The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants
Simple Summary
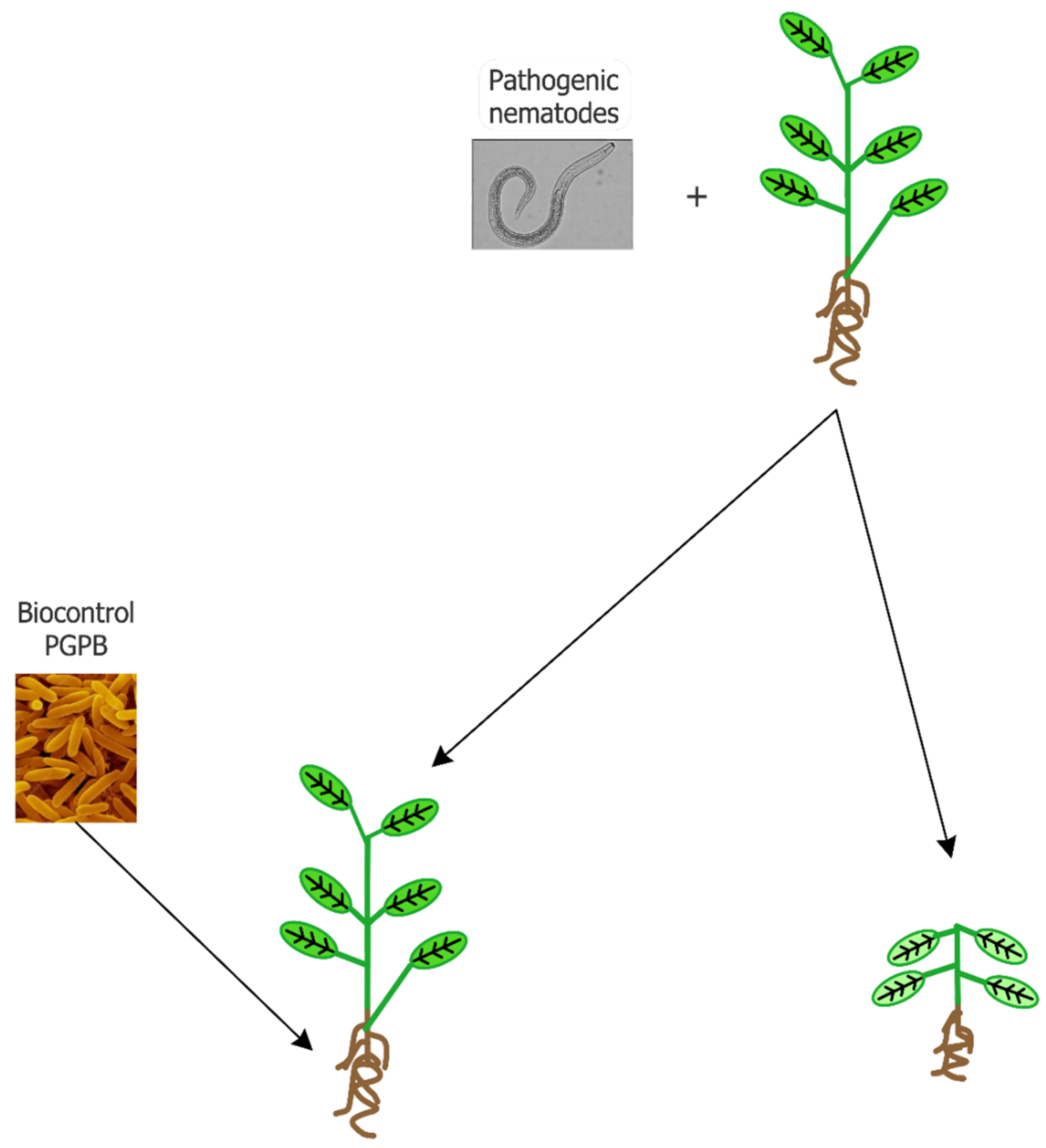
Abstract
1. Introduction
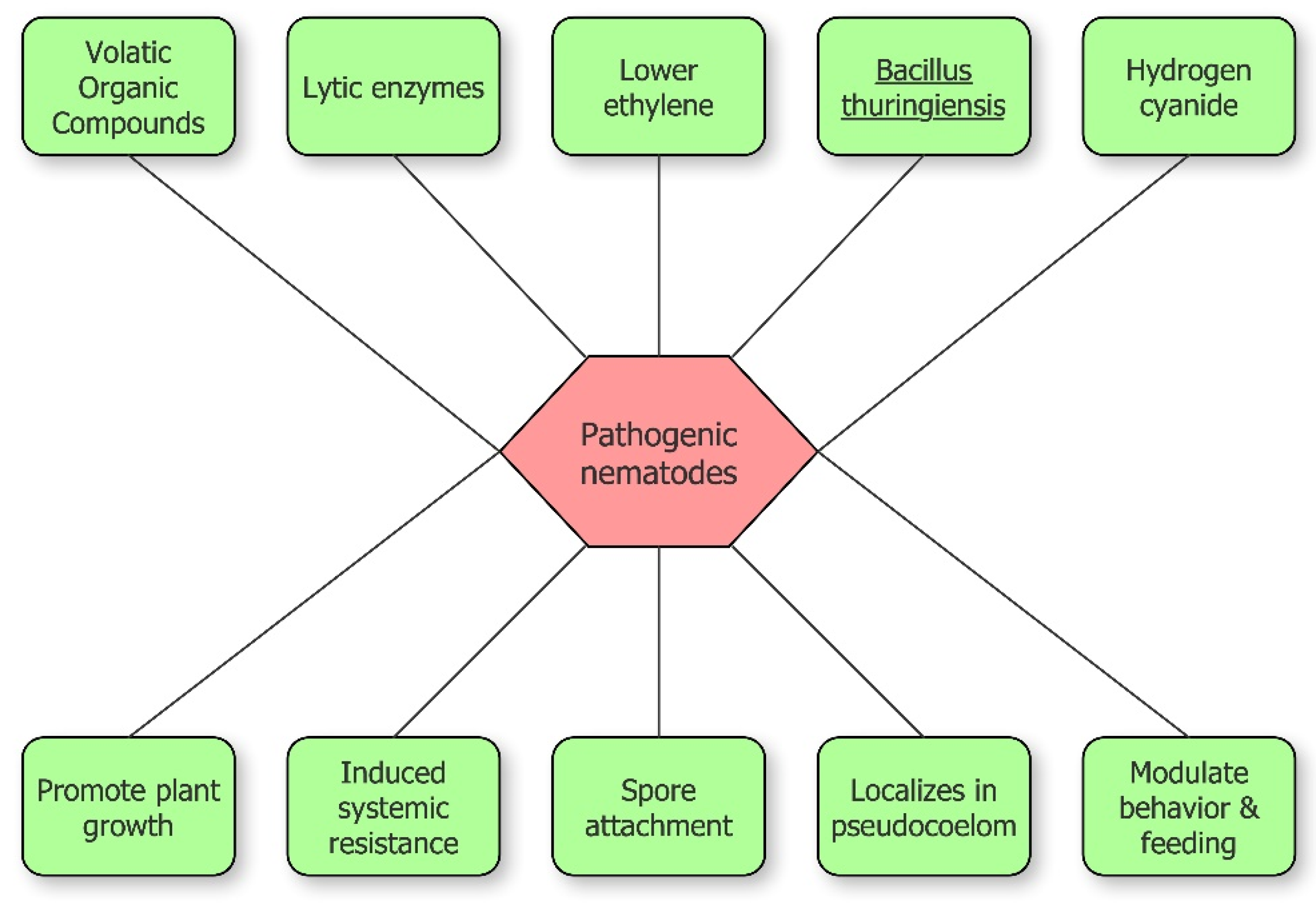
2. Mechanisms at the Base of Nematode Suppression by PGPB
2.1. Lytic Enzymes
2.2. Nematicidal Toxins
2.3. Volatile Organic Compounds (VOCs)
2.4. Bacterial Nematode Hyperparasitism: Pasteuria and Its Influence on Nematode Fertility
2.5. Induced Systemic Resistance
2.6. Modulation of Nematode Behavior, Feeding, and Movement
2.7. Alleviation of Nematode Induced Plant Stress through 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glick, B.R. Beneficial Plant-Bacterial Interactions, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2020; p. 383. [Google Scholar]
- Bardgett, R.D.; Van Der Putten, W.H. Below ground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Van Den Hoogen, J.; Geisen, S.; Routh, D.; Ferris, H.; Traunspurger, W.; Wardle, D.A.; De Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Andriuzzi, W.S.; et al. Soil nematode abundance and functional group composition at a global scale. Nature 2019, 572, 194–198. [Google Scholar] [CrossRef]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nemat. 2010, 42, 63–67. [Google Scholar]
- Hoogen, J.V.D.; Geisen, S.; Wall, D.H.; Wardle, D.A.; Traunspurger, W.; De Goede, R.G.M.; Adams, B.J.; Ahmad, W.; Ferris, H.; Bardgett, R.D.; et al. A global database of soil nematode abundance and functional group composition. Sci. Data 2020, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Pritchard, S. Soil organisms and global climate change. Plant Pathol. 2011, 60, 82–99. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, X.; Smith, P.; Li, L.; Filley, T.R.; Cheng, K.; Shen, M.; He, Y.; Pan, G. Size and variability of crop productivity both impacted by CO2 enrichment and warming—A case study of 4-year field experiment in a Chinese paddy. Agric. Ecosyst. Environ. 2016, 221, 40–46. [Google Scholar] [CrossRef]
- Thakur, M.P.; Reich, P.B.; Hobbie, S.E.; Stefanski, A.; Rich, R.; Rice, K.E.; Eddy, W.C.; Eisenhauer, N. Reduced feeding activity of soil detritivores under warmer and drier conditions. Nat. Clim. Chang. 2018, 8, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hasegawa, T.; Li, L.; Lam, S.K.; Zhang, X.; Liu, X.; Pan, G. Changes in grain protein and amino acids composition of wheat and rice under short term increased [CO2] and temperature of canopy air in a paddy from East China. New Phytol. 2019, 222, 726–734. [Google Scholar] [CrossRef]
- Gao, D.D.; Wang, F.M.; Li, J.; Yu, S.Q.; Li, Z.A.; Zhao, J. Soil nematode communities as indicators of soil health in different land use types in tropical area. Nematology 2020, 22, 595–610. [Google Scholar] [CrossRef]
