Time- and Zinc-Related Changes in Biomechanical Properties of Human Colorectal Cancer Cells Examined by Atomic Force Microscopy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Preparation of Zinc-Deficient Medium
2.3. Cell Culture
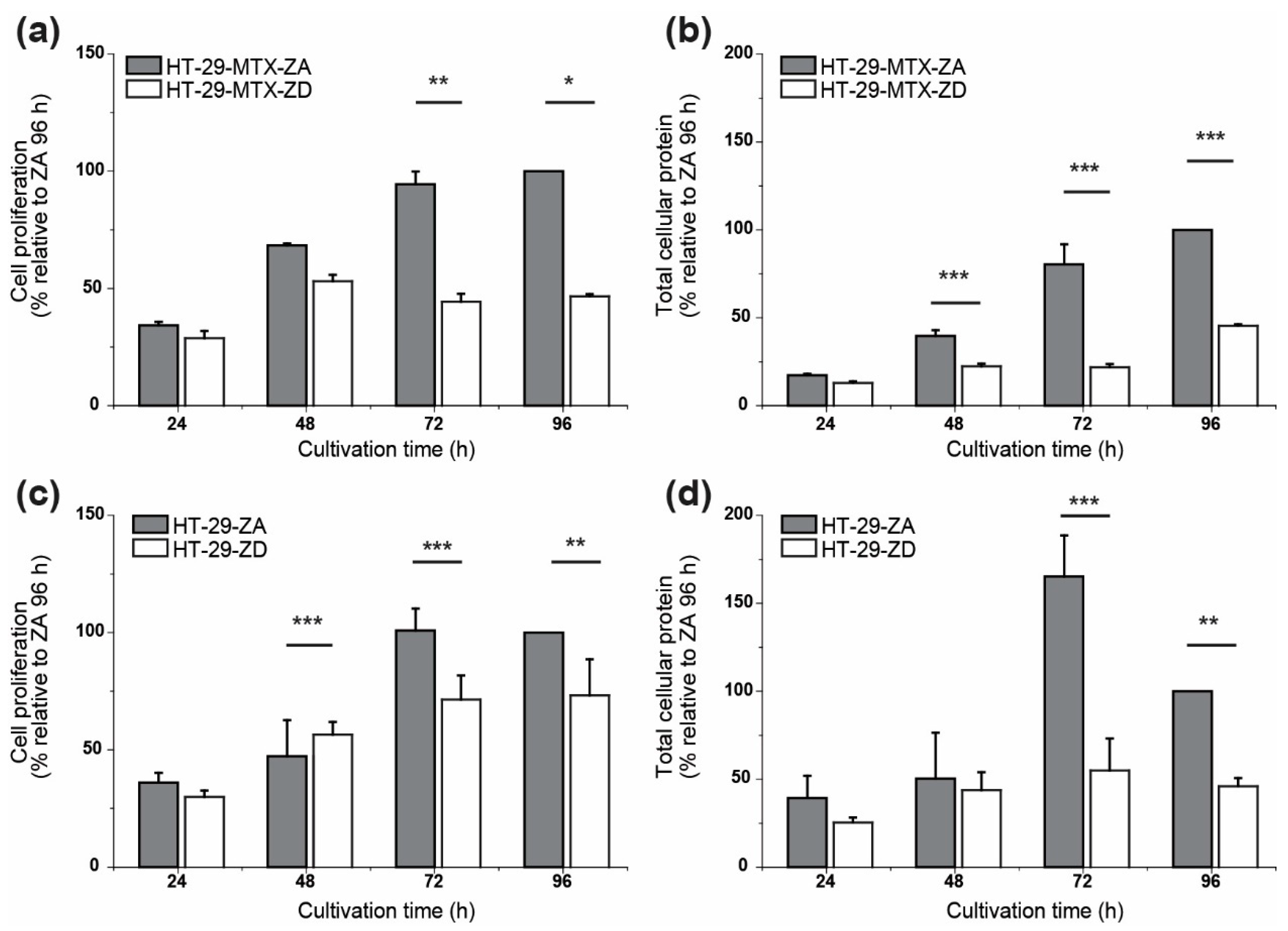
2.4. Cell Proliferation
2.5. Atomic Force Microscopy (AFM)
2.6. Data Analysis
2.6.1. Elastic Modulus E
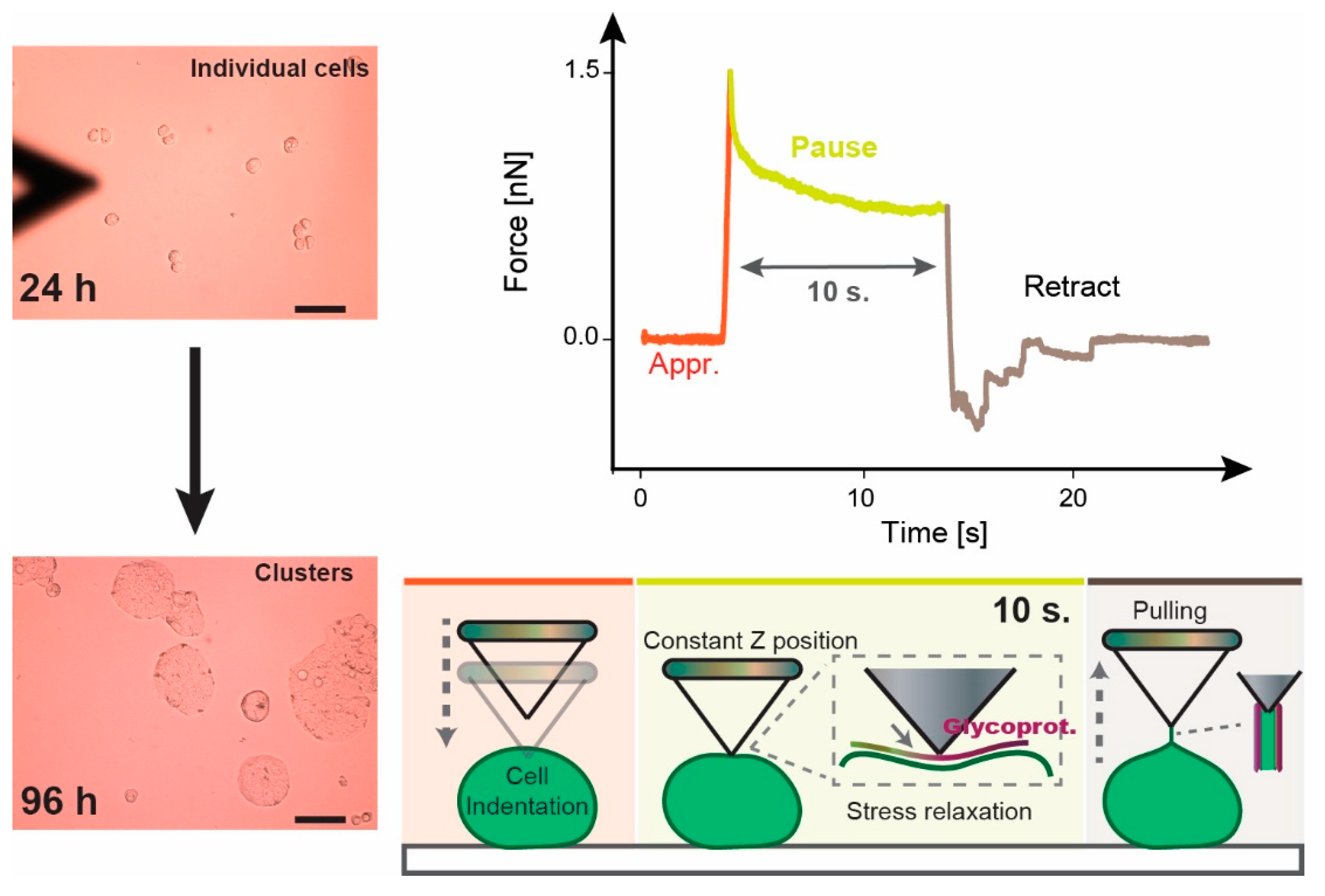
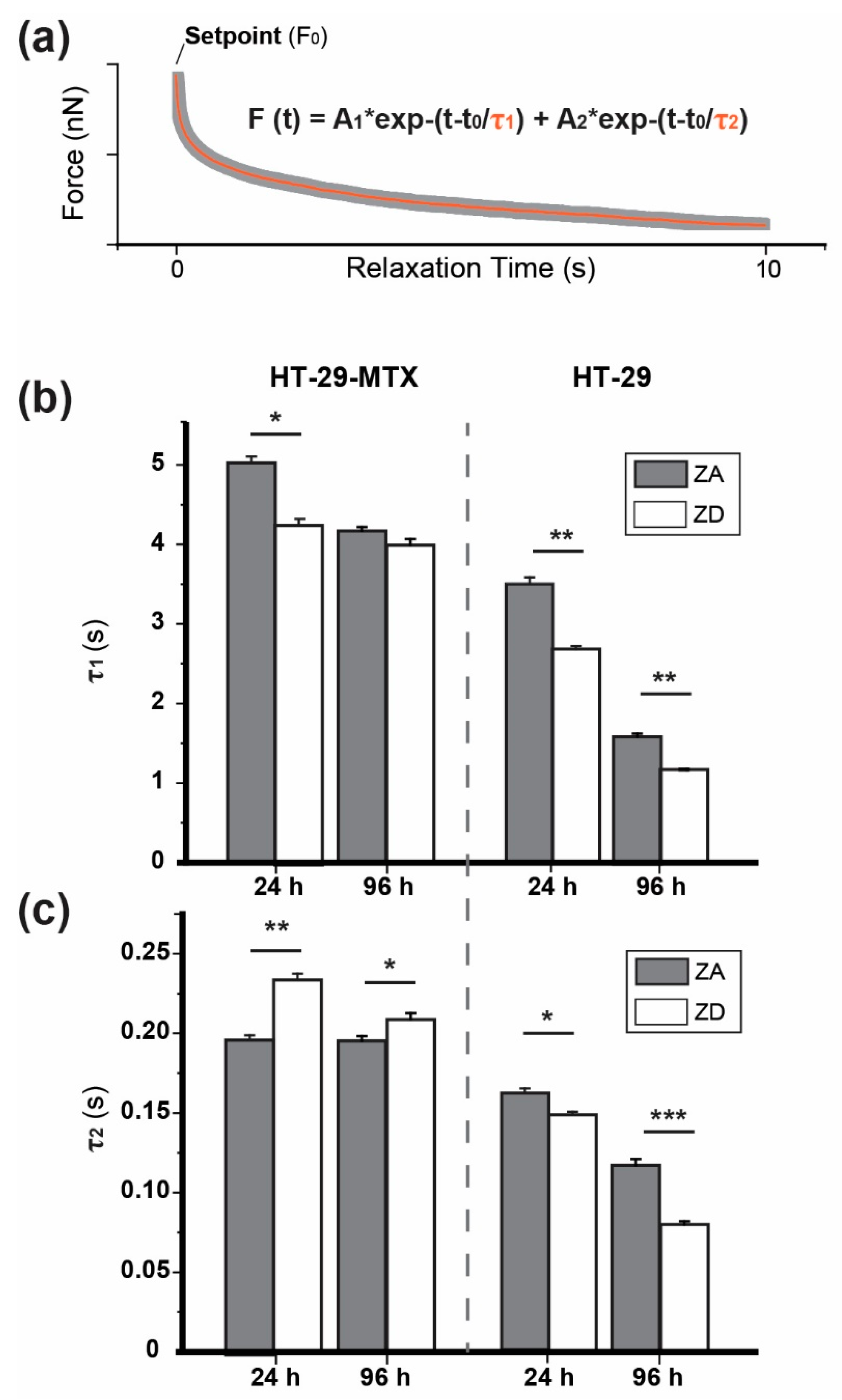
2.6.2. Stress Relaxation
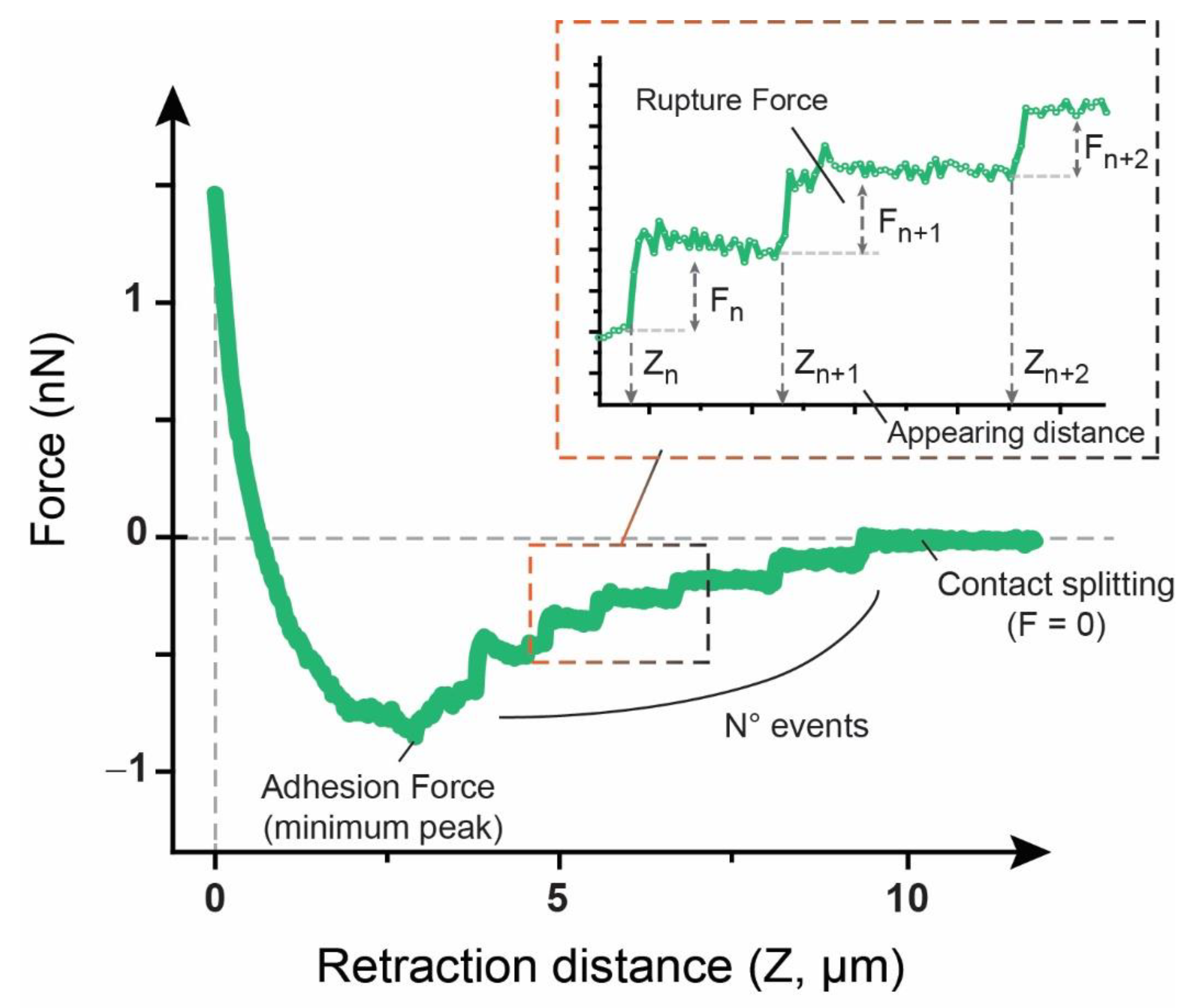
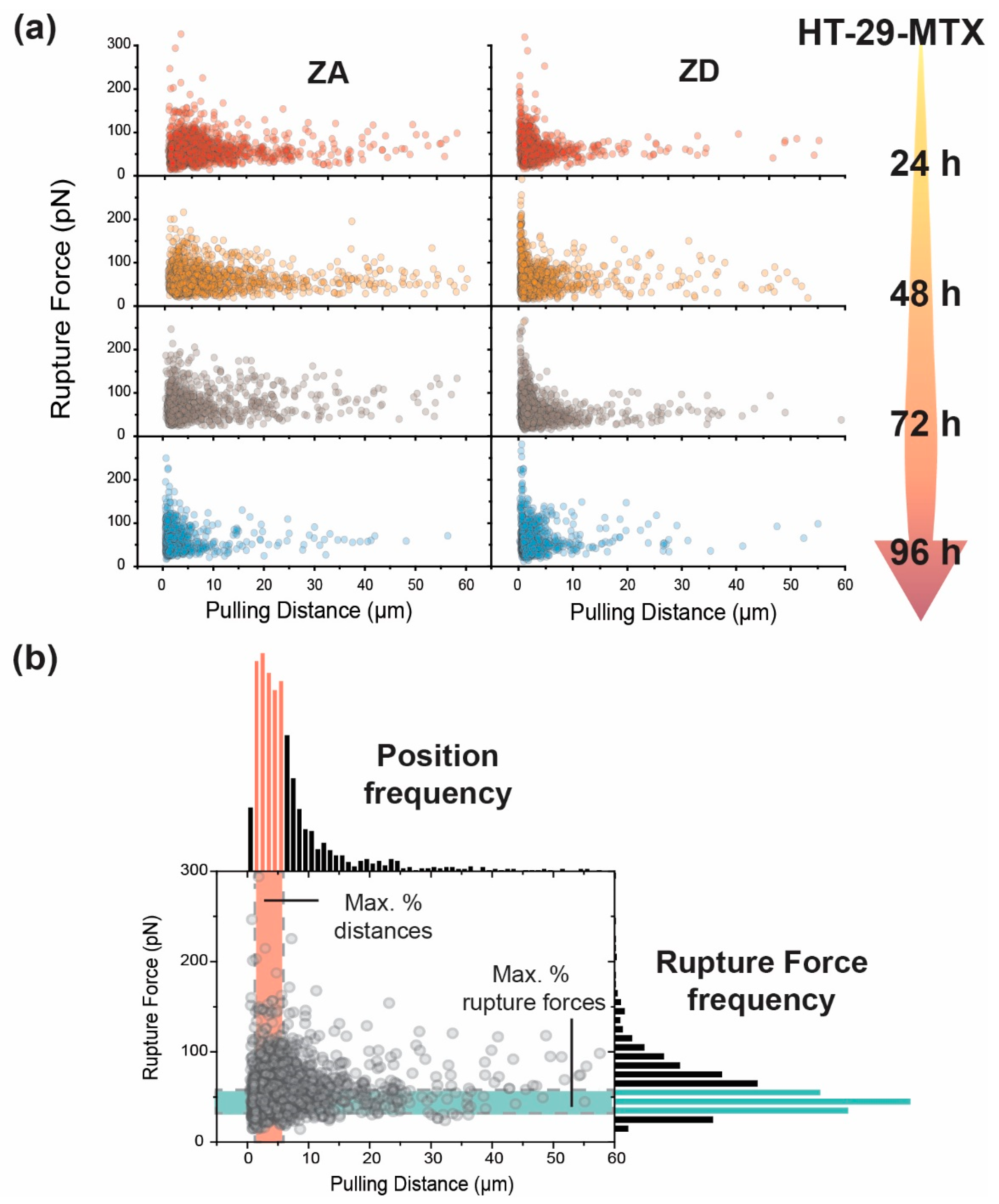
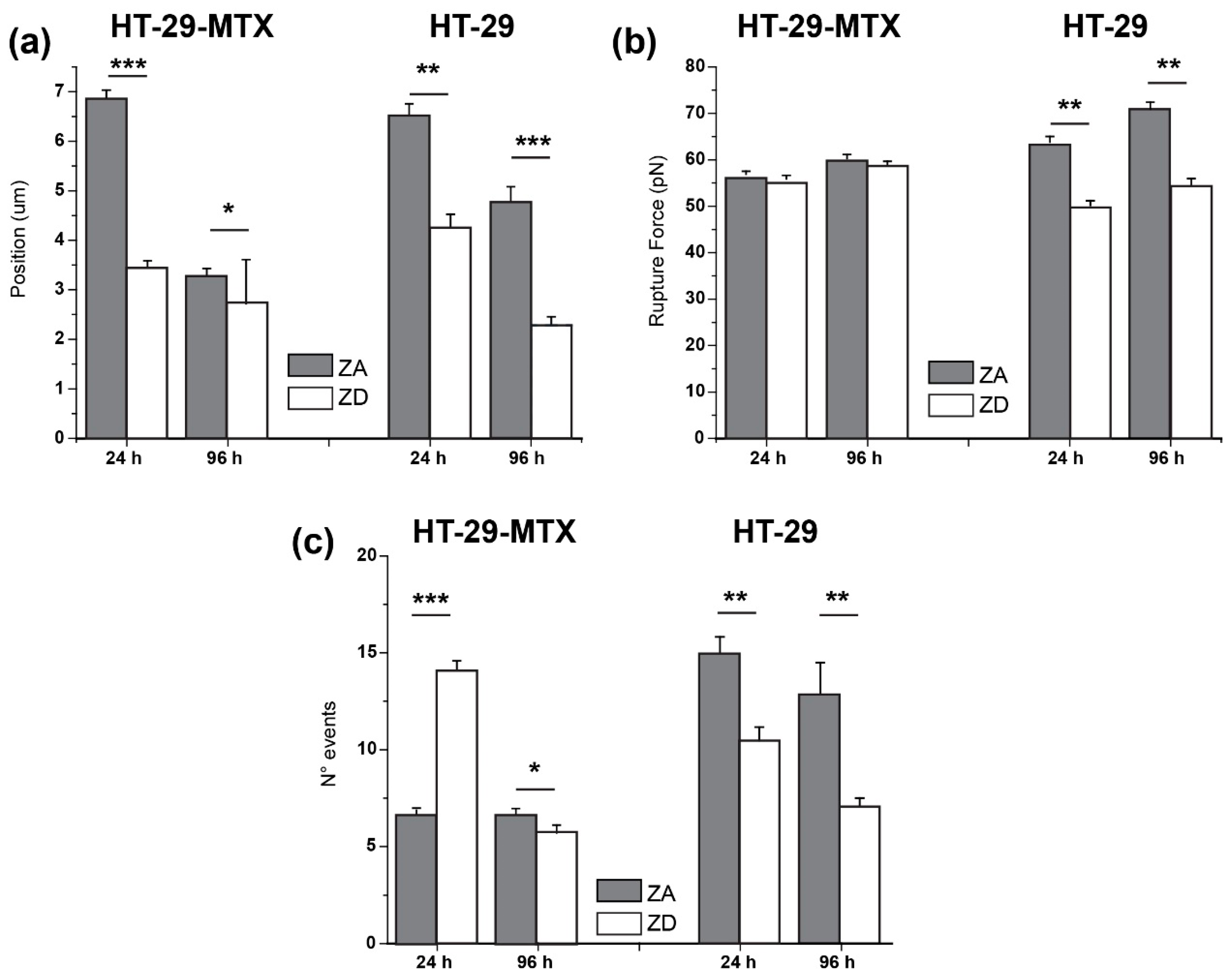
2.6.3. Adhesion Factors
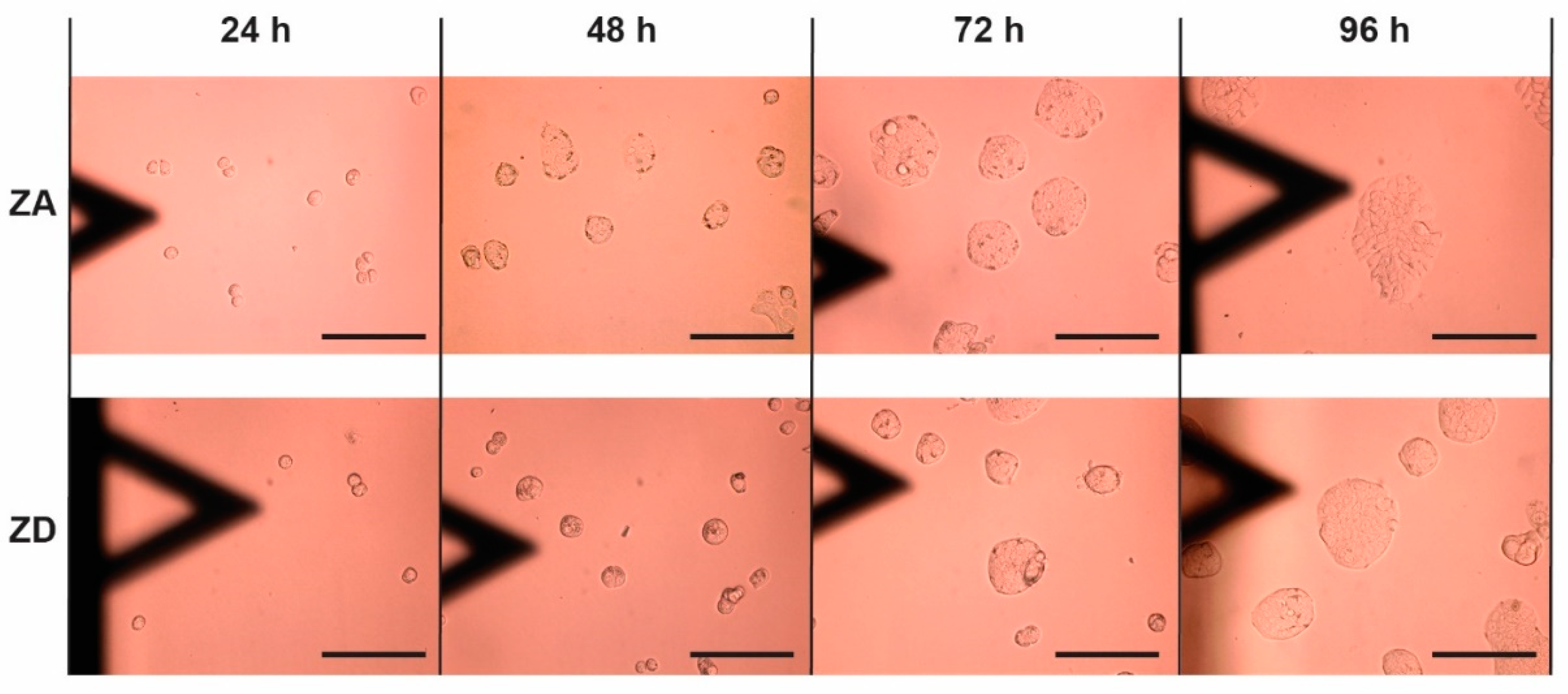
3. Results
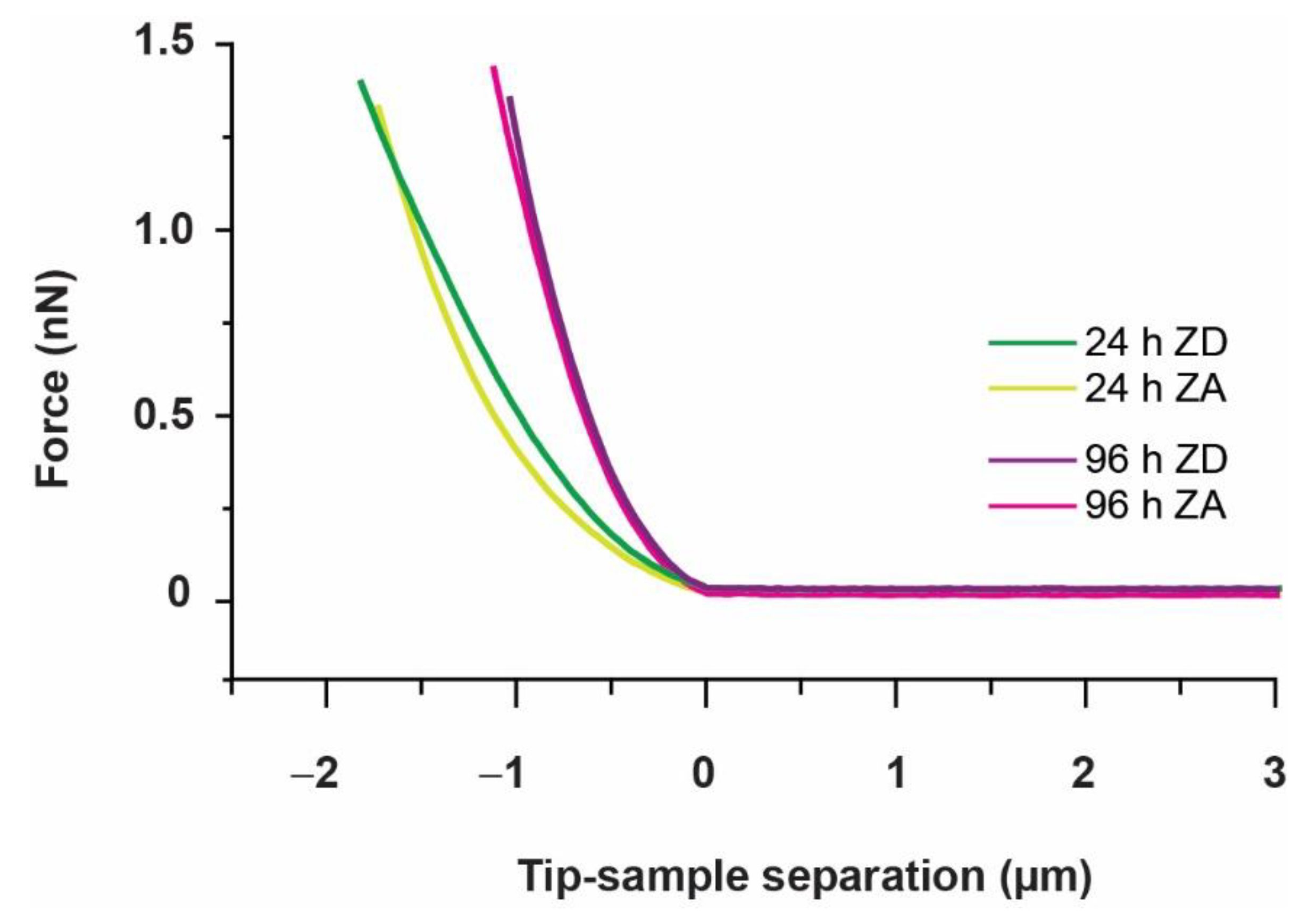
3.1. Approach and Pause: Elastic Modulus Determination and Stress Relaxation
3.2. Adhesion and Rupture Events
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A



| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| 24 h | 1.67 ± 0.08 kPa | 1.26 ± 0.05 kPa | 0.91 ± 0.05 kPa | 1.09 ± 0.06 kPa |
| 48 h | 1.25 ± 0.03 kPa | 1.37 ± 0.06 kPa | ||
| 72 h | 1.58 ± 0.07 kPa | 1.62 ± 0.08 kPa | ||
| 96 h | 2.85 ± 0.12 kPa | 2.62 ± 0.12 kPa | 1.28 ± 0.17 kPa | 0.85 ± 0.08 kPa |
| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| 24 h | τ1 = 5.02 ± 0.08 s | τ1 = 4.19 ± 0.06 s | τ1 = 3.51 ± 0.08 s | τ1 = 2.68 ± 0.04 s |
| τ2 = 0.19 ± 0.003 s | τ2 = 0.23 ± 0.003 s | τ2 = 0.16 ± 0.003 s | τ2 = 0.15 ± 0.002 s | |
| r2 = 0.9950 | r2 = 0.9958 | r2 = 0.9964 | r2 = 0.9976 | |
| 48 h | τ1 = 3.77 ± 0.05 s | τ1 = 4.33 ± 0.05 s | ||
| τ2 = 0.15 ± 0.004 s | τ2 = 0.16 ± 0.003 s | |||
| r2 = 0.9948 | r2 = 0.9957 | |||
| 72 h | τ1 = 5.83 ± 0.10 s | τ1 = 5.41 ± 0.12 s | ||
| τ2 = 0.24 ± 0.004 s | τ2 = 0.22 ± 0.005 s | |||
| r2 = 0.9958 | r2 = 0.9919 | |||
| 96 h | τ1 = 4.10 ± 0.05 s | τ1 = 3.99 ± 0.06 s | τ1 = 1.60 ± 0.08 s | τ1 = 1.13 ± 0.01 s |
| τ2 = 0.19 ± 0.004 s | τ2 = 0.21 ± 0.004 s | τ2 = 0.12 ± 0.004 s | τ2 = 0.08 ± 0.002 s | |
| r2 = 0.9962 | r2 = 0.9941 | r2 = 0.9839 | r2 = 0.9954 | |
| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| 24 h | 0.24 ± 0.02 nN | 0.59 ± 0.03 nN | 0.61 ± 0.03 nN | 0.37 ± 0.02 nN |
| 48 h | 0.34 ± 0.01 nN | 0.31 ± 0.01 nN | ||
| 72 h | 0.44 ± 0.02 nN | 0.39 ± 0.01 nN | ||
| 96 h | 0.32 ± 0.01 nN | 0.24 ± 0.01 nN | 0.47 ± 0.07 nN | 0.33 ± 0.02 nN |
| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| Mean Event Position | ||||
| 24 h | 6.86 ± 0.17 µm | 3.48 ± 0.14 µm | 6.49 ± 0.24 µm | 4.33 ± 0.23 µm |
| 48 h | 7.49 ± 0.22 µm | 3.74 ± 0.16 µm | ||
| 72 h | 7.23 ± 0.28 µm | 3.73 ± 0.14 µm | ||
| 96 h | 3.23 ± 0.15 µm | 2.69 ± 0.89 µm | 4.76 ± 0.31 µm | 2.28 ± 0.17 µm |
| Mean rupture Force | ||||
| 24 h | 56.9 ± 1.23 pN | 55.9 ± 0.76 pN | 63.8 ± 1.23 pN | 50.2 ± 1.01 pN |
