Fer1L5, a Dysferlin Homologue Present in Vesicles and Involved in C2C12 Myoblast Fusion and Membrane Repair
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Antibodies
2.2. Cell Culture
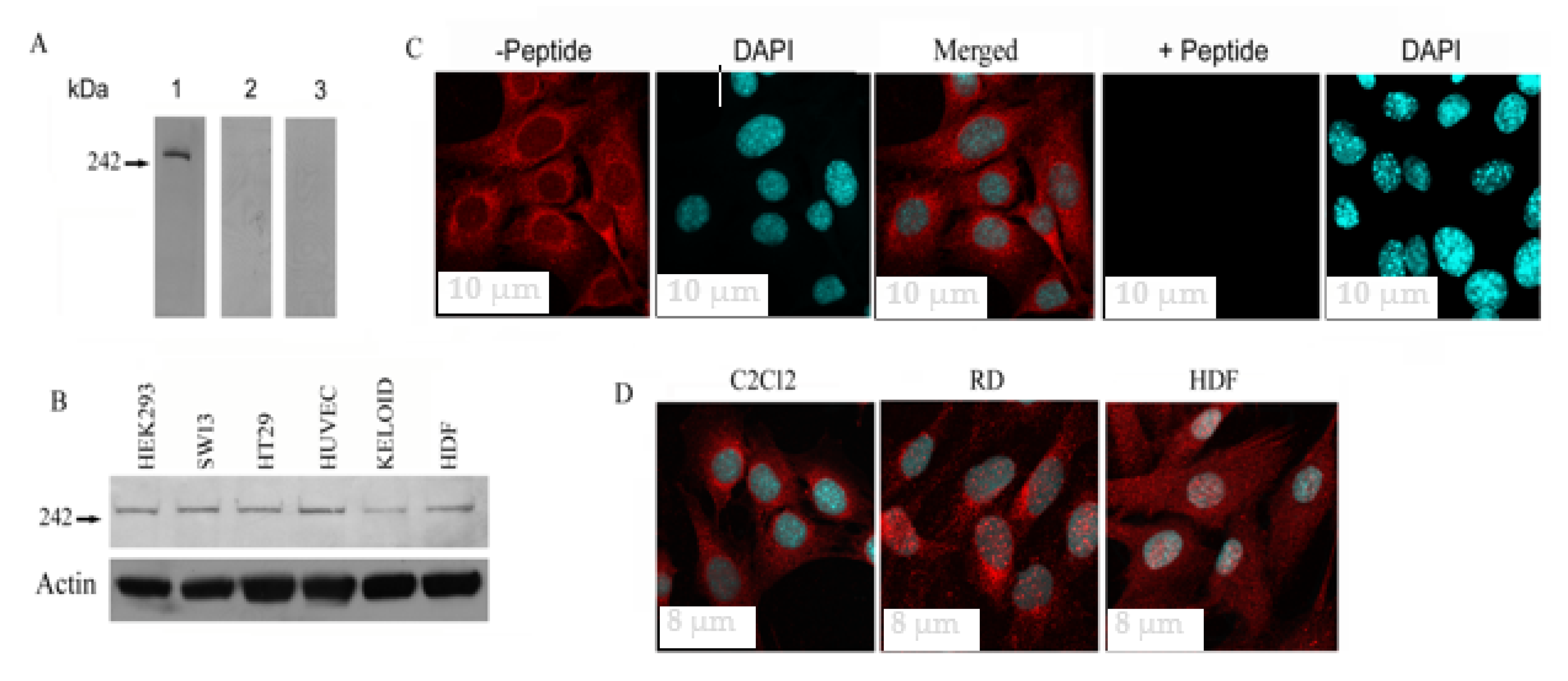
2.3. Immunofluorescence and Western Blotting
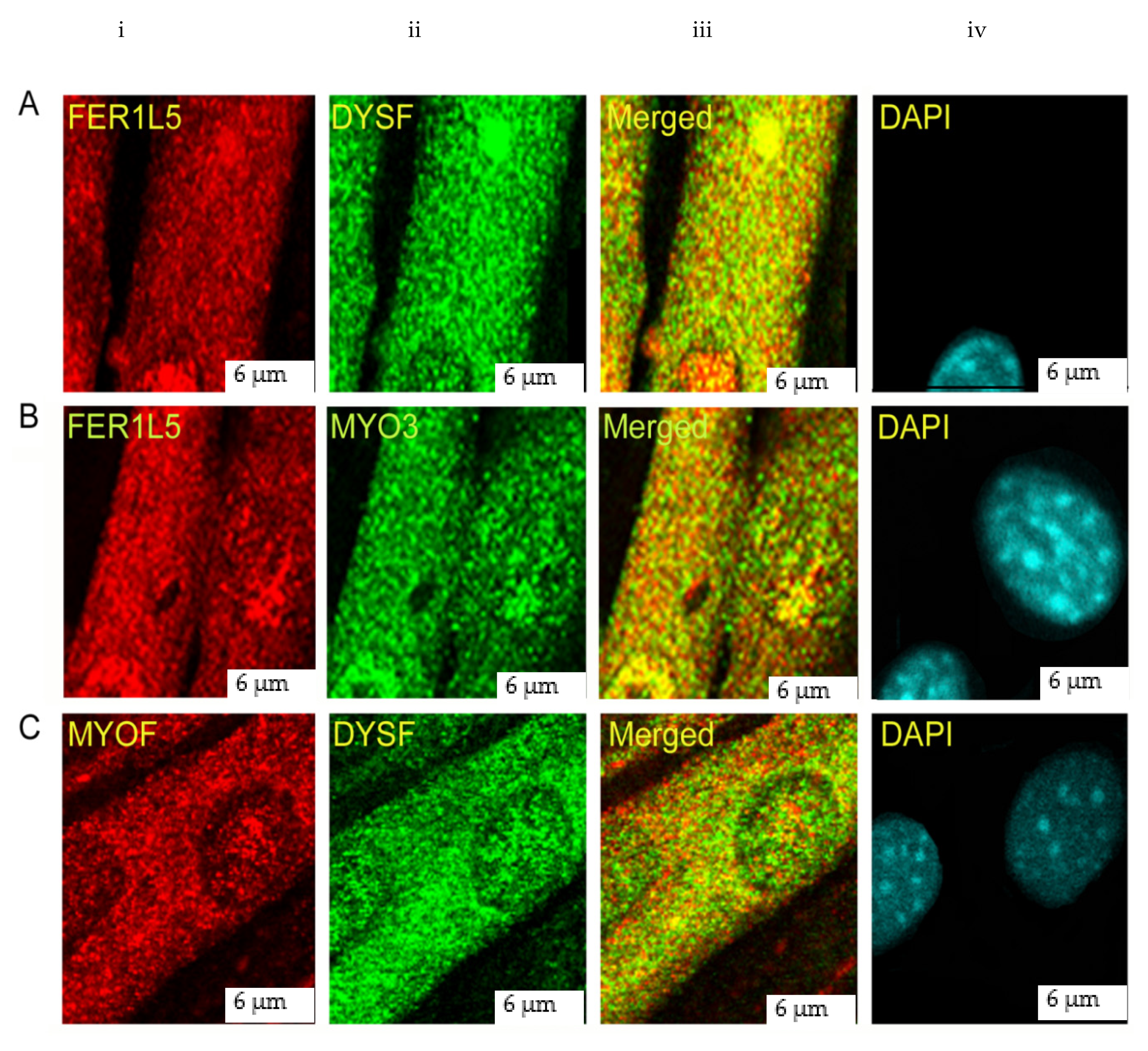
2.4. Confocal Microscopy
2.5. Densitometry
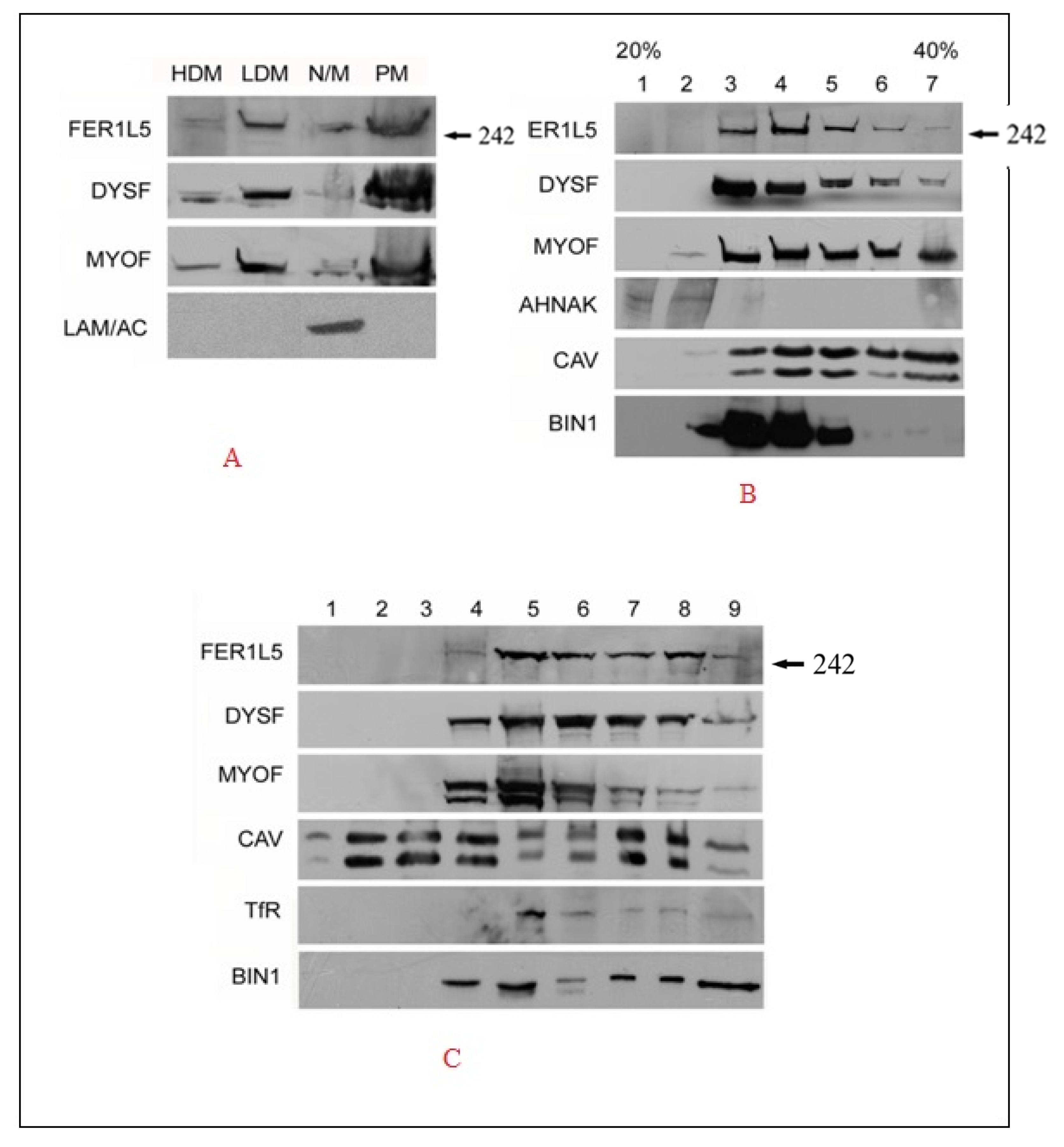
2.6. Biochemical Fractionation Studies
2.7. siRNA and shRNA Mediated Reduction
2.7.1. Generation of C2C12 Stably Expressing GAPDH siRNA
2.7.2. Fer1L5 shRNA Studies and Fer1L5 Inhibitory Antibody Loading Technique
2.8. Membrane Repair Analysis
Laser Injury Method to Analyze Membrane Resealing
3. Results
3.1. Fer1L5 Expression in Different Cell Lines
3.2. Overlapping Properties of Dysferlin Vesicles
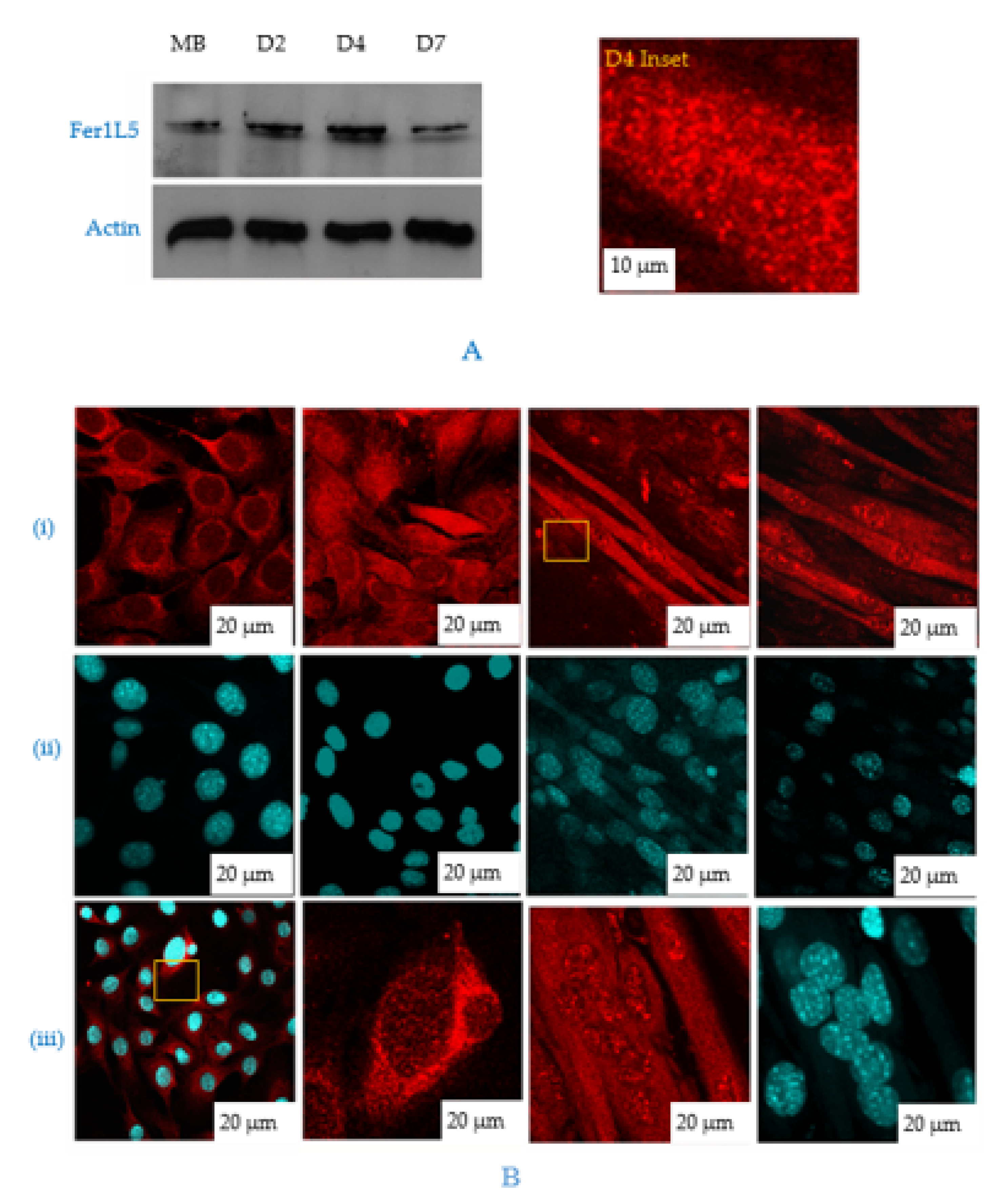
3.3. Fer1L5 is Elevated during C2C12 Myoblast Fusion
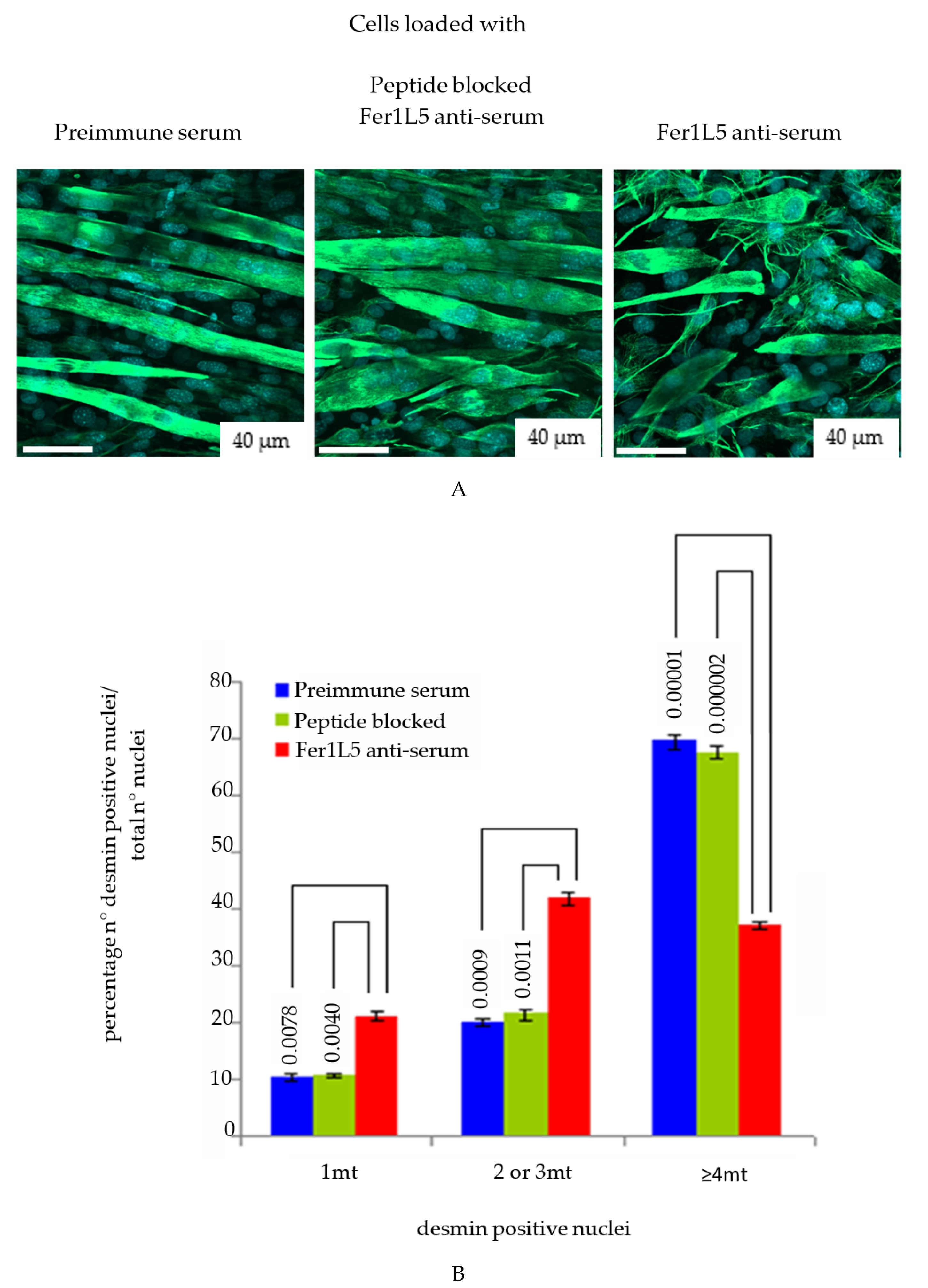
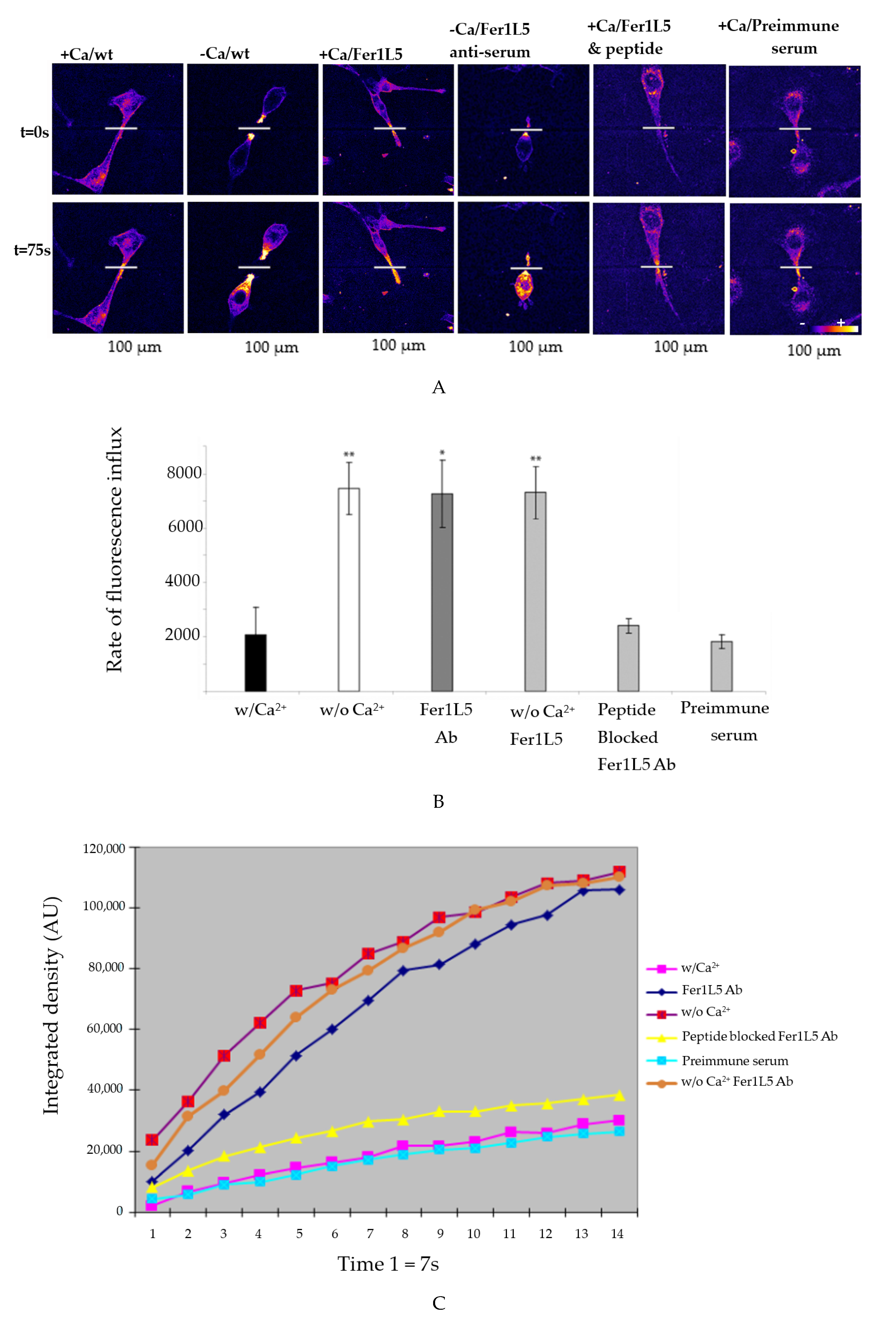
3.4. Inhibition of Fer1L5 Impairs Formation of Large Myotubes and Defective Membranerepair
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DysF | Dysferlin-like-Ferlin |
| EHD proteins | Eps15 Homology Domain |
| DMEM | Dulbeccos modified eagles medium |
| BIN 1 | Bridging integrator 1 |
| MG53 | Mitsugumin 53 (tripartite motif, TRIM72) |
References