- Hodda, M. Phylum Nematoda Cobb 1932. In: Animal Biodiversity: An outline of higher-level classification and survey of taxonomic richness (Ed. Zhang Z-Q). Zootaxa 2011, 3148, 63–95. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Den Nijs, L.; Hockland, S.; Tahna Maafi, Z. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Heidelberg, Germany, 2011; pp. 21–43. [Google Scholar]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Ebone, L.A.; Kovaleski, M.; Deuner, C.C. Review Article Nematicides: History, mode, and mechanism action. Plant Sci. Today 2019, 6, 91–97. [Google Scholar] [CrossRef]
- Singh, S.K.; Hodda, M.; Ash, G.J. Plant-parasitic nematodes of potential phytosanitary importance, their main hosts and reported yield losses. EPPO Bull. 2013, 43, 334–374. [Google Scholar] [CrossRef]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Manzanilla-López, R.H.; Costilla, M.; Doucet, M.; Franco, J.; Inserra, R.N.; Lehman, P.S.; Cid del Prado-Vera, I.; Souza, R.M.; Evans, K. The genus Nacobbus Thorne and Allen, 1944 (Nematoda: Pratylenchidae): Systematics, distribution, biology and management. Nematropica 2002, 32, 149–227. [Google Scholar]
- Sikder, M.M.; Vestergård, M. Impacts of root metabolites on soil nematodes. Front. Plant Sci. 2020, 10, 1792. [Google Scholar] [CrossRef]
- Torres-Acosta, J.F.J.; Mendoza-de-Gives, P.; Aguilar Caballero, A.J.; Cuéllar-Ordaz, J.A. Anthelmintic resistance in sheep farms: Update of the situation in the American continent. Vet. Parasitol. 2012, 189, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ramírez, G.S.; Torres-Acosta, J.F.D.J.; Sánchez, J.E.; Mendoza-De-Gives, P.; González-Cortázar, M.; Zamilpa, A.; Al-Ani, L.K.T.; Sandoval-Castro, C.; Soares, F.E.F.; Aguilar-Marcelino, L. The possible biotechnological use of edible mushroom bioproducts for controlling plant and animal parasitic nematodes. BioMed Res. Internat. 2020, 2020, 6078917. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Plant growth-promoting bacteria in agriculture and stressed environments. In Modern Soil Microbiology, 3rd ed.; Van Elsas, J.D., Trevors, J.T., Rosado, A.S., Nannipieri, P., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 361–380. [Google Scholar]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.; Venkatasalam, E.; Srinivasan, S.; Ramkumar, G.; Saranya, C.; Shanmuganathan, R. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocat. Agricul. Biotechnol. 2019, 17, 119–128. [Google Scholar] [CrossRef]
- Wharton, D. Nematode eggshells. Parasitology 1980, 81, 447–463. [Google Scholar] [CrossRef]
