Rapid Efficacy of Gemtuzumab Ozogamicin in Refractory AML Patients with Pulmonary and Kidney Failure
Abstract
1. Introduction
2. Clinical Cases
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rabbat, A.; Chaoui, D.; Montani, D.; Legrand, O.; Lefebvre, A.; Rio, B.; Roche, N.; Lorut, C.; Marie, J.P.; Huchon, G. Prognosis of patients with acute myeloid leukaemia admitted to intensive care. Br. J. Haematol. 2005, 129, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Mokart, D.; Pène, F.; Lambert, J.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Laisne, L.M. Outcomes of Critically Ill Patients with Hematologic Malignancies: Prospective Multicenter Data From France and Belgium—A Groupe de Recherche Respiratoire en Re´animation Onco-He´matologique Study. J. Clin. Oncol. 2013, 31, 2810–2818. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Schlenk, R.; Heuser, M.; Ganser, A. How I treat refractory and early relapsed acute myeloid leukemia. Blood 2015, 126, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Suciu, S.; Selleslag, D.; Aversa, F.; Gaidano, G.; Musso, M.; Annino, L.; Venditti, A.; Voso, M.T.; Mazzone, C. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J. Clin. Oncol. 2016, 34, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Medeiros, B.C.; Gardner, K.M.; Orlowski, K.F.; Gallegos, L.; Scott, B.L.; Hendrie, P.C.; Estey, E.H. Gemtuzumab ozogamicin in combination with vorinostat and azacitidine in older patients with relapsed or refractory acute myeloid leukemia: A phase I/II study. Haematologica 2014, 99, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.C.; Tanaka, T.N.; Balaian, L.; Bashey, A.; Guzdar, A.; Li, H.; Messer, K.; Ball, E.D. A Phase I/II