| 48 h | 61.9 ± 0.65 pN | 59.4 ± 0.87 pN | ||
| 72 h | 73.0 ± 0.99 pN | 58.1 ± 0.84 pN | ||
| 96 h | 60.3 ± 0.83 pN | 58.8 ± 0.89 pN | 70.9 ± 1.49 pN | 54.3 ± 1.66 pN |
References
- Weber, A.; Iturri, J.; Benitez, R.; Zemljic-Jokhadar, S.; Toca-Herrera, J.L. Microtubule disruption changes endothelial cell mechanics and adhesion. Sci. Rep. 2019, 9, 14903. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Aroush, D.R.B.; Asnacios, A.; Chen, W.C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef]
- Moeendarbary, E.; Harris, A.R. Cell mechanics: Principles, practices, and prospects. Wiley Interdiscip. Rev. Syst. Biol. Med. 2014, 6, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Katti, K.S.; Katti, D.R.; Mishra, S.R.; Khan, S.; Jaggi, M.; Chauhan, S.C. The Roles of Cellular Nanomechanics in Cancer. Med. Res. Rev. 2015, 35, 198–223. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.E.; Jin, Y.S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Lim, C.T. Biomechanics approaches to studying human diseases. Trends Biotechnol. 2007, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xiong, F.; Li, X.; Xiang, B.; Li, Z.; Wu, X.; Guo, C.; Li, X.; Li, Y.; Li, G.; et al. Application of atomic force microscopy in cancer research. J. Nanobiotechnol. 2018, 16, 102. [Google Scholar] [CrossRef]
- Lekka, M. Discrimination Between Normal and Cancerous Cells Using AFM. Bionanoscience 2016, 6, 65–80. [Google Scholar] [CrossRef]
- Stylianou, A.; Lekka, M.; Stylianopoulos, T. AFM assessing of nanomechanical fingerprints for cancer early diagnosis and classification: From single cell to tissue level. Nanoscale 2018, 10, 20930–20945. [Google Scholar] [CrossRef]
- Okegawa, T.; Pong, R.C.; Li, Y.; Hsieh, J.T. The role of cell adhesion molecule in cancer progression and its application in cancer therapy. Acta Biochim. Pol. 2004, 51, 445–457. [Google Scholar] [CrossRef]
- Bendas, G.; Borsig, L. Cancer Cell Adhesion and Metastasis: Selectins, Integrins, and the Inhibitory Potential of Heparins. Int. J. Cell Biol. 2012, 2012, 676731. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, M.; Primi, M.C.; Izard, T. Cell adhesion in cancer: Beyond the migration of single cells. J. Biol. Chem. 2020, 295, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Kampen, K.R. Membrane proteins: The key players of a cancer cell. J. Membr. Biol. 2011, 242, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, M.C.; de Geus, S.W.; Prevoo, H.A.; Hawinkels, L.J.; van de Velde, C.J.; Kuppen, P.J.; Vahrmeijer, A.L.; Sier, C.F. Selecting Targets for Tumor Imaging: An Overview of Cancer-Associated Membrane Proteins. Biomark Cancer 2016, 8, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Lee, C.H.; Chuang, Y.H.; Lee, J.Y.; Chiu, Y.Y.; Wu Lee, Y.H.; Jong, Y.J.; Hwang, J.K.; Huang, S.H.; Chen, L.C.; et al. Membrane protein-regulated networks across human cancers. Nat. Commun. 2019, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol. 2012, 7, 757–765. [Google Scholar] [CrossRef]
