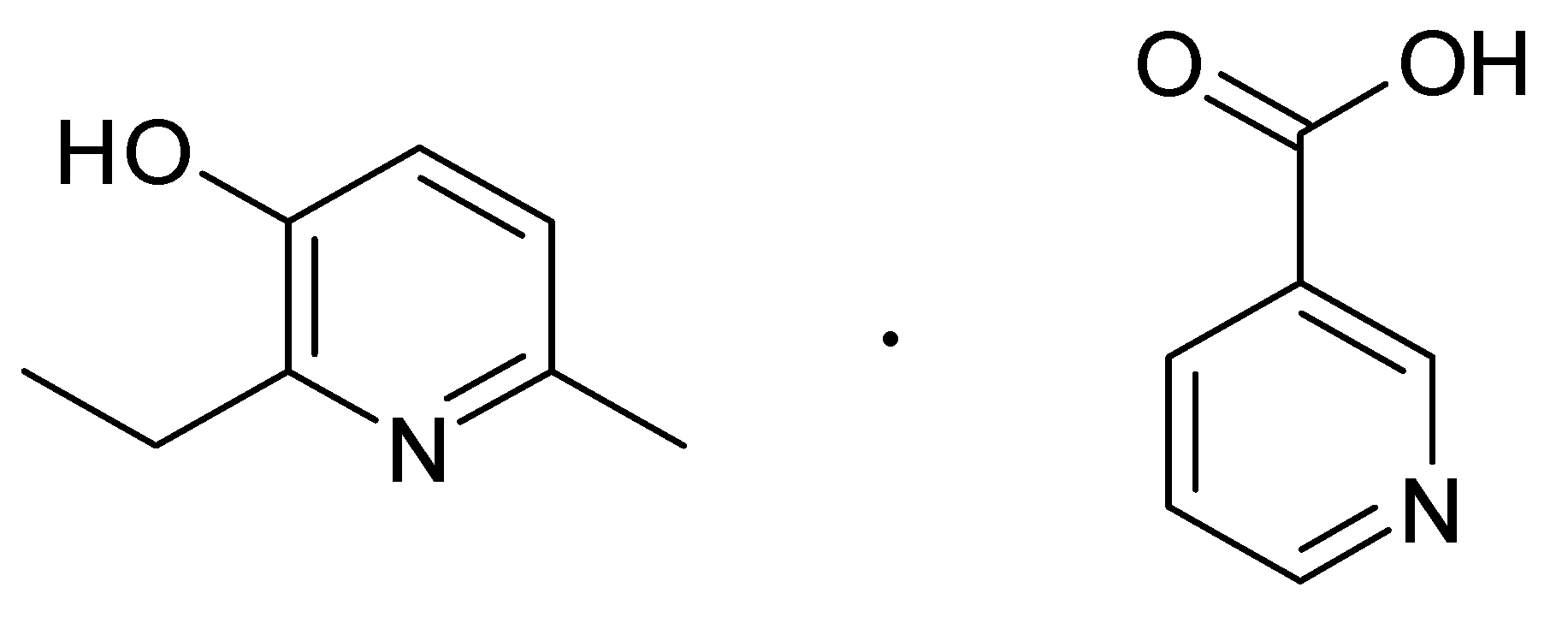
Retinoprotective Effect of 2-Ethyl-3-hydroxy-6-methylpyridine Nicotinate
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Design of the Experiment
2.3. Ophthalmoscopy
2.4. Laser Doppler Flowmetry
2.5. Electroretinography
2.6. Statistical Analysis
3. Results
3.1. Results of the Eye Fundus State Evaluation
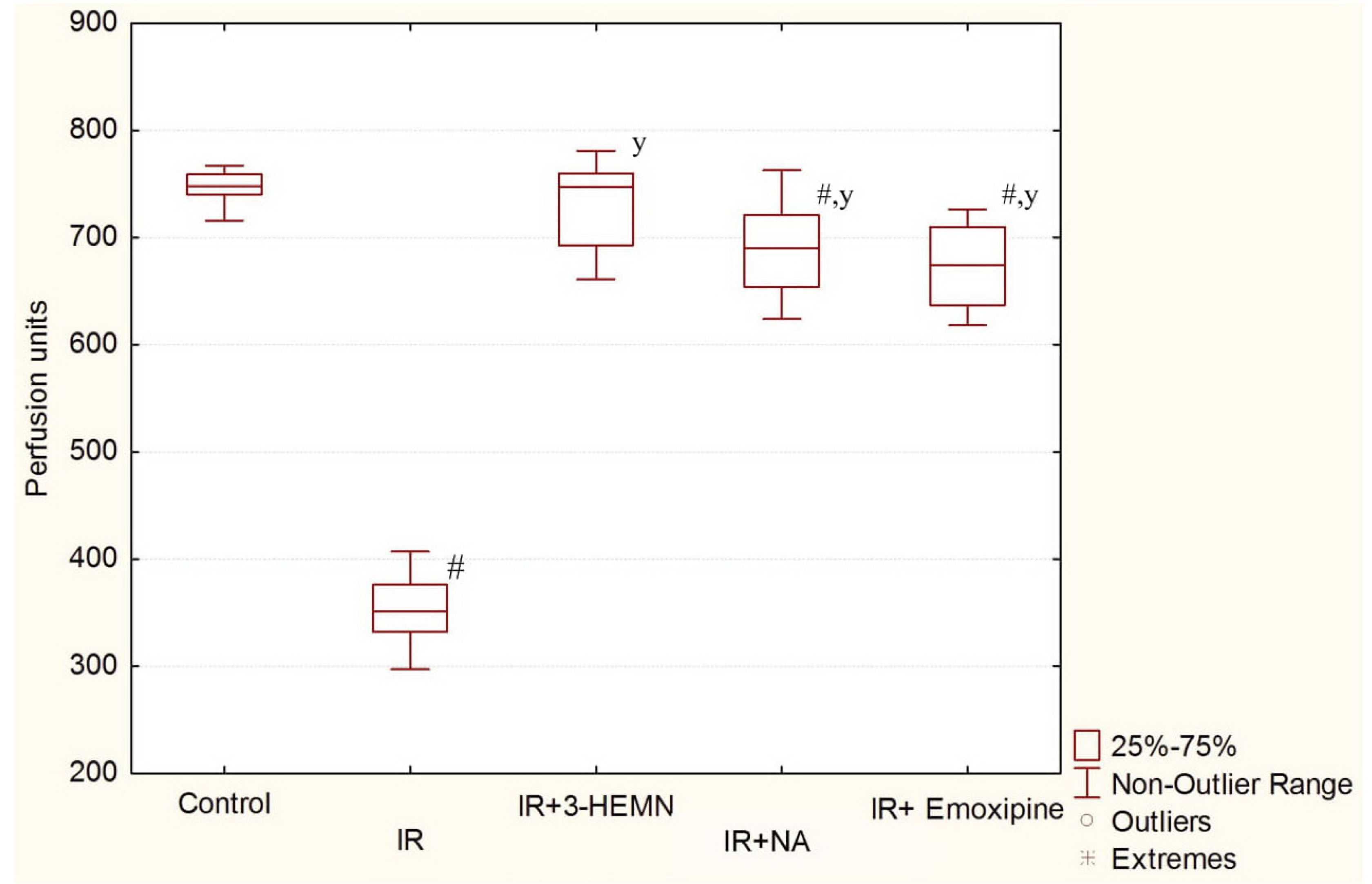
3.2. Results of the LDF
3.3. Results of the ERG
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luo, J.; He, T.; Yang, J.; Yang, N.; Li, Z.; Xing, Y. SIRT1 is required for the neuroprotection of resveratrol on retinal ganglion cells after retinal ischemia-reperfusion injury in mice. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, C.; Chen, Y.; Liang, J.J.; Xu, Y.; Chen, S.L.; Huang, S.; Yang, Q.; Cen, L.P.; Pang, C.P.; et al. Green Tea Extract Ameliorates Ischemia-Induced Retinal Ganglion Cell Degeneration in Rats. Oxid. Med. Cell. Longev. 2019, 2019, 8407206. [Google Scholar] [CrossRef] [PubMed]
- Shabelnikova, A.S.; Peresypkina, A.A.; Pokrovskii, M.V.; Shchegoleva, T.A.; Sernov, L.N.; Reznikov, K.M.; Nikolaev, S.B.; Shutov, V.I.; Lutsenko, V.D.; Philippenko, N.G. Pharmacological preconditioning by recombinant erythropoietin—A new way of treatment of retinal ischemia/reperfusion. IJPT 2016, 8, 26889–26896. [Google Scholar]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.; Chidlow, G.; Graham, M.; Melena, J. Retinal ischemia: Mechanisms of damage and potential therapeutic strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Huang, R.; Liang, S.; Fang, L.; Wu, M.; Cheng, H.; Mi, X.; Ding, Y. Low-dose minocycline mediated neuroprotection on retinal ischemia-reperfusion injury of mice. Mol. Vis. 2018, 24, 367–378. [Google Scholar]
- Palmhof, M.; Lohmann, S.; Schulte, D.; Stute, G.; Wagner, N.; Dick, H.B.; Joachim, S.C. Fewer functional deficits and reduced cell death after Ranibizumab treatment in a retinal ischemia model. Int. J. Mol. Sci. 2018, 19, 1636. [Google Scholar] [CrossRef]
