Simple Summary
Crops are the primary feedstock for the production of food/feed and energy, and our dependency on them has been increasing over the last decade. Climate change, reduced land for active agriculture, increased population, reduced amount of clean water, and high costs of chemical fertilizers and energy make modern agriculture unsustainable. Demands are rising for the next generation of cheap and sustainable feedstock, which could be productive under existing conditions. Aquatic plants, such as the free-floating species duckweed and Azolla, have started attracting attention because of their unique features, presenting advantages over terrestrial plants. Terrestrial plants grow on the land or need to be on dry land to survive. On the other hand, aquatic plants have the ability to thrive in ponds under controlled conditions using wastewaters as a source of nutrients. Over the last decade, Azolla species became an attractive feedstock for livestock because of their accumulation of valuable products, phenolic compounds, proteins, lipids, and carbohydrates. Stress-triggered changes in these nutritional components could have a significant impact on the nutritional value of Azolla, which is used as a sustainable food supplement for livestock, poultry, and fish industries. This paper aims to assess the effects of environmental and nutrient stresses on the biosynthesis of valuable metabolites in Azolla.
Abstract
The metabolic plasticity of shikimate and phenylpropanoid pathways redirects carbon flow to different sink products in order to protect sessile plants from environmental stresses. This study assessed the biochemical responses of two Azolla species, A. filiculoides and A. pinnata, to the combined effects of environmental and nutritional stresses experienced while growing outdoors under Australian summer conditions. These stresses triggered a more than 2-fold increase in the production of total phenols and their representatives, anthocyanins (up to 18-fold), flavonoids (up to 4.7-fold), and condensed tannins (up to 2.7-fold), which led to intense red coloration of the leaves. These changes were also associated with an increase in the concentration of carbohydrates and a decrease in concentrations of lipids and total proteins. Changes in lipid biosynthesis did not cause significant changes in concentrations of palmitoleic acid (C16:0), linolenic acid (C18:3), and linoleic acid (C18:2), the fatty acid signatures of Azolla species. However, a reduction in protein production triggered changes in biosynthesis of alanine, arginine, leucine, tyrosine, threonine, valine, and methionine amino acids. Stress-triggered changes in key nutritional components, phenolics, lipids, proteins, and carbohydrates could have a significant impact on the nutritional value of both Azolla species, which are widely used as a sustainable food supplement for livestock, poultry, and fish industries.
1. Introduction
Crops are the primary feedstock for the production of food/feed and energy, and our dependency on them has increased over the last decade. Climate change, reduced land for active agriculture, increased population, reduced amount of clean water, along with high costs of chemical fertilizers and energy, make modern agriculture unsustainable [1,2]. Demands are rising for the next generation of cheap and sustainable feedstock which could be productive under existing conditions. Microalgae tick all the boxes for producing a broad spectrum of nutrients and showing the capacity to grow rapidly on wastewater nutrients [3,4,5]. However, challenges with harvesting under large scale production make their technology expensive [6,7]. Aquatic plants, such as the free-floating species duckweed and Azolla, have started attracting attention because of their unique features, which present the advantages of microalgae with easy and cheap harvesting technologies [8,9,10,11,12,13]. Azolla species grow in a symbiotic association with Nostoc (Anabaena) azollae, a nitrogen-fixing cyanobacterium that allows its host plant to grow rapidly in nitrogen-free solutions [14,15,16]. The application of this species for wastewater treatment and the production of biomass for food/feed and as feedstock for bioenergy has been broadly investigated [9,13,17,18,19,20]. Azolla thrives in tropical to temperate regions of the world [15,21]. When grown under optimal conditions, this species can double its biomass every two to four days, with average annual productivity of 39 t/hectare [11]. Over the last decade, Azolla species became an attractive feedstock for livestock because of the accumulation of valuable products, such as proteins (up to 400 g/kg DW), lipids (160 g/kg DW), and phenolics comprising antioxidants such as flavonoids, tannins, and anthocyanins (50 g/kg DW in total) [11,13,20,22,23,24].
Fresh Azolla can replace up to 50% of feed concentrate for cows, decreasing feed and labor costs by 16.5% and the cost of milk production by 18.5% [25]. The addition of fresh Azolla to the diet of native chickens, laying hens, and grazing ducks has also been shown to reduce the feeding costs significantly without affecting egg weight and other quality parameters [26]. The use of dried Azolla at 7.5% resulted in a 2.6% increase in the bodyweight of broilers and contributed to a better immune response [27]. Moreover, Azolla has been widely used as a low-cost and a high nutritional supplement in fish meal [28].
Azolla’s protein level and composition of amino acids are the main nutritional components that make it a valuable feed supplement [11,29,30]. An average yield of dry Azolla biomass of 35 t DW/ha-year correlates with an annual protein production of 7–9 t DW/ha-year [29]. This is up to 3-fold higher than the soybean, which yields up to 2 t protein/ha-year [11,31]. An additional advantage of Azolla-based protein production is related to the fact that under favorable conditions these species can be grown and collected throughout the year, whereas the production of soybean grains is seasonal (once per year). Azolla can grow without nitrogen due to its nitrogen-fixing capacity, while commercial-scale soybean production still requires at least 150 kg of N fertilizer ha−1 per year [11,32]. However, Azolla has a lower protein content compared to Spirulina, a cyanobacterium commonly used as a food source. Spirulina (Arthrospira sp.) contains around 48.9% protein and can produce in semi-continuous cultures up to 25 t protein/ha-year [33]. However, harvesting and concentration of cyanobacteria and algae still account for 30–40% of the product’s cost [7].
