β2-Adrenergic Signalling Promotes Cell Migration by Upregulating Expression of the Metastasis-Associated Molecule LYPD3
Abstract
1. Introduction
2. Results
2.1. Basal-Type Breast Cancer Cell Lines Express Higher Levels of Functional β2-Adrenoceptor and Their Survival Is Not Significantly Altered Following Non-Selective ADRβ Activation
2.2. Substrate-Modulated Cell Adhesion/Migration/Invasion Responses Are ADRβ-Dependent
2.2.1. Cell Adhesion
2.2.2. Cell Migration
2.2.3. Cell Invasion
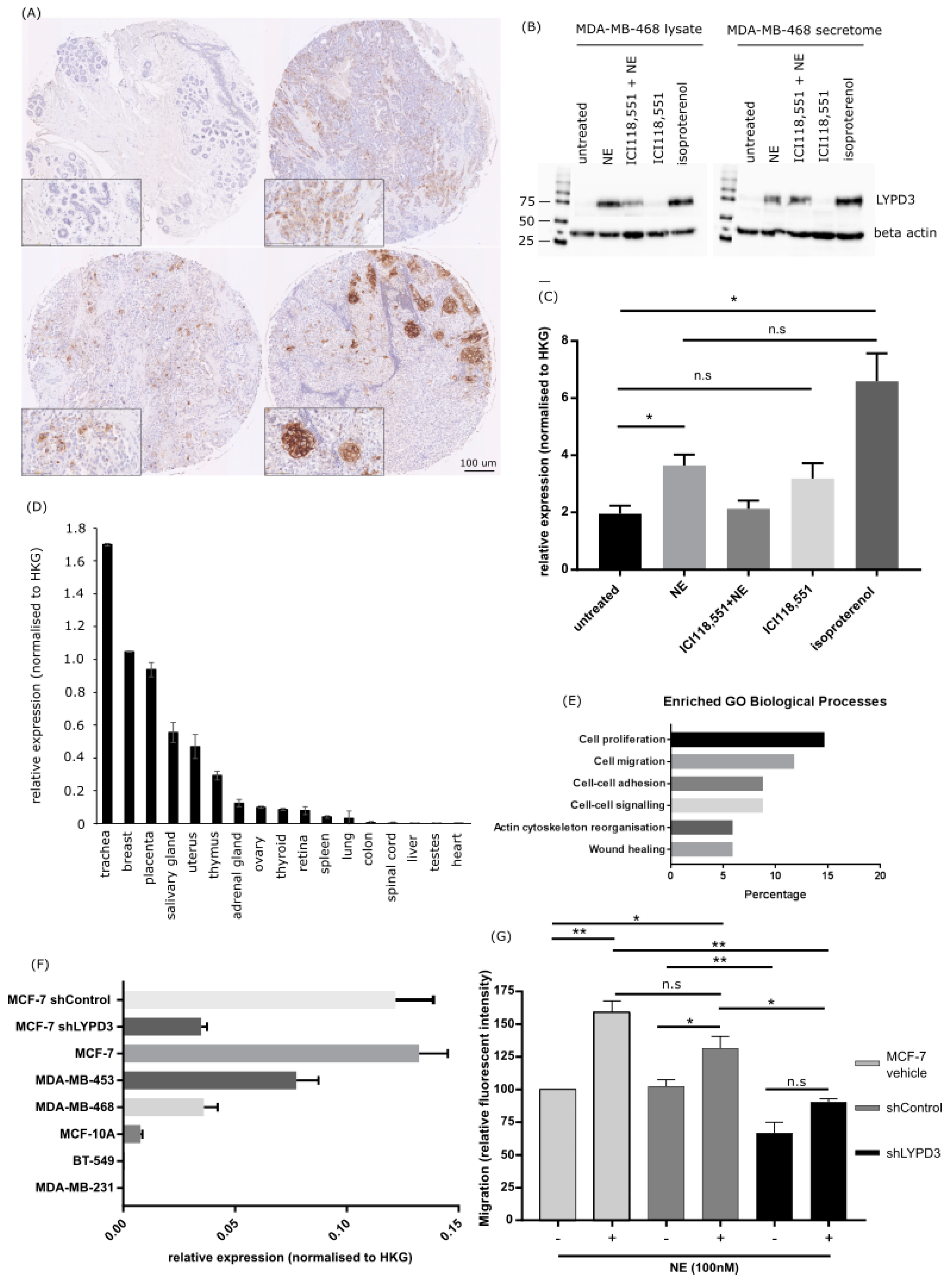
2.3. Protein Expression Changes in MDA-MB-468 and MDA-MB-231 Cells
2.4. Increased LYPD3 Protein Is Exclusively Expressed in Primary and Metastatic Breast Cancer
2.5. LYPD3 Knockdown Significantly Reduces the Migration of MCF-7 Cells In Vitro
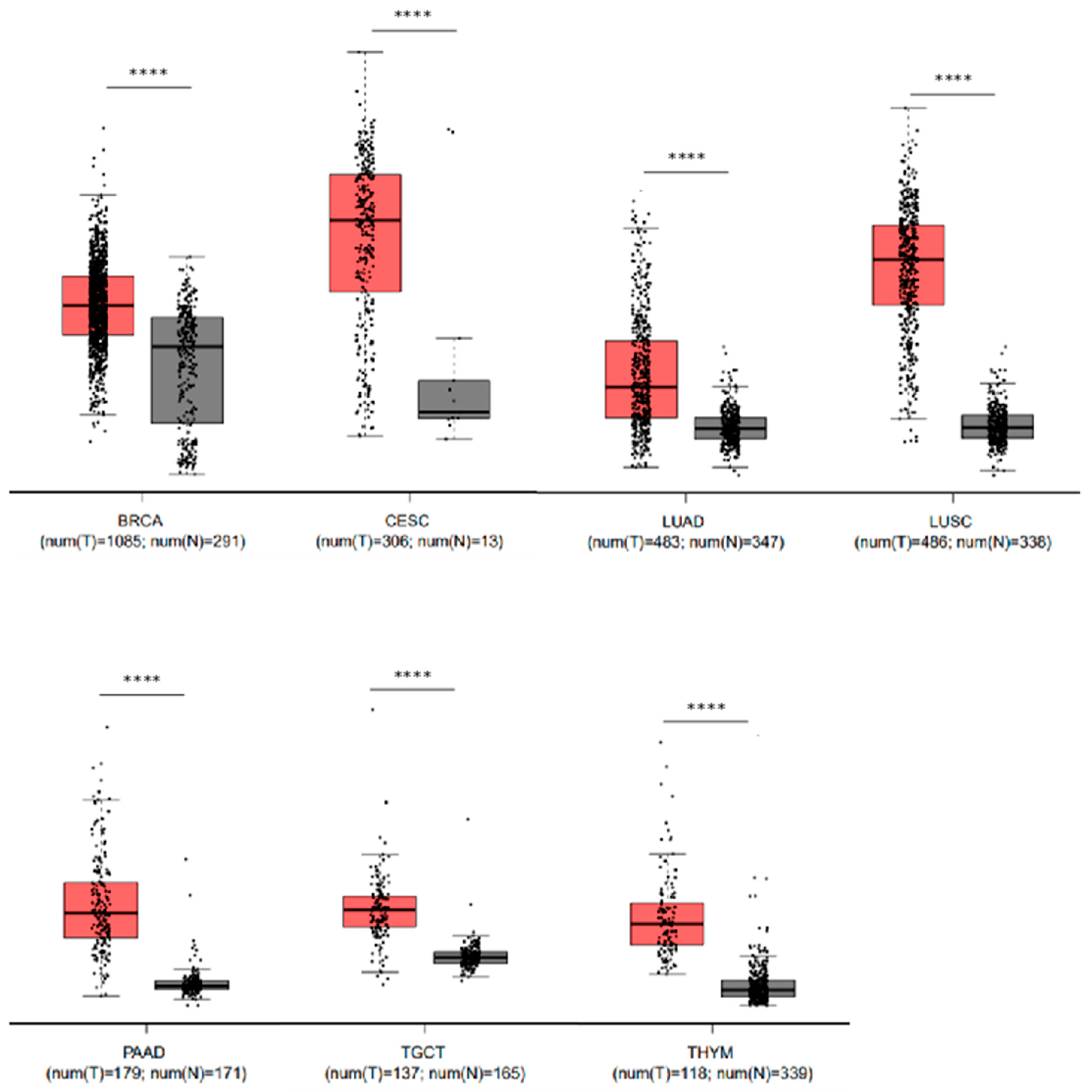
2.6. Elevated Levels of LYPD3 mRNA Are Present in Malignant Disease Compared to Their Non-Malignant Counterpart in Several Cancers
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. RNA Extraction, cDNA Synthesis and qRT-PCR
4.3. Flow Cytometry
4.4. cAMP Signalling
4.5. Cellular Survival
4.6. Adhesion Assays
4.7. Cultrex® Cell Migration Assay
4.8. CultreCoat® Medium BME Cell Invasion Assay
4.9. Proteomic Analysis
4.10. Mass Spectrometry
4.11. LYPD3 Protein Expression
4.12. Western Blot
4.13. Generation of LYPD3 Knockdown Cell Line
4.14. In Silico Gene Expression Profiling
5. Conclusions
Availability of Data and Materials
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GPCR | g-protein coupled receptor |
| qPCR | polymerase chain reaction |
| MFI | mean fluorescence intensity |
| FCS | foetal calf serum |
| PFS | progression free survival |
| ADRβ | beta-adrenergic receptor |
| BME | basement membrane extract |
| LCMS | liquid chromatography mass spectrometry |
| SWATH-MS | sequential window acquisition of all theoretical mass spectra |
| LYPD3 | ly6/PLAUR domain-containing protein 3 precursor |
| TMA | tumour microarray |
| cAMP | cyclic adenosine monophosphate |
| NE | norepinephrine |
| ISO | isoproterenol |
| UT | untreated |
| EMT | epithelial to mesenchymal transition |
| GPI | glycosyl-phosphatidyl-inositol |
| CREB | cAMP response element binding protein |
| ATP | adenosine triphosphate |
| PKA | protein kinase A |
| LAMC1 | laminin subunit gamma 1 |
| MMP | matrix metallopeptidase |
| uPAR | urokinase-type plasminogen activator receptor |
| NE | norepinephrine |
| SWATH | Sequential Window Acquisition of All Theoretical Mass Spectra |
| IDA | information dependent acquisition |
| HKG | house keeping gene |
| ANOVA | analysis of variance |
| EDTA | ethylenediaminetetraacetic acid |
| SDS | sodium dodecyl sulphate |
References
- Steeg, P.S. Targeting metastasis. Nat. Rev. Cancer 2016, 16, 201–218. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Cakir, Y.; Plummer, H.K.; Tithof, P.K.; Schuller, H.M. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int. J. Oncol. 2002, 21, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lüthy, I.A.; Bruzzone, A.; Piñero, C.P.; Castillo, L.F.; Chiesa, I.J.; Vázquez, S.M.; Sarappa, M.G. Adrenoceptors: Non conventional target for breast cancer? Curr. Med. Chem. 2009, 16, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Powe, D.G.; Entschladen, F. Targeted therapies: Using β-blockers to inhibit breast cancer progression. Nat. Rev. Clin. Oncol. 2011, 8, 511–512. [Google Scholar] [CrossRef]
