Impact of Isolation Procedures on the Development of a Preclinical Synovial Fibroblasts/Macrophages in an In Vitro Model of Osteoarthritis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
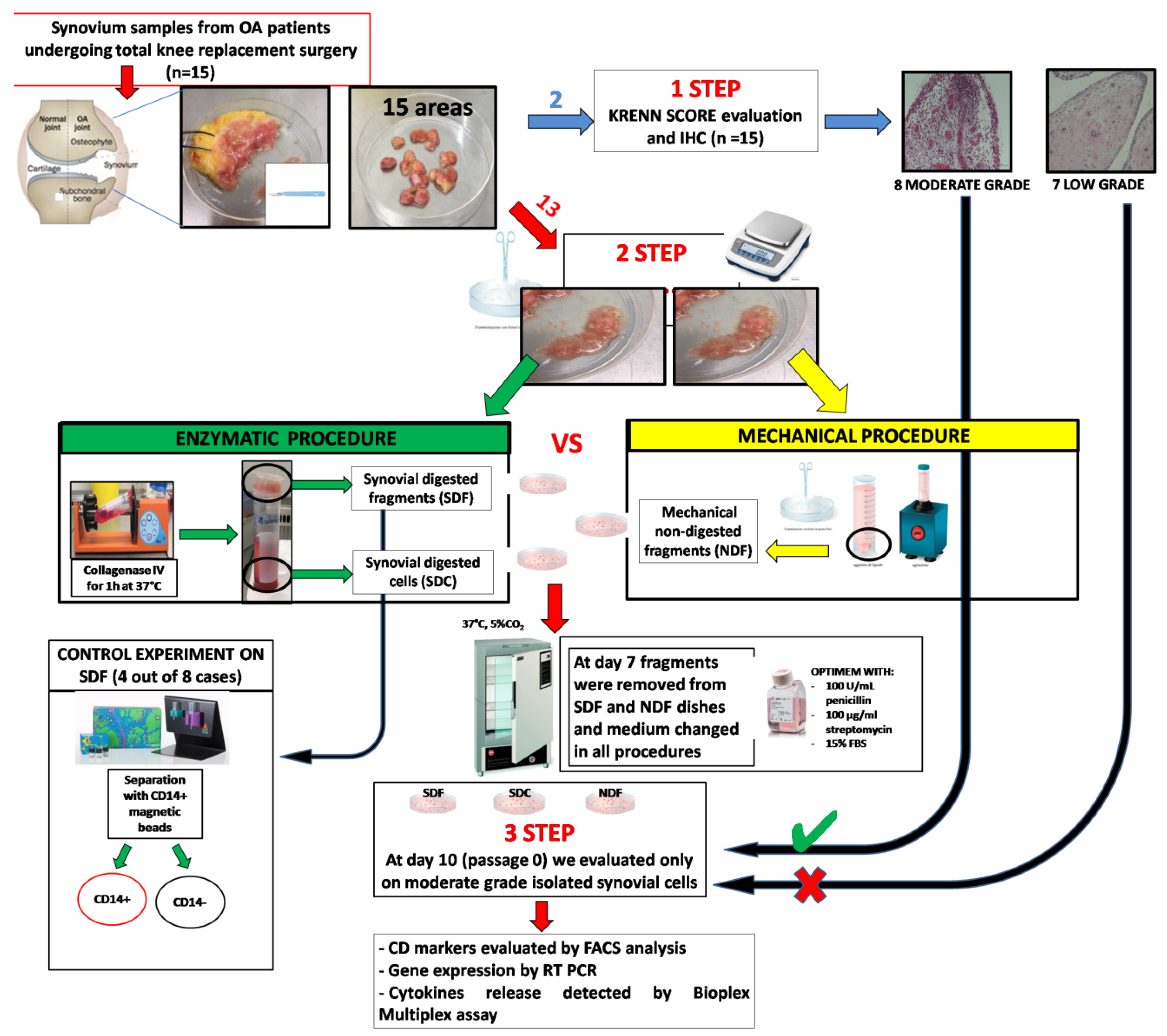
2.1. Patients Characterization
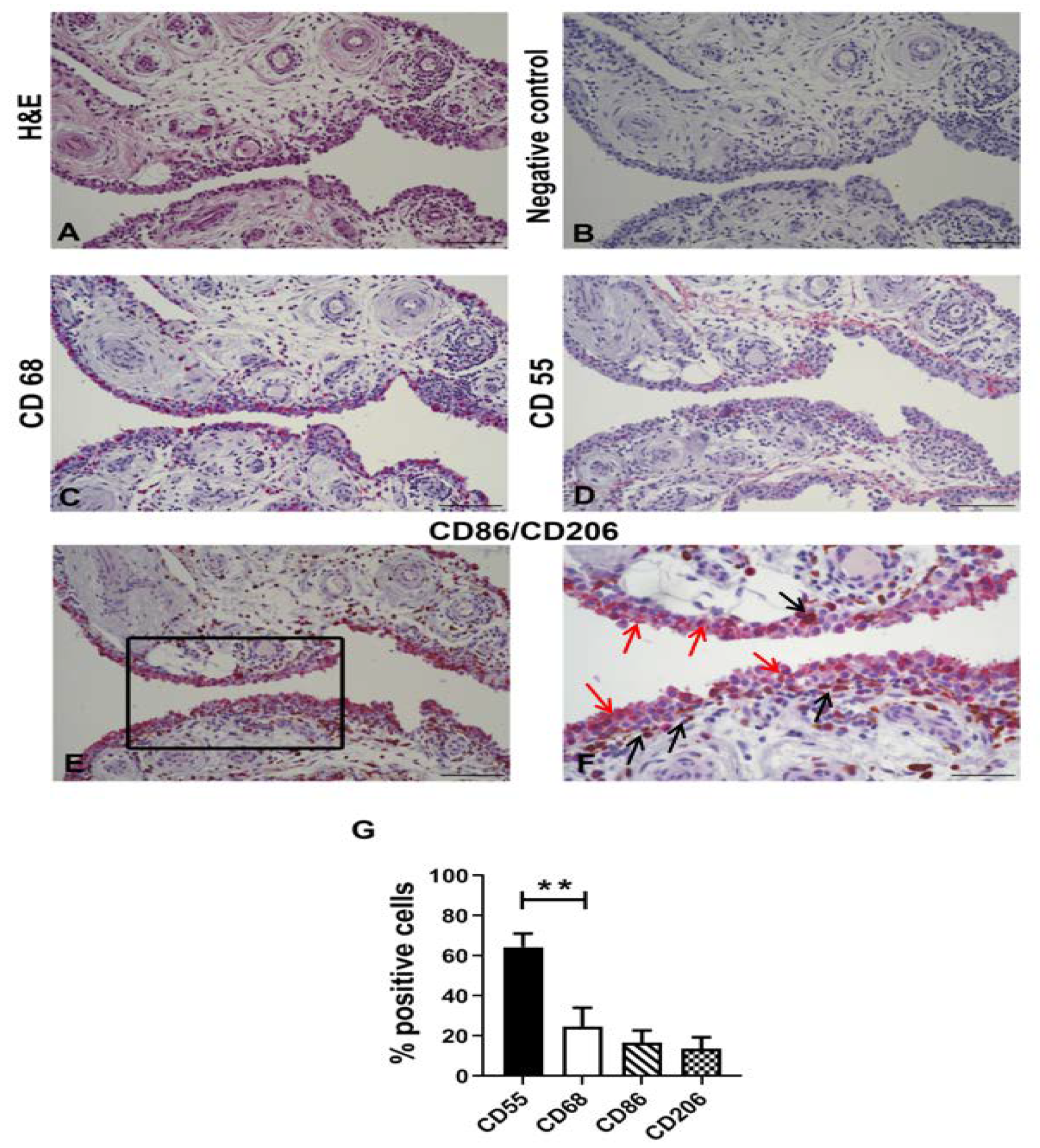
2.2. Synovial Tissues Characterization
2.3. Immunohistochemical Analysis of Synovial Tissue
2.4. Synovial Cell Isolation by Using Both Mechanical and Enzymatic Procedures
2.5. Characterization of Isolated Synovial Cells
2.6. Cytokine and Chemokine Release in Supernatants
2.7. Real Time-PCR Analysis
2.8. Statistical Analysis
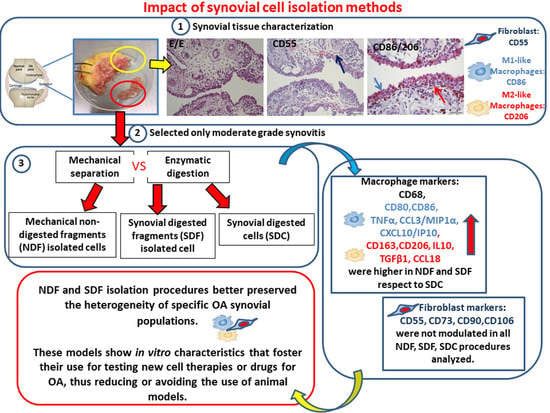
3. Results
3.1. Characterization of OA Synovial Tissue Samples
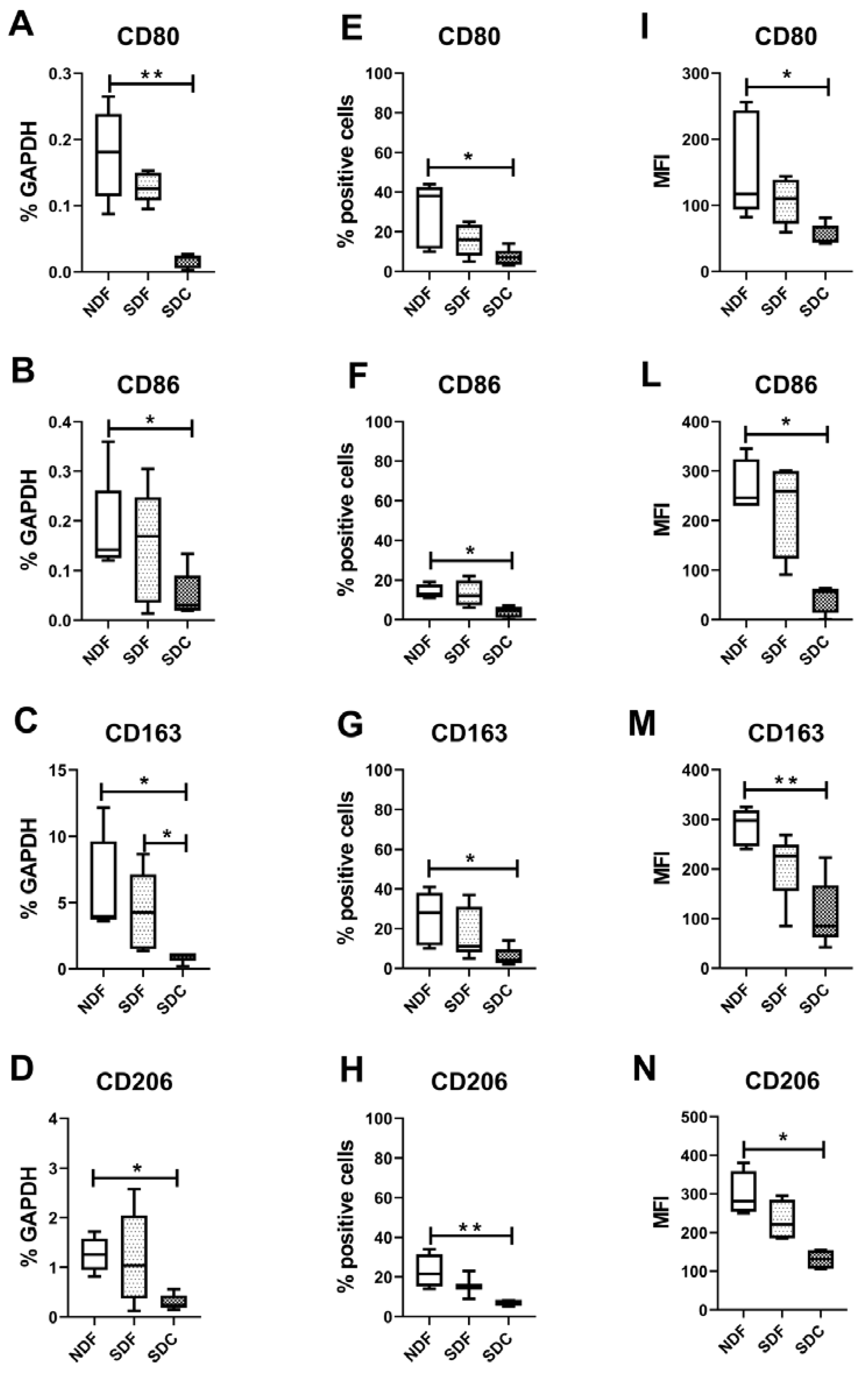
3.2. Synovial Cell Isolation Procedures Yield a Different Percentage of Macrophages
3.3. Synovial Cell Isolation Procedures Yield a Different Expression of Synovial M1- and M2-Like Macrophage Markers
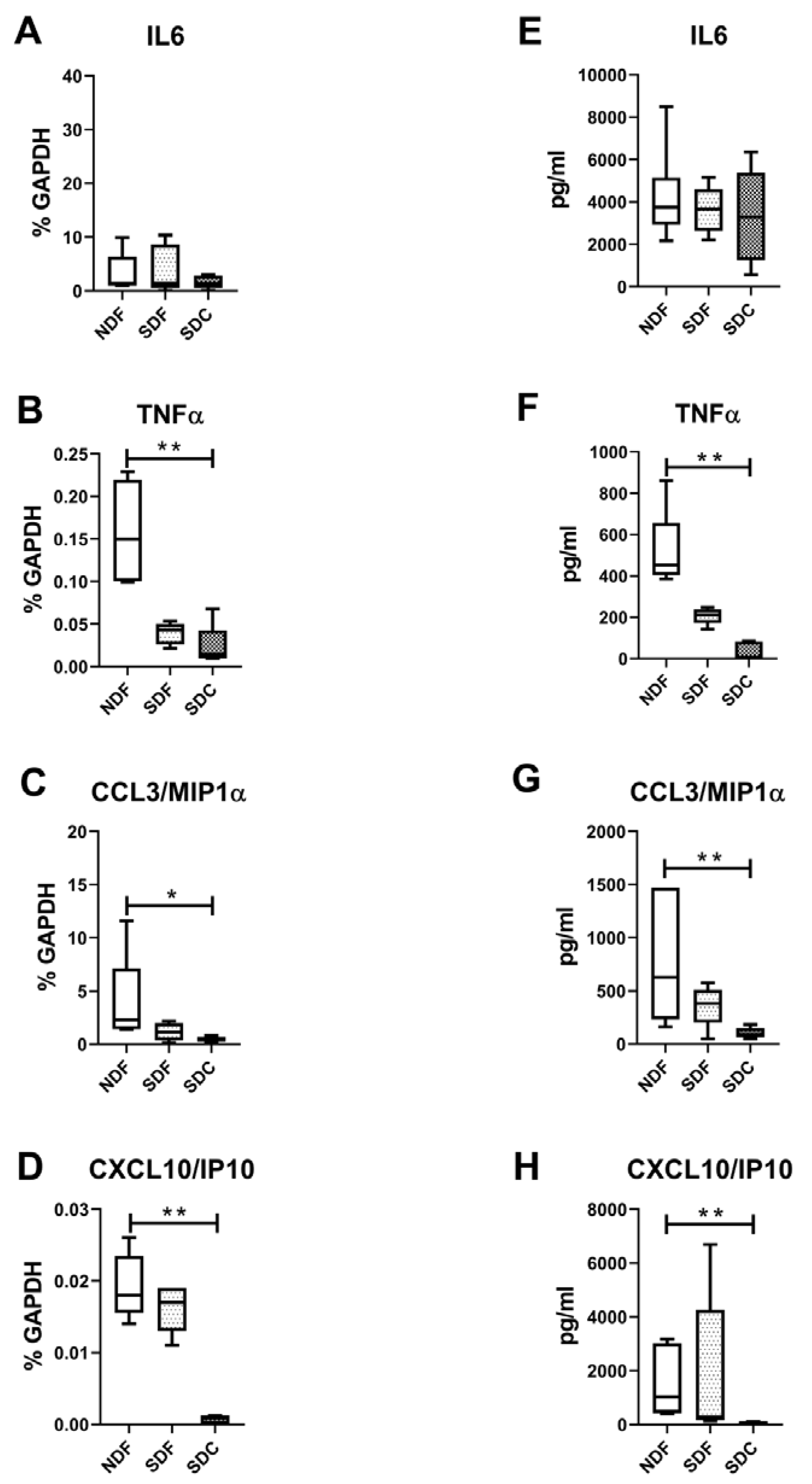
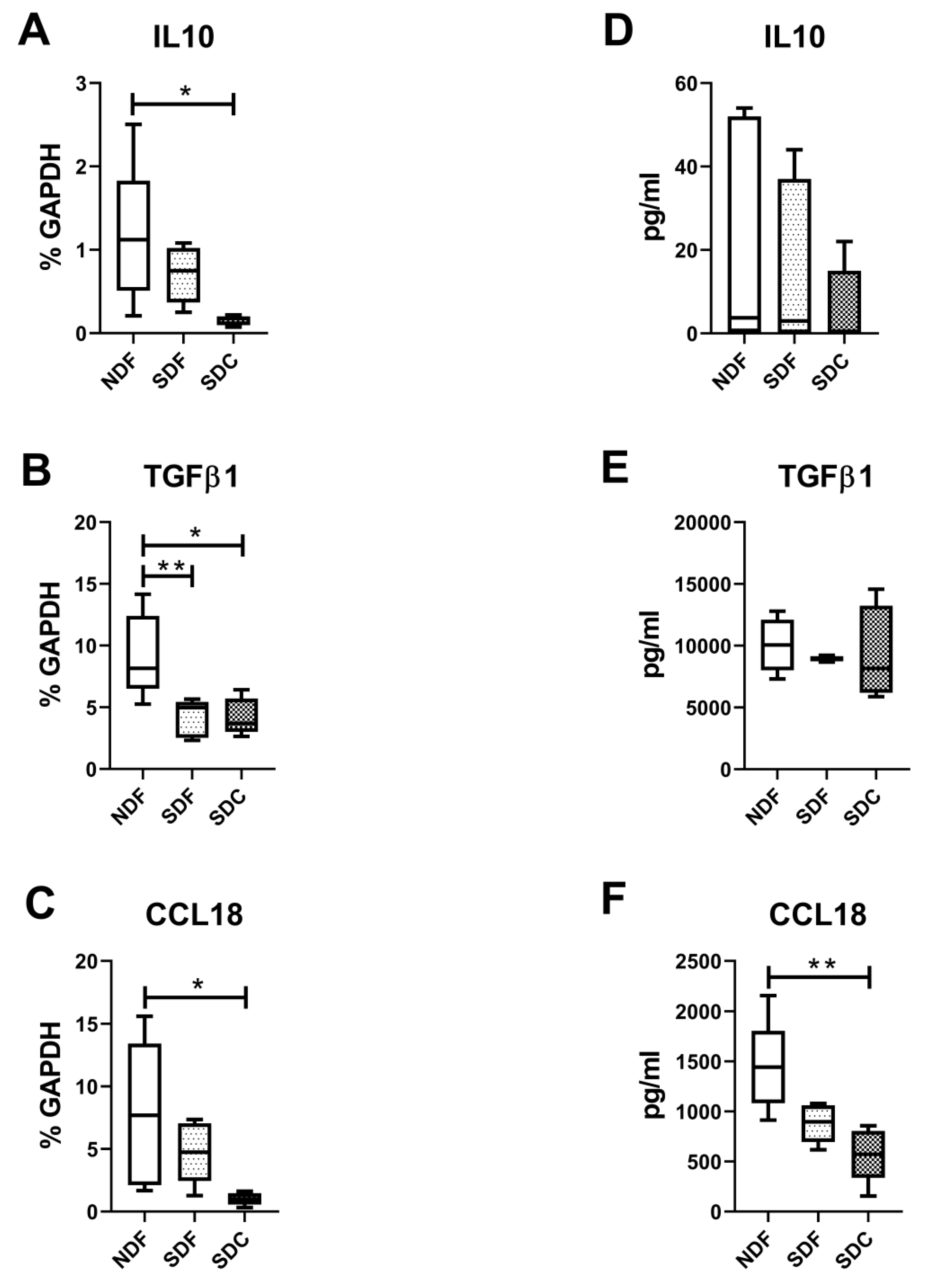
3.4. Synovial Cell Isolation Procedures Yield a Different Level of Synovial Cell Released Factors
3.5. Lower Amounts of All Measured Secreted Factors in Both CD14-Positive and CD14-Negative Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19, 18. [Google Scholar] [CrossRef]
- Zhao, X.; Gu, M.; Xu, X.; Wen, X.; Yang, G.; Li, L.; Sheng, P.; Meng, F. CCL3/CCR1 mediates CD14(+)CD16(−) circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr. Cartil. 2020, 28, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- De Lange-Brokaar, B.J.; Ioan-Facsinay, A.; van Osch, G.J.; Zuurmond, A.M.; Schoones, J.; Toes, R.E.; Huizinga, T.W.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef] [PubMed]