Phenolic compounds, the products of shikimate and phenylpropanoids pathways, comprise more than 8000 compounds, including flavonoids, flavonols, flavones, anthocyanins, tannins and condensed tannins (proanthocyanins), and other benzene (C6) ring-containing molecules [34,35]. The most abundant phenolic compounds identified in Azolla include anthocyanidins, coumarins, flavonols, flavones, and condensed tannins [23,35]. The antioxidation mechanism triggered by plant phenolics has been shown to benefit human health, including a reduced risk of developing cardiovascular diseases, cancer, diabetes type 2, and neurological diseases [36,37,38]. Condensed tannins are the most abundant phenolic component extracted from Azolla species [10,17]. Because of their health benefits, which include anticancer, antidiabetic, neuroprotective, and antimicrobial properties, they became popular as a food/feed supplement [39]. As livestock feed, low concentrations of these polymers can non-specifically bind food proteins, reducing their fermentation in gastrointestinal tracts and reducing ruminal bloats [17]. A high concentration of phenols and condensed tannins, however, can have a negative impact on the nutritional value of Azolla plants, reducing their digestibility [11,29,30,40,41,42]. Phenolics from Azolla have been extensively characterized previously [10,35,43].
The metabolic plasticity of shikimate and phenylpropanoid pathways redirecting carbon flow to the biosynthesis of different phenolic compounds and other carbon sinks, such as carbohydrates, lipids, and proteins, is a crucial evolutional factor developed by sessile plants to protect them against biotic and abiotic stresses [44,45,46]. Stressed Azolla plants show the intense red coloration of their leaves, indicating enhanced production of colored phenolic pigments (Figure S1). Earlier, we showed that this triggered differential accumulation of metabolites from the phenylpropanoid and fatty acid biosynthesis pathways when plants were grown indoors, in glasshouse conditions [20,47]. In this study, we assessed the biochemical responses of two Azolla species, A. filiculoides and A. pinnata, to nutrient and environmental stresses experienced while growing outdoors under summer conditions in Australia. These two species differ in habitat, growth rates, and genetic makeup (i.e., A. filiculoides grows in temperate climates in Europe and A. pinnata grows in tropical climates in Asia and Australia) [48,49]. To our knowledge, this is the first comprehensive study of biochemical responses of two Azolla species, showing differential accumulation of the key nutrients (anthocyanins, flavonoids, condensed tannins, carbohydrates, lipids, and proteins) in response to stressed conditions.
2. Materials and Methods
2.1. Growth Conditions
Two aquatic plants, A. filiculoides and A. pinnata, were obtained from the RMIT collection of aquatic plants and algal species. As a stock, both plants were grown in 250-mL plastic boxes (11 cm × 18 cm) in half strength (1/2SH) Schenk and Hildebrandt’s medium [49] in a glasshouse with a 16-h photoperiod, 25 °C, and a photosynthetic photon flux density of 100 μm/m2-s provided by fluorescent tubes. In further experiments, A. filiculoides and A. pinnata plants were grown in 1.3 m (length) × 0.7 m (width) × 0.50 m (depth) tanks in 1/2SH medium without nitrogen. Plants were stressed by starving and growing under direct exposure to sunlight during Australian summer conditions (light intensity 5–10 Klux daytime). In control experiments, plants were regularly fed and grown under 70% shade cloth conditions, reducing light intensity to 0.5 Klux.
2.2. Biochemical Analysis
2.2.1. Lipid Extraction of Fatty Acid Methyl Esters
Lipid extraction and fatty acid methyl esters (FAMEs) analysis was conducted according to Miranda et al. [20], with minor modifications. Briefly, 5 mL of solvent (chloroform/methanol 2:1) was added to a tube containing 50 mg of ground Azolla and was left to shake at 200 rpm overnight. Samples were then vortexed at the maximum speed for 1 min and centrifuged at 3000 rpm for 30 min. The resulting supernatant was transferred to a new tube, and 2 mL of NaCl was added before vortexing for 5 s. After that, the tubes were centrifuged at 3000 rpm for 3 min. The lower layer was transferred into the pre-weighed tube and dried under nitrogen flow at 70 °C.
2.2.2. Total Carbohydrates
Total carbohydrates were measured according to Chandran et al. [12], with minor modifications. Briefly, 2.5 mL of 2.5 N hydrochloric acid was added to 50 mg of sample. The sample was then hydrolyzed by boiling in a water bath for three hours. After cooling down, solid sodium carbonate was added to neutralize the reaction, and distilled water was added up to 50 mL. Samples were then centrifuged at 3000× g for 10 min at room temperature. The supernatant was then collected and 500 µL was added to a test tube. One milliliter of Milli-Q and 4 mL of anthrone reagent was added to each test tube, including the blank. After mixing well, the samples were kept in a boiling water bath for 8 min. After cooling, the absorbance of a blue-green solution was measured at 630 nm using a spectrophotometer (POLARstar Omega, BMG Labtech, Offenburg, Germany) and compared with a standard curve prepared with known amounts of glucose. The amount of total carbohydrates in the sample was calculated and expressed as g glucose equivalents/100 g sample.
