Prognostic Implication of SOX2 Expression Associated with p16 in Oropharyngeal Cancer: A Study of Consecutive Tissue Microarrays and TCGA
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Consideration
2.2. Patients
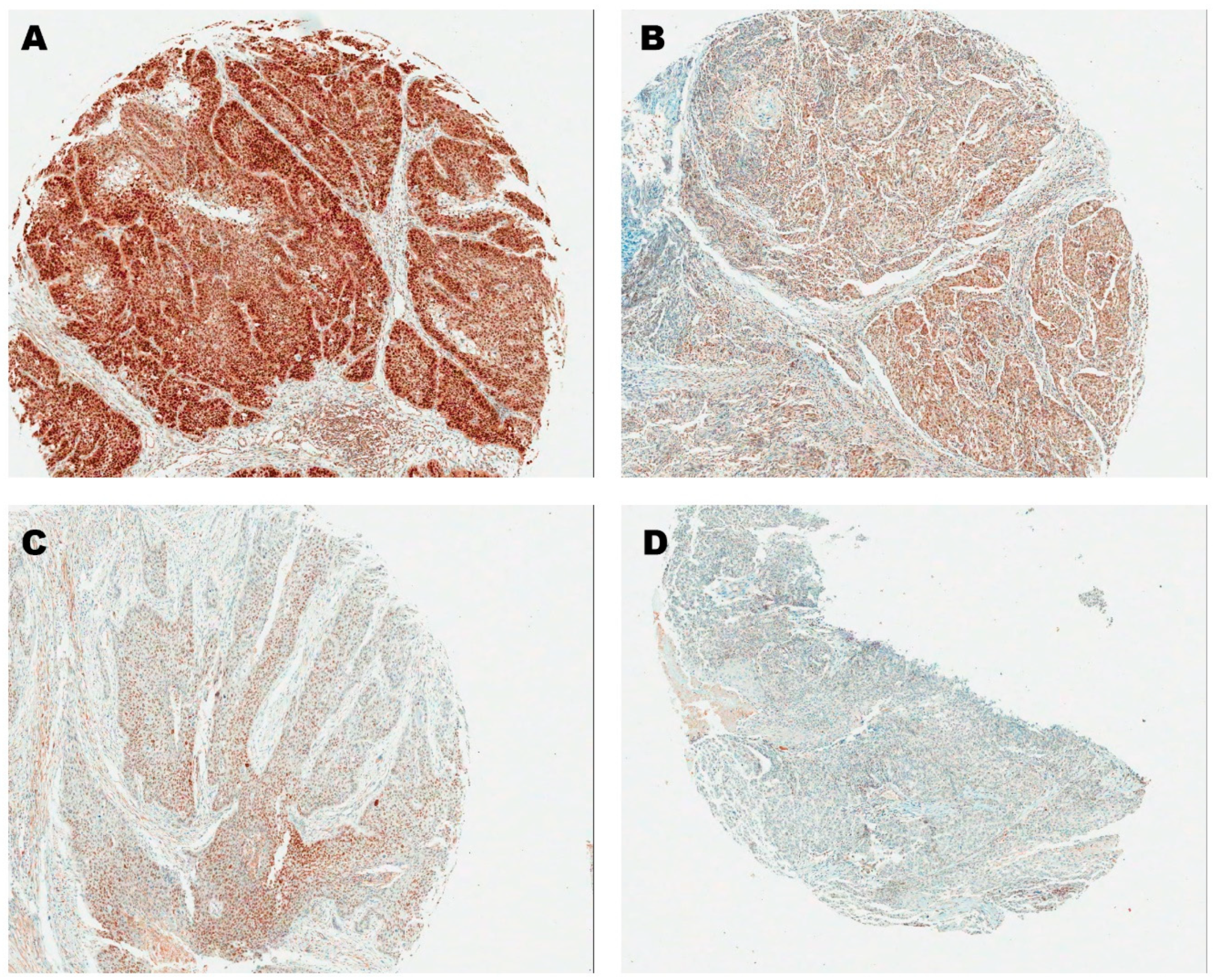
