Biochemical, Physiological, and Productive Response of Greenhouse Vegetables to Suboptimal Growth Environment Induced by Insect Nets
Abstract
Simple Summary
Abstract
1. Introduction
2. Technical Aspects of Anti-Insect Nets
3. Airflow Characterization of Screened Openings
- and
- = pressure drop across the unscreened opening, and
- = pressure drop across the screen
- and
4. Morphological, Physiological, and Biochemical Responses of Plants under Heat Stress
4.1. Effect of Heat Stress on Growth and Yield
4.2. Plant Physiological Response to Heat Stress
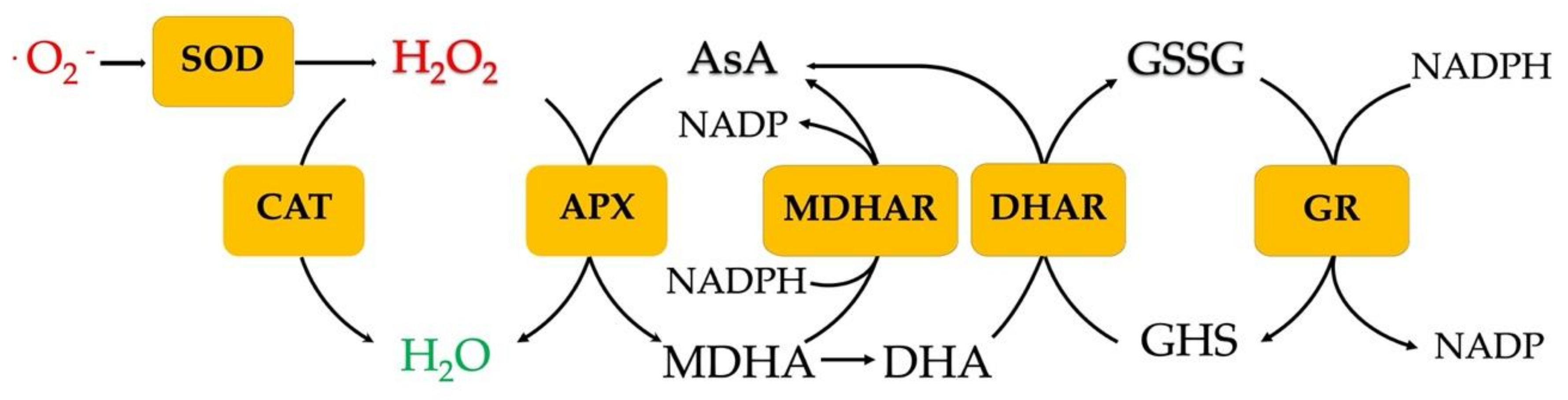
4.3. Biochemical Response to Heat Stress: The Role of Antioxidant Compounds
4.4. Heat Stress Impact on Product Quality
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Valenzano, V.; Santamaria, P.; Gonnella, M. Evolution of quality of leafy vegetables for fresh consumption in Italy. Italus Hortus 2003, 10, 184–188. [Google Scholar]
- Weng, C.J.; Yen, G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: Phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat. Rev. 2012, 38, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Harnafi, H.; Serghini Caid, H.; El Houda Bouanani, N.; Aziz, M.; Amrani, S. Hypolipemic activity of polyphenol-rich extracts from Ocimum basilicum in Triton WR-1339-induced hyperlipidemic mice. Food Chem. 2008, 108, 205–212. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant Res. 2007, 120, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.; Leonradi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- FAO. Global Agriculture toward 2050, High-Level Expert Forum, How to Feed the World 2050. Food and Agriculture Organization of United Nations (FAO): Rome, 12–13 October 2009. Available online: http://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 16 October 2020).