2.2.3. Total Protein
The total protein content was measured using the Kjeldahl method by using Foss™ Kjeldahl Digestion Systems. The nitrogen content of the samples was measured and converted to protein content by multiplying by 6.25, 4.59, and 5.0 [50].
2.2.4. Total Phenolics
Total phenolic concentration was estimated using the Folin–Ciocalteu method, according to Makkar [51], with minor modifications. To 1 g of powdered sample, 7 mL of extract buffer (80% methanol) was added. The mixture was kept shaking for 12 h at 4 °C in the dark before being centrifuged at 3000× g for 10 min at room temperature. The supernatant was collected, and 0.5 mL of Folin–Ciocalteu reagent (1 N) was added to each sample, including a blank. After mixing using a vortex, the samples were rested for 5 min, and 2.5 mL of 5% sodium carbonate was added to all tubes, except the blank. Finally, the samples were shaken and incubated for 40 min at room temperature. The absorbance of the blue color developed against the reagent blank at 725 nm was determined using a spectrophotometer (POLARstar Omega, BMG Labtech, Offenburg, Germany).
2.2.5. Total Flavonoids
The total amount of flavonoid was estimated according to Zhishen et al. [52] by using the aluminum chloride method. Briefly, 500 µL of extract (supernatant in 2.4) was diluted to 1 mL with Milli-Q water, and 150 µL of 5% sodium nitrite was added to all samples, including a blank. The tubes were then mixed and incubated at room temperature for 5 min before adding 150 µL of 10% aluminum chloride to all the test tubes, including the blank. The mixture was mixed and further incubated for 6 min before adding 2 mL of 4% sodium hydroxide to all the test tubes. The contents of the tubes were made up to 5 mL using Milli-Q water, and the contents were mixed well using a vortex. The samples were rested for 15 min at room temperature, and the absorbance of the pink color that had developed was measured against the blank at 510 nm using a spectrophotometer (POLARstar Omega, BMG. Labtech, Offenburg, Germany) and estimated based on the amounts of rutin as standard and expressed as mg/standard equivalent per gram dry powder for the samples.
2.2.6. Total Condensed Tannins
The amount of total condensed tannins was measured as described by Ray et al. [53], with minor modifications. To each gram of sample powder, 3 mL of extract buffer (80% methanol and 5.3 mM sodium bisulfite) was added in the dark. The mixture was kept shaking for 12 h at room temperature and centrifuged at 3000× g for 10 min at room temperature. The supernatant containing the methanol-soluble fractions was collected and analyzed according to Ray et al. [53] and using catechin as standard.
2.2.7. DMACA Staining of Condensed Tannins
The stain 4-dimethylaminocinnamaldehyde (DMACA) was used to detect condensed tannins in aquatic plants using the method described in Abeynayake et al. [54]. Briefly, plants were decolorized in absolute ethanol for 3 h and stained for the presence of proanthocyanidins and flavan-3-ols using 0.01% (w/v) 4-dimethylaminocinnamaldehyde (DMACA.) in absolute ethanol containing 0.8% w/v hydrochloric acid for 2 h [55]. After this, plants were transferred to 100% ethanol and further vacuum-infiltrated for 1 min with fixative (6% w/v glutaraldehyde, 4% w/v paraformaldehyde in 50 mM sodium phosphate buffer, pH 7.4) before being incubated for 2 h at 4 °C. Finally, plants were washed three times (5 min) in 50 mM sodium phosphate buffer, pH 7.4. For making sections, tissues were embedded in LR White or paraffin matrices, followed by DMACA staining [55]. Transverse and longitudinal sections of embedded samples in LR White resin (6–10 μm) and paraffin (8 μm) were generated using a microtome. Cell walls were stained with 0.05% toluidine blue (ProSciTech, Kirwan, QLD, Australia, Cat. # C078). Images were captured using a Leica MZFLIII light microscope (Leica, Wetzlar, Germany).
2.2.8. Anthocyanin Levels
Anthocyanin levels were measured as in Neff et al. [56], with minor modifications. To 1 g of powdered sample, 3 mL of methanol 1% HCl was added in the dark. The mixture was shaken overnight in a dark room at 4 °C. After that, 2 mL of Milli-Q water and 5 mL of chloroform was added to the samples. The samples were centrifuged at 8000× g for 5 min at 4 °C. The supernatant was collected, and anthocyanin levels were estimated following [56] and by measuring the absorbance at 530 and 657 nm using a spectrophotometer (POLARstar Omega, BMG Labtech, Offenburg, Germany). The relative amount of anthocyanin was calculated by subtracting the A657 from the A530.
2.3. Statistical Analysis
All experiments in this study were conducted in triplicate. All data are expressed as a mean ± standard deviation. The experimental data were subjected to the one-way analysis of variance (ANOVA) as implemented in the GraphPad InStat 3 statistics platform, San Diego, CA, USA. Tukey simultaneous tests were conducted to determine the statistical differences between treatments. To ascertain that the observed variations were statistically significant, the probability (P) values were determined. A 95% confidence level (p < 0.05) was applied for all analyses.