- Campbell, J.P.; Karolak, M.R.; Ma, Y.; Perrien, D.S.; Masood-Campbell, S.K.; Penner, N.L.; Munoz, S.A.; Zijlstra, A.; Yang, X.; Sterling, J.A.; et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012, 10, e1001363. [Google Scholar] [CrossRef]
- Strell, C.; Niggemann, B.; Voss, M.J.; Powe, D.G.; Zänker, K.S.; Entschladen, F. Norepinephrine promotes the β1-integrin-mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROα release. Mol. Cancer Res. 2012, 10, 197–207. [Google Scholar] [CrossRef]
- Kim, T.-H.; Gill, N.K.; Nyberg, K.D.; Nguyen, A.V.; Hohlbauch, S.V.; Geisse, N.A.; Nowell, C.J.; Sloan, E.K.; Rowat, A.C. Cancer cells become less deformable and more invasive with activation of β-adrenergic signaling. J. Cell Sci. 2016, 14, jcs.194803. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Zänker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef]
- Barron, T.I.; Connolly, R.M.; Sharp, L.; Bennett, K.; Visvanathan, K. Beta blockers and breast cancer mortality: A population- based study. J. Clin. Oncol. 2011, 29, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Melhem-Bertrandt, A.; Chavez-Macgregor, M.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011, 29, 2645–2652. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K. Study of Propranolol in Newly Diagnosed Breast Cancer Patients Undergoing Neoadjuvant Chemotherapy. Available online: https://clinicaltrials.gov (accessed on 1 December 2018).
- Allweiss, T. Perioperative Administration of COX2 Inhibitors and Beta-Blockers to Women Undergoing Breast Cancer Surgery. 2007. Available online: https://clinicaltrials.gov (accessed on 1 December 2018).
- Neoadjuvant Propranolol in Breast Cancer. 2015. Available online: https://clinicaltrials.gov1 (accessed on 1 December 2018).
- Carson, W. Propranolol Hydrochloride in Treating Patients with Locally Recurrent or Metastatic Solid Tumours That Cannot be Removed by Surgery. 2013. Available online: https://clinicaltrials.gov (accessed on 1 December 2018).
- Cardwell, C.R.; Coleman, H.G.; Murray, L.J.; Entschladen, F.; Powe, D.G. Beta-blocker usage and breast cancer survival: A nested case-control study within a UK clinical practice research datalink cohort. Int. J. Epidemiol. 2013, 42, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.; Lopez-Olivo, M.A.; Dubowitz, J.; Pratt, G.; Hiller, J.; Gottumukkala, V.; Sloan, E.; Riedel, B.; Schier, R. Effect of beta-blockers on cancer recurrence and survival: A meta-analysis of epidemiological and perioperative studies. Br. J. Anaesth. 2018, 121, 45–57. [Google Scholar] [CrossRef]
- Carie, A.E.; Sebti, S.M. A chemical biology approach identifies a beta-2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2 pathway. Oncogene 2007, 26, 3777–3788. [Google Scholar] [CrossRef]
- Paret, C.; Hildebrand, D.; Weitz, J.; Kopp-Schneider, A.; Kuhn, A.; Beer, A.; Hautmann, R.; Zöller, M. C4.4A as a candidate marker in the diagnosis of colorectal cancer. Br. J. Cancer 2007, 97, 1146–1156. [Google Scholar] [CrossRef][Green Version]