2.3. Immunohistochemistry of p16 and SOX2
2.4. The Cancer Genome Atlas (TCGA)
2.5. Statistical Analysis
3. Results
3.1. SOX2 Cut-Off Value and Correlation with p16
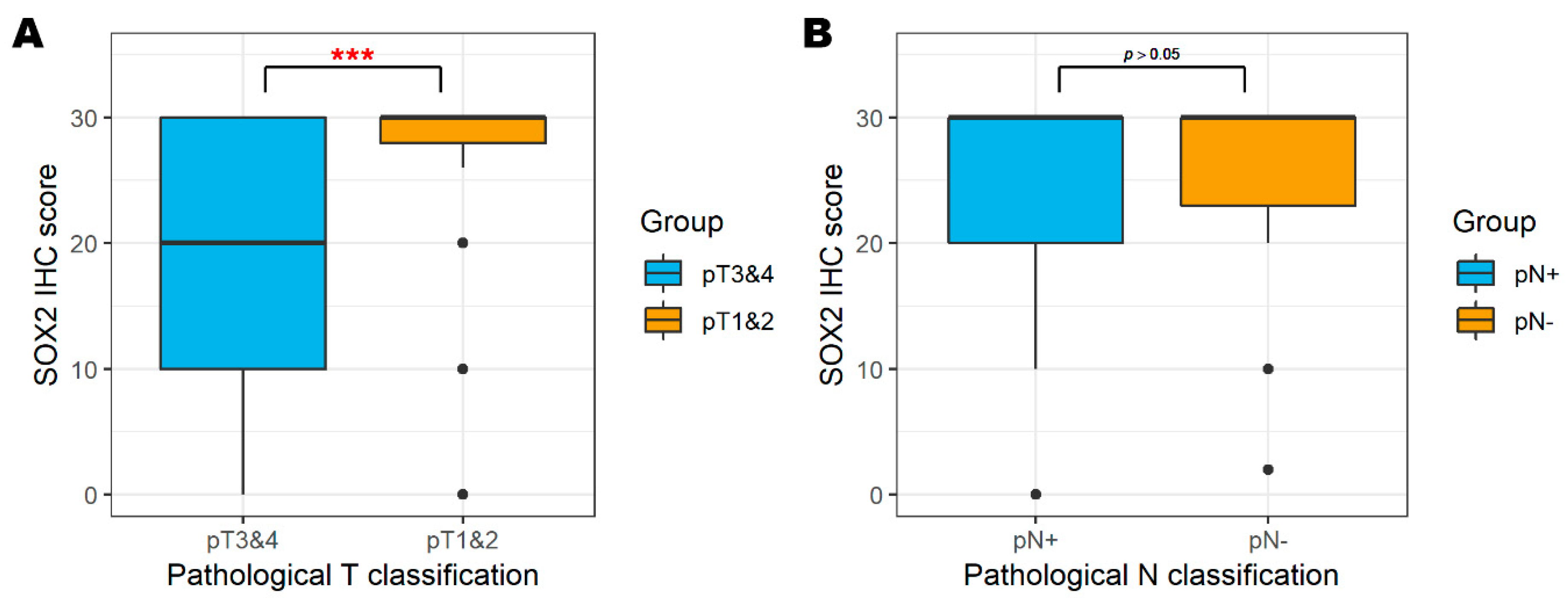
3.2. SOX2 and Pathological Classification