- Bhattaram, P.; Chandrasekharan, U. The joint synovium: A critical determinant of articular cartilage fate in inflammatory joint diseases. Semin. Cell Dev. Biol. 2017, 62, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.B.; McDaniel, G.; Huebner, J.L.; Stabler, T.V.; Pieper, C.F.; Shipes, S.W.; Petry, N.A.; Low, P.S.; Shen, J.; McNearney, T.A.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef]
- Lopes, E.B.P.; Filiberti, A.; Husain, S.A.; Humphrey, M.B. Immune Contributions to Osteoarthritis. Curr. Osteoporos. Rep. 2017, 15, 593–600. [Google Scholar] [CrossRef]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Silvestri, Y.; Gambari, L.; Cattini, L.; Filardo, G.; Fleury-Cappellesso, S.; Lisignoli, G. From osteoarthritic synovium to synovial-derived cells characterization: Synovial macrophages are key effector cells. Arthritis Res. Ther. 2016, 18, 83. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Allen, J.E.; Biswas, S.K.; Fisher, E.A.; Gilroy, D.W.; Goerdt, S.; Gordon, S.; Hamilton, J.A.; Ivashkiv, L.B.; Lawrence, T.; et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity 2014, 41, 14–20. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Lopa, S.; Leijs, M.J.C.; Moretti, M.; Lubberts, E.; van Osch, G.J.V.M.; Bastiaansen-Jenniskens, Y.M. Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes. Osteoarthr. Cartil. 2015, 23, 1853–1857. [Google Scholar] [CrossRef] [PubMed]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Gambari, L.; Piacentini, A.; Filardo, G.; Fleury-Cappellesso, S.; Barbero, A.; Murphy, M.; Lisignoli, G. Adipose stromal cells mediated switching of the pro-inflammatory profile of M1-like macrophages is facilitated by PGE2: In vitro evaluation. Osteoarthr. Cartil. 2017, 25, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.L.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Bueno, D.F.; Amano, M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020, 11, 111. [Google Scholar] [CrossRef]