- Jiménez, J.L.; Bashir, R. In silico functional and structural characterisation of ferlin proteins by mapping disease-causing mutations and evolutionary information onto three-dimensional models of their C2 domains. J. Neurol. Sci. 2007, 260, 114–123. [Google Scholar] [CrossRef]
- Chapman, E.R. Synaptotagmin: A Ca2+ sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 2002, 3, 498–508. [Google Scholar] [PubMed]
- Achanzar, W.E.; Ward, S. A nematode gene required for sperm vesicle fusion. J. Cell Sci. 1997, 110, 1073–1081. [Google Scholar] [PubMed]
- Redpath, G.M.I.; Sophocleous, R.A.; Turnbull, L.; Whitchurch, C.B.; Cooper, S.T. Ferlins Show Tissue-Specific Expression and Segregate as Plasma Membrane/Late Endosomal or Trans-Golgi/Recycling Ferlins. Traffic 2016, 17, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Rachubinski, D.A.; Joshi, S.; Rachubinski, R.A.; Subramani, S. Dysferlin domain-containing proteins, Pex30p and Pex31p, localized to two compartments, control the number and size of oleate-induced peroxisomes in Pichia pastoris. Mol. Biol. Cell 2008, 19, 885–898. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R.; Britton, S.; Strachan, T.; Keers, S.; Vafiadaki, E.; Lako, M.; Richard, I.; Marchand, S.; Bourg, N.; Argov, Z.; et al. A gene related to caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat. Genet. 1998, 20, 37–42. [Google Scholar] [CrossRef]