- Minhas, G.; Sharma, J.; Khan, N. Cellular stress response and immune signaling in retinal ischemia-reperfusion injury. Front. Immunol. 2016, 7, 444. [Google Scholar] [CrossRef]
- Peresypkina, A.A.; Pokrovskii, M.V.; Gubareva, V.O.; Dolzhikov, A.A. Protective effect of carbamylated darbepoetin on the model of ischemic neuropathy of the optic nerve in rats. Eksp. Klin. Farm. 2018, 81, 8–13. [Google Scholar] [CrossRef]
- Ju, W.K.; Shim, M.S.; Kim, K.Y.; Bu, J.H.; Park, T.L.; Ahn, S.; Weinreb, R.N. Ubiquinol promotes retinal ganglion cell survival and blocks the apoptotic pathway in ischemic retinal degeneration. Biochem. Biophys. Res. Commun. 2018, 503, 2639–2645. [Google Scholar] [CrossRef]
- Hayreh, S.S. Ischemic optic neuropathies—where are we now? Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 1873–1884. [Google Scholar] [CrossRef]
- Aly, H.A.; Domènech, O. Cytotoxicity and mitochondrial dysfunction of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in isolated rat hepatocytes. Toxicol. Lett. 2009, 191, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Patrushev, M.V.; Vinogradova, E.N.; Kamenski, P.A.; Mazunin, I.O. Mitochondrial fission and fusion. Biochem. Mosc. 2015, 80, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010, 38, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Bramanti, P.; Scimone, C.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. miRNAexpression profile of retinal pigment epithelial cells under oxidative stress conditions. FEBS Open Bio 2018, 8, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Scimone, C.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. Non-coding RNAome of RPE cells under oxidative stress suggests unknown regulative aspects of Retinitis pigmentosa etiopathogenesis. Sci. Rep. 2018, 8, 16638. [Google Scholar] [CrossRef] [PubMed]
- Peresypkina, A.A.; Gubareva, V.O.; Levkova, E.A.; Shabelnikova, A.S.; Pokrovskii, M.V. Pharmacological correction of retinal ischemia/reperfusion by minoxidil. Srp. Arh. Celok. Lek. 2018, 146, 530–533. [Google Scholar] [CrossRef]