- Xie, J.; Huang, Z.; Yu, X.; Zhou, L.; Pei, F. Clinical implications of macrophage dysfunction in the development of osteoarthritis of the knee. Cytokine Growth Factor Rev. 2019, 46, 36–44. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef]
- Bondeson, J.; Blom, A.B.; Wainwright, S.; Hughes, C.; Caterson, B.; van den Berg, W.B. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010, 62, 647–657. [Google Scholar] [CrossRef]
- Haraden, C.A.; Huebner, J.L.; Hsueh, M.-F.; Li, Y.-J.; Kraus, V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res. Ther. 2019, 21, 146. [Google Scholar] [CrossRef]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Mason, C.; Werre, S.R.; Luo, X.; Byron, C.R.; Kalbfleisch, T.S.; MacLeod, J.N.; Dahlgren, L.A. Inflamed synovial fluid induces a homeostatic response in bone marrow mononuclear cells in vitro: Implications for joint therapy. FASEB J. 2020, 34, 4430–4444. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.B.; Jones, E.A.; McGonagle, D.; Luyten, F.P.; Lories, R.J. Flow cytometric characterization of freshly isolated and culture expanded human synovial cell populations in patients with chronic arthritis. Arthritis Res. Ther. 2010, 12, R15. [Google Scholar] [CrossRef] [PubMed]
- Orr, C.; Vieira-Sousa, E.; Boyle, D.L.; Buch, M.H.; Buckley, C.D.; Canete, J.D.; Catrina, A.I.; Choy, E.H.S.; Emery, P.; Fearon, U.; et al. Synovial tissue research: A state-of-the-art review. Nat. Rev. Rheumatol. 2017, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Ferro, T.; Santhagunam, A.; Madeira, C.; Salgueiro, J.B.; da Silva, C.L.; Cabral, J.M.S. Successful isolation and ex vivo expansion of human mesenchymal stem/stromal cells obtained from different synovial tissue-derived (biopsy) samples. J. Cell. Physiol. 2019, 234, 3973–3984. [Google Scholar] [CrossRef]