- Roux, I.; Safieddine, S.; Nouvian, R.; Grati, M.; Simmler, M.C.; Bahloul, A.; Perfettini, I.; Le Gall, M.; Rostaing, P.; Hamard, G.; et al. Otoferlin, Defective in a Human Deafness Form, Is Essential for Exocytosis at the Auditory Ribbon Synapse. Cell 2006, 127, 277–289. [Google Scholar] [CrossRef]
- Bulankina, A.V.; Thoms, S. Functions of Vertebrate Ferlins. Cells 2020, 9, 534. [Google Scholar] [CrossRef]
- De Luna, N.; Gallardo, E.; Soriano, M.; Dominguez-Perles, R.; De La Torre, C.; Rojas-García, R.; García-Verdugo, J.M.; Illa, I. Absence of dysferlin alters myogenin expression and delays human muscle differentiation “in vitro”. J. Biol. Chem. 2006, 281, 17092–17098. [Google Scholar] [CrossRef] [PubMed]
- Klinge, L.; Laval, S.; Keers, S.; Haldane, F.; Straub, V.; Barresi, R.; Bushby, K. From T-tubule to sarcolemma: Damage-induced dysferlin translocation in early myogenesis. FASEB J. 2007, 21, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Doherty, K.R.; Cave, A.; Davis, D.B.; Delmonte, A.J.; Posey, A.; Early, J.U.; Hadhazy, M.; McNally, E.M. Normal myoblast fusion requires myoferlin. Development 2005, 132, 5565–5575. [Google Scholar] [CrossRef] [PubMed]
- Lennon, N.J.; Kho, A.; Bacskai, B.J.; Perlmutter, S.L.; Hyman, B.T.; Brown, R.H. Dysferlin Interacts with Annexins A1 and A2 and Mediates Sarcolemmal Wound-healing. J. Biol. Chem. 2003, 278, 50466–50473. [Google Scholar] [CrossRef]