- Fletcher, G.C.; Patel, S.; Tyson, K.; Adam, P.J.; Schenker, M.; Loader, J.A.; Daviet, L.; Legrain, P.; Parekh, R.; Harris, A.L.; et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4A and dystroglycan. Br. J. Cancer 2003, 88, 579–585. [Google Scholar] [CrossRef]
- Jacobsen, B.; Kriegbaum, M.C.; Santoni-Rugiu, E.; Ploug, M. C4.4A as a biomarker in pulmonary adenocarcinoma and squamous cell carcinoma. World J. Clin. Oncol. 2014, 5, 621–632. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Qin, J.; Jin, F.; Li, N.; Guan, H.; Lan, L.; Ni, H.; Wang, Y. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015, 48, 295–300. [Google Scholar] [CrossRef]
- Pon, C.K.; Lane, J.R.; Sloan, E.K.; Halls, M.L. The 2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J. 2016, 30, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Creed, S.J.; Le, C.P.; Hassan, M.; Pon, C.K.; Albold, S.; Chan, K.T.; Berginski, M.E.; Huang, Z.; Bear, J.E.; Lane, J.R. β2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 2015, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Le, C.P.; Walker, A.K.; Creed, S.J.; Pon, C.K.; Albold, S.; Carroll, D.; Halls, M.L.; Lane, J.R.; Riedel, B.; et al. β2 -Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav. Immun. 2016, 57, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.S.; Szpunar, M.J.; Brown, E.B. β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by divergent pathways in high β-AR-expressing breast cancer cell lines. Breast Cancer Res. Treat. 2011, 130, 747–758. [Google Scholar] [CrossRef]
- Pasquier, E.; Ciccolini, J.; Carre, M.; Giacometti, S.; Fanciullino, R.; Pouchy, C.; Montero, M.P.; Serdjebi, C.; Kavallaris, M.; Andre, N. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: Implication in breast cancer treatment. Oncotarget 2011, 2, 797–809. [Google Scholar] [CrossRef]
- Chen, H.; Liu, D.; Yang, Z.; Sun, L.; Deng, Q.; Yang, S.; Qian, L.; Guo, L.; Yu, M.; Hu, M.; et al. Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocr. Relat. Cancer 2014, 21, 783–795. [Google Scholar] [CrossRef]
- Sastry, K.S.R.; Karpova, Y.; Prokopovich, S.; Smith, A.J.; Essau, B.; Gersappe, A.; Carson, J.P.; Weber, M.J.; Register, T.C.; Chen, Y.Q.; et al. Epinephrine Protects Cancer Cells from Apoptosis via Activation of cAMP-dependent Protein Kinase and BAD Phosphorylation. J. Biol. Chem. 2007, 282, 14094–14100. [Google Scholar] [CrossRef]
- Lutgendorf, S.K.; Sood, A.K.; Antoni, M.H. Host factors and cancer progression: Biobehavioral signaling pathways and interventions. J. Clin. Oncol. 2010, 28, 4094–4099. [Google Scholar] [CrossRef]
- Reeder, A.; Attar, M.; Nazario, L.; Bathula, C.; Zhang, A.; Hochbaum, D.; Roy, E.; Cooper, K.L.; Oesterreich, S.; Davidson, N.E.; et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br. J. Cancer 2015, 112, 1461–1470. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Habashy, H.O.; Zänker, K.S.; Green, A.R.; Ellis, I.O.; Entschladen, F. Alpha- and beta-adrenergic receptor (AR) protein expression is associated with poor clinical outcome in breast cancer: An immunohistochemical study. Breast Cancer Res. Treat. 2011, 130, 457–463. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, K.; Lee, E.; Ahn, T.; Jung, H.H.; Lim, S.H.; Hong, M.; Do, I.G.; Cho, E.Y.; Kim, D.H.; et al. Gene Expression Profiling of Breast Cancer Brain Metastasis. Sci Rep. 2016, 6, 28623. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, L.; Copsel, S.; Rivero, E.M.; Galés, C.; Sénard, J.-M.; Lüthy, I.A.; Davio, C.; Bruzzone, A. Differential β2-adrenergic receptor expression defines the phenotype of non-tumorigenic and malignant human breast cell lines. Oncotarget 2014, 5, 10058–10069. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, L.; May, M.; Rivero, E.M.; Copsel, S.; Lamb, C.; Lydon, J.; Davio, C.; Lanari, C.; Lüthy, I.A.; Bruzzone, A. A Novel Effect of β-Adrenergic Receptor on Mammary Branching Morphogenesis and its Possible Implications in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2017, 22, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Zhang, J.; Dancel, R.; Garcia, S.J.; Willis, C.; Seidler, F.J. Beta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cells. Breast Cancer Res. Treat. 2000, 60, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Pérez Piñero, C.; Bruzzone, A.; Sarappa, M.G.; Castillo, L.F.; Lüthy, I.A. Involvement of α2- and β2-adrenoceptors on breast cancer cell proliferation and tumour growth regulation. Br. J. Pharmacol. 2012, 166, 721–736. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Drell, T.L.; Lindecke, A.; Niggemann, B.; Kaltschmidt, C.; Zaenker, K.S.; Entschladen, F. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int. J. Cancer 2004, 112, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, D.; Duan, H.; Qian, L.; Wang, L.; Niu, L.; Zhang, H.; Yong, Z.; Gong, Z.; Song, L.; et al. The β2-adrenergic receptor and Her2 comprise a positive feedback loop in human breast cancer cells. Breast Cancer Res. Treat. 2011, 125, 351–362. [Google Scholar] [CrossRef]
- Choy, C.; Raytis, J.L.; Smith, D.D.; Duenas, M.; Neman, J.; Jandial, R.; Lew, M.W. Inhibition of β2-adrenergic receptor reduces triple-negative breast cancer brain metastases: The potential benefit of perioperative β-blockade. Oncol. Rep. 2016, 35, 3135–3142. [Google Scholar] [CrossRef]
- Boulay, G.; Malaquin, N.; Loison, I.; Foveau, B.; Van Rechem, C.; Rood, B.R.; Pourtier, A.; Leprince, D. Loss of Hypermethylated in Cancer 1 (HIC1) in Breast Cancer Cells Contributes to Stress-induced Migration and Invasion through β-2 Adrenergic Receptor (ADRB2) Misregulation. J. Biol. Chem. 2012, 287, 5379–5389. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular pathways: Beta-adrenergic signaling in cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Entschladen, F.; Drell, T.L.; Lang, K.; Joseph, J.; Zaenker, K.S. Tumour-cell migration, invasion, and metastasis: Navigation by neurotransmitters. Lancet Oncol. 2004, 5, 254–258. [Google Scholar] [CrossRef]
- Lavoie, C.; Mercier, J.-F.; Salahpour, A.; Umapathy, D.; Breit, A.; Villeneuve, L.-R.; Zhu, W.Z.; Xiao, R.P.; Lakatta, E.G.; Bouvier, M.; et al. β1/ β2-adrenergic receptor heterodimerization regulates beta 2-adrenergic receptor internalization and ERK signaling efficacy. J. Biol. Chem. 2002, 277, 35402–35410. [Google Scholar] [CrossRef] [PubMed]
- Hague, C.; Uberti, M.A.; Chen, Z.; Hall, R.A.; Minneman, K.P. Cell surface expression of alpha1D-adrenergic receptors is controlled by heterodimerization with alpha1B-adrenergic receptors. J. Biol. Chem. 2004, 279, 15541–15549. [Google Scholar] [CrossRef] [PubMed]