- Park, J.W.; Sung, M.S.; Ha, J.Y.; Guo, Y.; Piao, H.; Heo, H.; Park, S.W. Neuroprotective Effect of Brazilian Green Propolis on Retinal Ganglion Cells in Ischemic Mouse Retina. Curr. Eye Res. 2019, 1–10. [Google Scholar] [CrossRef]
- Hui, Q.; Karlstetter, M.; Xu, Z.; Yang, J.; Zhou, L.; Eilken, H.M.; Terjung, C.; Cho, H.; Gong, J.; Lai, M.J.; et al. Inhibition of the Keap1-Nrf2 protein-protein interaction protects retinal cells and ameliorates retinal ischemia-reperfusion injury. Free Radic. Biol. Med. 2020, 146, 181–188. [Google Scholar] [CrossRef]
- Qin, X.; Li, N.; Zhang, M.; Lin, S.; Zhu, J.; Xiao, D.; Cui, W.; Zhang, T.; Lin, Y.; Cai, X. Tetrahedral framework nucleic acids prevent retina ischemia-reperfusion injury from oxidative stress via activating the Akt/Nrf2 pathway. Nanoscale 2019, 11, 20667–20675. [Google Scholar] [CrossRef]
- Chesnokova, N.B.; Beznos, O.V.; Pavlenko, T.A.; Zabozlaev, A.A.; Pavlova, M.V. Effects of hydroxypyridine derivatives mexidol and emoxypin on the reparative processes in rabbit eye on the models of corneal epithelial defect and conjunctival ischemia. Bull. Exp. Biol. Med. 2015, 158, 346–348. [Google Scholar] [CrossRef]
- Peresypkina, A.; Pazhinsky, A.; Pokrovskii, M.; Beskhmelnitsyna, E.; Pobeda, A.; Korokin, M. Correction of Experimental Retinal Ischemia by l-Isomer of Ethylmethylhydroxypyridine Malate. Antioxidants 2019, 8, 34. [Google Scholar] [CrossRef]
- Voronina, T.A. Mexidol: The spectrum of pharmacological effects. Zh. Nevrol. Psikhiatr. Im. SS Korsakova 2012, 112, 86–90. [Google Scholar]
- Avetisov, S.E.; Egorov, E.A.; Moshetova, L.K.; Neroev, V.V.; Tahchidi, H.P. Ophthalmology: National Guidance; GEOTAR-Media: Moscow, Russia, 2018; p. 904. (In Russian) [Google Scholar]
- Rao, K.R.; Mahesh, S.V.; Rao, P.N. Massive perfusion with vasodilators for ischaemic retinopathy and optic nerve diseases—A new approach. Indian J. Ophthalmol. 1987, 35, 91–93. [Google Scholar] [PubMed]