3. Results and Discussion
3.1. Accumulation of Phenolic Compounds in Green and Red A. filiculoides and A. pinnata Plants
A. filiculoides and A. pinnata plants growing outdoors under normal and stressed conditions (Material and Methods) were assessed for the concentration of phenolic compounds, total phenols, anthocyanins, flavonoids, and condensed tannins, which have a complex effect on the nutritional value of these plants [10,11]. In unstressed plants, the concentration of total phenols in A. pinnata was 1.7-fold higher than in A. filiculoides, producing 53.5 mg/g DW (5.1% DW) compared to 30.4 mg/g DW (3.1% DW) in A. filiculoides (p < 0.05) (Figure 1A). Stress increased the accumulation of phenols up to 2.6-fold in A. pinnata, and 2-fold in A. filiculoides, producing 139.5 mg/g DW (14.2% DW) and 61.4 mg/g DW (6.0% DW), respectively (Figure 1A). A higher concentration of phenolics in A. pinnata than in A. filiculoides was also shown for the same species by Brouwer et al. [11]. The enhancement of total phenolic compounds exposed to different abiotic stresses has also been shown for other Azolla species [57,58,59,60]. Unstressed A. filiculoides and A. pinnata showed similar very low concentrations of anthocyanins (Figure 1B). Stress significantly increased the concentration of anthocyanins in red A. filiculoides by up to 18-fold, with the concentration rising from 1.5 mg/g DW to 27.18 mg/g DW (Figure 1B). In red A. pinnata, the concentrations of anthocyanins showed a 15-fold increase (up to 23.8 mg/g DW) when compared to green plants. In terrestrial plants, an increased concentration of anthocyanins in response to biotic/abiotic stress conditions, such as high light intensity, cold, and pathogen infections, in order to protect from oxidative damage is a well-investigated phenomenon [61,62]. A much lower increase in the concentration of anthocyanins was observed in Azolla imbricata after nine days of exposure to 0.05–0.1 mg/L of cadmium [60].
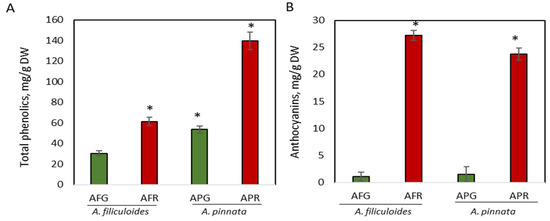
Figure 1.
Accumulation of total phenolics (A) and anthocyanins (B) in green and red A. filiculoides and A. pinnata. AFG: A. filiculoides_Green; AFR: A. filiculoides_Red; APG: A. pinnata_Green; APR: A. pinnata_Red. (ANOVA; * p < 0.05 compared to AFG).
It was shown that anthocyanins in frond extracts of Azolla are mainly represented by 3-deoxyanthocyanins and their acetylated glycosides, such as luteolinidin (m/z 475) and apigeninidin (m/z 459) [63,64]. High levels of deoxyanthocyanins in Azolla fronds negatively correlated with their palatability for adult Lymnaea swinhoei snails and Polypedates leucomystax tadpoles [65].
The concentration of another representative of phenolic compounds, flavonoids, were increased 3.6-fold and 4.7-fold, reaching concentrations of 109 mg/g DW and 218 mg/g DW, respectively, in stressed A. filiculoides and A. pinnata plants (Figure 2A). Both stressed and green A. pinnata plants contained a higher concentration of flavonoids than A. filiculoides. Earlier, we showed a detailed composition of flavonoids in Azolla grown on swine wastewater during the summer and winter in Australia [47]. An extensive list of the flavonoids identified in A. filiculoides and A. pinnata was previously shown [17,66]. Similarly to Azolla, the increase in the concentrations of flavonoid compounds was triggered in duckweed (Landoltia punctata) by nutrient starvation [67]. In terrestrial plants, the composition of flavonoids and their metabolic plasticity is mostly dependent on the plant species, their developmental stage, and environmental conditions, including both biotic and abiotic stresses [35].
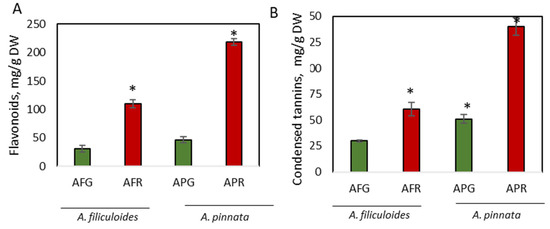
Figure 2.
Accumulation of flavonoids (A) and condensed tannins (B) in green and red A. filiculoides and A. pinnata. AFG: A. filiculoides_Green; AFR: A. filiculoides_Red; APG: A. pinnata_Green; APR: A. pinnata_Red. (ANOVA; * p < 0.05 compared to AFG).