- Mahmood, A.; Hu, Y.; Tanny, J.; Asante, E.A. Effects of shading and insect-proof screens on crop microclimate and production: A review of recent advances. Sci. Hortic. 2018, 241, 241–251. [Google Scholar] [CrossRef]
- Lacasa, A.; Contreras, J. Comportamiento de Frankliniella occidentalis en la transmisión del virus del bronceado del tomate: Planteamientos para su control en cultivos hortícolas. Phytoma España 1993, 50, 33–39. [Google Scholar]
- Hanafi, A.; Bouharroud, R.; Amouat, S.; Miftah, S. Efficiency of insect nets in excluding whiteflies and their impact on some natural biological control agents. Acta Hortic. 2007, 747, 383–387. [Google Scholar] [CrossRef]
- Berlinger, M.J.; Taylor, R.A.J.; Lebiush-Mordechi, S.; Shalhevet, S.; Spharim, I. Efficiency of insect exclusion screens for preventing whitefly transmission of tomato yellow leaf curl virus of tomatoes in Israel. Bull. Entomol. Res. 2002, 92, 367–373. [Google Scholar] [CrossRef]
- Teitel, M.; Shklyar, A. Pressure Drop Across Insect-Proof Screens. Am. Soc. Agric. Eng. 1998, 41, 1829–1834. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Ahmad, P.; Bhardwaj, R.; Tuteja, N. Plant signaling under abiotic stress environment. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 297–323. [Google Scholar]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunić, L.; Manojlović, M. Color Shade Nets Improve Vegetables Quality at Harvest and Maintain Quality During Storage. Contemp. Agric. 2018, 67, 9–19. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 208. [Google Scholar] [CrossRef]
- Arena, C.; Conti, S.; Francesca, S.; Melchionna, G.; Hájek, J.; Barták, M.; Barone, A.; Rigano, M.M. Eco-physiological screening of different tomato genotypes in response to high temperatures: A combined field-to-laboratory approach. Plants 2020, 9, 508. [Google Scholar] [CrossRef]
- Vollenweider, P.; Günthardt-Goerg, M.S. Diagnosis of abiotic and biotic stress factors using the visible symptoms in foliage. Environ. Pollut. 2005, 137, 455–465. [Google Scholar] [CrossRef]
- Peñaranda, A.; Payan, M.C.; Garrido, D.; Gómez, P.; Jamilena, M. Production of fruits with attached flowers in zucchini squash is correlated with the arrest of maturation of female flowers. J. Hortic. Sci. Biotechnol. 2007, 82, 579–584. [Google Scholar] [CrossRef]
- Dutta, S.; Mohanty, S.; Tripathy, B.C. Role of temperature stress on chloroplast biogenesis and protein import in pea1 [OA]. Plant Physiol. 2009, 150, 1050–1061. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Diwan, H.A.; Abrol, Y.P. Global climate change, stress and plant productivity. In Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genome Foundation; Pareek, A., Sopory, S.K., Bohnert, H., Govindjee, Eds.; Springer Science+Business Media B. V.: Dordrecht, The Netherlands, 2010; pp. 503–521. ISBN 978-90-481-3111-2. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. Abiotic Stress Responses Appl. Agric. 2013, 13, 169–205. [Google Scholar]
- Formisano, L.; Pannico, A.; El-nakhel, C.; Starace, G.; Poledica, M.; De Pascale, S.; Rouphael, Y. Improved Porosity of Insect Proof Screens Enhances Quality Aspects of Zucchini Squash without Compromising the Yield. Plants 2020, 9, 1264. [Google Scholar] [CrossRef]
- Castellano, S.; Scarascia Mugnozza, G.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waaijenberg, D. Plastic nets in agriculture: A general review of types and applications. Am. Soc. Agric. Biol. Eng. 2008, 24, 799–808. [Google Scholar] [CrossRef]
- NGMA (National Greenhouse Manufactures Associtation). Insect Screening Standards and Guidelines. Available online: https://ngma.com/wp-content/uploads/2018/05/InsectScreen2010.pdf (accessed on 16 September 2020).