- Chen, E.H.; Olson, E.N. Towards a molecular pathway for myoblast fusion in Drosophila. Trends Cell Biol. 2004, 14, 452–460. [Google Scholar] [PubMed]
- Davis, D.B.; Delmonte, A.J.; Ly, C.T.; McNally, E.M. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Hum. Mol. Genet. 2000, 9, 217–226. [Google Scholar] [CrossRef]
- Haslett, J.N.; Sanoudou, D.; Kho, A.T.; Bennett, R.R.; Greenberg, S.A.; Kohane, I.S.; Beggs, A.H.; Kunkel, L.M. Gene expression comparison of biopsies from Duchenne muscular dystrophy (DMD) and normal skeletal muscle. Proc. Natl. Acad. Sci. USA 2002, 99, 15000–15005. [Google Scholar] [CrossRef]
- Lostal, W.; Bartoli, M.; Roudaut, C.; Bourg, N.; Krahn, M.; Pryadkina, M.; Borel, P.; Suel, L.; Roche, J.A.; Stockholm, D.; et al. Lack of correlation between outcomes of membrane repair assay and correction of dystrophic changes in experimental therapeutic strategy in dysferlinopathy. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Posey, A.D.; Swanson, K.E.; Alvarez, M.G.; Krishnan, S.; Earley, J.U.; Band, H.; Pytel, P.; McNally, E.M.; Demonbreun, A.R. EHD1 mediates vesicle trafficking required for normal muscle growth and transverse tubule development. Dev. Biol. 2014, 387, 179–190. [Google Scholar] [CrossRef]