Condensed tannins were identified as the most abundant soluble phenolic compounds in both Azolla species, accounting for 30.2 mg/g DW (3.1% DW) and 51.1 mg/g DW (5.2% DW) of the biomasses of A. filiculoides and A. pinnata, respectively (Figure 2B). Their concentrations were further increased, up to 2.7-fold, in response to stress (Figure 2A). Staining with DMCA showed a blue coloration ubiquitously accumulating in vegetative and reproductive organs (Figure 3). In leaves, blue staining was observed mainly in dorsal compared to ventral lobes (Figure 3B,C; Figure S2). Intense blue staining was detected in leaf primordia (Figure 3D). At the cellular level, blue staining was mainly observed in cell walls (Figure 3E). Within leaf cavities, strong staining was detected in trichomes (Figure 3F). Accumulation of condensed tannins in trichomes was also shown by Pereira et al. [68]. In Azolla roots, DMACA staining was detected in the vascular cells around the xylem (Figure 3G,H). The accumulation of insoluble (poly)phenols around the vascular tissue of A. filiculoides was previously observed, using confocal imaging, in stems and leaves of A. filiculoides stained with propidium periodide [17]. In reproductive organs, a well-detectable level of condensed tannins was observed in microsporocarps and microspores (Figure 3I,J). In both species, stronger coloration was detected in stressed plants compared to green plants. White clover flowers were used as a positive control for DMACA activity, since they have a high level of condensed tannins [69]. Accumulation of condensed tannins in root vasculature cells resembles accumulation profiles of lignin in the roots of terrestrial plants. Existing data describing lignin content in Azolla species is controversial, with a broad range of concentrations observed at different developmental stages, from no lignin until 41% in mature plants [23,42,70,71].
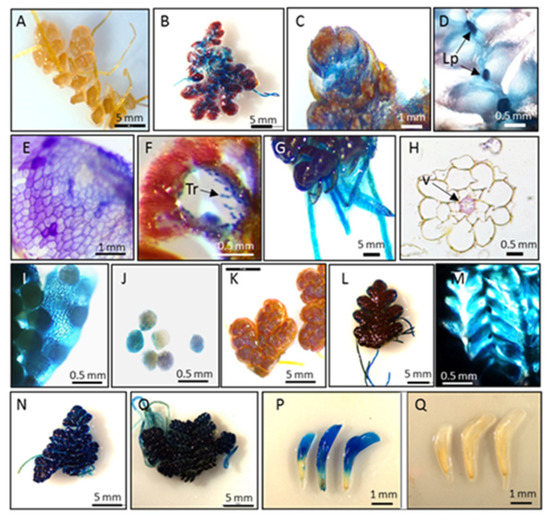
Figure 3.
Staining of condensed tannins in A. filiculoides and A. pinnata with 4-dimethylaminocinnamaldehyde (DMACA). A. filiculoides green, non-stained (A) and stained by DMACA (B–J); leaf (E); leaf cavity (F); roots (G–H); microsporocarps (I) microspores (J); A. filiculoides red, non-stained (K) and stained with DMACA (L,M); L. pinnata green, stained (N); L. pinnata red, stained (O); white clover flowers stained with DMACA ((P), positive control); white clover flowers not stained with DMACA ((Q), negative control). Lp: leaf primordia; Tr: trichomes; V: vasculature cells.
Based on the molecular analysis of flash pyrolysis and thermally assisted hydrolysis and methylation, the production of lignin was not predicted in Azolla caroliniana [23]. However, it is very likely that these strategies were not sensitive enough, since low levels of G-lignin-specific pyrolysis products, such as 4-(1-propenyl)guaiacol, cis-4-(2-propenyl)guaiacol, and trans-4-(2-propenyl)guaiacol, were identified in A. filiculoides [17]. Recent studies indicate that high concentrations of phenolic compounds, caffeoylquinic acids, and condensed tannins are responsible for reducing the nutritional value and digestibility of Azolla species [10].
However, low concentrations of condensed tannins (<3% DW) in a common forage plant, white clover, showed a beneficial effect on animal health, preventing pasture bloat and increasing nutrient uptake in ruminant livestock as a result of protection of proteins from fermentation [35,72]. Inconsistent data reflecting animal responses to the concentration of condensed tannins in the diet also correlates with their structures [73]. In terrestrial plants, the accumulation of protein-bound or fiber-bound condensed tannins protects them from vertebrates and insects [11,74]. Condensed tannins are built by two main monomers: 2,3-trans-flavan 3-ols (afzelechin, catechin, and gallocatechin) and 2,3-cis-flavan 3-ols (epiafzelechin, epicatechin, and epigallocatechin) [10,69].
3.2. Accumulation of Proteins, Lipids, and Carbohydrates in Green and Red A. filiculoides and A. pinnata
Green A. pinnata showed a significantly higher content of total carbohydrates than A. filiculoides, with 171 mg/g DW and 119 mg/g DW (12% DW and 17% DW), respectively (Figure 4A). Stress conditions increased the concentration of carbohydrates in both plants, with up to 164 mg/g DW and 195 mg/g DW (16% DW and 19% DW) in A. filiculoides and A. pinnata, respectively. These levels are similar to the sum of polysaccharides (19.8% DW) and soluble sugars (2.1% DW) but lower than the levels of carbohydrates observed for A. filiculoides (40% DW) collected from a lake in Iran [17,18].