- Von Zabeltitz, C. Insect Screening. In Integrated Greenhouse Systems for Mild Climates; Springer: Berlin/Heidelberg, Germany, 2011; pp. 233–250. ISBN 9783642145827. [Google Scholar]
- Alvarez, A.J. Estudio de las Características Geométricas y del Comportamiento Aerodinámico de las Mallas Antiinsectos Utilizadas en los Invernaderos Como Medida de Protección Vegetal. Ph.D. Thesis, University of Almería, Almería, Spain, 22 April 2010. [Google Scholar]
- Bethke, J.A.; Paine, T.D. Screen hole size and barriers for exclusion of insect pests of glasshouse crops. J. Entomol. Sci. 1991, 26, 169–177. [Google Scholar] [CrossRef]
- Bell, M.L.; Baker, J.R. Choose a greenhouse screen based on its pest exclusion efficiency. N. C. Flower Grow. Bull. 1997, 42, 7–13. [Google Scholar]
- Álvarez, A.J.; Oliva, R.M. Insect exclusion screens: The size of the holes from a three-dimensional perspective. Acta Hortic. 2017, 1170, 1035–1042. [Google Scholar] [CrossRef]
- Oliva, R.M.; Álvarez, A.J. Factors influencing the efficacy of insect-proof screens. Acta Hortic. 2017, 1170, 1027–1033. [Google Scholar] [CrossRef]
- Castellano, S.; Starace, G.; De Pascalis, L.; Lippolis, M.; Scarascia-Mugnozza, G. Experimental results on air permeability of agricultural nets. J. Agric. Eng. 2016, 47, 134–141. [Google Scholar] [CrossRef]
- Bell, M.L.; Baker, J.R. Comparison of greenhouse screening materials for excluding whitefly (Homoptera: Aleyrodidae) and thrips (Thysanoptera: Thripidae). J. Econ. Entomol. 2000, 93, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Ingwell, L.L.; Kaplan, I. Insect Exclusion Screens Reduce Cucumber Beetle Infestations in High Tunnels, Increasing Cucurbit Yield. J. Econ. Entomol. 2019, 112, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Doble, M.; Kumar, A. Degradation of polymers. In Biotreatment of Industrial Effluents, 1st ed.; Doble, M., Kumar, A., Eds.; Butterworth-Heinemann-Elsevier: Burlington, MA, USA, 2005; pp. 101–110. [Google Scholar] [CrossRef]
- Antignus, Y.; Nestel, D.; Cohen, S.; Lapidot, M. Ultraviolet-deficient greenhouse environment affects whitefly attraction and flight-behavior. Environ. Entomol. 2001, 30, 394–399. [Google Scholar] [CrossRef]
- Legarrea, S.; Weintraub, P.G.; Plaza, M.; Viñuela, E.; Fereres, A. Dispersal of aphids, whiteflies and their natural enemies under photoselective nets. BioControl 2012, 57, 523–532. [Google Scholar] [CrossRef]
- Ben-Yakir, D.; Antignus, Y.; Offir, Y.; Shahak, Y. Colored shading nets impede insect invasion and decrease the incidences of insect-transmitted viral diseases in vegetable crops. Entomol. Exp. Appl. 2012, 144, 249–257. [Google Scholar] [CrossRef]
- Dáder, B.; Legarrea, S.; Moreno, A.; Plaza, M.; Carmo-Sousa, M.; Amor, F.; Viñuela, E.; Fereres, A. Control of insect vectors and plant viruses in protected crops by novel pyrethroid-treated nets. Pest Manag. Sci. 2015, 71, 1397–1406. [Google Scholar] [CrossRef]
- Martin, T.; Assogba-Komlan, F.; Sidick, I.; Ahle, V.; Chandre, F. An acaricide-treated net to control phytophagous mites. Crop Prot. 2010, 29, 470–475. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Krauter, P.C.; Gilder, K.; Heinz, K.M. Evaluation of deltamethrin-impregnated nets as a protective barrier against Western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae) under laboratory and greenhouse conditions. Crop Prot. 2018, 112, 227–231. [Google Scholar] [CrossRef]
- Bethke, J.A. Considering installing screening? This is what you need to know. Greenh. Manag. 1994, 13, 34–37. [Google Scholar]
- Stanghellini, C.; Van’t Oosfer, B.; Heuvelink, E. Greenhouse Horticulture: Technology for Optimal Crop Production; Wageningen Academic Publishers: Wageningen, The Netherlands, 2019; ISBN 9789086863297. [Google Scholar]