- Iturri, J.; Weber, A.; Moreno-Cencerrado, A.; Vivanco, M.d.; Benítez, R.; Leporatti, S.; Toca-Herrera, J.L. Resveratrol-Induced Temporal Variation in the Mechanical Properties of MCF-7 Breast Cancer Cells Investigated by Atomic Force Microscopy. Int. J. Mol. Sci. 2019, 20, 3275. [Google Scholar] [CrossRef]
- Raudenska, M.; Kratochvilova, M.; Vicar, T.; Gumulec, J.; Balvan, J.; Polanska, H.; Pribyl, J.; Masarik, M. Cisplatin enhances cell stiffness and decreases invasiveness rate in prostate cancer cells by actin accumulation. Sci. Rep. 2019, 9, 1660. [Google Scholar] [CrossRef]
- Graham, H.K.; Hodson, N.W.; Hoyland, J.A.; Millward-Sadler, S.J.; Garrod, D.; Scothern, A.; Griffiths, C.E.M.; Watson, R.E.B.; Cox, T.R.; Erler, J.T.; et al. Tissue section AFM: In situ ultrastructural imaging of native biomolecules. Matrix Biol. 2010, 29, 254–260. [Google Scholar] [CrossRef]
- Brauchle, E.; Kasper, J.; Daum, R.; Schierbaum, N.; Falch, C.; Kirschniak, A.; Schäffer, T.E.; Schenke-Layland, K. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol. 2018, 68, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Sotres, J.; Jankovskaja, S.; Wannerberger, K.; Arnebrant, T. Ex-Vivo Force Spectroscopy of Intestinal Mucosa Reveals the Mechanical Properties of Mucus Blankets. Sci. Rep. 2017, 7, 7270. [Google Scholar] [CrossRef] [PubMed]
- Aghajanova, A.H.; Safarzadeh, A. Atomic Force Microscopy in the Study of Cell Membranes Normal Epithelium and Adenocarcinoma Cell of the Large Intestine. J. Cancer Sci. Ther. 2014, 2014. [Google Scholar] [CrossRef]
- Schimpel, C.; Werzer, O.; Frohlich, E.; Leitinger, G.; Absenger-Novak, M.; Teubl, B.; Zimmer, A.; Roblegg, E. Atomic force microscopy as analytical tool to study physico-mechanical properties of intestinal cells. Beilstein J. Nanotechnol. 2015, 6, 1457–1466. [Google Scholar] [CrossRef]
- Rodríguez-Nieto, M.; Mendoza-Flores, P.; García-Ortiz, D.; Montes-de-Oca, L.M.; Mendoza-Villa, M.; Barrón-González, P.; Espinosa, G.; Menchaca, J.L. Viscoelastic properties of doxorubicin-treated HT-29 cancer cells by atomic force microscopy: The fractional Zener model as an optimal viscoelastic model for cells. Biomech. Model. Mechanobiol. 2020, 19, 801–813. [Google Scholar] [CrossRef]
- Mayne, S.T.; Playdon, M.C.; Rock, C.L. Diet, nutrition, and cancer: Past, present and future. Nat. Rev. Clin. Oncol. 2016, 13, 504–515. [Google Scholar] [CrossRef]
- Maret, W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Ho, E. Zinc deficiency, DNA damage and cancer risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef]
