Spaghetti to a Tree: A Robust Phylogeny for Terebelliformia (Annelida) Based on Transcriptomes, Molecular and Morphological Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Transcriptome Sequencing and Bioinformatic Processing
2.2. Phylogenetic Analyses of the Transcriptome Dataset
2.3. Sanger Matrix and Morphological Characters
2.4. Phylogenetic Analysis of the Total Evidence Matrix and Ancestral State Reconstruction
3. Results
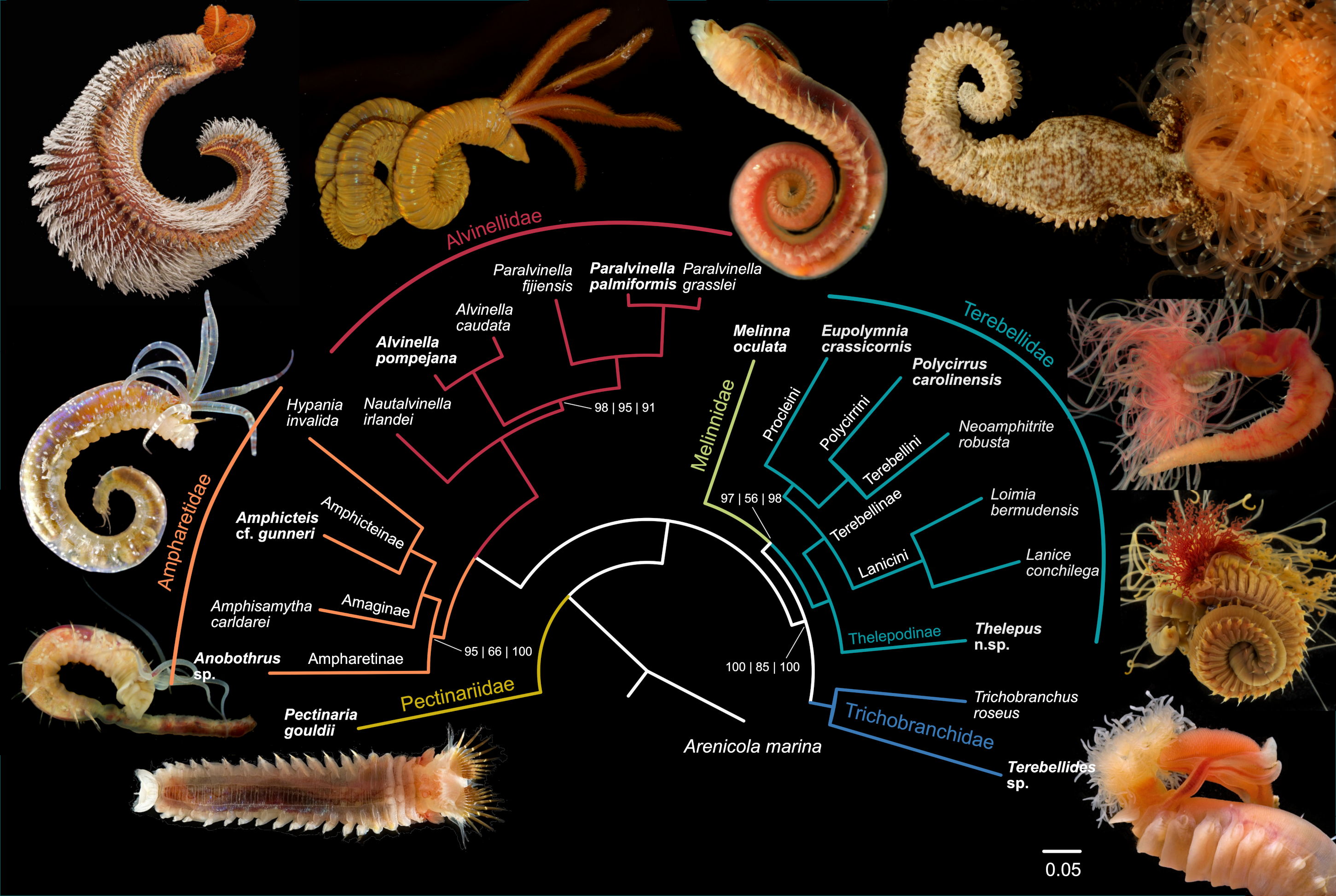
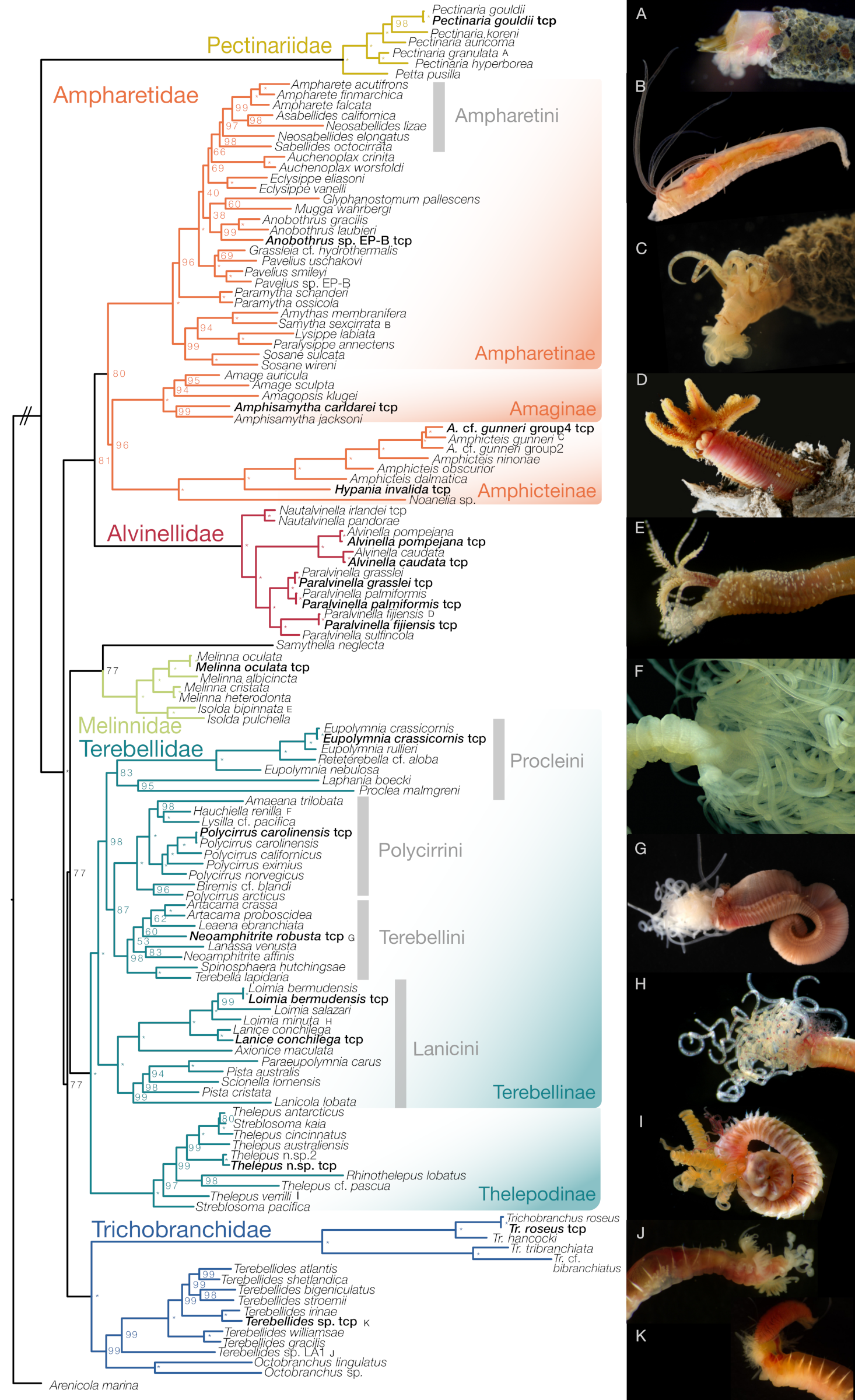
3.1. Phylogenomic Backbone Phylogeny
3.2. Total Evidence Dataset
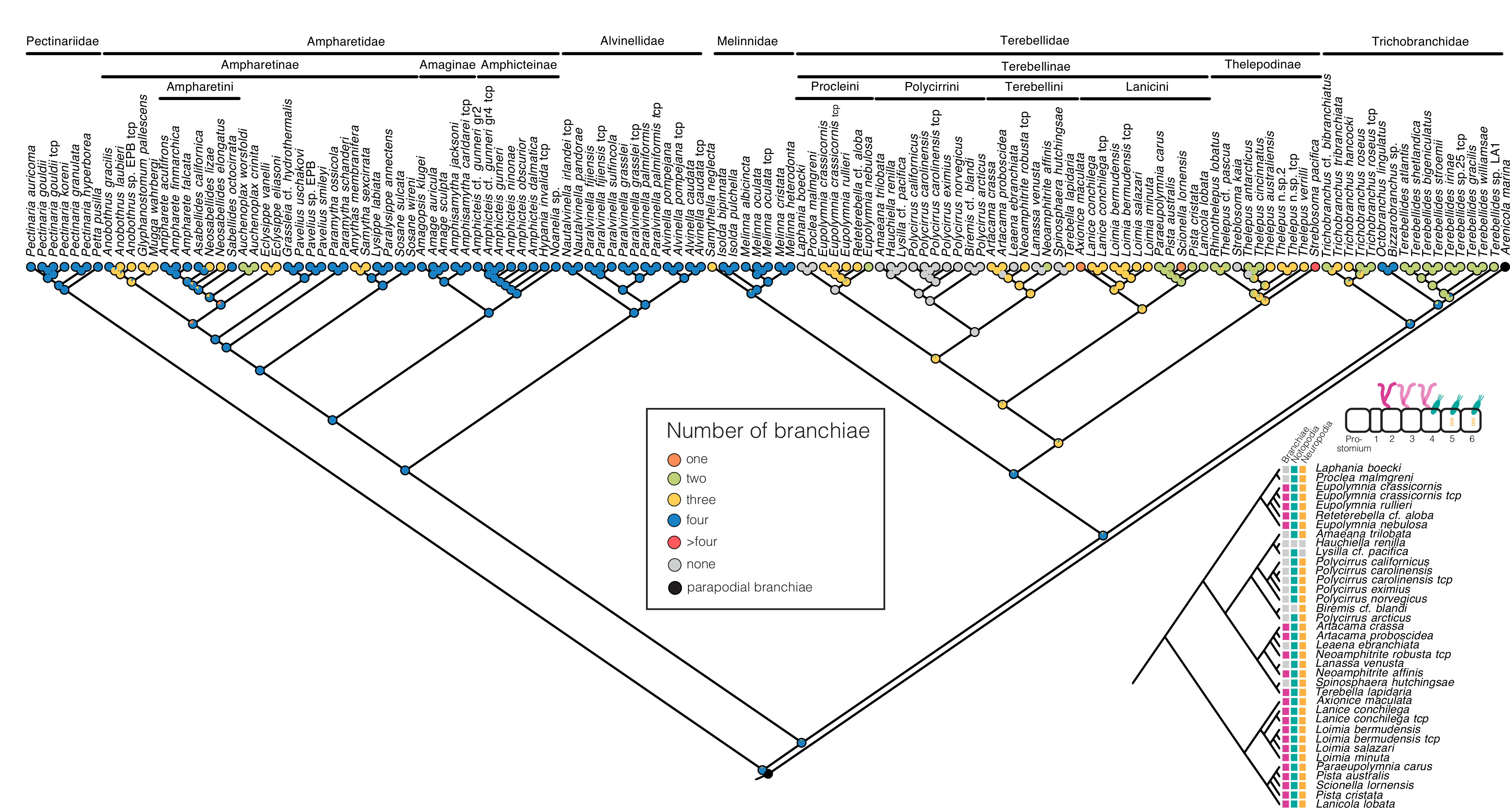
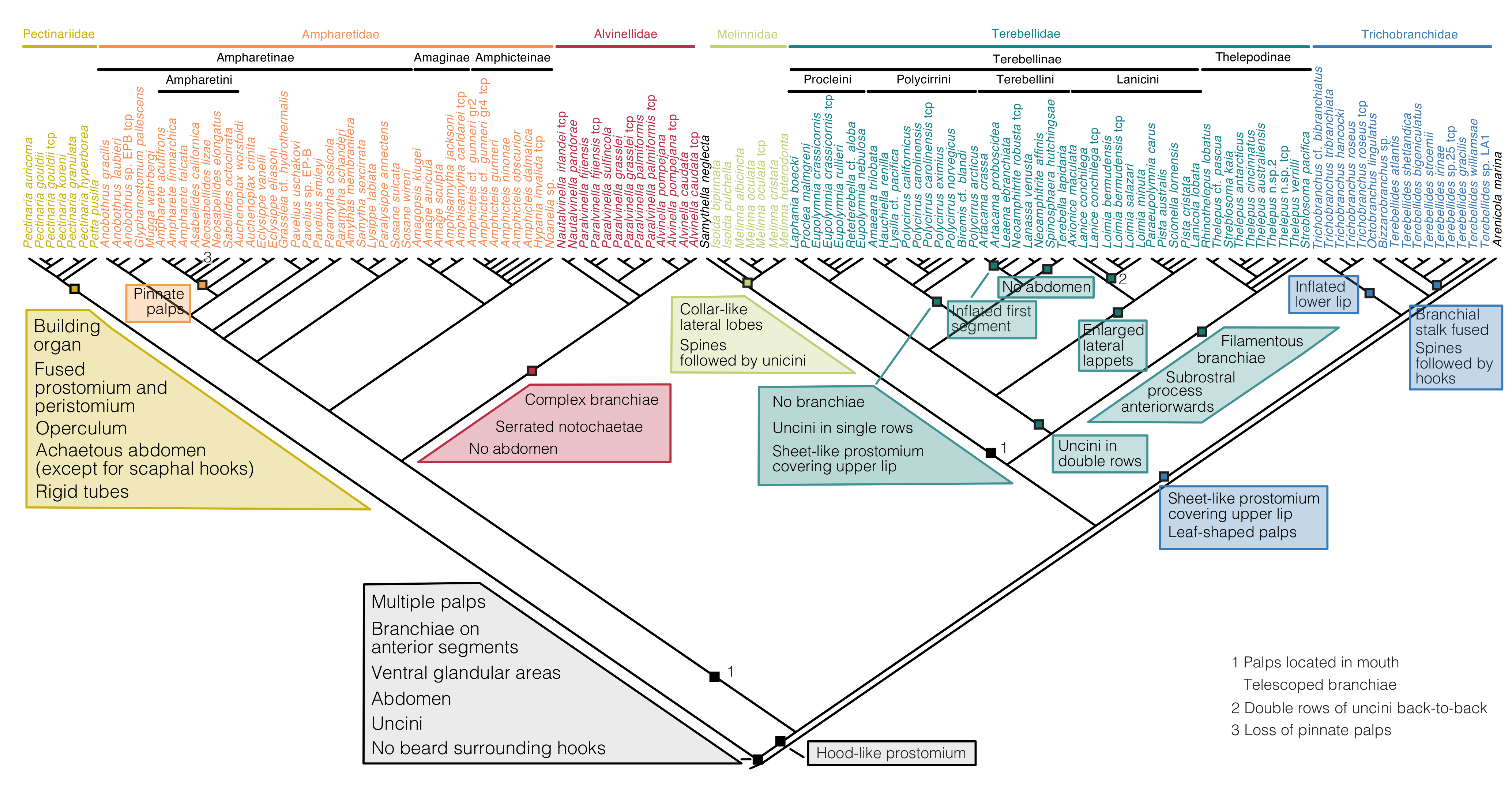
3.3. Character Evolution