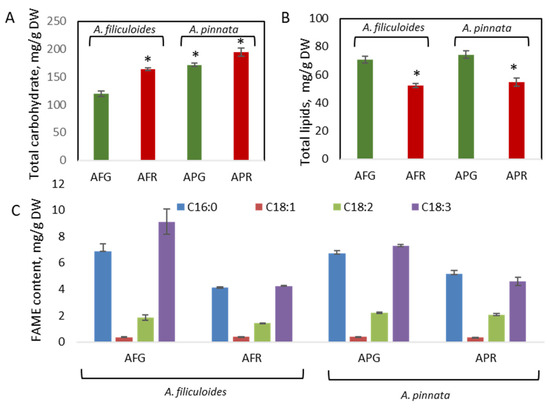
Figure 4.
Accumulation of carbohydrates (A) and total lipids (B) in green and red A. filiculoides and A. pinnata. Fatty acid methyl ester (FAME) composition of lipids (C). AFG: A. filiculoides_Green; AFR: A. filiculoides_Red; APG: A. pinnata_Green; APR: A. pinnata_Red. (ANOVA; * p < 0.05 compared to AFG).
The lipid content in green plants was around 7% DW (72 mg/g DW) and was significantly increased when plants were stressed (5% DW, 52 mg/g DW) (Figure 4B). High concentrations of palmitoleic acid (C16:0), linolenic acid (C18:3), and linoleic acid (C18:2) with lower levels of oleic acid (18:1) are FAME signatures for Azolla species (Figure 4C) [20]. In both Azolla species, stress conditions have not been shown to contribute to significant changes in the FAME profiles. Earlier, it was shown that, based on its FAME composition, Azolla could be a high-quality feedstock for biodiesel if an additional fractionation step could be used to decrease the cold filter plugging point [11,20].
Total nitrogen, protein, and amino acid contents of A. filiculoides and A. pinnata are shown in Table 1 and Table 2. Using the Kjeldahl analysis, stressed A. filiculoides and A. pinnata showed 1.4- and 1.6-fold lower concentrations of nitrogen than green plants, respectively. Table 1 shows total protein concentrations based on three known nitrogen-to-protein conversion factors: a standard nitrogen-to-protein conversion factor (6.25) based on albumin protein, as well as updated conversion factors, 4.59 and 5.0, used for marine and aquatic plants respectively [11,75,76].

Table 1.
Concentrations of nitrogen, total amino acids (AA) and proteins in A. filiculoides and A. pinnata.

Table 2.
Amino acid concentrations in biomass of A. filiculoides and A. pinnata grown under normal and stress conditions.
Based on our results, the sum of amino acids in green A. filiculoides and A. pinnata ranged from 205 to 265 mg/g DW, which is lower than the total protein value based on the 6.25 conversion factor, but higher than for conversion factors 4.59 and 5.0 and can be averaged as 5.3. Stressed plants showed lower protein concentrations than green plants, with 141 mg/g DW and 134 mg/g DW for A. filiculoides and A. pinnata, respectively, reflecting the differences in concentrations of total nitrogen. The fact that the 6.25 conversion factor is not accurate for nitrogen-fixing plants can be explained by the accumulation of nitrogen in the form of different nitrogen-containing compounds [11]. Conversion levels for nitrogen-to-protein lower than 6.25 were observed for other aquatic plants such as duckweed (4.8), water hyacinth (4.59), and Ulva lactuca (4.59) [11]. The FAME composition of Azolla plants is shown in Table 2. Stress led to changes in the profiles of some amino acids in both plants. In A. filiculoides, it caused an increase in levels of alanine, arginine, leucine, tyrosine, threonine, and valine (p ≤ 0.05), while the percentage of glycine was decreased. In A. pinnata, stress increased the contents of asparagine and glycine. In both species, the methionine biosynthesis was reduced. In terrestrial plants, the increased levels in some of these amino acids have previously been shown to be triggered by abiotic stress [77,78,79,80,81,82].
4. Conclusions
Azolla is a novel sustainable feed supplement, producing compounds that can be broadly assessed under fully controlled indoor conditions. However, globally, a large majority of farmers are growing plants outdoors, and as a result, the plant’s nutritional value can be affected by environmental and nutrient conditions. The changes in key components, phenolics, proteins, carbohydrates, and proteins were summarized in Figure 5. Anthocyanins, flavonoids, and condensed tannins were observed to be the most induced nutrients in response to stresses. Increased levels of these molecules, decreased concentrations of lipids and proteins, along with changes in amino acid profiles could have a complex impact on the nutritional value of these plants.
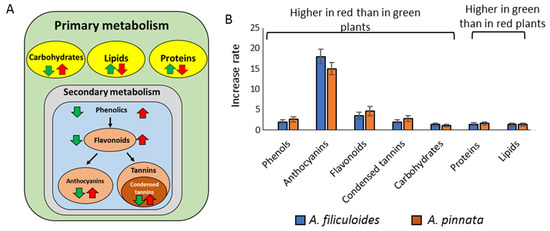
Figure 5.
(A) Representation of differential accumulation of cellular primary (carbohydrates, lipids, and proteins) and secondary (phenolics, anthocyanins, flavonoids, and condensed tannins) metabolites in red (red arrow) and greed (green arrow) plants; (B) comparative increase rates (fold increase, p < 0.05) in production of metabolites between red and green Azolla plants.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-7737/9/10/342/s1, Figure S1: (A) A. filiculoides green (a) and red (b) grown outdoors. (B) A. pinnata green (a) and red (b) grown outdoors. Figure S2. (A) Longitudinal sections of A. filiculoides plants. (B) Nostoc azollae in leaf cavities shown by arrow.