- Bailey, B.J.; Montero, J.I.; Parra, J.P.; Robertson, A.P.; Baeza, E.; Kamaruddin, R. Airflow resistance of greenhouse ventilators with and without insect screens. Biosyst. Eng. 2003, 86, 33–39. [Google Scholar] [CrossRef]
- Teitel, M. The effect of screens on the microclimate of greenhouses and screenhouses—A review. Acta Hortic. 2006, 719, 575–586. [Google Scholar] [CrossRef]
- Kittas, C.; Boulard, T.; Bartzanas, T.; Katsoulas, N.; Mermier, M. Influence of an insect screen on greenhouse ventilation. Am. Soc. Agric. Eng. 2002, 45, 1083–1090. [Google Scholar] [CrossRef]
- Fatnassi, H.; Boulard, T.; Bouirden, L. Simulation of climatic conditions in full-scale greenhouse fitted with insect-proof screens. Agric. For. Meteorol. 2003, 118, 97–111. [Google Scholar] [CrossRef]
- Teitel, M.; Barak, M.; Berlinger, M.J.; Lebiush-Mordechi, S. Insect-proof screens in greenhouses: Their effect on roof ventilation and insect penetration. Acta Hortic. 1999, 507, 29–37. [Google Scholar] [CrossRef]
- Miguel, A.F.; Van De Braak, N.J.; Silva, A.M.; Bot, G.P.A. Physical modelling of natural ventilation through screens and windows in greenhouses. J. Agric. Eng. Res. 1998, 70, 165–176. [Google Scholar] [CrossRef]
- Valera, D.L.; Molina, F.D.; Álvarez, A.J.; López, J.A.; Terrés-Nicoli, J.M.; Madueño, A. Contribution to characterisation of insect-proof screens: Experimental measurements in wind tunnel and CFD simulation. Acta Hortic. 2005, 691, 441–448. [Google Scholar] [CrossRef]
- Karava, P.; Stathopoulos, T.; Athienitis, A.K. Wind Driven Flow through Openings—A Review of Discharge Coefficients. Int. J. Vent. 2004, 3, 255–266. [Google Scholar] [CrossRef]
- Andersen, K. Friction and contraction by ventilation openings with movable flaps. In Proceedings of the Roomvent, Copenhagen, Denmark, 8–11 September 2002; p. 4. [Google Scholar]
- Teitel, M. The effect of screened openings on greenhouse microclimate. Agric. For. Meteorol. 2007, 143, 159–175. [Google Scholar] [CrossRef]
- Sase, S.; Christianson, L.L. Screening Greenhosues—Some Engineering Considerations. In Proceedings of the American Society of Agricultural Engineers/Northeast Agricultural in Biological Engineers Conference, Pennsylvania State University, PA, USA, 29 July–1 August 1990; Paper No. NABEC 90-201. ASABE: St. Joseph, MI, USA; pp. 1–13. [Google Scholar]
- Montero, J.I.; Muñoz, P.; Antón, A. Discharge coefficients of greenhouse windows with insect-proof screens. Acta Hortic. 1997, 443, 71–78. [Google Scholar] [CrossRef]
- Bot, G.P.A. Greenhouse Climate: From Physical Processes to a Dynamic Model. Ph.D. Thesis, University of Wageningen, Wageningen, The Netherlands, 1983. [Google Scholar]
- Miguel, A.F.; Van De Braak, N.J.; Bot, G.P.A. Analysis of the airflow characteristics of greenhouse screening materials. J. Agric. Eng. Res. 1997, 67, 105–112. [Google Scholar] [CrossRef]
- Pinker, R.A.; Herbert, M.V. Pressure loss Associated with Compressible flow through Square-Mesh wire Gauzes. J. Mech. Eng. Sci. 1967, 9, 11–23. [Google Scholar] [CrossRef]
- Ishizuka, M.; Nakagawa, S.; Koizumi, K.; Takegoshi, E. Measurement of Flow Resistance Coefficients for Wire Nets in Natural Air. Available online: https://www.semanticscholar.org/paper/MEASUREMENT-OF-FLOW-RESISTANCE-COEFFICIENTS-FOR-IN-Ishizuka-Nakagawa/c4eb967ba1416f19af4e9147d8b5b5842acf556e (accessed on 30 November 2020).
- Linker, R.; Tarnopolsky, M.; Seginer, I. Increased Resistance to Flow and Temperature-rise Resulting from Dust Accumulation on Greenhouse Insect-proof Screens. Available online: https://elibrary.asabe.org/abstract.asp?aid=10475 (accessed on 30 November 2020).