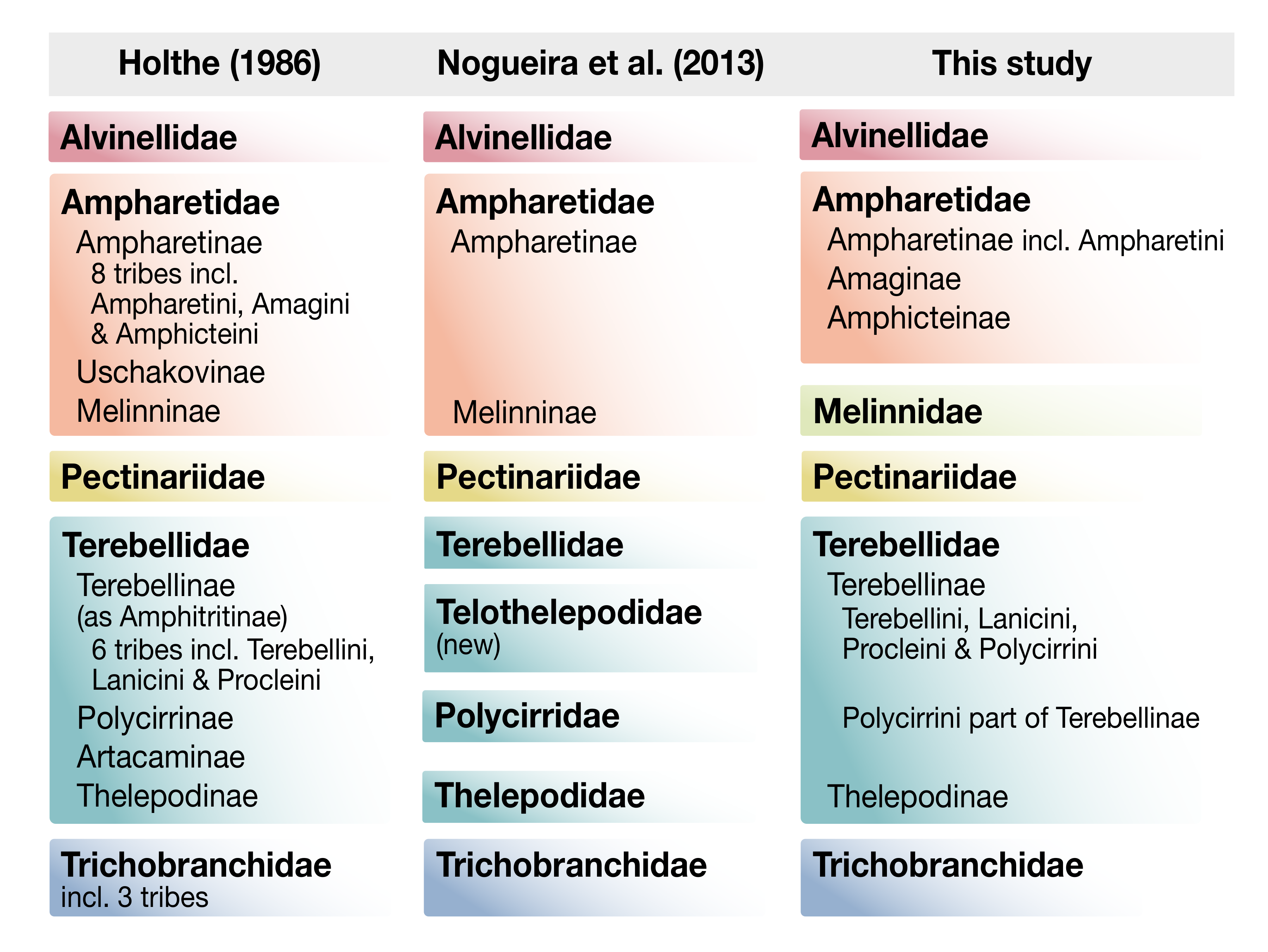
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Terminology and Homology of Head and Body Structures of Terebelliformia
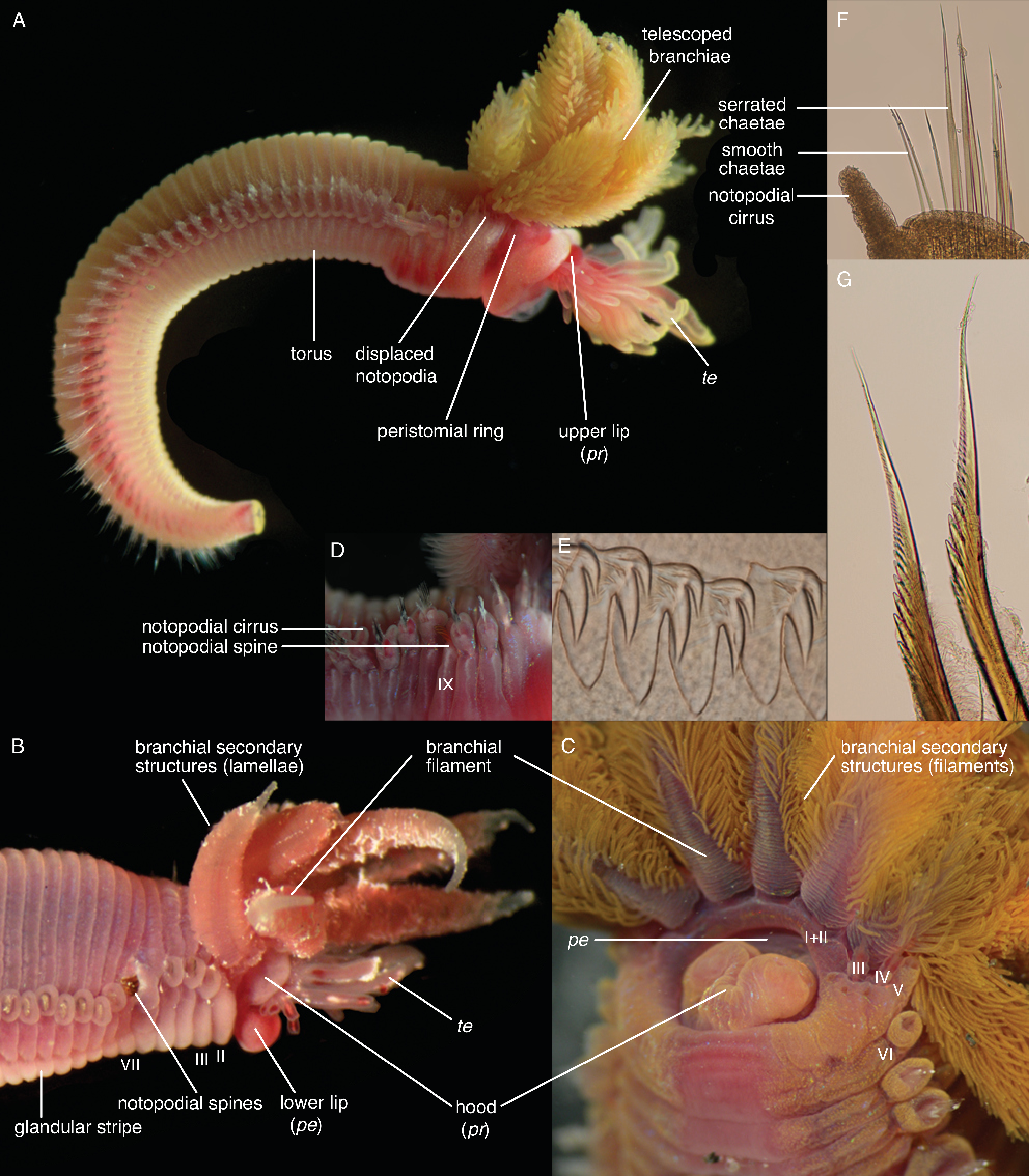
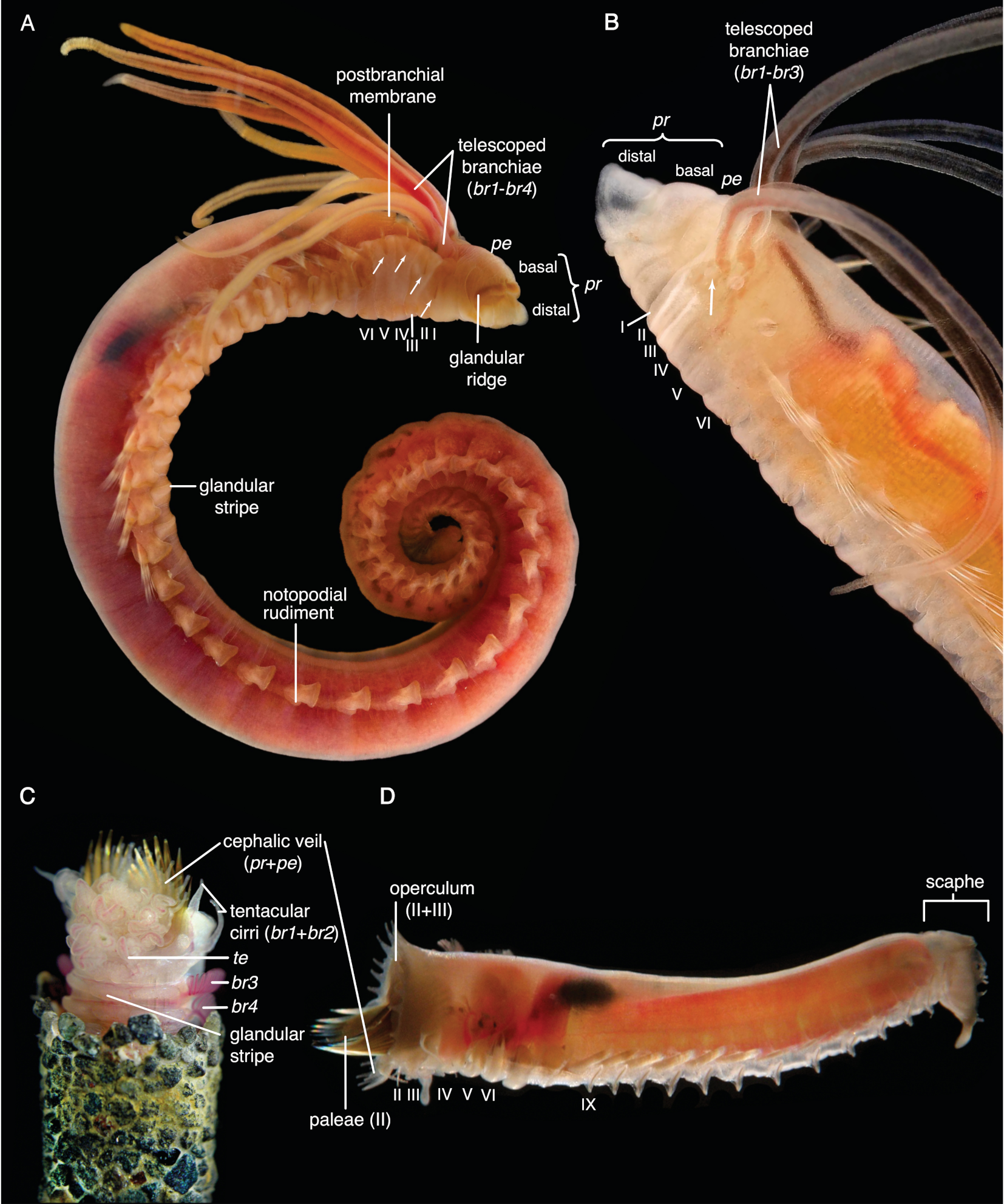

- Hooks are neurochaetae with a long manubrium. Neuropodial hooks are found in the outgroup and in the thorax of Trichobranchidae. Hooks are generally similar to the short-bodied uncini with most of the components also present: They have a pronounced rostrum surmounted by a capitium and a rounded occipitium present behind those. Because of the elongation of the entire basal part, the basis and the anterior process are not distinguishable. [25] calls this type manubriavicular.