- Posey, A.D.; Pytel, P.; Gardikiotes, K.; Demonbreun, A.R.; Rainey, M.; George, M.; Band, H.; McNally, E.M. Endocytic recycling proteins EHD1 and EHD2 interact with Fer-1-like-5 (Fer1L5) and mediate myoblast fusion. J. Biol. Chem. 2011, 286, 7379–7388. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, J.K.; Marlow, G.; Summerill, G.; Mahjneh, I.; Mueller, S.; Hill, M.; Miyake, K.; Haase, H.; Anderson, L.V.B.; Richard, I.; et al. Patients with a non-dysferlin miyoshi myopathy have a novel membrane repair defect. Traffic 2007, 8, 77–88. [Google Scholar] [CrossRef]
- Jasmer, D.P.; Kwak, D. Fusion and differentiation of murine C2C12 skeletal muscle cells that express Trichinella spiralis p43 protein. Exp. Parasitol. 2006, 112, 67–75. [Google Scholar] [CrossRef]
- Riquelme, C.; Barthel, K.K.B.; Qin, X.F.; Liu, X. Ubc9 expression is essential for myotube formation in C2C12. Exp. Cell Res. 2006, 312, 2132–2141. [Google Scholar] [CrossRef]
- Yaffe, D.; Saxel, O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 1977, 270, 725–727. [Google Scholar] [CrossRef]
- Cocucci, E.; Racchetti, G.; Podini, P.; Rupnik, M.; Meldolesi, J. Enlargeosome, an exocytic vesicle resistant to nonionic detergents, undergoes endocytosis via a nonacidic route. Mol. Biol. Cell 2004, 15, 5356–5368. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, B.; Cocucci, E.; Racchetti, G.; Podini, P.; Bachi, A.; Meldolesi, J. Regulated exocytosis: A novel, widely expressed system. Nat. Cell Biol. 2002, 4, 955–962. [Google Scholar] [CrossRef]