- Donlin, L.T.; Rao, D.A.; Wei, K.; Slowikowski, K.; McGeachy, M.J.; Turner, J.D.; Meednu, N.; Mizoguchi, F.; Gutierrez-Arcelus, M.; Lieb, D.J.; et al. Methods for high-dimensional analysis of cells dissociated from cryopreserved synovial tissue. Arthritis Res. Ther. 2018, 20, 139. [Google Scholar] [CrossRef]
- Labinsky, H.; Panipinto, P.M.; Ly, K.A.; Khuat, D.K.; Madarampalli, B.; Mahajan, V.; Clabeaux, J.; MacDonald, K.; Verdin, P.J.; Buckner, J.H.; et al. Multiparameter Analysis Identifies Heterogeneity in Knee Osteoarthritis Synovial Responses. Arthritis Rheumatol. 2020, 72, 598–608. [Google Scholar] [CrossRef]
- Stefani, R.M.; Halder, S.S.; Estell, E.G.; Lee, A.J.; Silverstein, A.M.; Sobczak, E.; Chahine, N.O.; Ateshian, G.A.; Shah, R.P.; Hung, C.T. A Functional Tissue-Engineered Synovium Model to Study Osteoarthritis Progression and Treatment. Tissue Eng. Part A 2019, 25, 538–553. [Google Scholar] [CrossRef]
- Culemann, S.; Grüneboom, A.; Nicolás-Ávila, J.; Weidner, D.; Lämmle, K.F.; Rothe, T.; Quintana, J.A.; Kirchner, P.; Krljanac, B.; Eberhardt, M.; et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019, 572, 670–675. [Google Scholar] [CrossRef]
- Alivernini, S.; MacDonald, L.; Elmesmari, A.; Finlay, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Di Mario, C.; Bui, L.; Perniola, S.; et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat. Med. 2020, 26, 1295–1306. [Google Scholar] [CrossRef]
- Zimmermann, T.; Kunisch, E.; Pfeiffer, R.; Hirth, A.; Stahl, H.D.; Sack, U.; Laube, A.; Liesaus, E.; Roth, A.; Palombo-Kinne, E.; et al. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture--primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2001, 3, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Utomo, L.; van Osch, G.J.; Bayon, Y.; Verhaar, J.A.; Bastiaansen-Jenniskens, Y.M. Guiding synovial inflammation by macrophage phenotype modulation: An in vitro study towards a therapy for osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Haltmayer, E.; Ribitsch, I.; Gabner, S.; Rosser, J.; Gueltekin, S.; Peham, J.; Giese, U.; Dolezal, M.; Egerbacher, M.; Jenner, F. Co-culture of osteochondral explants and synovial membrane as in vitro model for osteoarthritis. PLoS ONE 2019, 14, e0214709. [Google Scholar] [CrossRef] [PubMed]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Haupl, T.; Neidel, J.; Petersen, I.; Konig, A. Grading of chronic synovitis—A histopathological grading system for molecular and diagnostic pathology. Pathol. Res. Pract. 2002, 198, 317–325. [Google Scholar] [CrossRef]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Cattini, L.; Rojewski, M.; Schrezenmeier, H.; Addimanda, O.; Meliconi, R.; Lisignoli, G. Osteoarthritic Milieu Affects Adipose-Derived Mesenchymal Stromal Cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Pilling, D.; Fan, T.; Huang, D.; Kaul, B.; Gomer, R.H. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE 2009, 4, e7475. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- Samvelyan, H.J.; Hughes, D.; Stevens, C.; Staines, K.A. Models of Osteoarthritis: Relevance and New Insights. Calcif. Tissue Int. 2020. [Google Scholar] [CrossRef]
- Mucke, J.; Hoyer, A.; Brinks, R.; Bleck, E.; Pauly, T.; Schneider, M.; Vordenbäumen, S. Inhomogeneity of immune cell composition in the synovial sublining: Linear mixed modelling indicates differences in distribution and spatial decline of CD68+ macrophages in osteoarthritis and rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 170. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Morawietz, L.; Burmester, G.R.; Kinne, R.W.; Mueller-Ladner, U.; Muller, B.; Haupl, T. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Xu, X.; Wu, Y.; Ji, B.; Zhou, X.; Qin, L. Study of the mechanism underlying hsa-miR338-3p downregulation to promote fibrosis of the synovial tissue in osteoarthritis patients. Mol. Biol. Rep. 2019, 46, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Ikejima, K.; Enomoto, N.; Seabra, V.; Ikejima, A.; Brenner, D.A.; Thurman, R.G. Pronase destroys the lipopolysaccharide receptor CD14 on Kupffer cells. Am. J. Physiol. 1999, 276, G591–G598. [Google Scholar] [CrossRef]
- Wood, M.J.; Leckenby, A.; Reynolds, G.; Spiering, R.; Pratt, A.G.; Rankin, K.S.; Isaacs, J.D.; Haniffa, M.A.; Milling, S.; Hilkens, C.M.U. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight 2019, 4. [Google Scholar] [CrossRef]
- Van den Bosch, M.H.J.; van Lent, P.; van der Kraan, P.M. Identifying effector molecules, cells, and cytokines of innate immunity in OA. Osteoarthr. Cartil. 2020, 28, 532–543. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Yang, Z.B.; Zhang, Z.J.; Zhang, Z.Q.; Kang, Y.; Huang, G.X.; Wang, S.W.; Huang, H.; Liao, W.M. CCL3 serves as a potential plasma biomarker in knee degeneration (osteoarthritis). Osteoarthr. Cartil. 2015, 23, 1405–1411. [Google Scholar] [CrossRef]
- Beekhuizen, M.; Gierman, L.M.; van Spil, W.E.; Van Osch, G.J.V.M.; Huizinga, T.W.J.; Saris, D.B.F.; Creemers, L.B.; Zuurmond, A.M. An explorative study comparing levels of soluble mediators in control and osteoarthritic synovial fluid. Osteoarthr. Cartil. 2013, 21, 918–922. [Google Scholar] [CrossRef]
- Chintalacharuvu, S.R.; Wang, J.X.; Giaconia, J.M.; Venkataraman, C. An essential role for CCL3 in the development of collagen antibody-induced arthritis. Immunol. Lett. 2005, 100, 202–204. [Google Scholar] [CrossRef]
- Millerand, M.; Sudre, L.; Nefla, M.; Pène, F.; Rousseau, C.; Pons, A.; Ravat, A.; André-Leroux, G.; Akira, S.; Satoh, T.; et al. Activation of innate immunity by 14-3-3 ε, a new potential alarmin in osteoarthritis. Osteoarthr. Cartil. 2020, 28, 646–657. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhang, L.; Kong, W.; Liang, J.; Xu, X.; Wu, H.; Feng, X.; Hua, B.; Wang, H.; Sun, L. Umbilical Cord-Derived Mesenchymal Stem Cells Inhibit Cadherin-11 Expression by Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. J. Immunol. Res. 2015, 2015, 137695. [Google Scholar] [CrossRef]