- Gaevyĭ, M.D.; Sankina, T.V.; Nagornaia, G.V. Effect of pentoxyfylline and xanthinol nicotinate on the overall blood pressure and tonus of cerebral and peripheral vessels. Farmakol. Toksikol. 1984, 47, 53–57. [Google Scholar] [PubMed]
- Halder, S.K.; Matsunaga, H.; Ishii, K.J.; Akira, S.; Miyake, K.; Ueda, H. Retinal cell type-specific prevention of ischemia-induced damages by LPS-TLR4 signaling through microglia. J. Neurochem. 2013, 126, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, Q.; Tu, J.; Zhang, M.; Ji, B. Curcumin protects against hypertension aggravated retinal ischemia/reperfusion in a rat stroke model. Clin. Exp. Hypertens. 2017, 39, 711–717. [Google Scholar] [CrossRef]
- Peresypkina, A.A.; Pokrovskii, M.V.; Dolzhikov, A.A.; Levkova, E.A.; Pobeda, A.S. Correction of experimental ischemic neuropathy of the optic nerve by imidazoline receptor agonist type I and II. Eksp. Klin. Farm. 2018, 81, 12–17. [Google Scholar] [CrossRef]
- Sachidanandam, R.; Khetan, V.; Sen, P. Comparison between fullfield electroretinography obtained from handheld and tabletop devices in normal subjects. Can. J. Ophthalmol. 2015, 50, 166–171. [Google Scholar] [CrossRef]
- Burguera, J.A.; Vilela, C.; Traba, A.; Ameave, Y.; Vallet, M. The electroretinogram and visual evoked potentials in patients with Parkinson’s disease. Arch. Neurobiol. (Madr.) 1990, 53, 1–7. [Google Scholar]
- Korokin, M.; Gudyrev, O.; Gureev, V.; Korokina, L.; Peresypkina, A.; Pokrovskaia, T.; Lazareva, G.; Soldatov, V.; Zatolokina, M.; Pokrovskii, M. Studies to Elucidate the Effects of Furostanol Glycosides from Dioscorea deltoidea Cell Culture in a Rat Model of Endothelial Dysfunction. Molecules 2020, 25, 169. [Google Scholar] [CrossRef]
- Novikov, V.E.; Levchenkova, O.S. Promising directions of search for antihypoxants and targets of their action. Eksp. Klin. Farm. 2013, 76, 37–47. [Google Scholar]