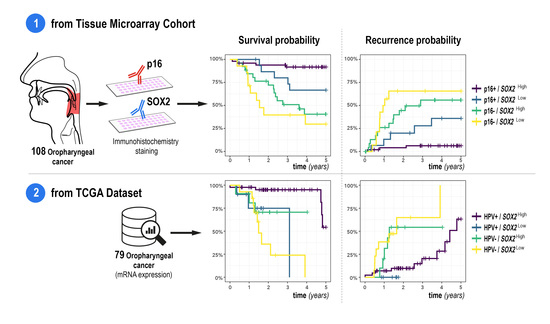
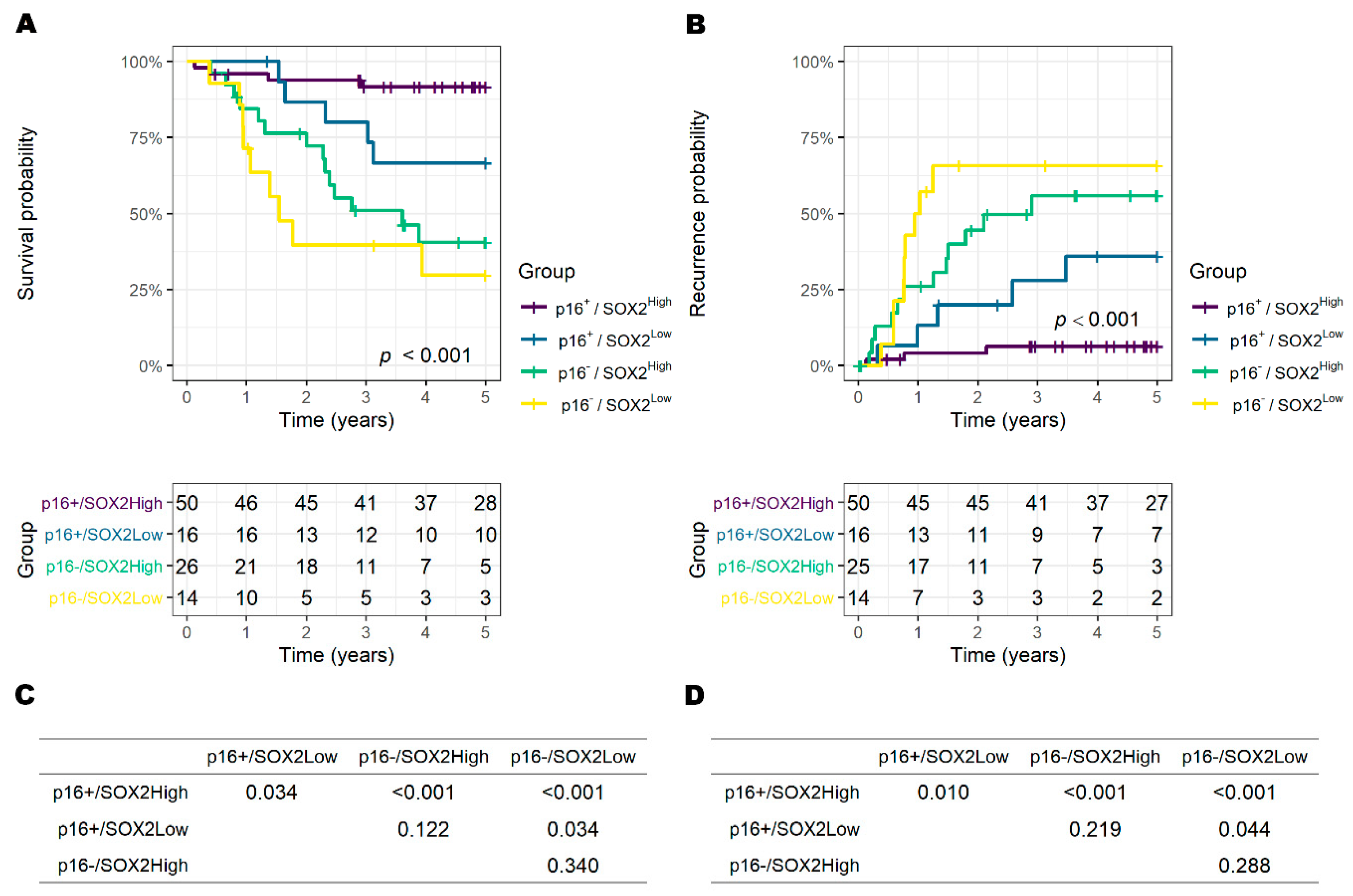
3.3. Risk Factors for Overall Survival and Recurrence
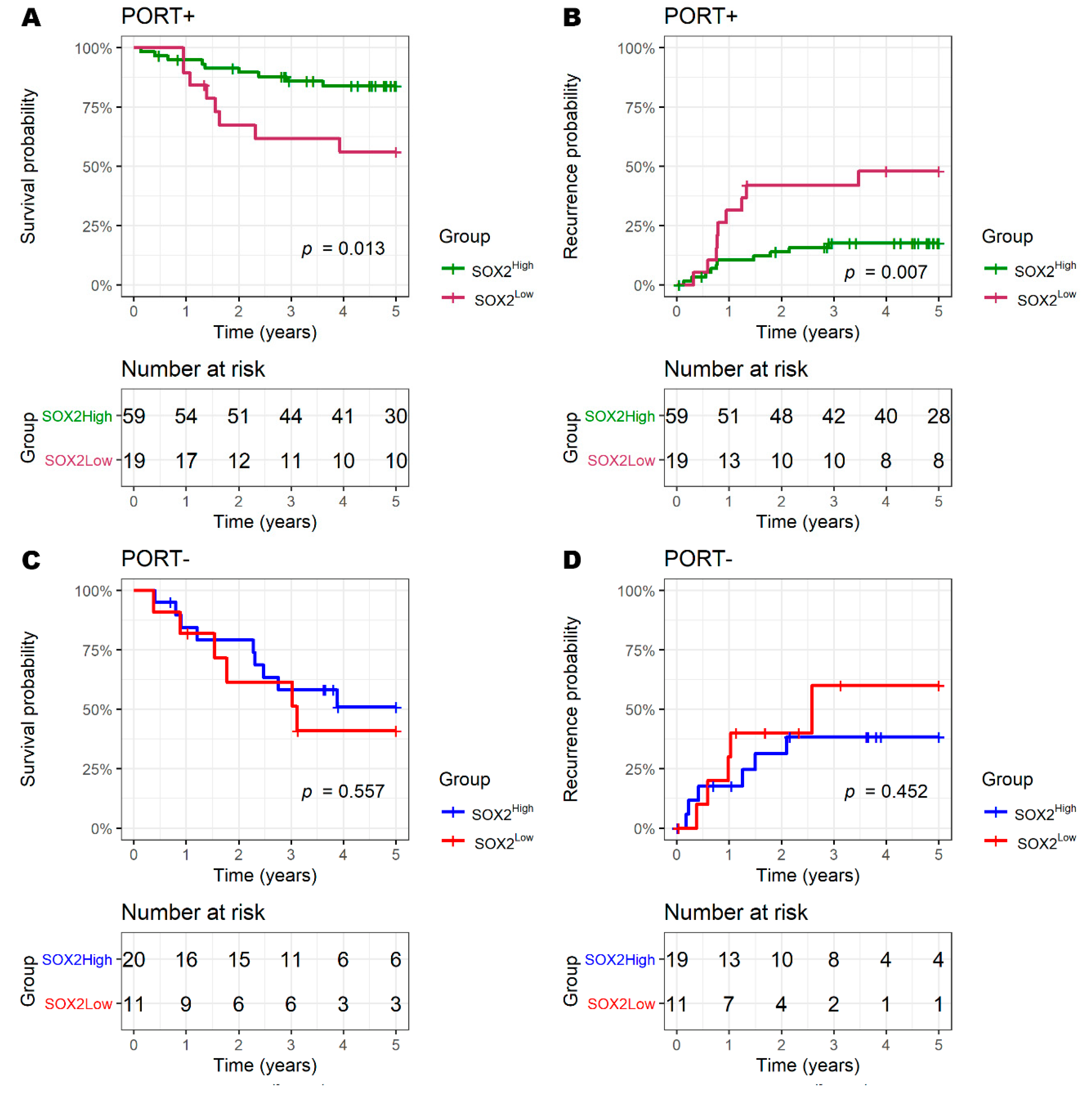
3.4. SOX2 and Radiotherapy
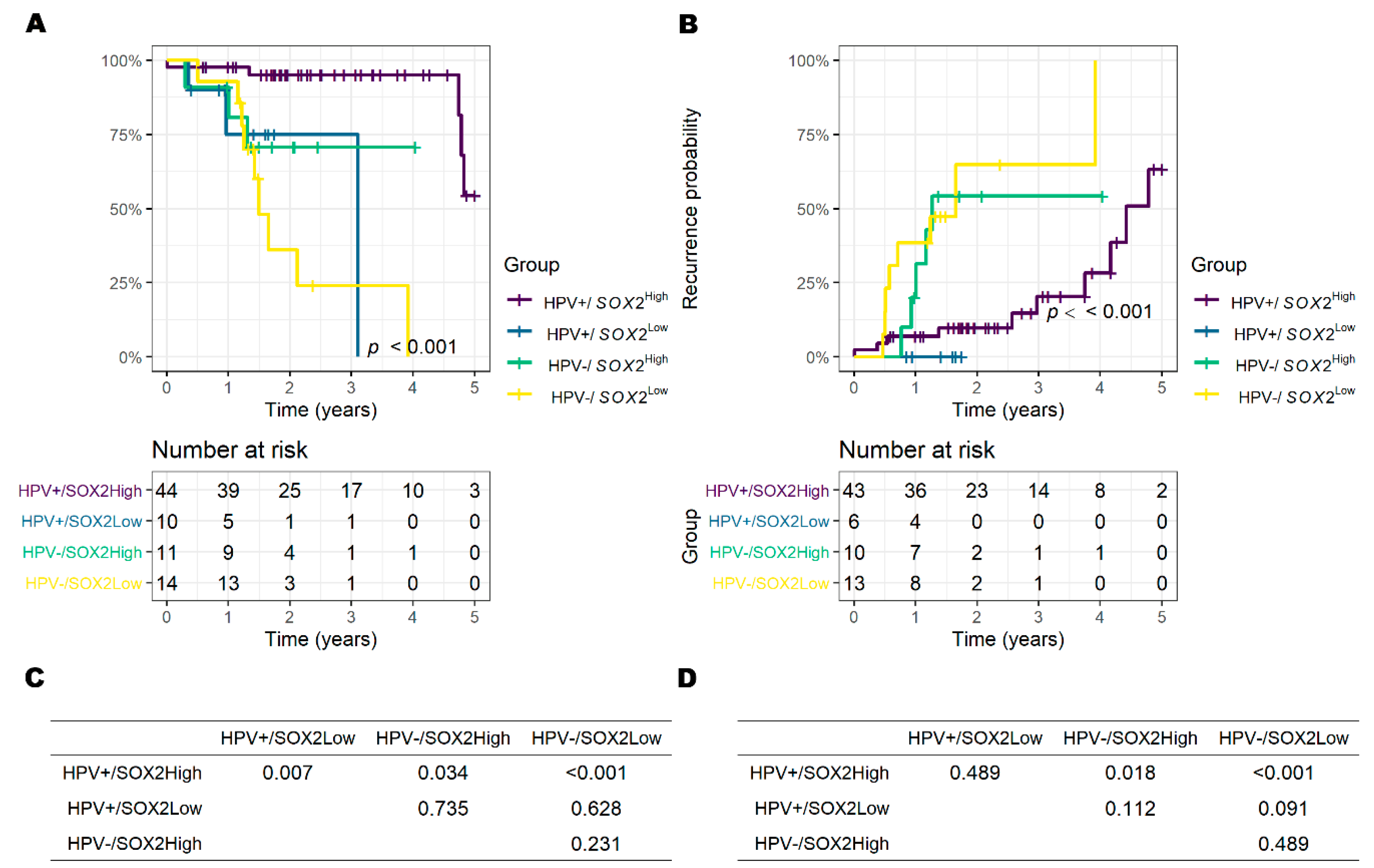
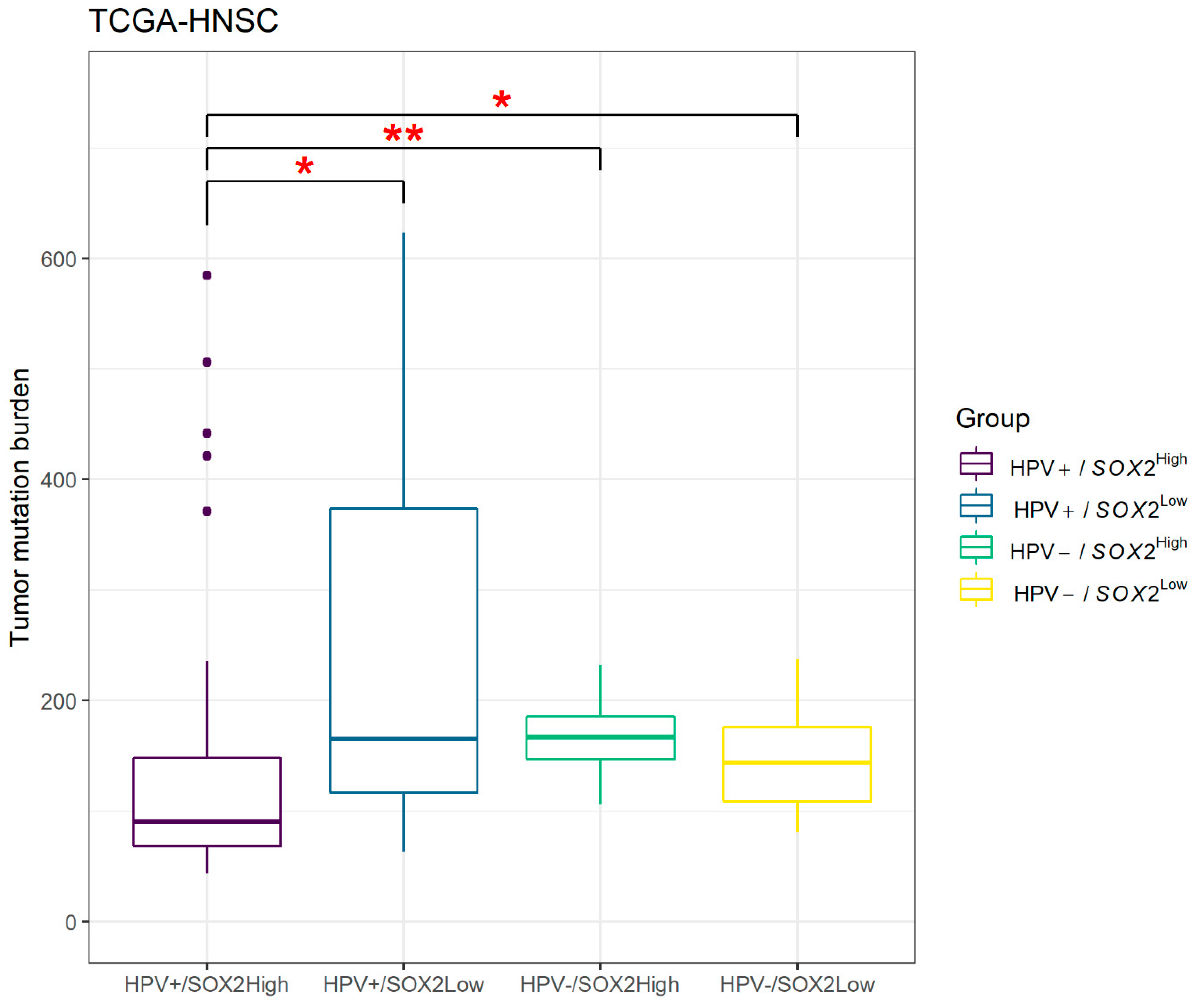
3.5. Results of TCGA Data Set Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A. Global Cancer Observatory: Cancer Today. Lyon Fr. Int. Agency Res. Cancer 2020. Available online: https://gco.iarc.fr/today (accessed on 17 March 2020).