- Dhawan, D.K.; Chadha, V.D. Zinc: A promising agent in dietary chemoprevention of cancer. Indian J. Med. Res. 2010, 132, 676–682. [Google Scholar]
- Dani, V.; Goel, A.; Vaiphei, K.; Dhawan, D.K. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicol. Lett. 2007, 171, 10–18. [Google Scholar] [CrossRef]
- Maares, M.; Haase, H. A Guide to Human Zinc Absorption: General Overview and Recent Advances of In Vitro Intestinal Models. Nutrients 2020, 12, 762. [Google Scholar] [CrossRef] [PubMed]
- Haase, H.; Ellinger, S.; Linseisen, J.; Neuhäuser-Berthold, M.; Richter, M. Revised D-A-CH-reference values for the intake of zinc. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2020, 61, 126536. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Giovannucci, E.L.; Smith-Warner, S.A.; Wu, K.; Fuchs, C.S.; Pollak, M.; Willett, W.C.; Ma, J. A prospective study of intakes of zinc and heme iron and colorectal cancer risk in men and women. Cancer Causes Control 2011, 22, 1627. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Jenab, M.; Freisling, H.; Becker, N.P.; Czuban, M.; Tjønneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.C.; Mancini, F.R.; et al. Pre-diagnostic copper and zinc biomarkers and colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Carcinogenesis 2017, 38, 699–707. [Google Scholar] [CrossRef]
- Li, P.; Xu, J.; Shi, Y.; Ye, Y.; Chen, K.; Yang, J.; Wu, Y. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clin. Nutr. 2014, 33, 415–420. [Google Scholar] [CrossRef]
- Barresi, V.; Valenti, G.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome analysis reveals an altered expression profile of zinc transporters in colorectal cancer. J. Cell. Biochem. 2018, 119, 9707–9719. [Google Scholar] [CrossRef]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 17029. [Google Scholar] [CrossRef]
- Shi, Y.; Amin, K.; Sato, B.G.; Samuelsson, S.J.; Sambucetti, L.; Haroon, Z.A.; Laderoute, K.; Murphy, B.J. The metal-responsive transcription factor-1 protein is elevated in human tumors. Cancer Biol. Ther. 2010, 9, 469–476. [Google Scholar] [CrossRef]
- Ninsontia, C.; Phiboonchaiyanan, P.P.; Chanvorachote, P. Zinc induces epithelial to mesenchymal transition in human lung cancer H460 cells via superoxide anion-dependent mechanism. Cancer Cell Int. 2016, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Hogstrand, C.; Kille, P.; Ackland, M.L.; Hiscox, S.; Taylor, K.M. A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3). Biochem. J. 2013, 455, 229–237. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Xu, Z.; Cheng, X. Zinc dysregulation in cancers and its potential as a therapeutic target. Cancer Biol. Med. 2020, 17, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Stovall, D.B.; Wang, W.; Sui, G. Advances of Zinc Signaling Studies in Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 667. [Google Scholar] [CrossRef] [PubMed]