- Small, K.M.; Schwarb, M.R.; Glinka, C.; Theiss, C.T.; Brown, K.M.; Seman, C.A.; Liggett, S.B. α2A- and α2C-adrenergic receptors form homo- and heterodimers: The heterodimeric state impairs agonist-promoted GRK phosphorylation and beta-arrestin recruitment. Biochemistry 2006, 45, 4760–4767. [Google Scholar] [CrossRef] [PubMed]
- Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Kanareikina, M.A.; Kalinina, N.I.; Sysoeva, V.Y.; Dyikanov, D.T.; Stambolsky, D.V.; Tkachuk, V.A. Activation of β-adrenergic receptors is required for elevated α1A-adrenoreceptors expression and signaling in mesenchymal stromal cells. Sci. Rep. 2016, 6, 32835. [Google Scholar] [CrossRef]
- Uberti, M.A.; Hague, C.; Oller, H.; Minneman, K.P.; Hall, R.A. Heterodimerization with beta2-adrenergic receptors promotes surface expression and functional activity of alpha1D-adrenergic receptors. J. Pharmacol. Exp. Ther. 2005, 313, 16–23. [Google Scholar] [CrossRef]
- Xu, J.; He, J.; Castleberry, A.M.; Balasubramanian, S.; Lau, A.G.; Hall, R.A. Heterodimerization of alpha 2A- and beta 1-adrenergic receptors. J. Biol. Chem. 2003, 278, 10770–10777. [Google Scholar] [CrossRef]
- Hague, C.; Lee, S.E.; Chen, Z.; Prinster, S.C.; Hall, R.A.; Minneman, K.P. Heterodimers of alpha1B- and alpha1D-adrenergic receptors form a single functional entity. Mol. Pharmacol. 2006, 69, 45–55. [Google Scholar] [CrossRef]
- Hansen, L.V.; Gårdsvoll, H.; Nielsen, B.S.; Lund, L.R.; Danø, K.; Jensen, O.N.; Ploug, M. Structural analysis and tissue localization of human C4.4A: A protein homologue of the urokinase receptor. Biochem. J. 2004, 380 Pt 3, 845–857. [Google Scholar] [CrossRef]
- Würfel, J.; Seiter, S.; Stassar, M.; Claas, A.; Kläs, R.; Rösel, M.; Marhaba, R.; Savelyeva, L.; Schwab, M.; Matzku, S.; et al. Cloning of the human homologue of the metastasis-associated rat C4.4A. Gene 2001, 262, 35–41. [Google Scholar] [CrossRef]
- Hansen, L.V.; Skov, B.G.; Ploug, M.; Pappot, H. Tumour cell expression of C4.4A, a structural homologue of the urokinase receptor, correlates with poor prognosis in non-small cell lung cancer. Lung Cancer 2007, 58, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.V.; Laerum, O.D.; Illemann, M.; Nielsen, B.S.; Ploug, M. Altered expression of the urokinase receptor homologue, C4.4A, in invasive areas of human esophageal squamous cell carcinoma. Int. J. Cancer 2008, 122, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.; Muley, T.; Meister, M.; Dienemann, H.; Christensen, I.J.; Santoni-Rugiu, E.; Lærum, O.D.; Ploug, M. Ly6/uPAR-related protein C4.4A as a marker of solid growth pattern and poor prognosis in lung adenocarcinoma. J. Thorac. Oncol. 2013, 8, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Ito, T.; Yanai, A.; Inoue, N.; Miyagawa, Y.; Murase, K.; Imamura, M.; Ichii, S.; Takatsuka, Y.; Nishizaki, T.; et al. C4.4A highly expressed in HER2-positive human breast cancers may indicate a good prognosis. Breast Cancer 2015, 22, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Ngora, H.; Galli, U.M.; Miyazaki, K.; Zöller, M. Membrane-bound and exosomal metastasis-associated C4.4A promotes migration by associating with the α(6)β(4) integrin and MT1-MMP. Neoplasia 2012, 14, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lin, S.; Li, J.-L.; Nakagawa, H.; Chen, Z.; Jin, B.; Tian, L.; Ucar, D.A.; Shen, H.; Lu, J.; et al. Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene 2012, 31, 469–479. [Google Scholar] [CrossRef]