- Succi, S.; Vulpiani, A. Dinamica dei Fluidi. In Enciclopedia Degli Idrocarburi; Istituto Della Enciclopedia Italiana Treccani: Rome, Italy, 2005; pp. 205–221. ISBN OCLC868526989. [Google Scholar]
- Teitel, M. The effect of insect-proof screens in roof openings on greenhouse microclimate. Agric. For. Meteorol. 2001, 110, 13–25. [Google Scholar] [CrossRef]
- Parra, J.P.; Baeza, E.; Montero, J.I.; Bailey, B.J. Natural ventilation of parral greenhouses. Biosyst. Eng. 2004, 87, 355–366. [Google Scholar] [CrossRef]
- Fatnassi, H.; Boulard, T.; Demrati, H.; Bouirden, L.; Sappe, G. Ventilation performance of a large Canarian-type greenhouse equipped with insect-proof nets. Biosyst. Eng. 2002, 82, 97–105. [Google Scholar] [CrossRef]
- Fatnassi, H.; Boulard, T.; Demrati, H.; Bouirden, L.; Sappe, G. Greenhouse insect screening optimized based on CFD studies. In Proceedings of the ISHS Intl Conference Sustainable Greenhouse System—Greensys 2004, Leuven, Belgium, 12–16 September 2004. [Google Scholar]
- Ajwang, P.O.; Tantau, H.J. Prediction of the effect of insect-proof screens on climate in a naturally ventilated greenhouse in humid tropical climates. Acta Hortic. 2005, 691, 449–456. [Google Scholar] [CrossRef]
- Soni, P.; Salokhe, V.M.; Tantau, H.J. Effect of screen mesh size on vertical temperature distribution in naturally ventilated tropical greenhouses. Biosyst. Eng. 2005, 92, 469–482. [Google Scholar] [CrossRef]
- Fatnassi, H.; Boulard, T.; Poncet, C.; Chave, M. Optimisation of greenhouse insect screening with computational fluid dynamics. Biosyst. Eng. 2006, 93, 301–312. [Google Scholar] [CrossRef]
- Harmanto; Tantau, H.J.; Salokhe, V.M. Microclimate and Air Exchange Rates in Greenhouses covered with Different Nets in the Humid Tropics. Biosyst. Eng. 2006, 94, 239–253. [Google Scholar] [CrossRef]
- Kittas, C.; Katsoulas, N.; Bartzanas, T.; Mermier, M.; Boulard, T. The impact of insect screens and ventilation openings on the greenhouse microclimate. Am. Soc. Agric. Biol. Eng. 2008, 51, 2151–2165. [Google Scholar] [CrossRef]
- Patel, D.; Franklin, K.A. Temperature-regulation of plant architecture. Plant. Signal. Behav. 2009, 4, 577–579. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Essemine, J.; Ammar, S.; Bouzid, S. Impact of heat stress on germination and growth in higher plants: Physiological, biochemical and molecular repercussions and mechanisms of defence. J. Biol. Sci. 2010, 10, 565–572. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Craufurd, P.Q.; Summerfield, R.J. Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Ann. Bot. 1999, 84, 381–386. [Google Scholar] [CrossRef]
- Rainey, K.M.; Griffiths, P.D. Evaluation of Phaseolus acutifolius A. Gray plant introductions under high temperatures in a controlled environment. Genet. Resour. Crop. Evol. 2005, 52, 117–120. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant. Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef]
- Johannsson, M.H.; Stephenson, A.G. Effects of temperature during microsporogenesis on pollen performance in Cucurbita pepo L. (Cucurbitaceae). Int. J. Plant. Sci. 1998, 159, 616–626. [Google Scholar] [CrossRef]
- Parish, R.W.; Phan, H.A.; Iacuone, S.; Li, S.F. Tapetal development and abiotic stress: A centre of vulnerability. Funct. Plant. Biol. 2012, 39, 553–559. [Google Scholar] [CrossRef]
- Ismail, A.M.; Hall, A.E. Reproductive-stage heat tolerance, leaf membrane thermostability and plant morphology in cowpea. Crop. Sci. 1999, 39, 1762–1768. [Google Scholar] [CrossRef]
- Sakata, T.; Oshino, T.; Miura, S.; Tomabechi, M.; Tsunaga, Y.; Higashitani, N.; Miyazawa, Y.; Takahashi, H.; Watanabe, M.; Higashitani, A. Auxins reverse plant male sterility caused by high temperatures. Proc. Natl. Acad. Sci. USA 2010, 107, 8569–8574. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Stress physiology. In Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2006; pp. 671–681. [Google Scholar]