- Vigneswaran, N.; Williams, M.D. Epidemiologic Trends in Head and Neck Cancer and Aids in Diagnosis. Oral Maxillofac. Surg. Clin. North Am. 2014, 26, 123–141. [Google Scholar] [CrossRef] [PubMed]
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef]
- Jou, A.; Hess, J. Epidemiology and Molecular Biology of Head and Neck Cancer. Oncol. Res. Treat. 2017, 40, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Hüser, L.; Novak, D.; Umansky, V.; Altevogt, P.; Utikal, J.S. Targeting SOX2 in anticancer therapy. Expert Opin. Ther. Targets 2018, 22, 983–991. [Google Scholar] [CrossRef]
- Bass, A.J.; Watanabe, H.; Mermel, C.H.; Yu, S.; Perner, S.; Verhaak, R.G.; Kim, S.Y.; Wardwell, L.; Tamayo, P.; Gat-Viks, I.; et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 2009, 41, 1238–1242. [Google Scholar] [CrossRef]
- Wuebben, E.L.; Rizzino, A. The dark side of SOX2: Cancer-a comprehensive overview. Oncotarget 2017, 8, 44917–44943. [Google Scholar] [CrossRef]
- Bayo, P.; Jou, A.; Stenzinger, A.; Shao, C.; Gross, M.; Jensen, A.; Grabe, N.; Herold-Mende, C.; Rados, P.V.; Debus, J.; et al. Loss of SOX2 expression induces cell motility via vimentin up-regulation and is an unfavorable risk factor for survival of head and neck squamous cell carcinoma. Mol. Oncol. 2015, 9, 1704–1719. [Google Scholar] [CrossRef] [PubMed]
- Piva, M.; Domenici, G.; Iriondo, O.; Rábano, M.; Simões, B.M.; Comaills, V.; Barredo, I.; López-Ruiz, J.A.; Zabalza, I.; Kypta, R.; et al. Sox2 promotes tamoxifen resistance in breast cancer cells. EMBO Mol. Med. 2013, 6, 66–79. [Google Scholar] [CrossRef]
- Lundberg, I.V.; Edin, S.; Eklöf, V.; Öberg, Å.; Palmqvist, R.; Wikberg, M.L. SOX2 expression is associated with a cancer stem cell state and down-regulation of CDX2 in colorectal cancer. BMC Cancer 2016, 16, 1–11. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Zhu, L.; Chen, M.; Huang, Y.; Zhang, J.; He, S.; Li, A.; Chen, R.; Zhou, J. SOX2 inhibits metastasis in gastric cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1221–2130. [Google Scholar] [CrossRef] [PubMed]
- Dogan, S.; Xu, B.; Middha, S.; Vanderbilt, C.M.; Bowman, A.S.; Migliacci, J.; Morris, L.G.; Seshan, V.E.; Ganly, I. Identification of prognostic molecular biomarkers in 157 HPV-positive and HPV-negative squamous cell carcinomas of the oropharynx. Int. J. Cancer 2019, 145, 3152–3162. [Google Scholar] [CrossRef]
- Vokač, N.K.; Ćižmarević, B.; Zagorac, A.; Zagradišnik, B.; Lanišnik, B. An evaluation of SOX2 and hTERC gene amplifications as screening markers in oral and oropharyngeal squamous cell carcinomas. Mol. Cytogenet. 2014, 7, 5. [Google Scholar] [CrossRef]
- Freier, K.; Knoepfle, K.; Flechtenmacher, C.; Pungs, S.; Devens, F.; Toedt, G.; Hofele, C.; Joos, S.; Lichter, P.; Radlwimmer, B. Recurrent copy number gain of transcription factorSOX2and corresponding high protein expression in oral squamous cell carcinoma. Genes Chromosom. Cancer 2010, 49, 9–16. [Google Scholar] [CrossRef]
- Tang, X.-B.; Shen, X.-H.; Li, L.; Zhang, Y.-F.; Chen, G. SOX2 overexpression correlates with poor prognosis in laryngeal squamous cell carcinoma. Auris Nasus Larynx 2013, 40, 481–486. [Google Scholar] [CrossRef]
- Fu, T.-Y.; Hsieh, I.-C.; Cheng, J.-T.; Tsai, M.-H.; Hou, Y.-Y.; Lee, J.-H.; Liou, H.-H.; Huang, S.-F.; Chen, H.-C.; Yen, L.-M.; et al. Association of OCT4, SOX2, and NANOG expression with oral squamous cell carcinoma progression. J. Oral Pathol. Med. 2015, 45, 89–95. [Google Scholar] [CrossRef]
- Pradhan, S.; Guddattu, V.; Solomon, M. Association of the co-expression of SOX2 and Podoplanin in the progression of oral squamous cell carcinomas-an immunohistochemical study. J. Appl. Oral Sci. 2019, 27, e20180348. [Google Scholar] [CrossRef]
- Chung, J.H.; Jung, H.R.; Jung, A.R.; Lee, Y.C.; Kong, M.; Lee, J.-S.; Eun, Y.-G. SOX2 activation predicts prognosis in patients with head and neck squamous cell carcinoma. Sci. Rep. 2018, 8, 1677. [Google Scholar] [CrossRef]
- Gillison, M.L. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin. Oncol. 2004, 31, 744–754. [Google Scholar] [CrossRef]
- Preti, M.; Rotondo, J.C.; Holzinger, D.; Micheletti, L.; Gallio, N.; McKay-Chopin, S.; Carreira, C.; Privitera, S.S.; Watanabe, R.; Ridder, R.; et al. Role of human papillomavirus infection in the etiology of vulvar cancer in Italian women. Infect. Agents Cancer 2020, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef]
- Vogel, U.F.; Bueltmann, B.D. Simple, inexpensive, and precise paraffin tissue microarrays constructed with a conventional microcompound table and a drill grinder. Am. J. Clin. Pathol. 2006, 126, 342–348. [Google Scholar] [CrossRef]
- Park, W.S.; Ryu, J.; Cho, K.H.; Choi, M.K.; Moon, S.H.; Yun, T.; Chun, B.-S.; Lee, G.K.; Ahn, H.J.; Lee, J.H.; et al. Human papillomavirus in oropharyngeal squamous cell carcinomas in korea: Use of G1 cycle markers as new prognosticators. Head Neck 2011, 34, 1408–1417. [Google Scholar] [CrossRef]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Liu, Z.; Li, M.; Jiang, Z.; Wang, X. A Comprehensive Immunologic Portrait of Triple-Negative Breast Cancer. Transl. Oncol. 2018, 11, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, P.J.; Saha-Chaudhuri, P.; Saha-Chaudhuri, M.P. Package ‘survivalROC’. 2013. Available online: http://www2.uaem.mx/r-mirror/web/packages/survivalROC/survivalROC.pdf (accessed on 15 February 2013).