- Esselens, C.W.; Malapeira, J.; Colomé, N.; Moss, M.; Canals, F.; Arribas, J. Metastasis-associated C4.4A, a GPI-anchored protein cleaved by ADAM10 and ADAM17. Biol Chem. 2008, 389, 1075–1084. [Google Scholar] [CrossRef][Green Version]
- Thuma, F.; Ngora, H.; Zöller, M. The metastasis-associated molecule C4.4A promotes tissue invasion and anchorage independence by associating with the alpha6beta4 integrin. Mol Oncol. 2013, 7, 917–928. [Google Scholar] [CrossRef]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Harner-Foreman, N.; Vadakekolathu, J.; Laversin, S.A.; Mathieu, M.G.; Reeder, S.; Pockley, A.G.; Rees, R.C.; Boocock, D.J. A novel spontaneous model of epithelial-mesenchymal transition (EMT) using a primary prostate cancer derived cell line demonstrating distinct stem-like characteristics. Sci. Rep. 2017, 7, 40633. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, R.; Yamamoto, H.; Takahashi, H.; Ohtsuka, M.; Wu, X.; Nishimura, J.; Takemasa, I.; Mizushima, T.; Ikeda, M.; Sekimoto, M.; et al. C4.4A is associated with tumor budding and epithelial-mesenchymal transition of colorectal cancer. Cancer Sci. 2012, 103, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Vyas, F.S.; Hargreaves, A.J.; Bonner, P.L.R.; Boocock, D.J.; Coveney, C.; Dickenson, J.M. A1 adenosine receptor-induced phosphorylation and modulation of transglutaminase 2 activity in H9c2 cells: A role in cell survival. Biochem. Pharmacol. 2016, 107, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Balahmar, R.M.; Boocock, D.J.; Coveney, C.; Ray, S.; Vadakekolathu, J.; Regad, T.; Ali, S.; Sivasubramaniam, S. Identification and characterisation of NANOG+/ OCT-4high/SOX2+ doxorubicin-resistant stem-like cells from transformed trophoblastic cell lines. Oncotarget 2018, 9, 7054. [Google Scholar] [CrossRef]
- Available online: https://www.cancer.gov/tcga (accessed on 14 March 2018).
- Available online: https://gtexportal.org/home/ (accessed on 14 March 2018).
- Deutsch, E.W.; Csordas, A.; Sun, Z.; Jarnuczak, A.; Perez-Riverol, Y.; Ternent, T.; Ternent, T.; Campbell, D.S.; Bernal-Llinares, M.; Okuda, S.; et al. The ProteomeXchange consortium in 2017: Supporting the cultural change in proteomics public data deposition. Nucleic Acids Res. 2017, 45, D1100–D1106. [Google Scholar] [CrossRef]
- Vizcaíno, J.A.; Csordas, A.; Del-Toro, N.; Dianes, J.A.; Griss, J.; Lavidas, I.; Mayer, G.; Perez-Riverol, Y.; Reisinger, F.; Ternent, T.; et al. 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 2016, 44, D447–D456. [Google Scholar] [CrossRef]


| Lysate | Secretome | |||||
|---|---|---|---|---|---|---|
| Treatment (vs UT) | Protein | Swiss-Prot ID | Log2 Fold Change | Protein | SwissProt ID | Log2 Fold Change |
| NE | LYPD3 | O95274 | 2.235 | IF5A1 | P63241 | 2.181 |
| CRYAB | P02511 | 1.342 | H2BFS | P57053 | 2.066 | |
| NDRG1 | Q92597 | 1.021 | LYPD3 | O95274 | 1.577 | |