- Uncini are neurochaetae with a short manubrium. Uncini are found in most chaetae-bearing Terebelliformia. Uncini can be regarded as being homologous to hooks [108] by reducing their manubria in length and therefore having a distinct basis. Uncini, in our definition, are always short-bodied, even though they can have prolonged elements but never prolonged bases. This led to confusing terminology when elongate uncini are described with the same terms as the structures we call hooks. Elongate uncini are of two types:
- (i)
- In Pectinariidae, the uncini of the anterior body have a broadly elongated posterior process including posterior parts of the occipitium (see figure 4D–F in [108]). The basis of the uncinus is not elongated.
- (ii)
- In certain Terebellinae, only the posterior process is prolonged forming a thin extension. This opisthavicular uncinus is probably derived from a “normal” avicular uncinus characteristic of Terebellidae [25]. Even though the manubrium (handle, shaft) is not prolonged in this type, they are often described as “long-handled” or “long-shafted” [26], a term, which is also confusingly used for trichobranchid hooks (where the term is appropriate because the manubrium is elongated). Several authors have pointed out that these two types are not homologous, as only the posterior process, not the entire body of the uncinus, is elongated [25,26,77,109].
- Aciculae are simple, pointed neurochaetae that are present in the anterior body of Melinnidae, the first neuropodium of Terebellides, and the posterior body of Amaeana. They are often called “acicular spines” but we reserve the term spine for notopodial structures. We also consider the scaphal “hooks” of Pectinariidae as aciculae of neuropodial origin [60]. Neuropodial aciculae are straight or bent distally and most of the components of hooks and uncini are reduced. We treat them here as consisting of only an elongate manubrium until studies address their formation. Terebellides has both aciculae and hooks in its anterior body which is an apomorphy of this genus [99]: the first neuropodium carries geniculate aciculae that only have the rostral tooth present and no capitium, while the following ones are manubriavicular hooks with a capitium atop the rostrum (Figure A3D).
References
- Horton, T.; Kroh, A.; Ahyong, S.; Bailly, N.; Boyko, C.B.; Brandão, S.N.; Gofas, S.; Hooper, J.N.A.; Hernandez, F.; Holovachov, O.; et al. World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org (accessed on 13 March 2020).
- Eilertsen, M.H.; Kongsrud, J.A.; Alvestad, T.; Stiller, J.; Rouse, G.W.; Rapp, H.T. Do ampharetids take sedimented steps between vents and seeps? Phylogeny and habitat-use of Ampharetidae (Annelida, Terebelliformia) in chemosynthesis-based ecosystems. BMC Evol. Biol. 2017, 17, 222. [Google Scholar]
- Reuscher, M.; Fiege, D.; Wehe, T. Terebellomorph polychaetes from hydrothermal vents and cold seeps with the description of two new species of Terebellidae (Annelida: Polychaeta) representing the first records of the family from deep-sea vents. J. Mar. Biol. Assoc. UK 2012, 92, 997–1012. [Google Scholar]
- Kongsrud, J.A.; Eilertsen, M.H.; Alvestad, T.; Kongshavn, K.; Rapp, H.T. New species of Ampharetidae (Annelida: Polychaeta) from the Arctic Loki Castle vent field. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2017, 137, 232–245. [Google Scholar]
- Oug, E.; Bakken, T.; Kongsrud, J.A.; Alvestad, T. Polychaetous annelids in the deep Nordic Seas: Strong bathymetric gradients, low diversity and underdeveloped taxonomy. Deep Sea Res. Part 2 Top. Stud. Oceanogr. 2017, 137, 102–112. [Google Scholar]
- Nygren, A.; Parapar, J.; Pons, J.; Meißner, K.; Bakken, T.; Kongsrud, J.A.; Oug, E.; Gaeva, D.; Sikorski, A.; Johansen, R.A.; et al. A mega-cryptic species complex hidden among one of the most common annelids in the North East Atlantic. PLoS ONE 2018, 13, e0198356. [Google Scholar]
- Merz, R.A. Textures and traction: How tube-dwelling polychaetes get a leg up. Invertebr. Biol. 2015, 134, 61–77. [Google Scholar] [PubMed]
- Andrade, S.C.S.; Novo, M.; Kawauchi, G.Y.; Worsaae, K.; Pleijel, F.; Giribet, G.; Rouse, G.W. Articulating “Archiannelids”: Phylogenomics and annelid relationships, with emphasis on meiofaunal taxa. Mol. Biol. Evol. 2015, 32, 2860–2875. [Google Scholar]
- Helm, C.; Beckers, P.; Bartolomaeus, T.; Drukewitz, S.H.; Kourtesis, I.; Weigert, A.; Purschke, G.; Worsaae, K.; Struck, T.H.; Bleidorn, C. Convergent evolution of the ladder-like ventral nerve cord in Annelida. Front. Zool. 2018, 15, 36. [Google Scholar]
- Weigert, A.; Helm, C.; Meyer, M.; Nickel, B.; Arendt, D.; Hausdorf, B.; Santos, S.R.; Halanych, K.M.; Purschke, G.; Bleidorn, C.; et al. Illuminating the base of the annelid tree using transcriptomics. Mol. Biol. Evol. 2014, 31, 1391–1401. [Google Scholar]
- Zhong, M.; Hansen, B.; Nesnidal, M.; Golombek, A.; Halanych, K.M.; Struck, T.H. Detecting the symplesiomorphy trap: A multigene phylogenetic analysis of terebelliform annelids. BMC Evol. Biol. 2011, 11, 369. [Google Scholar]
- Colgan, D.J.; Hutchings, P.A.; Braune, M. A multigene framework for polychaete phylogenetic studies. Org. Divers. Evol. 2006, 6, 220–235. [Google Scholar] [CrossRef]
- de Nogueira, J.M.M.; Fitzhugh, K.; Hutchings, P. The continuing challenge of phylogenetic relationships in Terebelliformia (Annelida: Polychaeta). Invertebr. Syst. 2013, 27, 186–238. [Google Scholar] [CrossRef]
- Glasby, C.J.; Hutchings, P.A.; Hall, K. Assessment of monophyly and taxon affinities within the polychaete clade Terebelliformia (Terebellida). J. Mar. Biol. Assoc. UK 2004, 84, 961–971. [Google Scholar] [CrossRef]
- Hall, K.A.; Hutchings, P.A.; Colgan, D.J. Further phylogenetic studies of the Polychaeta using 18S rDNA sequence data. J. Mar. Biol. Assoc. UK 2004, 84, 949–960. [Google Scholar] [CrossRef]
- Rousset, V.; Pleijel, F.; Rouse, G.W.; Erséus, C.; Siddall, M.E. A molecular phylogeny of annelids. Cladistics 2007, 23, 41–63. [Google Scholar] [CrossRef]
- Desbruyères, D.; Laubier, L. Alvinella pompejana gen. sp. nov., Ampharetidae aberrant des sources hydrothermales de la ride Est-Pacifique. Oceanol. Acta 1980, 3, 267–274. [Google Scholar]
- Desbruyères, D.; Laubier, L. Les Alvinellidae, une famille nouvelle d’annélides polychètes inféodées aux sources hydrothermales sous-marines: systématique, biologie et écologie. Can. J. Zool. 1986, 64, 2227–2245. [Google Scholar] [CrossRef]