- Gumulec, J.; Masarik, M.; Adam, V.; Eckschlager, T.; Provaznik, I.; Kizek, R. Serum and Tissue Zinc in Epithelial Malignancies: A Meta-Analysis. PLoS ONE 2014, 9, e99790. [Google Scholar] [CrossRef] [PubMed]
- Grattan, B.J.; Freake, H.C. Zinc and cancer: Implications for LIV-1 in breast cancer. Nutrients 2012, 4, 648–675. [Google Scholar] [CrossRef] [PubMed]
- Christudoss, P.; Selvakumar, R.; Pulimood, A.B.; Fleming, J.J.; Mathew, G. Zinc and zinc related enzymes in precancerous and cancerous tissue in the colon of dimethyl hydrazine treated rats. Asian Pac. J. Cancer Prev. 2012, 13, 487–492. [Google Scholar] [CrossRef][Green Version]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef]
- Finamore, A.; Massimi, M.; Conti Devirgiliis, L.; Mengheri, E. Zinc deficiency induces membrane barrier damage and increases neutrophil transmigration in Caco-2 cells. J. Nutr. 2008, 138, 1664–1670. [Google Scholar] [CrossRef]
- Tejeda-Mora, H.; Stevens, L.; Gröllers, M.; Katan, A.; van de Steeg, E.; van der Heiden, M. AFM based elasticity of intestinal epithelium correlate with barrier function under drug action. bioRxiv 2019. [Google Scholar] [CrossRef]
- Ciasca, G.; Papi, M.; Minelli, E.; Palmieri, V.; De Spirito, M. Changes in cellular mechanical properties during onset or progression of colorectal cancer. World J. Gastroenterol. 2016, 22, 7203–7214. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Ophir, I.; Shaul, Y.B. Induced differentiation in HT29, a human colon adenocarcinoma cell line. J. Cell Sci. 1999, 112, 2657–2666. [Google Scholar] [PubMed]
- Fogh, J. Human tumor lines for cancer research. Cancer Investig. 1986, 4, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Schroy, P.C.; Rustgi, A.K.; Ikonomu, E.; Liu, X.P.; Polito, J.; Andry, C.; O’Keane, J.C. Growth and intestinal differentiation are independently regulated in HT29 colon cancer cells. J. Cell. Physiol. 1994, 161, 111–123. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Porchet, N.; Aubert, J.P.; Swallow, D.; Gum, J.R.; Kim, Y.S.; Real, F.X.; Zweibaum, A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J. Cell Sci. 1993, 106, 771–783. [Google Scholar]
- Behrens, I.; Stenberg, P.; Artursson, P.; Kissel, T. Transport of Lipophilic Drug Molecules in a New Mucus-Secreting Cell Culture Model Based on HT29-MTX Cells. Pharm. Res. 2001, 18, 1138–1145. [Google Scholar] [CrossRef]