- Huang, Y.; Laval, S.H.; Remoortere, A.; Baudier, J.; Benaud, C.; Anderson, L.V.B.; Straub, V.; Deelder, A.; Frants, R.R.; Dunnen, J.T.; et al. AHNAK a novel component of the dysferlin protein complex, redistributes to the cytoplasm with dysferlin during skeletal muscle regeneration. FASEB J. 2007, 21, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Scherer, P.E.; Tang, Z.; Okamoto, T.; Li, S.; Chafel, M.; Chu, C.; Kohtz, D.S.; Lisanti, M.P. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells: Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 1996, 271, 15160–15165. [Google Scholar] [CrossRef]
- Lee, E.; Marcucci, M.; Daniell, L.; Pypaert, M.; Weisz, O.A.; Ochoa, G.C.; Farsad, K.; Wenk, M.R.; De Camilli, P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science 2002, 297, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Nicot, A.S.; Toussaint, A.; Tosch, V.; Kretz, C.; Wallgren-Pettersson, C.; Iwarsson, E.; Kingston, H.; Garnier, J.M.; Biancalana, V.; Oldfors, A.; et al. Mutations in amphiphysin 2 (BIN1) disrupt interaction with dynamin 2 and cause autosomal recessive centronuclear myopathy. Nat. Genet. 2007, 39, 1134–1139. [Google Scholar] [CrossRef]