- Feral, J.-P.; Philippe, H.; Desbruyères, D.; Laubier, L.; Derelle, E.; Chenuil, A. Phylogénie moléculaire de polychètes Alvinellidae des sources hydrothermales actives de l’océan Pacifique. C.R. Acad. Sci. III-Vie 1994, 317, 771–779. [Google Scholar]
- Rousset, V.; Rouse, G.W.; Feral, J.-P.; Desbruyères, D.; Pleijel, F. Molecular and morphological evidence of Alvinellidae relationships (Terebelliformia, Polychaeta, Annelida). Zool. Scr. 2003, 32, 185–197. [Google Scholar] [CrossRef]
- Stiller, J.; Rousset, V.; Pleijel, F.; Chevaldonné, P.; Vrijenhoek, R.C.; Rouse, G.W. Phylogeny, biogeography and systematics of hydrothermal vent and methane seep Amphisamytha (Ampharetidae, Annelida), with descriptions of three new species. Syst. Biodivers. 2013, 11, 35–65. [Google Scholar] [CrossRef]
- Zrzavý, J.; Ríha, P.; Piálek, L.; Janouskovec, J. Phylogeny of Annelida (Lophotrochozoa): Total-evidence analysis of morphology and six genes. BMC Evol. Biol. 2009, 9, 189. [Google Scholar] [CrossRef] [PubMed]
- Garraffoni, A.R.S.; Lana, P.C. Phylogenetic relationships within the Terebellidae (Polychaeta: Terebellida) based on morphological characters. Invertebr. Syst. 2008, 22, 605–626. [Google Scholar] [CrossRef]
- McHugh, D. Phylogenetic analysis of the Amphitritinae (Polychaeta: Terebellidae). Zool. J. Linn. Soc. 1995, 114, 405–429. [Google Scholar] [CrossRef]
- Holthe, T. Evolution, systematics, and distribution of the Polychaeta Terebellomorpha, with a catalogue of the taxa and a bibliography. Gunneria 1986, 55, 1–236. [Google Scholar]
- de Nogueira, J.M.M.; Hutchings, P.A.; Fukuda, M.V. Morphology of terebelliform polychaetes (Annelida: Polychaeta: Terebelliformia), with a focus on Terebellidae. Zootaxa 2010, 2460, 1–185. [Google Scholar] [CrossRef]
- Glasby, C.J.; Hutchings, P. Revision of the taxonomy of Polycirrus Grube, 1850 (Annelida: Terebellida: Polycirridae). Zootaxa 2014, 3877, 1–117. [Google Scholar] [CrossRef]
- Rouse, G.; Pleijel, F. Polychaetes; Oxford University Press: New York, NY, USA, 2001; pp. 1–354. [Google Scholar]
- Faircloth, B.C.; Glenn, T.C. Not all sequence tags are created equal: Designing and validating sequence identification tags robust to indels. PLoS ONE 2012, 7, e42543. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dunn, C.W.; Howison, M.; Zapata, F. Agalma: An automated phylogenomics workflow. BMC Bioinform. 2013, 14, 330. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Borowiec, M.L. AMAS: A fast tool for alignment manipulation and computing of summary statistics. PeerJ 2016, 4, e1660. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.K.; Warnow, T. To include or not to include: The impact of gene filtering on species tree estimation methods. Syst. Biol. 2018, 67, 285–303. [Google Scholar] [CrossRef]
- Nute, M.; Chou, J.; Molloy, E.K.; Warnow, T. The performance of coalescent-based species tree estimation methods under models of missing data. BMC Genomics 2018, 19, 286. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Zhang, C.; Rabiee, M.; Sayyari, E.; Mirarab, S. ASTRAL-III: Polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinform. 2018, 19, 153. [Google Scholar] [CrossRef]
- Sayyari, E.; Mirarab, S. Fast coalescent-based computation of local branch support from quartet frequencies. Mol. Biol. Evol. 2016, 33, 1654–1668. [Google Scholar] [CrossRef]
- Naser-Khdour, S.; Minh, B.Q.; Zhang, W.; Stone, E.A.; Lanfear, R. The prevalence and impact of model violations in phylogenetic analysis. Genome Biol. Evol. 2019, 11, 3341–3352. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.; Chernomor, O.; Schrempf, D.; Woodhams, M.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. bioRxiv 2019, 849372. [Google Scholar] [CrossRef]
- Struck, T.H. TreSpEx–-Detection of misleading signal in phylogenetic reconstructions based on tree information. Evol. Bioinform. Online 2014, 10, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Walker, J.F.; Smith, S.A. Phyx: Phylogenetic tools for unix. Bioinformatics 2017, 33, 1886–1888. [Google Scholar] [CrossRef] [PubMed]
- Mai, U.; Mirarab, S. TreeShrink: Fast and accurate detection of outlier long branches in collections of phylogenetic trees. BMC Genomics 2018, 19, 272. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Edgecombe, G.D.; Giribet, G.; Wheeler, W.C. Phylogeny of Henicopidae (Chilopoda: Lithobiomorpha): A combined analysis of morphology and five molecular loci. Syst. Entomol. 2002, 27, 31–64. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Martin, A.P.; Romano, S.; McMillan, W.O.; Stice, L.; Grabowski, G. Simple Fool’s Guide to PCR; Department of Zoology Special Publication, University of Hawaii: Honolulu, HI, USA, 1991; pp. 1–15. [Google Scholar]
- Sjölin, E.; Erséus, C.; Källersjö, M. Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Mol. Phylogenet. Evol. 2005, 35, 431–441. [Google Scholar] [CrossRef]
- Giribet, G.; Carranza, S.; Baguna, J. First molecular evidence for the existence of a Tardigrada+Arthropoda clade. Biol. Evolut. 1996. [Google Scholar] [CrossRef]
- Giribet, G.; Carranza, S.; Riutort, M.; Baguñà, J.; Ribera, C. Internal phylogeny of the Chilopoda (Myriapoda, Arthropoda) using complete 18S rDNA and partial 28S rDNA sequences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 215–222. [Google Scholar] [CrossRef]
- Norén, M.; Jondelius, U. Phylogeny of the Prolecithophora (Platyhelminthes) inferred from 18S rDNA sequences. Cladistics 1999, 15, 103–112. [Google Scholar] [CrossRef]
- Le, H.L.; Lecointre, G.; Perasso, R. A 28S rRNA-based phylogeny of the gnathostomes: First steps in the analysis of conflict and congruence with morphologically based cladograms. Mol. Phylogenet. Evol. 1993, 2, 31–51. [Google Scholar] [CrossRef]
- Brown, S.; Rouse, G.; Hutchings, P.; Colgan, D. Assessing the usefulness of histone H3, U2 snRNA and 28S rDNA in analyses of polychaete relationships. Aust. J. Zool. 1999, 47, 499–516. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.-I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Day, J.H. A review of the family Ampharetidae (Polychaeta). Ann. S. Afr. Mus. 1964, 48, 97–120. [Google Scholar]
- Fauvel, P. Faune de France: Polychètes sédentaires; Paul Lechevalier: Paris, France, 1927; pp. 1–494. [Google Scholar]
- Hessle, C. Zur Kenntnis der terebellomorphen Polychaeten. Zool. Bidr. Upps. 1917, 5, 39–258. [Google Scholar]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis, version 3.31; Available online: http://www.mesquiteproject.org (accessed on 13 March 2020).