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Economopoulou, P.; Perisanidis, C.; Giotakis, E.I.; Psyrri, A. The emerging role of immunotherapy in head and neck squamous cell carcinoma (HNSCC): Anti-tumor immunity and clinical applications. Ann. Transl. Med. 2016, 4, 173. [Google Scholar] [CrossRef]
- Yamano, Y.; Uzawa, K.; Saito, K.; Nakashima, D.; Kasamatsu, A.; Koike, H.; Kouzu, Y.; Shinozuka, K.; Nakatani, K.; Negoro, K.; et al. Identification of cisplatin-resistance related genes in head and neck squamous cell carcinoma. Int. J. Cancer 2010, 126, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.B.; Liu, I.Y.; Gornbein, J.A.; Nguyen, C.T. HPV-Positive Oropharyngeal Carcinoma: A Systematic Review of Treatment and Prognosis. Otolaryngol. Neck Surg. 2015, 153, 758–769. [Google Scholar] [CrossRef]
- Moshi, J.M.; Hoogduin, K.J.; Ummelen, M.; Henfling, M.E.R.; Van Engeland, M.; Wouters, K.A.D.; Stoop, H.; Demers, I.; Looijenga, L.H.J.; Ramaekers, F.C.S.; et al. Switches of SOX17 and SOX2 expression in the development of squamous metaplasia and squamous intraepithelial lesions of the uterine cervix. Cancer Med. 2020, 9, 6330–6343. [Google Scholar] [CrossRef]
- Gut, A.; Moch, H.; Choschzick, M. SOX2 Gene Amplification and Overexpression is Linked to HPV-positive Vulvar Carcinomas. Int. J. Gynecol. Pathol. 2018, 37, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, I.; Del-Castillo-Falconi, V.; Mitre-Aguilar, I.B.; Amador-Molina, A.; Carrillo-García, A.; Langley, E.; Zentella-Dehesa, A.; Soto-Reyes, E.; García-Carrancá, A.; Herrera, L.A.; et al. SOX2 as a New Regulator of HPV16 Transcription. Viruses 2017, 9, 175. [Google Scholar] [CrossRef]
- Kenudson, M.M. Immunohistochemistry for predictive biomarkers in non-small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 570–587. [Google Scholar] [CrossRef]
- Khotskaya, Y.B.; Mills, G.B.; Shaw, K.R.M. Next-Generation Sequencing and Result Interpretation in Clinical Oncology: Challenges of Personalized Cancer Therapy. Annu. Rev. Med. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Novak, D.; Hüser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in development and cancer biology. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, P.; Hollmann, A.; Kitz, J.; Afthonidou, A.; Simon, F.; Shakhtour, J.; Mack, B.; Kranz, G.; Libl, D.; Leu, M.; et al. High Expression of EpCAM and Sox2 is a Positive Prognosticator of Clinical Outcome for Head and Neck Carcinoma. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Schröck, A.; Bode, M.; Göke, F.J.M.; Bareiss, P.M.; Schairer, R.; Wang, H.; Weichert, W.; Franzen, A.; Kirsten, R.; Van Bremen, T.; et al. Expression and role of the embryonic protein SOX2 in head and neck squamous cell carcinoma. Carcinogenesis 2014, 35, 1636–1642. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, G.; Huang, B.; Sun, J.; Wu, D. Prognostic significance of SOX2 in head and neck cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 5010–5020. [Google Scholar]
- Du, L.; Yang, Y.; Xiao, X.; Wang, C.; Zhang, X.; Wang, L.; Zhang, X.; Li, W.; Zheng, G.; Wang, S.; et al. Sox2 nuclear expression is closely associated with poor prognosis in patients with histologically node-negative oral tongue squamous cell carcinoma. Oral Oncol. 2011, 47, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Züllig, L.; Roessle, M.; Weber, C.; Graf, N.; Haerle, S.; Jochum, W.; Stoeckli, S.; Moch, H.; Huber, G. High sex determining region Y-box 2 expression is a negative predictor of occult lymph node metastasis in early squamous cell carcinomas of the oral cavity. Eur. J. Cancer 2013, 49, 1915–1922. [Google Scholar] [CrossRef]