- Ray, S.; Reddigarim, S.R.; Jansma, P.L.; Allen, R.; Hussey, R.S. Immunocytochemical analysis of the stage-specific distribution of collagen in the cuticle of Meloidogyne incognita. Fund. Appl. Nematol. 1996, 19, 71–78. [Google Scholar]
- Andragi, S.; Faramarzi, M.A. From bacteria to human: A journey into the world of chitinases. Biotechnol. Adv. 2013, 31, 1786–1795. [Google Scholar]
- Lee, Y.S.; Nguyen, X.H.; Naing, K.W.; Park, Y.S.; Kim, K.Y. Role of lytic enzymes secreted by Lysobacter capsici YS1215 in the control of Root-Knot Nematode of tomato plants. J. Microbiol. 2015, 55, 74–80. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, S.; Khajura, A.; Ohri, P.; Kaur, R.; Kaur, R. Biocontrol potential of chitinases produced by newly isolated Chitinophaga sp. S167. World J. Microbiol. Biotechnol. 2020, 36, 90. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.J.; Li, J.Y.; Wang, C.; Chen, S.L.; Yan, S.Z. Effects of the endophytic bacteria Bacillus cereus BCM2 on tomato root exudates and Meloidogyne incognita infection. Plant Dis. 2019, 103, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.J.; Gao, Y.; Li, X.; Chen, S.L.; Yan, S.Z.; Tian, X.J. Identification and nematicidal characterization of proteases secreted by endophytic bacteria Bacillus cereus BCM2. Phytopathology 2020, 110, 336–344. [Google Scholar] [CrossRef]
- Heckel, D.G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect Biochem. Phisiol. 2020, 104, e21673. [Google Scholar] [CrossRef] [PubMed]
- Jouzani, G.S.; Valijanian, E.; Sharafi, R. Bacillus thuringiensis: A successful insecticide with new environmental features and tidings. Appl. Microbiol. Biotechnol. 2017, 101, 2691–2711. [Google Scholar] [CrossRef]
- Hu, Y.; Nguyen, T.-T.; Lee, A.; Urban, J.F.; Miller, M.M.; Zhan, B.; Koch, D.J.; Noon, J.B.; Abraham, A.; Fujiwara, R.T.; et al. Bacillus thuringiensis Cry5B protein as a new pan-hookworm cure. Intern. J. Parasitol. Drugs Drug Resist. 2018, 8, 287–294. [Google Scholar] [CrossRef]
- Martinez-Zavala, S.A.; Barboza-Perez, U.E.; Hernandez-Guzman, G.; Bideshi, D.K.; Barboza-Corona, J.E. Chitinases of Bacillus thuringiensis: Phylogeny, modular structure, and applied potentials. Front. Microbiol. 2020, 10, 3032. [Google Scholar] [CrossRef]
- Peng, D.; Lin, J.; Huang, Q.; Zheng, W.; Liu, G.; Zheng, J.; Zhu, L.; Sun, M. A novel metalloproteinase virulence factor is involved in Bacillus thuringiensis pathogenesis in nematodes and insects. Environ. Microbiol. 2016, 18, 846–862. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Xiong, J.; Zhou, Q.; Luo, H.; Hu, S.; Xia, L.; Sun, M.; Li, L.; Yu, Z. The diverse nematicidal properties and biocontrol efficacy of Bacillus thuringiensis Cry6A against the root-knot nematode Meloidogyne hapla. J. Invertebr. Pathol. 2015, 125, 73–80. [Google Scholar] [CrossRef]