Trial of the Combination of Azacitidine and Gemtuzumab Ozogamicin for Treatment of Relapsed Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2018, 18, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Balaian, L.; Ball, E.D. Cytotoxic activity of gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia correlates with the expression of protein kinase Syk. Leukemia 2006, 20, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Ganzel, C.; Manola, J.; Douer, D.; Rowe, J.M.; Fernandez, H.F.; Paietta, E.M.; Litzow, M.R.; Lee, J.W.; Luger, S.M.; Lazarus, H.M. Extramedullary Disease in Adult Acute Myeloid Leukemia Is Common but Lacks Independent Significance: Analysis of Patients in ECOG-ACRIN Cancer Research Group Trials, 1980–2008. J. Clin. Oncol. 2016, 34, 3544–3553. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.J.; Parisi, M.T.; Hijiya, N.; Meshinchi, S.; Cooper, T.; Tarlock, K. Clinical and Radiographic Response of Extramedullary Leukemia in Patients Treated With Gemtuzumab Ozogamicin. J. Pediatr. Hematol. Oncol. 2019, 41, e174–e176. [Google Scholar] [CrossRef]
- Owonikoko, T.; Agha, M.; Balassanian, R.; Smith, R.; Raptis, A. Gemtuzumab therapy for isolated extramedullary AML relapse following allogeneic stem-cell transplant. Nature Clinical Practice. Oncology 2007, 4, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Mitani, N.; Matsunaga, K.; Nakazora, T.; Gondo, T.; Yujiri, T.; Tanizawa, Y. Gemtuzumab ozogamicin therapy for isolated extramedullary AML relapse after allogeneic hematopoietic stem-cell transplantation. J. Exp. Med. 2010, 220, 121–126. [Google Scholar] [CrossRef]
- Amadori, S. New agents for the treatment of acute myeloid leukemia: Gemtuzumab ozogamicin. Hematol. Meet. Rep. 2008, 2, 69–71. [Google Scholar]