- Bansal, D.; Miyake, K.; Vogel, S.S.; Groh, S.; Chen, C.C.; Williamson, R.; McNeil, P.L.; Campbell, K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature 2003, 423, 168–172. [Google Scholar] [CrossRef]
- Carozzi, A.J.; Ikonen, E.; Lindsay, M.R.; Parton, R.G. Role of cholesterol in developing T-tubules: Analogous mechanisms for T-tubule and caveolae biogenesis. Traffic 2000, 1, 326–341. [Google Scholar] [CrossRef]
- Toussaint, A.; Cowling, B.S.; Hnia, K.; Mohr, M.; Oldfors, A.; Schwab, Y.; Yis, U.; Maisonobe, T.; Stojkovic, T.; Wallgren-Pettersson, C.; et al. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol. 2011, 121, 253–266. [Google Scholar] [CrossRef]
- Naslavsky, N.; Caplan, S. EHD proteins: Key conductors of endocytic transport. Trends Cell Biol. 2011, 21, 122–131. [Google Scholar] [CrossRef]
- McNeil, P.L.; Steinhardt, R.A. PLasma Membrane Disruption: Repair, Prevention, Adaptation. In Proceedings of the Annual Review of Cell and Developmental Biology. Annu. Rev. Cell Dev. Biol. 2003, 19, 697–731. [Google Scholar] [CrossRef]
- McNeil, P.L.; Kirchhausen, T. An emergency response team for membrane repair. Nat. Rev. Mol. Cell Biol. 2005, 6, 499–505. [Google Scholar] [CrossRef]