Author Contributions
Conceptualization: A.M., methodology: T.L.N.T., A.F.M., and S.W.A.; writing—original draft preparation: T.L.N.T. and A.F.M.; writing—review and editing: A.M., A.F.M., and S.W.A., supervision: A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The first author acknowledges scholarship support from the Vietnamese government and RMIT University. The authors thank Jacinta Colvin for proofreading this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ritchie, H.; Roser, M. Environmental impacts of food production. Our World Data. 2020. Published online at OurWorldInData.org. Available online: https://ourworldindata.org/environmental-impacts-of-food (accessed on 5 September 2020).
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Miranda, A.; Mouradov, A. Microalgae as Bio-Converters of Wastewater into Biofuel and Food. In Water Scarcity and Ways to Reduce the Impact: Management Strategies and Technologies for Zero Liquid Discharge and Future Smart Cities; Pannirselvam, M., Shu, L., Griffin, G., Philip, L., Natarajan, A., Hussain, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 75–94. [Google Scholar]
- Miranda, A.F.; Ramkumar, N.; Andriotis, C.; Höltkemeier, T.; Yasmin, A.; Rochfort, S.; Wlodkowic, D.; Morrison, P.; Roddick, F.; Spangenberg, G. Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol. Biofuels 2017, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbio. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A.; Moheimani, N.R. Sustainable biofuels from algae. Mitiga. Adapt. Strat. Glob. Chang. 2013, 18, 13–25. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Wrede, D.; Kadali, K.; Gujar, A.; Stevenson, T.; Ball, A.S.; Mouradov, A. Fungal-assisted algal flocculation: Application in wastewater treatment and biofuel production. Biotechnol. Biofuels 2015, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.L. The Unique Symbiotic System Between a Fern and a Cyanobacterium, Azolla-Anabaena azollae: Their potential as biofertilizer, feed, and remediation. In Symbiosis; IntechOpen: London, UK, 2017. [Google Scholar]
- Costa, M.; Santos, M.; Carrapiço, F.; Pereira, A. Azolla–Anabaena’s behaviour in urban wastewater and artificial media—Influence of combined nitrogen. Water Res. 2009, 43, 3743–3750. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, P.; Nierop, K.G.; Huijgen, W.J.; Schluepmann, H. Aquatic weeds as novel protein sources: Alkaline extraction of tannin-rich Azolla. Biotechnol. Rep. 2019, 24, e00368. [Google Scholar] [CrossRef]
- Brouwer, P.; Schluepmann, H.; Nierop, K.G.; Elderson, J.; Bijl, P.K.; van der Meer, I.; de Visser, W.; Reichart, G.-J.; Smeekens, S.; van der Werf, A. Growing Azolla to produce sustainable protein feed: The effect of differing species and CO2 concentrations on biomass productivity and chemical composition. J. Sci. Food Agric. 2018, 98, 4759–4768. [Google Scholar] [CrossRef]
- Chandran, R.; Nivedhini, V.; Parimelazhagan, T. Nutritional composition and antioxidant properties of Cucumis dipsaceus ehrenb. ex spach leaf. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Miranda, A.F.; Biswas, B.; Ramkumar, N.; Singh, R.; Kumar, J.; James, A.; Roddick, F.; Lal, B.; Subudhi, S.; Bhaskar, T.; et al. Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnol. Biofuels 2016, 9, 221. [Google Scholar] [CrossRef]
- Watanabe, I.; Liu, C.C. Improving nitrogen-fixing systems and integrating them into sustainable rice farming. Plant Soil 1992, 141, 57–67. [Google Scholar] [CrossRef]
- van Hove, C.; Lejeune, A. The Azolla: Anabaena Symbiosis. Biol. Environ. Proceed. R. Ir. Acad. 2002, 102B, 23–26. [Google Scholar] [CrossRef]
- Carrapico, F. The Azolla–Anabaena–Bacteria association: A case of symbiotic abduction? In Algal and Cyanobacteria Symbioses; World Scientific: Hackensack, NJ, USA, 2017; pp. 329–345. [Google Scholar]
- Brouwer, P. Turning the Aquatic Weed Azolla Into a Sustainable Crop. Ph.D. Thesis, Universiteit Utrecht, Utrecht, The Netherlands, 7 July 2017. [Google Scholar]
- Dohaei, M.; Karimi, K.; Rahimmalek, M.; Satari, B. Integrated biorefinery of aquatic fern Azolla filiculoides for enhanced extraction of phenolics, protein, and lipid and methane production from the residues. J. Clean. Prod. 2020, 276, 123175. [Google Scholar] [CrossRef]
- Miranda, A.F.; Kumar, N.R.; Spangenberg, G.; Subudhi, S.; Lal, B.; Mouradov, A. Aquatic Plants, Landoltia punctata, and Azolla filiculoides as Bio-Converters of Wastewater to Biofuel. Plants 2020, 9, 437. [Google Scholar] [CrossRef]