- Ramalakshmi, A.; Sharmila, R.; Iniyakumar, M.; Gomathi, V. Nematicidal activity of native Bacillus thuringiensis against the root knot nematode, Meloidogyne incognita (Kofoid and White). Egyp. J. Biol. Pest Control 2020, 30, 1–9. [Google Scholar] [CrossRef]
- Aballay, E.; Prodan, S.; Correa, P.; Allende, J. Assessment of rhizobacterial consortia to manage plant parasitic nematodes of grapevine. Crop Protect. 2020, 131, 105103. [Google Scholar] [CrossRef]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef]
- Effmert, U.; Kalderas, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef]
- Chernin, L.; Toklikishvili, N.; Ovadis, M.; Kim, S.; Ben-Ari, J.; Khmel, I.; Vainstein, A. Quorum-sensing quenching by rhizobacterial volatiles. Environ. Microbiol. Rep. 2011, 3, 698–704. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- Popova, A.A.; Koksharova, O.A.; Lipasova, V.A.; Zaitseva, J.V.; Katkova-Zhukotskava, O.A.; Eremina, S.I.; Mironov, A.S.; Chernin, L.S.; Khmel, I.A. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. Biomed. Res. Internat. 2014, 2014, 125704. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Lu, H.; Wang, X.; Zhang, K.Q.; Li, G.-H. Effect of volatile organic compounds from bacteria on nematodes. Chem. Biodiv. 2015, 12, 1415–1421. [Google Scholar] [CrossRef]
- Bui, H.X.; Buyung, A.R.H.; Oliva, R.; Schroedere, N.E. Beneficial bacterial volatile compounds for the control of root-knot nematode and bacterial leaf blight on rice. Crop Protect. 2020, 135, 104792–104799. [Google Scholar] [CrossRef]
- Davies, K.G.; Curtis, R.H.C. Cuticle surface coat of plant-parasitic nematodes. Ann. Rev. Phytopathol. 2011, 49, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Phani, V.; Rao, U. Revisiting the life-cycle of Pasteuria penetrans infecting Meloidogyne incognita under soil-less medium, and effect of streptomycin sulfate on its development. J. Nematol. 2018, 50, 91–98. [Google Scholar] [CrossRef]
- Chen, Z.X.; Dickson, D.W.; McSorley, R.; Mitchell, D.J.; Hewlett, T.E. Suppression of Meloidogyne arenaria race 1 by soil application of endospores of Pasteuria penetrans. J. Nematol. 1996, 28, 159–168. [Google Scholar]
- Mohan, S.; Mauchline, T.H.; Rowe, J.; Hirsch, P.R.; Davies, K.G. Pasteuria endospores from Heterodera cajani (Nematoda: Heteroderidae) exhibit inverted attachment and altered germination in cross-infection studies with Globodera pallida (Nematoda: Heteroderidae). FEMS Microbiol. Ecol. 2012, 79, 675–684. [Google Scholar] [CrossRef]