- Wunderlich, M.; Stockman, C.; Devarajan, M.; Ravishankar, N.; Sexton, C.; Kumar, A.R.; Mizukawa, B.; Mulloy, J.C. A xenograft model of macrophage activation syndrome amenable to anti-CD33 and anti-IL-6R treatment. JCI Insight 2016, 1, e88181. [Google Scholar] [CrossRef] [PubMed]
- Karakike, E.; Giamarellos-Bourboulis, E.J. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis. Front. Immunol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Sievers, E.L.; Stadtmauer, E.A.; Löwenberg, B.; Estey, E.H.; Dombret, H.; Theobald, M.; Voliotis, D.; Bennett, J.M.; Richie, M. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005, 104, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef] [PubMed]
| Sex Age | Male Patient. 74 Years Patient No. 2 | Male Patient. 54 Years Patient No. 3 |
|---|---|---|
| Diagnosis | AML without maturation (NOS, WHO 2016). Intermediate risk group (ELN2017). Normal karyotype. Primary refractory disease. | AML with maturation (NOS, WHO 2016). Add (21) (q22). Intermediate risk group (ELN2017). Primary refractory disease. |
| Clinical data | A patient with a progression disease during the treatment by azacitidine. Increasing blast cells in blood and marrow by more than 50% from baseline after 2 cycles of azacytidine therapy. | A patient with a progression disease during the treatment by azacitidine. Increasing blast cells in blood and marrow by more than 50% from baseline after 2 cycles of azacytidine therapy. |
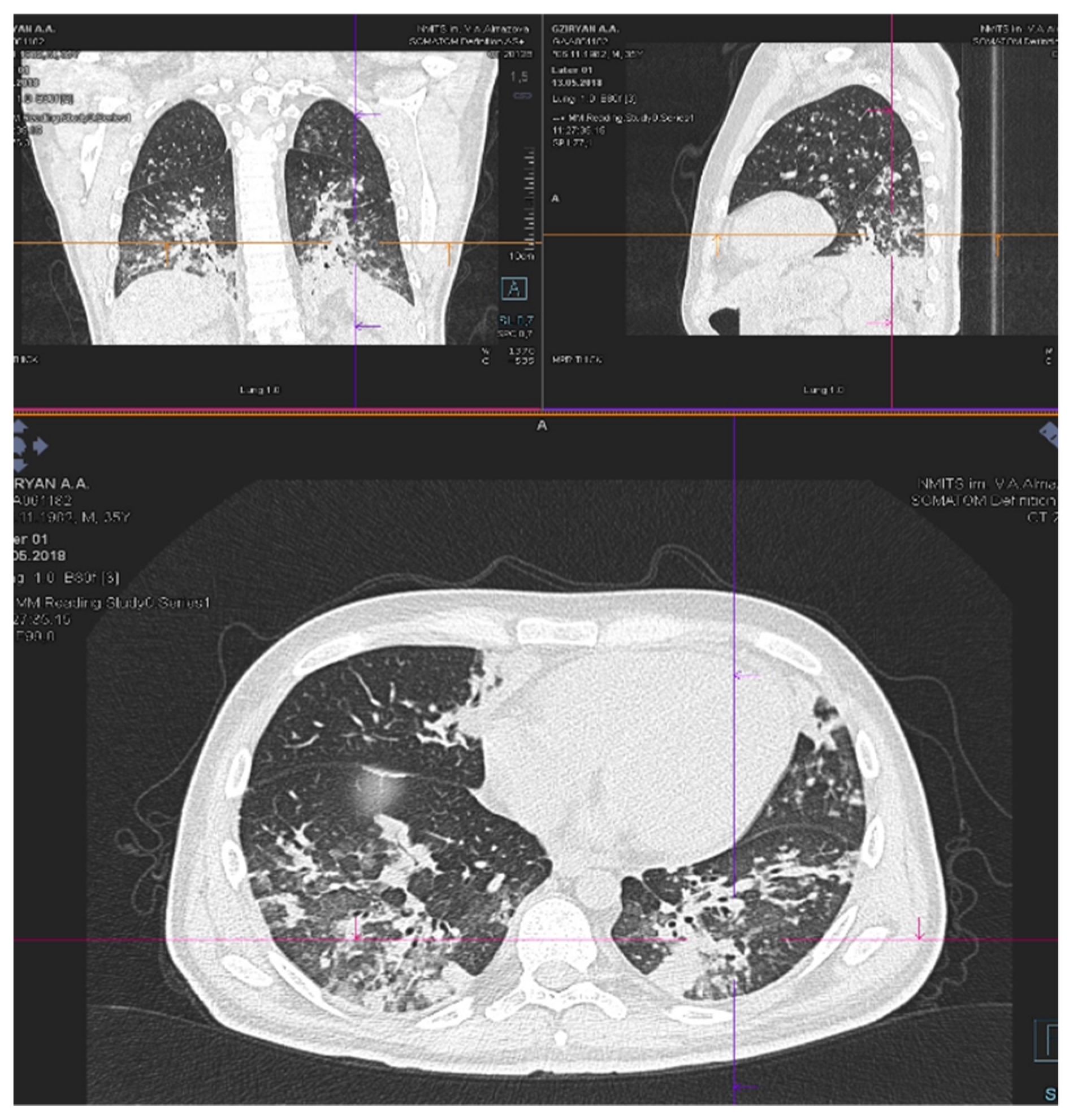
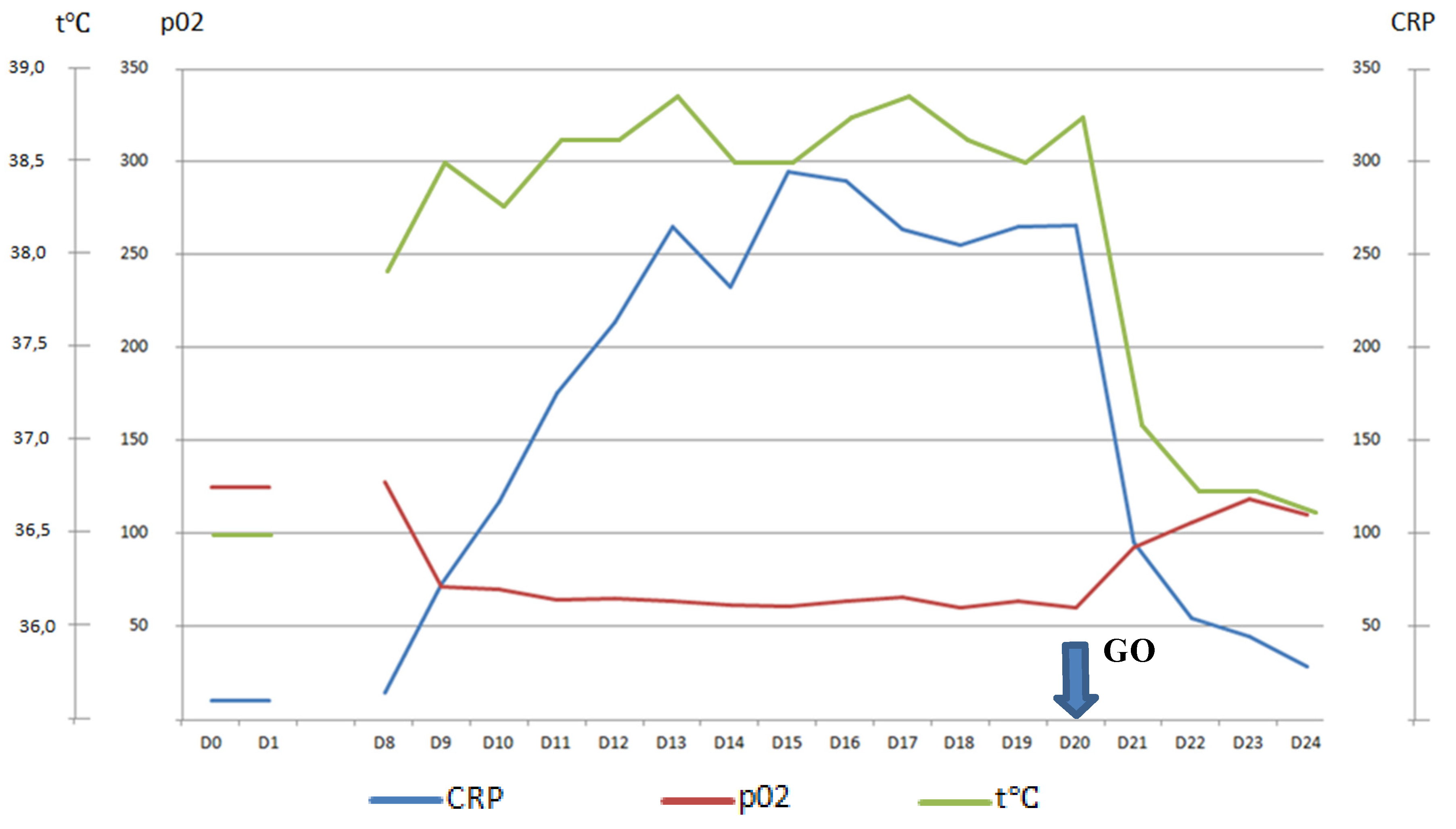
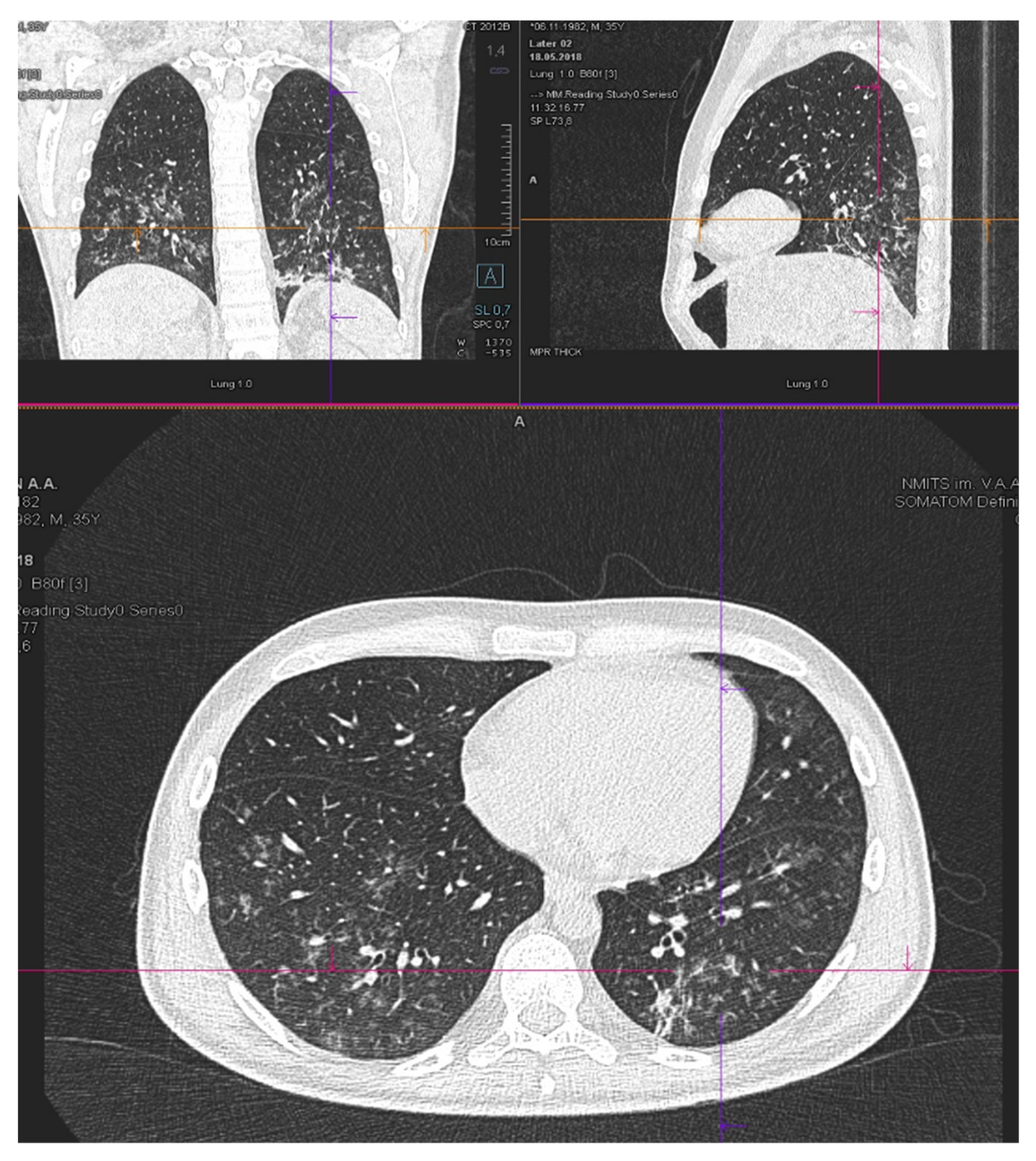