- Morales, D.; Rodríguez, P.; Dell’Amico, J.; Nicolás, E.; Torrecillas, A.; Sánchez-Blanco, M.J. High-temperature preconditioning and thermal shock imposition affects water relations, gas exchange and root hydraulic conductivity in tomato. Biol. Plant. 2003, 47, 203–208. [Google Scholar] [CrossRef]
- Klimenko, S.B.; Peshkova, A.A.; Dorofeev, N.V. Nitrate reductase activity during heat shock in winter wheat. J. Stress Physiol. Biochem. 2006, 2, 50–55. [Google Scholar]
- Dinar, M.; Rudich, J. Effect of heat stress on assimilate partitioning in tomato. Ann. Bot. 1985, 56, 239–248. [Google Scholar] [CrossRef]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant. Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Maestri, E.; Klueva, N.; Perrotta, C.; Gulli, M.; Nguyen, H.T.; Marmiroli, N. Maestri2002-MolGen Heat Cereals. Plant. Mol. Biol. 2002, 48, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant. Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef]
- Crafts-Brandner, S.J.; Salvucci, M.E. Sensitivity of Photosynthesis in a C4 Plant, Maize, to Heat stress. Plant. Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef]
- Camejo, D.; Jiménez, A.; Alarcón, J.J.; Torres, W.; Gómez, J.M.; Sevilla, F. Changes in photosynthetic parameters and antioxidant activities following heat-shock treatment in tomato plants. Funct. Plant. Biol. 2006, 33, 177–187. [Google Scholar] [CrossRef]
- Tewari, A.K.; Tripathy, B.C. Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant. Physiol. 1998, 117, 851–858. [Google Scholar] [CrossRef]
- Mohanty, S.; Grimm, B.; Tripathy, B.C. Light and dark modulation of chlorophyll biosynthetic genes in response to temperature. Planta 2006, 224, 692–699. [Google Scholar] [CrossRef]
- Sakamoto, A.; Murata, N. Genetic engineering of glycinebetaine synthesis in plants: Current status and implications for enhancement of stress tolerance. J. Exp. Bot. 2000, 51, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Cheema, Z.A.; Cheema, M.A.; Khaliq, A. Physiological role of exogenously applied glycinebetaine to improve drought tolerance in fine grain aromatic rice (Oryza sativa L.). J. Agron. Crop. Sci. 2008, 194, 325–333. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Close, T.J. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Huang, B.; Xu, C. Identification and characterization of proteins associated with plant tolerance to heat stress. J. Integr. Plant. Biol. 2008, 50, 1230–1237. [Google Scholar] [CrossRef]
- Roitsch, T.; González, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant. Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Carbohydrate accumulation in relation to heat stress tolerance in two creeping bentgrass cultivars. J. Am. Soc. Hortic. Sci. 2000, 125, 442–447. [Google Scholar] [CrossRef]
- Firon, N.; Shaked, R.; Peet, M.M.; Pharr, D.M.; Zamski, E.; Rosenfeld, K.; Althan, L.; Pressman, E. Pollen grains of heat tolerant tomato cultivars retain higher carbohydrate concentration under heat stress conditions. Sci. Hortic. 2006, 109, 212–217. [Google Scholar] [CrossRef]
- Sugio, A.; Dreos, R.; Aparicio, F.; Maule, A.J. The cytosolic protein response as a subcomponent of the wider heat shock response in arabidopsis. Plant. Cell. 2009, 21, 642–654. [Google Scholar] [CrossRef]
- Lang-Mladek, C.; Popova, O.; Kiok, K.; Berlinger, M.; Rakic, B.; Aufsatz, W.; Jonak, C.; Hauser, M.T.; Luschnig, C. Transgenerational inheritance and resetting of stress-induced loss of epigenetic gene silencing in arabidopsis. Mol. Plant. 2010, 3, 594–602. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant. Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant. Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant. Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Adriano, S. Analisi Delle Attività di Enzimi Antiossidanti e dei Livelli di Molecole Indicatrici Dello Stress Idrico E Ossidativo in Olivo (Olea europaea L.). Ph.D. Thesis, University of Basilicata, Potenza, Italy, 2010. [Google Scholar]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant. Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Meiri, D.; Tazat, K.; Cohen-Peer, R.; Farchi-Pisanty, O.; Aviezer-Hagai, K.; Avni, A.; Breiman, A. Involvement of Arabidopsis ROF2 (FKBP65) in thermotolerance. Plant. Mol. Biol. 2010, 72, 191. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vidigal, P.; Amâncio, S. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front. Environ. Sci. 2015, 3, 20. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food—The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Laber, H.; Lattauschke, G. Vegetable Production, 2nd ed.; Verlag, E.U., Ed.; Eugen Ulmer Verlag: Stuttgart, Germany, 2014. [Google Scholar]