- Hennebicq-Reig, S.; Tetaert, D.; Soudan, B.; Kim, I.; Huet, G.; Briand, G.; Richet, C.; Demeyer, D.; Degand, P. O-Glycosylation and cellular differentiation in a subpopulation of mucin-secreting HT-29 cell line. Exp. Cell Res. 1997, 235, 100–107. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Straubing, S.; Robbe-Masselot, C.; Haase, H. Zinc Deficiency Disturbs Mucin Expression, O-Glycosylation and Secretion by Intestinal Goblet Cells. Int. J. Mol. Sci. 2020, 21, 6149. [Google Scholar] [CrossRef]
- Kindermann, B.; Doöring, F.; Pfaffl, M.; Daniel, H. Identification of Genes Responsive to Intracellular Zinc Depletion in the Human Colon Adenocarcinoma Cell Line HT-291. J. Nutr. 2004, 134, 57–62. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006, 11, 1049–1062. [Google Scholar] [CrossRef]
- Maares, M.; Keil, C.; Koza, J.; Straubing, S.; Schwerdtle, T.; Haase, H. In Vitro Studies on Zinc Binding and Buffering by Intestinal Mucins. Int. J. Mol. Sci. 2018, 19, 2662. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Keil, C.; Thomsen, S.; Gunzel, D.; Wiesner, B.; Haase, H. Characterization of Caco-2 cells stably expressing the protein-based zinc probe eCalwy-5 as a model system for investigating intestinal zinc transport. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. 2018, 49, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Iturri, J.; Benitez, R.; Toca-Herrera, J.L. Measuring biomaterials mechanics with atomic force microscopy. 1. Influence of the loading rate and applied force (pyramidal tips). Microsc. Res. Tech. 2019, 82, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Flores, S.; Benitez, R.; Vivanco, M.; Toca-Herrera, J.L. Stress relaxation and creep on living cells with the atomic force microscope: A means to calculate elastic moduli and viscosities of cell components. Nanotechnology 2010, 21, 445101. [Google Scholar] [CrossRef] [PubMed]
- Alker, W.; Schwerdtle, T.; Schomburg, L.; Haase, H. A Zinpyr-1-based Fluorimetric Microassay for Free Zinc in Human Serum. Int. J. Mol. Sci. 2019, 20, 4006. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, A.; Divieto, C.; Mortati, L.; Pavarelli, S.; Sassi, G.; Sassi, M.P. Toward the realization of reproducible Atomic Force Microscopy measurements of elastic modulus in biological samples. J. Biomech. 2015, 48, 1099–1104. [Google Scholar] [CrossRef]
- Sumarokova, M.; Iturri, J.; Weber, A.; Maares, M.; Keil, C.; Luis Toca-Herrera, J. Influencing the adhesion properties and wettability of mucin protein films by variation of the environmental pH. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Lawson, M.J.; Butler, R.N.; Goland, G.J.; Jarrett, I.G.; Roberts-Thomson, I.C.; Partick, E.J.; Dreosti, I.E. Zinc deficiency is associated with suppression of colonocyte proliferation in the distal large bowel of rats. Biol. Trace Elem. Res. 1988, 18, 115–121. [Google Scholar] [CrossRef]