- Nguyen-Tan, P.F.; Zhang, Q.; Ang, K.K.; Weber, R.S.; Rosenthal, D.I.; Soulieres, D.; Kim, H.; Silverman, C.; Raben, A.; Galloway, T.J.; et al. Randomized Phase III Trial to Test Accelerated Versus Standard Fractionation in Combination with Concurrent Cisplatin for Head and Neck Carcinomas in the Radiation Therapy Oncology Group 0129 Trial: Long-Term Report of Efficacy and Toxicity. J. Clin. Oncol. 2014, 32, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Qualliotine, J.R.; Nishant, A.; Califano, J.A.; Kim, Y.; Saunders, J.R.; Blanco, R.G.; D’Souza, G.; Zhang, Z.; Chung, C.H.; et al. Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 2015, 121, 1977–1984. [Google Scholar] [CrossRef]
- Sinha, P.; Thorstad, W.; Nussenbaum, B.; Haughey, B.; Adkins, D.R.; Kallogjeri, D.; Lewis, J.S., Jr. Distant metastasis in p16-positive oropharyngeal squamous cell carcinoma: A critical analysis of patterns and outcomes. Oral Oncol. 2014, 50, 45–51. [Google Scholar] [CrossRef]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Ioue, Y.; Miki, C.; Kusunoki, M. Correlation of CD133, OCT4, and SOX2 in Rectal Cancer and Their Association with Distant Recurrence after Chemoradiotherapy. Ann. Surg. Oncol. 2009, 16, 3488–3498. [Google Scholar] [CrossRef]
- Chou, M.-Y.; Hu, F.-W.; Yu, C.-H.; Yu, C.-C. Sox2 expression involvement in the oncogenicity and radiochemoresistance of oral cancer stem cells. Oral Oncol. 2015, 51, 31–39. [Google Scholar] [CrossRef]
- Huang, C.; Lu, H.; Li, J.; Xie, X.; Fan, L.; Wang, N.; Tan, W.; Wang, Y.; Lin, Z.; Yao, T. SOX2 regulates radioresistance in cervical cancer via the hedgehog signaling pathway. Gynecol. Oncol. 2018, 151, 533–541. [Google Scholar] [CrossRef]
- Shen, L.; Huang, X.; Xie, X.; Su, J.; Yuan, J.; Chen, X. High Expression of SOX2 and OCT4 Indicates Radiation Resistance and an Independent Negative Prognosis in Cervical Squamous Cell Carcinoma. J. Histochem. Cytochem. 2014, 62, 499–509. [Google Scholar] [CrossRef]
- Lassen, P.; Primdahl, H.; Johansen, J.; Kristensen, C.A.; Andersen, E.; Andersen, L.J.; Evensen, J.F.; Eriksen, J.G.; Overgaard, J. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother. Oncol. 2014, 113, 310–316. [Google Scholar] [CrossRef]
- Hay, A.; Nixon, I.J. Recent advances in the understanding and management of oropharyngeal cancer. F1000Research 2018, 7. [Google Scholar] [CrossRef]
- Steuer, C.E.; Ramalingam, S.S. Tumor Mutation Burden: Leading Immunotherapy to the Era of Precision Medicine? J. Clin. Oncol. 2018, 36, 631–632. [Google Scholar] [CrossRef]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed]
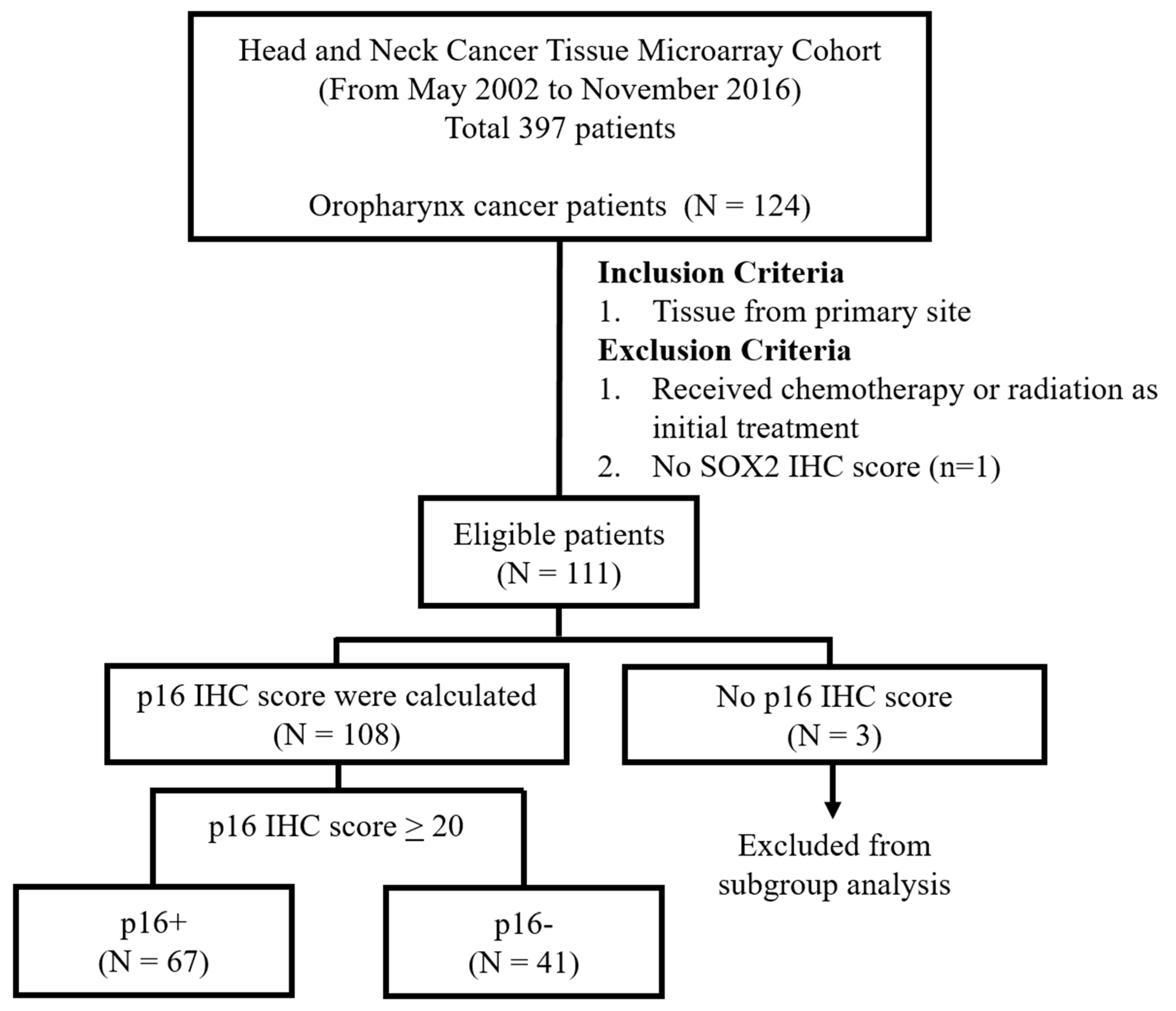
| Total (n = 111) | p16+ (n = 67) | p16− (n = 41) | p | |||
|---|---|---|---|---|---|---|
| Age at operation (years) | Avg. ± SD | 59.5 ± 10.8 | 57.0 ± 9.3 | 64.3 ± 11.3 | <0.001 * | |
| Sex | Male | 97 (87.4%) | 57 (85.1%) | 38 (92.7%) | 0.363 † | |
| Female | 14 (12.6%) | 10 (14.9%) | 3 (7.3%) | |||
| Smoking | Yes | 85 (76.6%) | 46 (68.7%) | 37 (90.2%) | 0.010 †† | |
| No | 26 (23.4%) | 21 (31.3%) | 4 (9.8%) | |||
| Subsite | Tonsil | 89 (80.2%) | 59 (88.1%) | 27 (65.9%) | 0.007 †† | |
| Base of tongue | 13 (11.7%) | 7 (10.4%) | 6 (14.6%) | |||
| Soft palate | 6 (5.4%) | 1 (1.5%) | 5 (12.2%) | |||
| Uvula | 3 (2.7%) | 0 (0.0%) | 3 (7.3%) | |||
| T classification | pT3 and -4 | 19 (17.1%) | 6 (9.0%) | 13 (31.7%) | 0.003 †† | |
| pT1 and -2 | 92 (82.9%) | 61 (91.0%) | 28 (68.3%) | |||
| Nodal status | pN+ | 68 (61.3%) | 47 (70.1%) | 20 (48.8%) | 0.060 †† | |
| pN− | 35 (31.5%) | 18 (26.9%) | 17 (41.5%) | |||
| pNx (cN0) | 8 (7.2%) | 2 (3.0%) | 4 (9.8%) | |||