- Han, R.; Bansal, D.; Miyake, K.; Muniz, V.P.; Weiss, R.M.; McNeil, P.L.; Campbell, K.P. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J. Clin. Invest. 2007, 117, 1805–1813. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usha Kalyani, R.; Perinbam, K.; Jeyanthi, P.; Al-Dhabi, N.A.; Valan Arasu, M.; Esmail, G.A.; Kim, Y.O.; Kim, H.; Kim, H.-J. Fer1L5, a Dysferlin Homologue Present in Vesicles and Involved in C2C12 Myoblast Fusion and Membrane Repair. Biology 2020, 9, 386. https://doi.org/10.3390/biology9110386
Usha Kalyani R, Perinbam K, Jeyanthi P, Al-Dhabi NA, Valan Arasu M, Esmail GA, Kim YO, Kim H, Kim H-J. Fer1L5, a Dysferlin Homologue Present in Vesicles and Involved in C2C12 Myoblast Fusion and Membrane Repair. Biology. 2020; 9(11):386. https://doi.org/10.3390/biology9110386
Chicago/Turabian StyleUsha Kalyani, R., K. Perinbam, P. Jeyanthi, Naif Abdullah Al-Dhabi, Mariadhas Valan Arasu, Galal Ali Esmail, Young Ock Kim, Hyungsuk Kim, and Hak-Jae Kim. 2020. "Fer1L5, a Dysferlin Homologue Present in Vesicles and Involved in C2C12 Myoblast Fusion and Membrane Repair" Biology 9, no. 11: 386. https://doi.org/10.3390/biology9110386
APA StyleUsha Kalyani, R., Perinbam, K., Jeyanthi, P., Al-Dhabi, N. A., Valan Arasu, M., Esmail, G. A., Kim, Y. O., Kim, H., & Kim, H.-J. (2020). Fer1L5, a Dysferlin Homologue Present in Vesicles and Involved in C2C12 Myoblast Fusion and Membrane Repair. Biology, 9(11), 386. https://doi.org/10.3390/biology9110386