- Gasperi, V.; Sibilano, M.; Savini, I.; Catani, M.V. Niacin in the Central Nervous System: An Update of Biological Aspects and Clinical Applications. Int. J. Mol. Sci. 2019, 20, 974. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chong, Z.Z.; Maiese, K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front. Biosci. 2004, 9, 2500–2520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, Y.; Wang, T.; Bi, J.; Li, H.; Zhang, L.Q.; Ye, S.Q.; Ding, S. Neuronal Protective Role of PBEF in a Mouse Model of Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2010, 30, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Bao, R.; Zhang, N.; Wang, Y.; Polo-Parada, L.; Tarim, A.; Alemifar, A.; Han, X.; Wilkins, H.M.; et al. Deletion of Nampt in Projection Neurons of Adult Mice Leads to Motor Dysfunction, Neurodegeneration, and Death. Cell Rep. 2017, 20, 2184–2200. [Google Scholar] [CrossRef]
- Liu, L.; Wang, P.; Liu, X.; He, D.; Liang, C.; Yu, Y. Exogenous NAD(+) supplementation protects H9c2 cardiac myoblasts against hypoxia/reoxygenation injury via Sirt1-p53 pathway. Fundam. Clin. Pharmacol. 2014, 28, 180–189. [Google Scholar] [CrossRef]
- Yang, T.; Sauve, A.A. NAD metabolism and sirtuins: Metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006, 8, E632–E643. [Google Scholar] [CrossRef]
- Kulikova, V.; Shabalin, K.; Nerinovski, K.; Dolle, C.; Niere, M.; Yakimov, A.; Redpath, P.; Khodorkovskiy, M.; Migaud, M.E.; Ziegler, M.; et al. Generation, Release, and Uptake of the NAD Precursor Nicotinic Acid Riboside by Human Cells. J. Biol. Chem. 2015, 290, 27124–27137. [Google Scholar] [CrossRef]
- Yang, Y.; Sauve, A.A. NAD(+) metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta 2016, 1864, 1787–1800. [Google Scholar] [CrossRef]
- Luo, H.; Zhuang, J.; Hu, P.; Ye, W.; Chen, S.; Pang, Y.; Li, N.; Deng, C.; Zhang, X. Resveratrol Delays Retinal Ganglion Cell Loss and Attenuates Gliosis-Related Inflammation from Ischemia-Reperfusion Injury. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3879–3888. [Google Scholar] [CrossRef]