- Colgan, D.J.; Hutchings, P.A.; Brown, S. Phylogenetic relationships within the Terebellomorpha. J. Mar. Biol. Assoc. UK 2001, 81, 765–773. [Google Scholar] [CrossRef]
- Fauchald, K.; Rouse, G. Polychaete systematics: Past and present. Zool. Scr. 1997, 26, 71–138. [Google Scholar] [CrossRef]
- Salazar-Vallejo, S.I.; Hutchings, P. A review of characters useful in delineating ampharetid genera (Polychaeta). Zootaxa 2012, 3402, 45–53. [Google Scholar] [CrossRef]
- Jirkov, I.A.; Leontovich, M.K. Review of genera within the Axionice/Pista complex (Polychaeta, Terebellidae), with discussion of the taxonomic definition of other Terebellidae with large lateral lobes. J. Mar. Biol. Assoc. UK 2017, 97, 911–934. [Google Scholar] [CrossRef]
- de Nogueira, J.M.M.; Fitzhugh, K.; Hutchings, P.; Carrerette, O. Phylogenetic analysis of the family Telothelepodidae Nogueira, Fitzhugh & Hutchings, 2013 (Annelida: Terebelliformia). Mar. Biol. Res. 2017, 13, 671–692. [Google Scholar]
- Alvestad, T.; Budaeva, N. Neosabellides lizae, a new species of Ampharetidae (Annelida) from Lizard Island, Great Barrier Reef, Australia. Zootaxa 2015, 4019, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Reuscher, M.; Fiege, D.; Wehe, T. Four new species of Ampharetidae (Annelida: Polychaeta) from Pacific hot vents and cold seeps, with a key and synoptic table of characters for all genera. Zootaxa 2009, 2191, 1–40. [Google Scholar]
- Fontanillas, E.; Galzitskaya, O.V.; Lecompte, O.; Lobanov, M.Y.; Tanguy, A.; Mary, J.; Girguis, P.R.; Hourdez, S.; Jollivet, D. Proteome evolution of deep-sea hydrothermal vent alvinellid polychaetes supports the ancestry of thermophily and subsequent adaptation to cold in some lineages. Genome Biol. Evol. 2017, 9, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Desbruyères, D.; Laubier, L. New species of Alvinellidae (Polychaeta) from the North Fiji back-arc basin hydrothermal vents (southwestern Pacific). Proc. Biol. Soc. Wash. 1993, 106, 225–236. [Google Scholar]
- Capa, M.; Hutchings, P.A. Terebellidae (Polychaeta) from Coiba National Park, Panamanian Pacific, including description of four new species and synonymy of the genus Paraeupolymnia with Lanicola. Zootaxa 2006, 1375, 1–29. [Google Scholar] [CrossRef]
- Jirkov, I. Three new species of Thelepus Leuckart, 1849 from Europe and a re-description of T. cincinnatus (Fabricius, 1780) (Annelida, Terebellidae). Zookeys 2018, 759, 29. [Google Scholar] [CrossRef]
- Fitzhugh, K.; de Nogueira, J.M.M.; Carrerette, O.; Hutchings, P. An assessment of the status of Polycirridae genera (Annelida: Terebelliformia) and evolutionary transformation series of characters within the family. Zool. J. Linn. Soc. 2015. [Google Scholar] [CrossRef]
- Rouse, G.W.; Fauchald, K. Cladistics and polychaetes. Zool. Scr. 1997, 26, 139–204. [Google Scholar] [CrossRef]
- Fauchald, K. The polychaete worms. Definitions and keys to the orders, families and genera. Nat. Hist. Mus. Los Angel. Cty. Sci. Ser. 1977, 28, 1–190. [Google Scholar]
- Zhadan, A.E.; Tzetlin, A.B. Comparative morphology of the feeding apparatus in the Terebellida (Annelida: Polychaeta). Cah. Biol. Mar. 2002. [Google Scholar]
- Garraffoni, A.R.S.; Lana, P.C. A critical review of ontogenetic development in Terebellidae (Polychaeta). Acta Zool. 2010, 91, 390–401. [Google Scholar] [CrossRef]
- Keßler, M. Die Entwicklung von Lanice conchilega (Pallas) mit besonderer Berücksichtigung der Lebensweise. Helgol. Wiss. Meeresunters. 1963, 8, 425. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Amaral, A.C.Z. Postlarval development of Nicolea uspiana (Polychaeta: Terebellidae). Zoologia 2009, 26, 61–66. [Google Scholar] [CrossRef]
- Blake, J.A. Larval development of Polychaeta from the Northern California coast V. Ramex californiensis Hartman (Polychaeta: Terebellidae). Bull. Mar. Sci. 1991, 48, 448–460. [Google Scholar]
- Bhaud, M.; Gremare, A. Larval development of the terebellid polychaete Eupolymnia nebulosa (Montagu) in the Mediterranean Sea. Zool. Scr. 1988, 17, 347–356. [Google Scholar] [CrossRef]
- Wilson, D.P. The post-larval development of Loimia medusa Sav. J. Mar. Biol. Assoc. UK 1928, 15, 129–149. [Google Scholar] [CrossRef]
- Eckelbarger, K.J. Population biology and larval development of the terebellid polychaete Nicolea zostericola. Mar. Biol. 1974, 27, 101–113. [Google Scholar]
- Orrhage, L. On the anatomy of the central nervous system and the morphological value of the anterior end appendages of Ampharetidae, Pectinariidae and Terebellidae (Polychaeta). Acta Zool. 2001, 82, 57–71. [Google Scholar] [CrossRef]
- Zottoli, R.A. Reproduction and larval development of the ampharetid polychaete Amphicteis floridus. Trans. Am. Microsc. Soc. 1974, 93, 78–89. [Google Scholar] [CrossRef]
- Zottoli, R. Amphisamytha galapagensis, a new species of ampharetid polychaete from the vicinity of abyssal hydrothermal vents in the Galapagos Rift, and the role of this species in rift ecosystems. Proc. R. Soc. Lond. B Biol. Sci. 1983, 96, 379–391. [Google Scholar]
- Zottoli, R. Early development of the deep-sea ampharetid (Polychaeta: Ampharetidae) Decemunciger apalea Zottoli. Proc. Biol. Soc. Wash. 1999, 112, 199–209. [Google Scholar]
- Okuda, S. On an ampharetid worm, Schistocomus sovjeticus Annenkova, with some notes on its larval development. J. Fac. Sc. Hokkaido Imp. Univ. Ser. VI 1947, 9, 321–329. [Google Scholar]