- Van der Kraan, P.M. The changing role of TGFβ in healthy, ageing and osteoarthritic joints. Nat. Rev. Rheumatol. 2017, 13, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Remst, D.F.; Blaney Davidson, E.N.; Vitters, E.L.; Bank, R.A.; van den Berg, W.B.; van der Kraan, P.M. TGF-ß induces Lysyl hydroxylase 2b in human synovial osteoarthritic fibroblasts through ALK5 signaling. Cell Tissue Res. 2014, 355, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ni, Z.; Wang, J.; Zhu, S.; Ye, H. Overexpression of Lymphocyte Activation Gene-3 Inhibits Regulatory T Cell Responses in Osteoarthritis. DNA Cell Biol. 2017, 36, 862–869. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Song, W.; Yin, L.; Wang, G.; Yu, D.; Zhang, Q.; Yan, X.; Li, S. IgM(+)CD27(+) B cells possessed regulatory function and represented the main source of B cell-derived IL-10 in the synovial fluid of osteoarthritis patients. Hum. Immunol. 2019, 80, 263–269. [Google Scholar] [CrossRef]
- Takano, S.; Uchida, K.; Itakura, M.; Iwase, D.; Aikawa, J.; Inoue, G.; Mukai, M.; Miyagi, M.; Murata, K.; Sekiguchi, H.; et al. Transforming growth factor-β stimulates nerve growth factor production in osteoarthritic synovium. BMC Musculoskelet. Disord. 2019, 20, 204. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, Y.R.; Lee, Y.H.; Kim, K.; Lee, C.K. Production of TGF-beta1 as a Mechanism for Defective Antigen-presenting Cell Function of Macrophages Generated in vitro with M-CSF. Immune Netw. 2009, 9, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, J.; Yang, G. Serum and synovial fluid levels of CCL18 are correlated with radiographic grading of knee osteoarthritis. Med Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 840–844. [Google Scholar] [CrossRef][Green Version]