- Seong, H.; Ryu, J.; Yoo, W.S.; Kim, S.J.; Han, Y.S.; Park, J.M.; Kang, S.S.; Seo, S.W. Resveratrol Ameliorates Retinal Ischemia/Reperfusion Injury in C57BL/6J Mice via Downregulation of Caspase-3. Curr. Eye Res. 2017, 42, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hartnett, M.E. Roles of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) Oxidase in Angiogenesis: Isoform-Specific Effects. Antioxidants 2017, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Nor Arfuzir, N.N.; Agarwal, R.; Iezhitsa, I.; Agarwal, P.; Sidek, S.; Spasov, A.; Ozerov, A.; Mohd Ismail, N. Effect of Magnesium Acetyltaurate and Taurine on Endothelin1-Induced Retinal Nitrosative Stress in Rats. Curr. Eye Res. 2018, 43, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Arfuzir, N.N.; Lambuk, L.; Jafri, A.J.; Agarwal, R.; Iezhitsa, I.; Sidek, S.; Agarwal, P.; Bakar, N.S.; Kutty, M.K.; Yusof, A.P.; et al. Protective effect of magnesium acetyltaurate against endothelin-induced retinal and optic nerve injury. Neuroscience 2016, 325, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Howell, G.R.; Macalinao, D.G.; Sousa, G.L.; Walden, M.; Soto, I.; Kneeland, S.C.; Barbay, J.M.; King, B.L.; Marchant, J.K.; Hibbs, M.; et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Invest. 2011, 121, 1429–1444. [Google Scholar] [CrossRef]
- Bosco, A.; Steele, M.R.; Vetter, M.L. Early microglia activation in a mouse model of chronic glaucoma. J. Comp. Neurol. 2011, 519, 599–620. [Google Scholar] [CrossRef]
- Todd, L.; Palazzo, I.; Suarez, L.; Liu, X.; Volkov, L.; Hoang, T.V.; Campbell, W.A.; Blackshaw, S.; Quan, N.; Fischer, A.J. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflamm. 2019, 16, 118. [Google Scholar] [CrossRef]
- Casson, R.J. Possible role of excitotoxicity in the pathogenesis of glaucoma. Clin. Exp. Ophthalmol. 2006, 34, 54–63. [Google Scholar] [CrossRef]

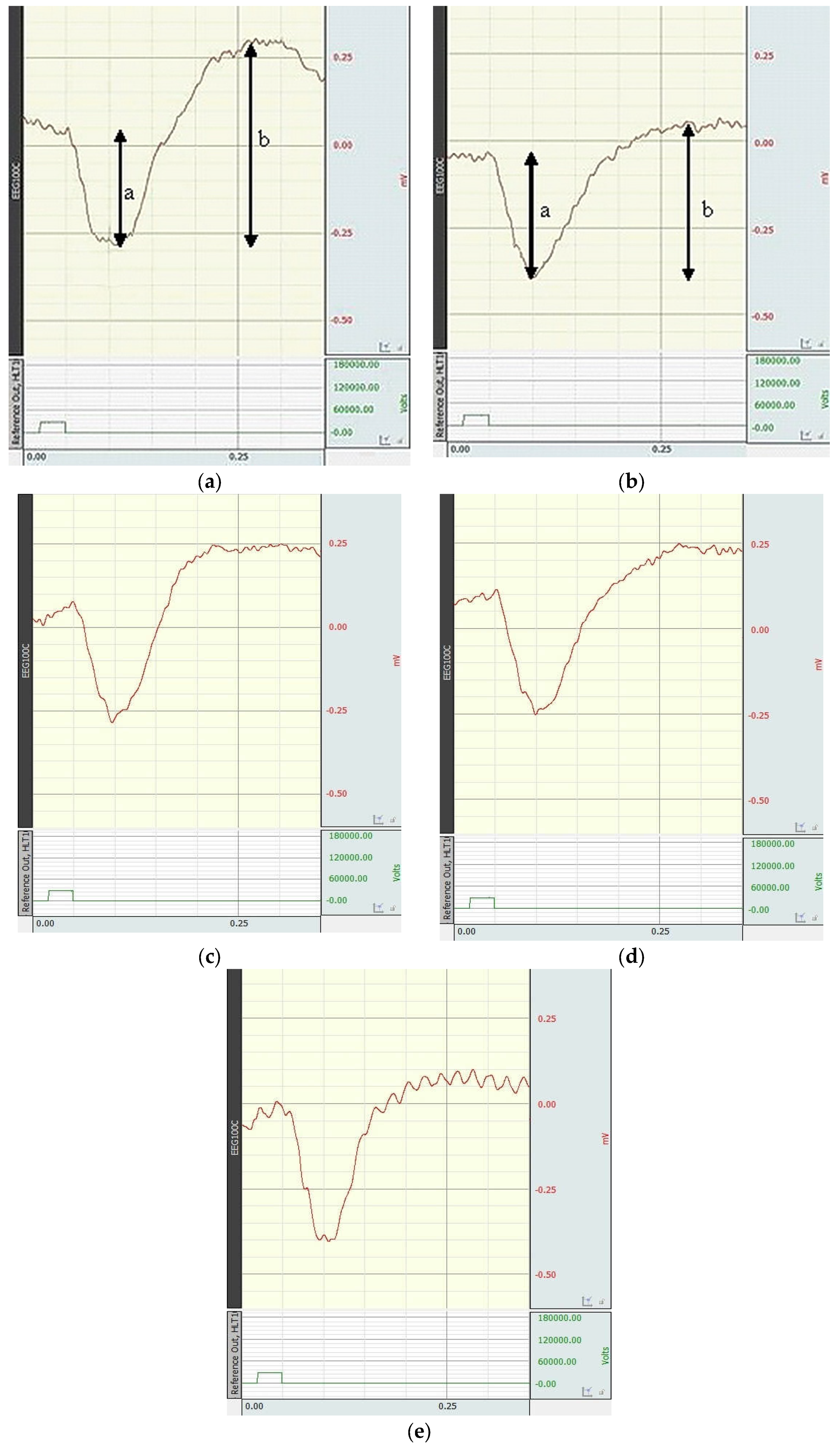