| CASPE | P31944 | 1.015 | H4 | P62805 | 1.489 | |
| AATM | P10809 | −1.010 | STC1 | P52823 | 1.077 | |
| CH60 | P10809 | −1.141 | LIF | P15018 | −2.090 | |
| HINT2 | Q9BX68 | −1.206 | ||||
| MDHM | P40926 | −1.315 | ||||
| ECH1 | Q13011 | −1.431 | ||||
| H14 | P10412 | −1.515 | ||||
| ODO4 | P36957 | −1.520 | ||||
| H12 | P16403 | −1.574 | ||||
| ISO | IF172 | Q9UG01 | 2.684 | DPY30 | Q9C005 | 1.320 |
| LYPD3 | O95274 | 2.564 | ADML | P35318 | 1.142 | |
| CRYAB | P02511 | 1.413 | H4 | P62805 | 1.078 | |
| ANXA1 | P04083 | 1.065 | ||||
| CTGF | P29279 | −1.151 | ||||
| UBA3 | Q8TBC4 | −1.995 | ||||
| LAMC1 | P11047 | −3.499 | ||||
| ICI 118,551 | S10-A7 | P31151 | 1.271 | NDKA | P15531 | 3.784 |
| IF5A1 | P63241 | 2.252 | ||||
| S10A6 | P06703 | 1.651 | ||||
| ANXA2 | P07355 | 1.311 | ||||
| DPY30 | Q9C005 | 1.286 | ||||
| EDF1 | O60869 | −2.432 | ||||
| ICI 118,551 + NE | KRT36 | O76013 | 2.160 | CRK | P46108 | 2.591 |
| CRYAB | P62750 | 1.776 | NUCL | P19338 | 1.947 | |
| NDRG1 | P02768 | −1.067 | HS90A | P07900 | 1.887 | |
| APOA1 | P02647 | 1.769 | ||||
| ANXA2 | P07355 | 1.694 | ||||
| CUTA | O60888 | 1.642 | ||||
| STC1 | P52823 | 1.474 | ||||
| FETUA | P02765 | 1.418 | ||||
| LYPD3 | O95274 | 1.308 | ||||
| H4 | P62805 | 1.140 | ||||
| S100P | P25815 | 1.139 | ||||
| SFRP1 | Q8N474 | 1.136 | ||||
| TSP1 | P07996 | −1.280 | ||||
| EDF1 | O60869 | −4.362 | ||||
| BSSP4 | Q0GZN4 | −4.870 | ||||
| Lysate | Secretome | |||||
|---|---|---|---|---|---|---|
| Treatment (vs UT) | Protein | Swiss-Prot ID | Log2 Fold Change | Protein | Swiss-Prot ID | Log2 Fold Change |
| NE | No significantly changed proteins | STC1 | P52823 | 1.385 | ||
| PPI1A | P62937 | 1.106 | ||||
| CSF1 | P09603 | −1.025 | ||||
| B2MG | P61769 | −1.145 | ||||
| LFNG | Q8NES3 | −1.146 | ||||
| PTX3 | P26022 | −1.151 | ||||
| CUL5 | Q93034 | −1.160 | ||||
| HUWE1 | Q7Z6Z7 | −1.172 | ||||
| ANR28 | O15084 | −1.226 | ||||
| UROK | P00749 | −1.364 | ||||
| ITIH2 | P19823 | −1.383 | ||||
| CTGF | P29279 | −1.443 | ||||
| TSP1 | P07996 | −1.461 | ||||
| KI13B | Q9NQT8 | −2.489 | ||||
| ISO | G45IP | Q8TAE8 | 3.552 | STC1 | P52823 | 1.097 |
| ODBA | P12694 | 3.331 | KI13B | Q9NQT8 | −1.048 | |
| BAF | O75531 | −1.013 | PPT1 | P50897 | −1.062 | |
| DBNL | Q9UJU6 | −1.025 | CAD11 | P55287 | −1.088 | |
| S10A4 | P26447 | −1.037 | CSF1 | P09603 | −1.109 | |
| THIO | P10599 | −1.074 | HUWE1 | Q7Z6Z7 | −1.164 | |
| NEDD8 | Q15843 | −1.117 | TSP1 | P07996 | −1.219 | |
| ANXA2 | P07355 | −1.129 | CTGF | P29279 | −1.446 | |
| CYTB | P04080 | −1.171 | UROK | P00749 | −1.564 | |
| PEBP1 | P30086 | −1.240 | CATD | P07339 | −2.309 | |
| PRDX3 | P30048 | −1.273 | ANR28 | O15084 | −2.313 | |
| LEG1 | P09382 | −1.329 | ||||
| GLRX3 | O76003 | −1.526 | ||||
| ANXA5 | P08758 | −2.254 | ||||
| TGM2 | P21980 | −4.288 | ||||
| ICI 118,551 | GLSK | O94925 | 1.757 | HUWE1 | Q7Z6Z7 | −1.053 |
| 2A5E | Q16537 | 1.179 | ANR28 | O15084 | −1.581 | |
| LEG1 | P09382 | −1.017 | ||||
| PRDX3 | P30048 | −1.027 | ||||
| ANX11 | P50995 | −1.097 | ||||
| OST48 | P39656 | −1.141 | ||||
| ANXA2 | P07355 | −1.491 | ||||
| CALX | P27824 | −1.565 | ||||
| S10AA | P60903 | −1.615 | ||||
| ANXA4 | P09525 | −1.670 | ||||
| PEBP1 | P30086 | −1.788 | ||||
| GALT2 | Q10471 | −1.927 | ||||