- Mohan, S.; Kiran Kumar, K.; Sutar, V.; Saha, S.; Rowe, J.; Davies, K.G. Plant root-exudates recruit hyperparasitic bacteria of phytonematodes by altered cuticle aging: Implications for biological control strategies. Front. Plant Sci. 2020, 11, 763. [Google Scholar] [CrossRef] [PubMed]
- Topalovic, O.; Heuer, H.; Reineke, A.; Zinkernagel, J.; Hallmann, J. Antagonistic role of the microbiome from a Meloidogyne hapla-suppressive soil against species of plant-parasitic nematodes with different life strategies. Nematology 2019, 22, 75–86. [Google Scholar] [CrossRef]
- Bhuiyan, S.A.; Garlick, K.; Anderson, J.M.; Wickramasinghe, P.; Stirling, G.R. Biological control of root-knot nematode on sugarcane in soil naturally or artificially infested with Pasteuria penetrans. Austr. Plant Pathol. 2018, 47, 45–52. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zhamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Sikora, R.A. Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Annu. Rev. Phytopathol. 1992, 30, 245–270. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S. Rhizobacteria-mediated Induction of systemic resistance (ISR) in tomato against Meloidogyne javanica. J. Phytopathol. 2002, 150, 469–473. [Google Scholar] [CrossRef]
- Soler, A.; Marie-Alphonsine, P.A.; Corbion, C.; Queneherve, P. Differential response of two pineapple cultivars (Ananas comosus (L.) Merr.) to SAR and ISR inducers against the nematode Rotylenchulus reniformis. Crop Protect. 2013, 54, 48–54. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Role of plant growth promoting bacteria (PGPRs) as biocontrol agents of Meloidogyne incognita through improved plant defense of Lycopersicon esculentum. Plant Soil 2019, 436, 325–345. [Google Scholar] [CrossRef]
- Xing, Z.; Wu, X.; Zhao, J.; Zhao, X.; Zhu, X.; Wang, Y.; Fan, H.; Chen, L.; Liu, X.; Duan, Y. Isolation and identification of induced systemic resistance determinants from Bacillus simplex Sneb545 against Heterodera glycines. Sci. Rep. 2020, 10, 11586. [Google Scholar] [CrossRef]
- Kaminaga, Y.; Schnepp, J.; Peel, G.; Kish, C.M. Plant phenylacetaldehyde synthase is a bifunctional homotetrameric enzyme that catalyzes phenylalanine decarboxylation and oxidation. J. Biol. Chem. 2006, 281, 23357–23366. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, R.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef]
- Beale, E.; Li, G.; Tan, M.-W.; Rumbaugh, K.P. Caenorhabditis elegans senses bacterial autoinducers. Appl. Environ. Microbiol. 2006, 7, 5135–5137. [Google Scholar] [CrossRef]