| Groups of Animals | The a-wave Amplitudes (n = 10) | The b-wave Amplitudes (n = 10) | The b/a Coefficient (n = 10) |
|---|---|---|---|
| Control | 0.36 (0.29;0.42) | 0.98 (0.67;1.07) | 2.60 (2.28;2.77) |
| Retinal ischemia–reperfusion | 0.36 (0.29;0.47) | 0.42 (0.33;0.52) # | 1.19 (1.10;1.32) # |
| Retinal ischemia–reperfusion + 2-ethyl-3-hydroxy-6-methylpyridine nicotinate 3.8 mg/kg | 0.33 (0.27;0.47) | 0.83 (0.76;1.01) y | 2.25 (2.09;2.69) y |
| Retinal ischemia–reperfusion + Nicotinic acid 2 mg/kg | 0.37 (0.28;0.48) | 0.77 (0.57;0.95) y | 2.06 (1.93;2.16) #y |
| Retinal ischemia–reperfusion + Emoxipine 2 mg/kg | 0.32 (0.27;0.50) | 0.77 (0.57;1.01) y | 2.18 (2.00;2.33) #y |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peresypkina, A.; Pazhinsky, A.; Danilenko, L.; Lugovskoy, S.; Pokrovskii, M.; Beskhmelnitsyna, E.; Solovev, N.; Pobeda, A.; Korokin, M.; Levkova, E.; et al. Retinoprotective Effect of 2-Ethyl-3-hydroxy-6-methylpyridine Nicotinate. Biology 2020, 9, 45. https://doi.org/10.3390/biology9030045
Peresypkina A, Pazhinsky A, Danilenko L, Lugovskoy S, Pokrovskii M, Beskhmelnitsyna E, Solovev N, Pobeda A, Korokin M, Levkova E, et al. Retinoprotective Effect of 2-Ethyl-3-hydroxy-6-methylpyridine Nicotinate. Biology. 2020; 9(3):45. https://doi.org/10.3390/biology9030045
Chicago/Turabian StylePeresypkina, Anna, Anton Pazhinsky, Lyudmila Danilenko, Sergey Lugovskoy, Mikhail Pokrovskii, Evgeniya Beskhmelnitsyna, Nikolai Solovev, Anna Pobeda, Mikhail Korokin, Elena Levkova, and et al. 2020. "Retinoprotective Effect of 2-Ethyl-3-hydroxy-6-methylpyridine Nicotinate" Biology 9, no. 3: 45. https://doi.org/10.3390/biology9030045
APA StylePeresypkina, A., Pazhinsky, A., Danilenko, L., Lugovskoy, S., Pokrovskii, M., Beskhmelnitsyna, E., Solovev, N., Pobeda, A., Korokin, M., Levkova, E., Gubareva, V., Korokina, L., Martynova, O., Soldatov, V., & Pokrovskii, V. (2020). Retinoprotective Effect of 2-Ethyl-3-hydroxy-6-methylpyridine Nicotinate. Biology, 9(3), 45. https://doi.org/10.3390/biology9030045