| Patient status before GO therapy initiation | The WHO performance status of 3. Marrow blast cells 88.6%, peripheral blood blast cells 60%, pancytopenia grade 3–4. CRP was slightly increased to 20 mg/L. Acute kidney failure grade 2 with no prior history of chronic kidney disease dehydration, use of nephrotoxic agents or tumor lysis syndrome signs. Creatinine increasing up to 2.8xULN and GFR decline to 15 mL/min. | The WHO performance status of 3. Marrow blasts cells 68%. High fever and elevated CRP level up to 332 mg/L with no response to escalated antibiotics/antimycotics combination. The patient had respiratory failure grade 2 with massive bilateral polysegmental lung infiltrates according to a chest CT scan. |
| Regimen of therapy with GO | «GO » 1 cycle «GO+Aza» 1 cycle | «GO » 1 cycle |
| Response to therapy | The WHO performance status improved to grade 2. | The WHO performance status improved to grade 2. Apyrexia was achieved on day 3 of the GO therapy. |
| Kidney function began to improve immediately after GO infusion. Creatinine started to decrease on day 1 of the therapy and returned to normal value on day 6 (GFR elevated up to 72 mL/min on day 6). Thus, recovery after acute kidney injury occurred on day 6. There was blast clearance in peripheral blood on day 5 after GO therapy. | CRP level started to drop on day 1 of the therapy (CRP on day 2—250 mg/L, on day 7—60 mg/L) | |
| On day 5 after GO infusion «GO+Aza» therapy was initiated. No laboratory signs of kidney injury were noticed during whole period of therapy in the «GO+Aza» regimen. | A chest CT scan on day 6 of the GO therapy showed a significant regression of pulmonary infiltrates in the size. | |
| Day 14 marrow blast cells 16.1%. | Day 7 marrow blast cells 46%. Thus, blast cell reduction was achieved on day 7 after GO infusion. | |