- Tzetlin, A.B. Ultrastructural study of the jaw structures in two species of Ampharetidae (Annelida: Polychaeta). Acta Zool. 2004, 85, 171–180. [Google Scholar] [CrossRef]
- Hutchings, P.; Peart, R. A review of the genera of Pectinariidae (Polychaeta) together with a description of the Australian fauna. Rec. Aust. Mus. 2002, 54, 99–127. [Google Scholar] [CrossRef][Green Version]
- Vovelle, J. Organes constructeurs et matériaux sécrétés chez les Polychètes tubicoles: homologies et convergences. Bull. Soc. Zool. Fr. 1997, 122, 59–66. [Google Scholar]
- Nilsson, D. Beiträge zur Kenntnis des Nervensystems der Polychaeten. Zool. Bidr. Upps. 1912, 1, 85–161. [Google Scholar]
- Cazaux, C. Larval development of the lagunar Ampharetidae Alkmaria rominjni Horst 1919. Cah. Biol. Mar. 1982, 23, 143–157. [Google Scholar]
- Nyholm, K.-G. Contributions to the life history of the ampharetid, Melinna cristata. Zool. Bidr. Upps. 1950, 29, 80–91. [Google Scholar]
- Jouin-Toulmond, C.; Augustin, D.; Desbruyères, D.; Toulmond, A. The gas transfer system in alvinellids (Annelida Polychaeta, Terebellida): Anatomy and ultrastructure of the anterior circulatory system and characterization of a coelomic, intracellular, haemoglobin. Cah. Biol. Mar. 1996, 37, 135–152. [Google Scholar]
- Pradillon, F.; Gaill, F. Oogenesis characteristics in the hydrothermal vent polychaete Alvinella pompejana. Invertebr. Reprod. Dev. 2003, 43, 223–235. [Google Scholar] [CrossRef]
- Storch, V.; Gaill, F. Ultrastructural observations on feeding appendages and gills of Alvinella pompejana (Annelida, Polychaeta). Helgoländ. Meeresun. 1986, 40, 309. [Google Scholar] [CrossRef]
- Jouin, C.; Gaill, F. Gills of hydrothermal vent annelids: Structure, ultrastructure and functional implications in two alvinellid species. Prog. Oceanogr. 1990, 24, 59–69. [Google Scholar] [CrossRef]
- Jouin-Toulmond, C.; Hourdez, S. Morphology, ultrastructure and functional anatomy of the branchial organ of Terebellides stroemii (Polychaeta: Trichobranchidae) and remarks on the systematic position of the genus Terebellides. Cah. Biol. Mar. 2006, 47, 287–299. [Google Scholar]
- Garraffoni, A.R.S.; Lana, P.C. Cladistic analysis of the subfamily Trichobranchinae (Polychaeta: Terebellidae). J. Mar. Biol. Assoc. UK 2004, 84, 973–982. [Google Scholar] [CrossRef]
- Jirkov, I.A. Discussion of taxonomic characters and classification of Ampharetidae (Polychaeta). Ital. J. Zool. 2011, 78, 78–94. [Google Scholar] [CrossRef]
- Schüller, M.; Hutchings, P.A. New species of Terebellides (Polychaeta: Trichobranchidae) from the deep Southern Ocean, with a key to all described species. Zootaxa 2013, 3619, 1–45. [Google Scholar] [CrossRef]
- Zhadan, A.E.; Tzetlin, A.B. Comparative study of the diaphragm (gular membrane) in Terebelliformia (Polychaeta, Annelida). In Advances in Polychaete Research; Sigvaldadóttir, E., Ed.; Springer: Dordrecht, The Netherlands, 2003; Volume 170, pp. 269–278. [Google Scholar]
- Kremer, P.; Fiege, D.; Wehe, T. Morphology and ultrastructure of the nephridial system of Hypania invalida (Grube, 1860) (Annelida, Polychaeta, Ampharetidae). J. Morphol. 2011, 272, 1–11. [Google Scholar] [CrossRef]
- Smith, R.I. Three nephromixial patterns in polychaete species currently assigned to the genus Pista (Annelida, Terebellidae). J. Morphol. 1992, 213, 365–393. [Google Scholar] [CrossRef]
- Bartolomaeus, T. Structure, function and development of segmental organs in Annelida. In Reproductive Strategies and Developmental Patterns in Annelids; Dorresteijn, A.W.C., Westheide, W., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 21–37. [Google Scholar]
- Hausen, H. Chaetae and chaetogenesis in polychaetes (Annelida). In Morphology, Molecules, Evolution and Phylogeny in Polychaeta and Related Taxa; Bartolomaeus, T., Purschke, G., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 37–52. [Google Scholar]
- Bartolomaeus, T. Structure and formation of the uncini in Pectinaria koreni, Pectinaria auricoma (Terebellida) and Spirorbis spirorbis (Sabellida): Implications for annelid phylogeny and the position of the Pogonophora. Zoomorphology 1995, 115, 161–177. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; de Camargo, M.G. A new application of morphometrics in a study of the variation in uncinal shape present within the Terebellidae (Polychaeta): A reevaluation from digital images. Cah. Biol. Mar. 2007, 48, 229. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiller, J.; Tilic, E.; Rousset, V.; Pleijel, F.; Rouse, G.W. Spaghetti to a Tree: A Robust Phylogeny for Terebelliformia (Annelida) Based on Transcriptomes, Molecular and Morphological Data. Biology 2020, 9, 73. https://doi.org/10.3390/biology9040073
Stiller J, Tilic E, Rousset V, Pleijel F, Rouse GW. Spaghetti to a Tree: A Robust Phylogeny for Terebelliformia (Annelida) Based on Transcriptomes, Molecular and Morphological Data. Biology. 2020; 9(4):73. https://doi.org/10.3390/biology9040073
Chicago/Turabian StyleStiller, Josefin, Ekin Tilic, Vincent Rousset, Fredrik Pleijel, and Greg W. Rouse. 2020. "Spaghetti to a Tree: A Robust Phylogeny for Terebelliformia (Annelida) Based on Transcriptomes, Molecular and Morphological Data" Biology 9, no. 4: 73. https://doi.org/10.3390/biology9040073
APA StyleStiller, J., Tilic, E., Rousset, V., Pleijel, F., & Rouse, G. W. (2020). Spaghetti to a Tree: A Robust Phylogeny for Terebelliformia (Annelida) Based on Transcriptomes, Molecular and Morphological Data. Biology, 9(4), 73. https://doi.org/10.3390/biology9040073