- Wang, X.; Valenzano, M.C.; Mercado, J.M.; Zurbach, E.P.; Mullin, J.M. Zinc supplementation modifies tight junctions and alters barrier function of Caco-2 human intestinal epithelial layers. Dig. Dis. Sci. 2012, 58, 77–87. [Google Scholar] [CrossRef]
- Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014, 5, e1307. [Google Scholar] [CrossRef]
- Park, K.S.; Lee, N.G.; Lee, K.H.; Seo, J.T.; Choi, K.Y. The ERK pathway involves positive and negative regulations of HT-29 colorectal cancer cell growth by extracellular zinc. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1181–G1188. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Ahn, Y.; Kim, J.A.; Yun, M.S.; Seong, B.L.; Choi, K.Y. Extracellular zinc stimulates ERK-dependent activation of p21(Cip/WAF1) and inhibits proliferation of colorectal cancer cells. Br. J. Pharmacol. 2002, 137, 597–607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oh, S.Y.; Park, K.S.; Kim, J.A.; Choi, K.Y. Differential modulation of zinc-stimulated p21(Cip/WAF1) and cyclin D1 induction by inhibition of PI3 kinase in HT-29 colorectal cancer cells. Exp. Mol. Med. 2002, 34, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Kwon, Y.; Jang, M.; Park, M.; Kim, J.; Cho, S.; Jang, D.G.; Lee, W.B.; Jung, S.H.; Choi, H.J.; et al. β-catenin activation down-regulates cell-cell junction-related genes and induces epithelial-to-mesenchymal transition in colorectal cancers. Sci. Rep. 2019, 9, 18440. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Zhang, Y.; Niu, J.; Nie, Z.; Liu, Q.; Lv, C. Zinc promotes cell apoptosis via activating the Wnt-3a/β-catenin signaling pathway in osteosarcoma. J. Orthop. Surg. Res. 2020, 15, 57. [Google Scholar] [CrossRef]
- Zhao, J.; Han, J.; Jiang, J.; Shi, S.; Ma, X.; Liu, X.; Wang, C.; Nie, X.; He, Y.; Jiang, S.; et al. The downregulation of Wnt/β-catenin signaling pathway is associated with zinc deficiency-induced proliferative deficit of C17.2 neural stem cells. Brain Res. 2015, 1615, 61–70. [Google Scholar] [CrossRef]
- Medico, E.; Russo, M.; Picco, G.; Cancelliere, C.; Valtorta, E.; Corti, G.; Buscarino, M.; Isella, C.; Lamba, S.; Martinoglio, B.; et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat. Commun. 2015, 6, 7002. [Google Scholar] [CrossRef]
- Dutton, J.S.; Hinman, S.S.; Kim, R.; Wang, Y.; Allbritton, N.L. Primary Cell-Derived Intestinal Models: Recapitulating Physiology. Trends Biotechnol. 2019, 37, 744–760. [Google Scholar] [CrossRef]



| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| 24 h | 210.7 ± 14.7 µm2 | 111.0 ± 9.6 µm2 | 203.7 ± 26.2 µm2 | 231.3 ± 71.2 µm2 |
| 48 h | 648.9 ± 54.2 µm2 | 334.8 ± 25.6 µm2 | ||
| 72 h | 1721.8 ± 227.2 µm2 | 751.2 ± 100.1 µm2 | ||
| 96 h | 3390.9 ± 526.4 µm2 | 3108.1 ± 344.2 µm2 | 2286 ± 571.7 µm2 | 2064.9 ± 923.5 µm2 |
| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| 24 h | 1.67 ± 0.08 kPa | 1.26 ± 0.05 kPa | 0.91 ± 0.05 kPa | 1.09 ± 0.06 kPa |
| 96 h | 2.85 ± 0.28 kPa | 2.62 ± 0.12 kPa | 1.28 ± 0.17 kPa | 0.85 ± 0.08 kPa |
| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| 24 h | τ1 = 5.02 ± 0.08 s | τ1 = 4.19 ± 0.06 s | τ1 = 3.51 ± 0.08 s | τ1 = 2.68 ± 0.04 s |
| τ2 = 0.19 ± 0.003 s | τ2 = 0.23 ± 0.003 s | τ2 = 0.16 ± 0.003 s | τ2 = 0.15 ± 0.002 s | |
| r2 = 0.9950 | r2 = 0.9958 | r2 = 0.9964 | r2 = 0.9976 | |
| 96 h | τ1 = 4.10 ± 0.05 s | τ1 = 3.99 ± 0.06 s | τ1 = 1.60 ± 0.08 s | τ1 = 1.13 ± 0.01 s |
| τ2 = 0.19 ± 0.004 s | τ2 = 0.21 ± 0.004 s | τ2 = 0.12 ± 0.004 s | τ2 = 0.08 ± 0.002 s | |
| r2 = 0.9962 | r2 = 0.9941 | r2 = 0.9839 | r2 = 0.9954 | |
| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| 24 h | 0.29 ± 0.01 nN | 0.59 ± 0.03 nN | 0.71 ± 0.24 nN | 0.38 ± 0.09 nN |
| 96 h | 0.31 ± 0.02 nN | 0.24 ± 0.01 nN | 0.60 ± 0.07 nN | 0.33 ± 0.02 nN |
| Incubation | HT-29-MTX | HT-29 | ||
|---|---|---|---|---|
| ZA | ZD | ZA | ZD | |
| Mean Event Position | ||||
| 24 h | 6.86 ± 0.17 µm | 3.48 ± 0.14 µm | 6.49 ± 0.24 µm | 4.33 ± 0.23 µm |
| 96 h | 3.23 ± 0.15 µm | 2.69 ± 0.89 µm | 4.76 ± 0.31 µm | 2.28 ± 0.17 µm |
| Mean Rupture Force | ||||
| 24 h | 56.9 ± 1.23 pN | 55.9 ± 0.76 pN | 63.8 ± 1.23 pN | 50.2 ± 1.01 pN |
| 96 h | 60.3 ± 0.83 pN | 58.8 ± 0.89 pN | 70.9 ± 1.49 pN | 54.3 ± 1.66 pN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maares, M.; Keil, C.; Löher, L.; Weber, A.; Andorfer-Sarr, A.; Haase, H.; Iturri, J.; Toca-Herrera, J.L. Time- and Zinc-Related Changes in Biomechanical Properties of Human Colorectal Cancer Cells Examined by Atomic Force Microscopy. Biology 2020, 9, 468. https://doi.org/10.3390/biology9120468
Maares M, Keil C, Löher L, Weber A, Andorfer-Sarr A, Haase H, Iturri J, Toca-Herrera JL. Time- and Zinc-Related Changes in Biomechanical Properties of Human Colorectal Cancer Cells Examined by Atomic Force Microscopy. Biology. 2020; 9(12):468. https://doi.org/10.3390/biology9120468
Chicago/Turabian StyleMaares, Maria, Claudia Keil, Leif Löher, Andreas Weber, Amsatou Andorfer-Sarr, Hajo Haase, Jagoba Iturri, and José L. Toca-Herrera. 2020. "Time- and Zinc-Related Changes in Biomechanical Properties of Human Colorectal Cancer Cells Examined by Atomic Force Microscopy" Biology 9, no. 12: 468. https://doi.org/10.3390/biology9120468
APA StyleMaares, M., Keil, C., Löher, L., Weber, A., Andorfer-Sarr, A., Haase, H., Iturri, J., & Toca-Herrera, J. L. (2020). Time- and Zinc-Related Changes in Biomechanical Properties of Human Colorectal Cancer Cells Examined by Atomic Force Microscopy. Biology, 9(12), 468. https://doi.org/10.3390/biology9120468