| Peripheral blood cell recovery was achieved on day 40 of the «GO+Aza» therapy. On day 40 the marrow blast cell was 1%. Thus, complete remission with peripheral blood cell recovery was achieved. | Patient became eligible for chemo intensification. On day 12 of the therapy, “7+3” was initiated. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaytsev, D.; Girshova, L.; Ivanov, V.; Budaeva, I.; Motorin, D.; Badaev, R.; Mirolubova, J.; Grobovenko, E.; Chitanava, T.; Zaykova, E.; et al. Rapid Efficacy of Gemtuzumab Ozogamicin in Refractory AML Patients with Pulmonary and Kidney Failure. Biology 2020, 9, 28. https://doi.org/10.3390/biology9020028
Zaytsev D, Girshova L, Ivanov V, Budaeva I, Motorin D, Badaev R, Mirolubova J, Grobovenko E, Chitanava T, Zaykova E, et al. Rapid Efficacy of Gemtuzumab Ozogamicin in Refractory AML Patients with Pulmonary and Kidney Failure. Biology. 2020; 9(2):28. https://doi.org/10.3390/biology9020028
Chicago/Turabian StyleZaytsev, Daniil, Larisa Girshova, Vladimir Ivanov, Irina Budaeva, Dmitri Motorin, Renat Badaev, Julia Mirolubova, Evgeni Grobovenko, Tamara Chitanava, Ekaterina Zaykova, and et al. 2020. "Rapid Efficacy of Gemtuzumab Ozogamicin in Refractory AML Patients with Pulmonary and Kidney Failure" Biology 9, no. 2: 28. https://doi.org/10.3390/biology9020028
APA StyleZaytsev, D., Girshova, L., Ivanov, V., Budaeva, I., Motorin, D., Badaev, R., Mirolubova, J., Grobovenko, E., Chitanava, T., Zaykova, E., Alexeeva, J., & Zaritskey, A. (2020). Rapid Efficacy of Gemtuzumab Ozogamicin in Refractory AML Patients with Pulmonary and Kidney Failure. Biology, 9(2), 28. https://doi.org/10.3390/biology9020028