- Broeren, M.G.A.; Waterborg, C.E.J.; Wiegertjes, R.; Thurlings, R.M.; Koenders, M.I.; Van Lent, P.; Van der Kraan, P.M.; Van de Loo, F.A.J. A three-dimensional model to study human synovial pathology. Altex 2019, 36, 18–28. [Google Scholar] [CrossRef]
- Manferdini, C.; Maumus, M.; Gabusi, E.; Piacentini, A.; Filardo, G.; Peyrafitte, J.A.; Jorgensen, C.; Bourin, P.; Fleury-Cappellesso, S.; Facchini, A.; et al. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013, 65, 1271–1281. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manferdini, C.; Saleh, Y.; Dolzani, P.; Gabusi, E.; Trucco, D.; Filardo, G.; Lisignoli, G. Impact of Isolation Procedures on the Development of a Preclinical Synovial Fibroblasts/Macrophages in an In Vitro Model of Osteoarthritis. Biology 2020, 9, 459. https://doi.org/10.3390/biology9120459
Manferdini C, Saleh Y, Dolzani P, Gabusi E, Trucco D, Filardo G, Lisignoli G. Impact of Isolation Procedures on the Development of a Preclinical Synovial Fibroblasts/Macrophages in an In Vitro Model of Osteoarthritis. Biology. 2020; 9(12):459. https://doi.org/10.3390/biology9120459
Chicago/Turabian StyleManferdini, Cristina, Yasmin Saleh, Paolo Dolzani, Elena Gabusi, Diego Trucco, Giuseppe Filardo, and Gina Lisignoli. 2020. "Impact of Isolation Procedures on the Development of a Preclinical Synovial Fibroblasts/Macrophages in an In Vitro Model of Osteoarthritis" Biology 9, no. 12: 459. https://doi.org/10.3390/biology9120459
APA StyleManferdini, C., Saleh, Y., Dolzani, P., Gabusi, E., Trucco, D., Filardo, G., & Lisignoli, G. (2020). Impact of Isolation Procedures on the Development of a Preclinical Synovial Fibroblasts/Macrophages in an In Vitro Model of Osteoarthritis. Biology, 9(12), 459. https://doi.org/10.3390/biology9120459