzalez, A. Encouraging results with the compassionate use of hydralazine/valproate (TRANSKRIP) as epigenetic treatment for myelodysplastic syndrome (MDS). Ann. Hematol. 2017, 96, 1825–1832. [Google Scholar] [CrossRef]
- Schcolnik-Cabrera, A.; Dominguez-Gomez, G.; Duenas-Gonzalez, A. Comparison of DNA demethylating and histone deacetylase inhibitors hydralazine-valproate versus vorinostat-decitabine incutaneous t-cell lymphoma in HUT78 cells. Am. J. Blood Res. 2018, 8, 5–16. [Google Scholar] [PubMed]
- Duenas-Gonzalez, A.; Coronel, J.; Cetina, L.; Gonzalez-Fierro, A.; Chavez-Blanco, A.; Taja-Chayeb, L. Hydralazine-valproate: A repositioned drug combination for the epigenetic therapy of cancer. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.H.; Cheng, C.C.; Chen, Y.C.; Chung, C.C.; Lee, T.I.; Chen, S.A.; Chen, Y.J. Hydralazine-induced promoter demethylation enhances sarcoplasmic reticulum Ca2+ -ATPase and calcium homeostasis in cardiac myocytes. Lab. Investig. 2011, 91, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Tampe, B.; Steinle, U.; Tampe, D.; Carstens, J.L.; Korsten, P.; Zeisberg, E.M.; Muller, G.A.; Kalluri, R.; Zeisberg, M. Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney Int. 2017, 91, 157–176. [Google Scholar] [CrossRef]
- Mann, J.; Oakley, F.; Akiboye, F.; Elsharkawy, A.; Thorne, A.W.; Mann, D.A. Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: Implications for wound healing and fibrogenesis. Cell Death Differ. 2007, 14, 275–285. [Google Scholar] [CrossRef]
- Mann, J.; Chu, D.C.; Maxwell, A.; Oakley, F.; Zhu, N.L.; Tsukamoto, H.; Mann, D.A. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 2010, 138, 705–714.e4. [Google Scholar] [CrossRef]
- Tao, H.; Huang, C.; Yang, J.J.; Ma, T.T.; Bian, E.B.; Zhang, L.; Lv, X.W.; Jin, Y.; Li, J. MeCP2 controls the expression of RASAL1 in the hepatic fibrosis in rats. Toxicology 2011, 290, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Bian, E.B.; Huang, C.; Ma, T.T.; Tao, H.; Zhang, H.; Cheng, C.; Lv, X.W.; Li, J. DNMT1-mediated PTEN hypermethylation confers hepatic stellate cell activation and liver fibrogenesis in rats. Toxicol. Appl. Pharmacol. 2012, 264, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.M.; Issa, J.P. Decitabine dosing schedules. Semin. Hematol. 2005, 42, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Okochi-Takada, E.; Kikuyama, M.; Wakabayashi, M.; Yamashita, S.; Ushijima, T. Methylation silencing of angiopoietin-like 4 in rat and human mammary carcinomas. Cancer Sci. 2011, 102, 1337–1343. [Google Scholar] [CrossRef]
- Yu, F.; Lin, Z.; Zheng, J.; Gao, S.; Lu, Z.; Dong, P. Suppression of collagen synthesis by Dicer gene silencing in hepatic stellate cells. Mol. Med. Rep. 2014, 9, 707–714. [Google Scholar] [CrossRef]
- Archer, R.G.; Pitelka, V.; Hammond, J.R. Nucleoside transporter subtype expression and function in rat skeletal muscle microvascular endothelial cells. Br. J. Pharmacol. 2004, 143, 202–214. [Google Scholar] [CrossRef]
- Momparler, R.L. Pharmacology of 5-Aza-2′-deoxycytidine (decitabine). Semin. Hematol. 2005, 42 (Suppl. 2), S9–S16. [Google Scholar] [CrossRef]
- Candelaria, M.; de la Cruz-Hernandez, E.; Taja-Chayeb, L.; Perez-Cardenas, E.; Trejo-Becerril, C.; Gonzalez-Fierro, A.; Chavez-Blanco, A.; Soto-Reyes, E.; Dominguez, G.; Trujillo, J.E.; et al. DNA methylation-independent reversion of gemcitabine resistance by hydralazine in cervical cancer cells. PLoS ONE 2012, 7, e29181. [Google Scholar] [CrossRef]
- Hummel-Eisenbeiss, J.; Hascher, A.; Hals, P.A.; Sandvold, M.L.; Muller-Tidow, C.; Lyko, F.; Rius, M. The role of human equilibrative nucleoside transporter 1 on the cellular transport of the DNA methyltransferase inhibitors 5-azacytidine and CP-4200 in human leukemia cells. Mol. Pharmacol. 2013, 84, 438–450. [Google Scholar] [CrossRef]
- Wu, L.; Shi, W.; Li, X.; Chang, C.; Xu, F.; He, Q.; Wu, D.; Su, J.; Zhou, L.; Song, L.; et al. High expression of the human equilibrative nucleoside transporter 1 gene predicts a good response to decitabine in patients with myelodysplastic syndrome. J. Transl. Med. 2016, 14, 66. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asada, K.; Kaji, K.; Sato, S.; Seki, K.; Shimozato, N.; Kawaratani, H.; Takaya, H.; Sawada, Y.; Nakanishi, K.; Furukawa, M.; et al. Hydralazine Sensitizes to the Antifibrotic Effect of 5-Aza-2′-deoxycytidine in Hepatic Stellate Cells. Biology 2020, 9, 117. https://doi.org/10.3390/biology9060117
Asada K, Kaji K, Sato S, Seki K, Shimozato N, Kawaratani H, Takaya H, Sawada Y, Nakanishi K, Furukawa M, et al. Hydralazine Sensitizes to the Antifibrotic Effect of 5-Aza-2′-deoxycytidine in Hepatic Stellate Cells. Biology. 2020; 9(6):117. https://doi.org/10.3390/biology9060117
Chicago/Turabian StyleAsada, Kiyoshi, Kosuke Kaji, Shinya Sato, Kenichiro Seki, Naotaka Shimozato, Hideto Kawaratani, Hiroaki Takaya, Yasuhiko Sawada, Keisuke Nakanishi, Masanori Furukawa, and et al. 2020. "Hydralazine Sensitizes to the Antifibrotic Effect of 5-Aza-2′-deoxycytidine in Hepatic Stellate Cells" Biology 9, no. 6: 117. https://doi.org/10.3390/biology9060117
APA StyleAsada, K., Kaji, K., Sato, S., Seki, K., Shimozato, N., Kawaratani, H., Takaya, H., Sawada, Y., Nakanishi, K., Furukawa, M., Kitade, M., Moriya, K., Namisaki, T., Noguchi, R., Akahane, T., & Yoshiji, H. (2020). Hydralazine Sensitizes to the Antifibrotic Effect of 5-Aza-2′-deoxycytidine in Hepatic Stellate Cells. Biology, 9(6), 117. https://doi.org/10.3390/biology9060117