| DNJA2 | O60884 | −2.297 | ||||
| CP1B1 | Q16678 | −3.311 | ||||
| ICI 118,551 + NE | ODBA | P12694 | 3.341 | RLA2 | P62805 | 1.019 |
| S10AA | P60903 | −1.077 | CUL5 | Q93034 | −1.049 | |
| ANX11 | P50995 | −1.223 | H4 | P62805 | −1.071 | |
| ANXA2 | P07355 | −1.460 | TCRG1 | O14776 | −1.078 | |
| ANXA4 | P09525 | −1.779 | BGH3 | Q15582 | −1.079 | |
| CP013 | Q96S19 | −2.699 | PPT1 | P50897 | −1.175 | |
| CYTS | P01037 | −1.180 | ||||
| TSP1 | P07996 | −1.230 | ||||
| CYTN | P01037 | −1.298 | ||||
| SRPX | P78539 | −1.345 | ||||
| HUWE1 | Q7Z6Z7 | −1.454 | ||||
| ITIH2 | P19823 | −1.509 | ||||
| FINC | P02751 | −1.510 | ||||
| ANR28 | O15084 | −1.930 | ||||
| KI13B | Q9NQT8 | −2.080 | ||||
| LYPD3 Score | |||
|---|---|---|---|
| Negative | Positive | Chi Square (p-Value) | |
| Age | |||
| <40 | 55 (91.7%) | 5 (8.3%) | 2.885 (0.236) |
| 40–59 | 179 (84.8%) | 32 (15.2%) | |
| >60 | 43 (91.5%) | 4 (8.5%) | |
| Tumour Grade | |||
| 1 | 39 (14.1%) | 2 (4.9%) | 10.034 (0.074) |
| 1–2 | 4 (1.4%) | 1 (2.4%) | |
| 2 | 105 (37.9%) | 19 (46.3%) | |
| 2–3 | 0 (0) | 1 (2.4%) | |
| 3 | 64 (23.1%) | 9 (22%) | |
| Tumour stage | |||
| I | 18 (6.5%) | 2 (4.9%) | 15.712 (0.028) |
| IIA | 102 (36.8%) | 8 (19.5%) | |
| IIB | 32 (11.6%) | 4 (9.8%) | |
| IIIA | 5 (1.8%) | 3 (7.3%) | |
| IIIB | 19 (6.9%) | 3 (7.3%) | |
| IV | 5 (1.8%) | 1 (2.4%) | |
| Oestrogen receptor status | |||
| Negative | 137 (69.2%) | 16 (76.2%) | 0.442 (0.506) |
| Positive | 61 (30.8%) | 5 (23.8%) | |
| HER2 status | |||
| Negative | 162 (81.4%) | 14 (66.7%) | 2.580 (0.108) |
| Positive | 37 (18.6%) | 7 (33.3%) | |
| Tissue pathology status | |||
| Malignant primary breast tumour | 219 (86.9%) | 33 (13.1%) | 3.535 (0.171) |
| Metastasis | 39 (83%) | 8 (17%) | |
| Adjacent normal breast tissue | 19 (100%) | 0 (0%) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruet, M.; Cotton, D.; Coveney, C.; Boocock, D.J.; Wagner, S.; Komorowski, L.; Rees, R.C.; Pockley, A.G.; Garner, A.C.; Wallis, J.D.; et al. β2-Adrenergic Signalling Promotes Cell Migration by Upregulating Expression of the Metastasis-Associated Molecule LYPD3. Biology 2020, 9, 39. https://doi.org/10.3390/biology9020039
Gruet M, Cotton D, Coveney C, Boocock DJ, Wagner S, Komorowski L, Rees RC, Pockley AG, Garner AC, Wallis JD, et al. β2-Adrenergic Signalling Promotes Cell Migration by Upregulating Expression of the Metastasis-Associated Molecule LYPD3. Biology. 2020; 9(2):39. https://doi.org/10.3390/biology9020039
Chicago/Turabian StyleGruet, Michael, Daniel Cotton, Clare Coveney, David J. Boocock, Sarah Wagner, Lucie Komorowski, Robert C. Rees, A. Graham Pockley, A. Christopher Garner, John D. Wallis, and et al. 2020. "β2-Adrenergic Signalling Promotes Cell Migration by Upregulating Expression of the Metastasis-Associated Molecule LYPD3" Biology 9, no. 2: 39. https://doi.org/10.3390/biology9020039
APA StyleGruet, M., Cotton, D., Coveney, C., Boocock, D. J., Wagner, S., Komorowski, L., Rees, R. C., Pockley, A. G., Garner, A. C., Wallis, J. D., Miles, A. K., & Powe, D. G. (2020). β2-Adrenergic Signalling Promotes Cell Migration by Upregulating Expression of the Metastasis-Associated Molecule LYPD3. Biology, 9(2), 39. https://doi.org/10.3390/biology9020039