- Peet, M.M.; Wolfe, D.W. Crop Ecosystem Responses to Climate Change: Vegetable Crops; CABI Publishing: New York, NY, USA; Wallingford, UK, 2000; pp. 213–243. [Google Scholar]
- Coolong, T.W.; Randle, W.M. Temperature Influences Flavor Intensity and Quality inGranex 33’Onion. J. Am. Soc. Hortic. Sci. 2003, 128, 176–181. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Nissinen, A.; Prozherina, N.; Oksanen, E.J.; Holopainen, J.K. The influence of exogenous monoterpene treatment and elevated temperature on growth, physiology, chemical content and headspace volatiles of two carrot cultivars (Daucus carota L.). Environ. Exp. Bot. 2006, 56, 95–107. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Krzesiński, W.; Knaflewski, M. Effect of temperature on the yield and quality of broccoli heads. Veg. Crop. Res. Bull. 2009, 71, 51–58. [Google Scholar] [CrossRef]
- Mølmann, J.A.B.; Steindal, A.L.H.; Bengtsson, G.B.; Seljåsen, R.; Lea, P.; Skaret, J.; Johansen, T.J. Effects of temperature and photoperiod on sensory quality and contents of glucosinolates, flavonols and vitamin C in broccoli florets. Food Chem. 2015, 172, 47–55. [Google Scholar] [CrossRef]
- Dixon, G.R.; Aldous, D.E. Horticulture: Plants for People and Places, Volume 1: Production Horticulture; Springer: Dordrecht, The Netherlands, 2014; Volume 1, ISBN 9401785783. [Google Scholar]
- Wien, H.C. The Physiology of Vegetable Crops; Cab International: Wallingford, UK, 1997; ISBN 0851991467. [Google Scholar]
- Rosales, M.A.; Cervilla, L.M.; Sánchez-Rodríguez, E.; del Rubio-Wilhelmi, M.M.; Blasco, B.; Ríos, J.J.; Soriano, T.; Castilla, N.; Romero, L.; Ruiz, J.M. The effect of environmental conditions on nutritional quality of cherry tomato fruits: Evaluation of two experimental Mediterranean greenhouses. J. Sci. Food Agric. 2011, 91, 152–162. [Google Scholar] [CrossRef]
- Chakraborty, U.; Pradhan, D. High temperature-induced oxidative stress in Lens culinaris, role of antioxidants and amelioration of stress by chemical pre-treatments. J. Plant. Interact. 2011, 6, 43–52. [Google Scholar] [CrossRef]
- Kocsy, G.; Szalai, G.; Galiba, G. Effect of heat stress on glutathione biosynthesis in wheat. Acta Biol. Szeged. 2002, 46, 71–72. [Google Scholar]
- Balla, K.; Bencze, S.; Janda, T.; Veisz, O. Analysis of heat stress tolerance in winter wheat. Acta Agron. Hung. 2009, 57, 437–444. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.; Sairam, R. High temperature stress tolerance in wheat genotypes: Role of antioxidant defence enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
| Insect Species | Screen Hole Size | Average Thorax Width 4 (μm) | ||||||
|---|---|---|---|---|---|---|---|---|
| Microns | Mesh | Male | Female | Male | Female | Male | Female | |
| Frankliniella occidentalis 2 | 192 | 132 | 190.6 | 258.0 | 184.4 | 245.5 | 215 | |
| Bemisia argentifolii | 239 | --- | --- | --- | --- | --- | 239 | |
| Trialeurodes vaporarium | 288 | --- | --- | --- | --- | --- | 288 | |
| Aphis gossypii | 340 | 78 | 486.3 | 355 | 355 | |||
| Bemisia tabaci | 462 3 | 52 | 241.7 | 277.5 | 215.8 | 261.3 | --- | |
| Myzus persicae | --- | --- | --- | --- | 433.8 | --- | ||
| Liriomyza trifolii | 640 | 40 | --- | --- | 562.5 | 653.8 | 608 | |
| Reference | [46] | [35] | [32] | [46] | ||||
| Experimental Conditions | Treatments | Effect on Microclimate | Reference |
|---|---|---|---|