- Yu, L.; Yan, X.; Ye, C.; Zhao, H.; Chen, X.; Hu, F.; Li, H. Bacterial respiration and growth rates affect the feeding preferences, brood size and lifespan of Caenorhabditis elegans. PLoS ONE 2015, 10, e0134401. [Google Scholar] [CrossRef]
- Lee, J.H.; Wood, T.K.; Lee, J. Roles of indole as an interspecies and interkingdom signaling molecule. Trends Microbiol. 2015, 23, 707–718. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Kim, M.; Kim, E.; Choi, H.; Kim, Y.; Lee, J. Indole-associated predator-prey interactions between the nematode Caenorhabditis elegans and bacteria. Environ. Microbiol. 2017, 19, 1776–1790. [Google Scholar] [CrossRef]
- Kim, D.H.; Flavell, S.W. Host-microbe interactions and the behavior of Caenorhabditis elegans. J. Neurogen. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Kirienko, N.V.; Kirienko, D.R.; Larkins-Ford, J.; Wählby, C.; Ruvkun, G.; Ausubel, F.M. Pseudomonas aeruginosa disrupts Caenorhabditis elegans iron homeostasis, causing a hypoxic response and death. Cell Host Microbe 2013, 13, 406–416. [Google Scholar] [CrossRef]
- Darby, C.; Cosma, C.L.; Thomas, J.H.; Manoil, C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 15202–15207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, H.; Bargmann, C.I. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 2005, 438, 179–184. [Google Scholar] [CrossRef]
- Chan, S.Y.; Liu, S.Y.; Seng, Z. Biofilm matrix disrupts nematode motility and predatory behavior. ISME J. 2020. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Shimomura, T. Metabolism of 1-aminocyclopropane-1- carboxylic acid. Agric. Biol. Chem. 1978, 42, 1825–1831. [Google Scholar]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-containing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.; Rossi, M.; Glick, B.R. Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in plant-bacterial interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef]
- Nascimento, F.; Vicente, C.S.L.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. BioControl 2013, 58, 427–433. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gamalero, E.; Glick, B.R. The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants. Biology 2020, 9, 381. https://doi.org/10.3390/biology9110381
Gamalero E, Glick BR. The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants. Biology. 2020; 9(11):381. https://doi.org/10.3390/biology9110381
Chicago/Turabian StyleGamalero, Elisa, and Bernard R. Glick. 2020. "The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants" Biology 9, no. 11: 381. https://doi.org/10.3390/biology9110381
APA StyleGamalero, E., & Glick, B. R. (2020). The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants. Biology, 9(11), 381. https://doi.org/10.3390/biology9110381