| Postoperative radiotherapy | Yes | 78 (70.5%) | 55 (80.9%) | 22 (53.7%) | 0.003 †† | |
| No | 33 (29.5%) | 13 (19.1%) | 19 (46.3%) | |||
| Follow-up duration (year) | Median [IQR] | 4.6 [2.3–6.8] | 5.4 [3.5–7.3] | 2.3 [1.1–4.1] | <0.001 § | |
| Recurrence | Recurred | 28 (25.2%) | 7 (10.4%) | 20 (48.8%) | 0.007 †† | |
| NED ≥ 3 years | 80 (72.1%) | 59 (88.1%) | 19 (46.3%) | |||
| PD or f/u loss | 3 (2.7%) | 1 (1.5%) | 2 (2.9%) | |||
| Survival | NED | 63 (56.8%) | 51 (76.1%) | 10 (24.4%) | ||
| AWD | 6 (5.4%) | 0 (0.0%) | 5 (12.2%) | |||
| DOD | 23 (20.8%) | 7 (10.5%) | 16 (3.9%) | |||
| DOC | 17 (15.2%) | 8 (11.9%) | 9 (22.0%) | |||
| f/u loss | 2 (1.3%) | 1 (1.5%) | 1 (2.5%) | |||
| SOX2 IHC score | High | 30 | 74 (66.7%) | 47 (70.1%) | 24 (58.5%) | 0.157 †† |
| 25–29 | 6 (5.4%) | 4 (6.0%) | 2 (4.9%) | |||
| Low | 20 | 15 (13.5%) | 10 (14.9%) | 5 (12.2%) | ||
| 10 | 13 (11.7%) | 4 (6.0%) | 9 (22.0%) | |||
| <5 | 3 (2.7%) | 2 (3.0%) | 1 (2.4%) | |||
| p16+ Oropharyngeal Cancer (n = 67) | p16− Oropharyngeal Cancer (n = 41) | |||||
|---|---|---|---|---|---|---|
| pT1 and -2 | pT3 and -4 | p * | pT1 and -2 | pT3 and -4 | p * | |
| SOX2High | 50 (82.0%) | 1 (16.7%) | 0.001 | 20 (71.4%) | 6 (46.2%) | 0.169 |
| SOX2Low | 11 (18.0%) | 5 (83.3%) | 8 (28.6%) | 7 (53.8%) | ||
| 5-Year Overall Survival | 5-Year Recurrence | |||
|---|---|---|---|---|
| HR (95% CI) | p * | HR (95% CI) | p * | |
| Univariate Analysis | ||||
| p16− (vs. p16+) | 6.16 (2.83–13.4) | <0.001 | 6.79 (2.98–15.5) | <0.001 |
| SOX2Low (vs. SOX2High) | 2.39 (1.19–4.81] | 0.015 | 2.72 (1.32–5.59) | 0.005 |
| PORT− (vs. PORT+) | 2.70 (1.34–5.41) | 0.004 | 2.02 (0.96–4.24) | 0.065 |
| Multivariate Analysis | ||||
| p16− (vs. p16+) | 5.87 (2.69–12.8) | <0.001 | 6.26 (2.73–14.4) | <0.001 |
| SOX2Low (vs. SOX2High) | 1.99 (0.99–4.04) | 0.054 | 2.33 (1.11–4.89) | 0.025 |
| PORT− (vs. PORT+) | >0.05 | >0.05 | ||
| 5-Year Overall Survival | 5-Year Recurrence | |||
|---|---|---|---|---|
| HR (95% CI) | p * | HR (95% CI) | p * | |
| Univariate Analysis | ||||
| HPV− (vs. HPV+) | 7.11 (2.45–20.6) | <0.001 | 5.97 (2.33–15.3) | <0.001 |
| SOX2Low (vs. SOX2High) | 8.98 (3.08–26.2) | <0.001 | 2.82 (1.12–7.09) | 0.028 |
| RT− (vs. RT+) | >0.05 | >0.05 | ||
| Multivariate Analysis | ||||
| HPV − (vs. HPV +) | 3.24 (0.96–10.9) | 0.059 | 5.56 (1.95–15.9) | 0.001 |
| SOX2Low (vs. SOX2High) | 4.87 (1.44–16.5) | 0.011 | 1.17 (0.43–3.22) | 0.759 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, J.; Ryu, C.H.; Ryu, J.; Kim, J.-H.; Lee, S.-J.; Park, W.S.; Jung, Y.-S. Prognostic Implication of SOX2 Expression Associated with p16 in Oropharyngeal Cancer: A Study of Consecutive Tissue Microarrays and TCGA. Biology 2020, 9, 387. https://doi.org/10.3390/biology9110387
Seok J, Ryu CH, Ryu J, Kim J-H, Lee S-J, Park WS, Jung Y-S. Prognostic Implication of SOX2 Expression Associated with p16 in Oropharyngeal Cancer: A Study of Consecutive Tissue Microarrays and TCGA. Biology. 2020; 9(11):387. https://doi.org/10.3390/biology9110387
Chicago/Turabian StyleSeok, Jungirl, Chang Hwan Ryu, Junsun Ryu, Ji-Hyun Kim, Sang-Jin Lee, Weon Seo Park, and Yuh-Seog Jung. 2020. "Prognostic Implication of SOX2 Expression Associated with p16 in Oropharyngeal Cancer: A Study of Consecutive Tissue Microarrays and TCGA" Biology 9, no. 11: 387. https://doi.org/10.3390/biology9110387
APA StyleSeok, J., Ryu, C. H., Ryu, J., Kim, J.-H., Lee, S.-J., Park, W. S., & Jung, Y.-S. (2020). Prognostic Implication of SOX2 Expression Associated with p16 in Oropharyngeal Cancer: A Study of Consecutive Tissue Microarrays and TCGA. Biology, 9(11), 387. https://doi.org/10.3390/biology9110387