| Simulation model | Evaluation of a model to predict the effect of screen area/opening area ratio on ΔT (inside/outside). Net radiation and wind velocity were set to 500 Wm−2 and 1 ms−1, respectively. | For a screen area/opening area ratio of one, the nets with a discharge coefficient of 0.1 and 0.5 resulted in a ΔT of 0.75 °C and 4.5 °C, respectively. | [58] |
| Multi-span greenhouse | Effect on inner temperature and humidity of two insect screens with different porosities (ε = 0.5 and ε = 0.6) | Anti-insect nets with porosity of 0.5 and 0.6 resulted in 2.5 and 2-fold increase in ΔT, respectively, compared to the unscreened greenhouse. | [50] |
| Four-span greenhouse | Effect on inner temperature and humidity of two insect screens with different porosities (ε = 0.2 and ε = 0.4) mounted on the roof and side openings of a four-span greenhouse. | Anti-insect nets with porosity of 0.2 and 0.4 resulted in 3 and 2-fold increases in air temperature and humidity, respectively, compared to the unscreened greenhouse. | [69] |
| Greenhouse | Effect of anti-thrips net (Cd = 0.22) on air temperature in a greenhouse in the tropical region with small plants and low transpiration rate. | Unripe plants (low transpiration rate) grown under the anti-thrips net led to a temperature increase of 5 °C. Differently, mature plants (high transpiration) under anti-thrips net showed a temperature of 3 °C. | [70] |
| Greenhouse | Effects of insect nets with different porosities (53%, 34%, 33%, and 19%) on vertical temperature distribution in greenhouses with tomato crops at two different growth stages and two densities. | Fine net porosity resulted in a higher air temperature. The highest temperature peak was recorded at the eaves height of the greenhouse. Taller plants and higher plant density resulted in lower air temperatures at all vertical points. | [71] |
| CFD simulation model | Evaluation of anti-Bemisia (ε = 0.41) and anti-thrips (ε = 0.2) nets positioned on the roof alone and roof and side openings of a multi-span greenhouse on the inner microclimate. | Both nets led to a significant increase in temperature, as compared to the unscreened control. Specifically, unscreened control, anti-Bemisia, and anti-thrips nets resulted in ΔT of 2.4 7.1, and 5.1 °C, respectively. | [72] |
| Greenhouse | Effects of different mesh sizes of nets (40, 52, and 78 mesh) on microclimate and air exchange rates in the humid tropics. | The 78 and 52-mesh nets increased air temperatures of 1–3 °C. In addition, the 78-mesh net determined an increase in humidity of about twice as much as observed with the 40-mesh net, while 52-mesh net led to a rise of 50%. | [73] |
| Mono-span greenhouse | Influence of different vent opening positions (side-only, roof-only, and combined roof and side openings) and anti-aphid insect screens on the microclimate. | The combined application of roof and side openings resulted in a reduction of the air temperature in the greenhouse compared to the roof or side vents alone. | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formisano, L.; El-Nakhel, C.; Corrado, G.; De Pascale, S.; Rouphael, Y. Biochemical, Physiological, and Productive Response of Greenhouse Vegetables to Suboptimal Growth Environment Induced by Insect Nets. Biology 2020, 9, 432. https://doi.org/10.3390/biology9120432
Formisano L, El-Nakhel C, Corrado G, De Pascale S, Rouphael Y. Biochemical, Physiological, and Productive Response of Greenhouse Vegetables to Suboptimal Growth Environment Induced by Insect Nets. Biology. 2020; 9(12):432. https://doi.org/10.3390/biology9120432
Chicago/Turabian StyleFormisano, Luigi, Christophe El-Nakhel, Giandomenico Corrado, Stefania De Pascale, and Youssef Rouphael. 2020. "Biochemical, Physiological, and Productive Response of Greenhouse Vegetables to Suboptimal Growth Environment Induced by Insect Nets" Biology 9, no. 12: 432. https://doi.org/10.3390/biology9120432
APA StyleFormisano, L., El-Nakhel, C., Corrado, G., De Pascale, S., & Rouphael, Y. (2020). Biochemical, Physiological, and Productive Response of Greenhouse Vegetables to Suboptimal Growth Environment Induced by Insect Nets. Biology, 9(12), 432. https://doi.org/10.3390/biology9120432