- Miranda, A.F.; Liu, Z.; Rochfort, S.; Mouradov, A. Lipid production in aquatic plant Azolla at vegetative and reproductive stages and in response to abiotic stress. Plant Physiol. Biochem. 2018, 124, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, T.A.; Plucknett, D.L. Azolla: Botany, physiology, and use as a green manure. Econ. Bot. 1980, 34, 111–153. [Google Scholar] [CrossRef]
- Anitha, K.C.; Rajeshwari, Y.B.; Prasanna, S.B.; Shilpashree, J. Nutritive evaluation of azolla as livestock feed. J. Exp. Biol. Agric. Sci. 2016, 4, 670–674. [Google Scholar] [CrossRef]
- Nierop, K.G.J.; Speelman, E.N.; de Leeuw, J.W.; Reichart, G.-J. The omnipresent water fern Azolla caroliniana does not contain lignin. Org. Geochem. 2011, 42, 846–850. [Google Scholar] [CrossRef]
- Kadir, A.A.; Abdullah, S.R.S.; Othman, B.A.; Hasan, H.A.; Othman, A.R.; Imron, M.F.; Ismail, N.I.; Kurniawan, S.B. Dual function of Lemna minor and Azolla pinnata as phytoremediator for Palm Oil Mill Effluent and as feedstock. Chemosphere 2020, 259, 127468. [Google Scholar] [CrossRef]
- Murthy, T.; Ashok, M.; Thirumalesh, T.; Umesh, B.; Nataraju, O. Effect of partial replacement of Azolla for concentrate supplement on lactating crossbred cows. Environ. Ecol. 2013, 31, 415–417. [Google Scholar]
- Sujatha, T.; Kundu, A.; Jeyakumar, S.; Kundu, M. Azolla supplementation: Feed cost benefit in duck ration in andaman islands. Tamilnadu J. Vet. Anim. Sci. 2013, 9, 130–136. [Google Scholar]
- Prabina, B.J.; Kumar, K. Dried Azolla as a nutritionally rich cost effective and immuno-modulatory feed supplement for broilers. Asian J. Anim. Sci. 2010, 5, 20–22. [Google Scholar]
- Sithara, K.; Kamalaveni, K. Formulation of low-cost feed using azolla as a protein supplement and its influence on feed utilization in fishes. Curr. Biot. 2008, 2, 212–219. [Google Scholar]
- Becerra, M.; Murgueitio, E.; Reyes, G.; Preston, T.R. Azolla filiculoides as partial replacement for traditional protein supplements in diets for growing-fattening pigs based on sugar cane juice. Livest. Res. Rural Dev. 1990, 2, 15–22. [Google Scholar]
- Leterme, P.; Londoño, A.M.; Ordoñez, D.C.; Rosales, A.; Estrada, F.; Bindelle, J.; Buldgen, A. Nutritional value and intake of aquatic ferns (Azolla filiculoides Lam. and Salvinia molesta Mitchell.) in sows. Anim. Feed Sci. Technol. 2010, 155, 55–64. [Google Scholar] [CrossRef]
- Pugesgaard, S.; Schelde, K.; Larsen, S.U.; Lærke, P.E.; Jørgensen, U. Comparing annual and perennial crops for bioenergy production–influence on nitrate leaching and energy balance. GCB Bioenerg. 2015, 7, 1136–1149. [Google Scholar] [CrossRef]
- Anglade, J.; Billen, G.; Garnier, J. Relationships for estimating N2 fixation in legumes: Incidence for N balance of legume-based cropping systems in Europe. Ecosphere 2015, 6, 1–24. [Google Scholar] [CrossRef]
- Olguín, E.J.; Galicia, S.; Mercado, G.; Pérez, T. Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J. Appl. Phycol. 2003, 15, 249–257. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant. Pathol. 2010, 11, 705–719. [Google Scholar] [CrossRef]
- Mouradov, A.; Spangenberg, G. Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front. Plant Sci. 2014, 5, 620. [Google Scholar] [CrossRef]
- Rupasinghe, H.V.; Arumuggam, N. Health Benefits of Anthocyanins. In Anthocyanins from Natural Sources; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 121–158. [Google Scholar]
- Ballard, C.R.; Junior, M.R.M. Health Benefits of Flavonoids. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 185–201. [Google Scholar]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharm. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Basak, B.; Pramanik, M.A.H.; Rahman, M.S.; Tarafdar, S.U.; Roy, B.C. Azolla (Azolla pinnata) as a feed ingredient in broiler ration. Int. J. Poult. Sci. 2002, 1, 29–34. [Google Scholar]
- Naghshi, H.; Khojasteh, S.; Jafari, M. Investigation the Effect of Different Levels of Azolla (Azolla pinnata) on Performance and Carcass Characteristics of Cobb Broiler Chicks. Int. J. Farm. Allied Sci. 2014, 1, 45–49. [Google Scholar]
- Alalade, O.A.; Iyayi, E.A. Chemical composition and the feeding value of Azolla (Azolla pinnata) meal for egg-type chicks. Int. J. Poult. Sci. 2006, 2, 137–141. [Google Scholar]
- Güngör, E.; Brouwer, P.; Dijkhuizen, L.W.; Shaffar, D.C.; Nierop, K.G.J.; de Vos, R.C.H.; Sastre Toraño, J.; van der Meer, I.M.; Schluepmann, H. Azolla ferns testify: Seed plants and ferns share a common ancestor for leucoanthocyanidin reductase enzymes. New Phytol. 2020. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef]
- Pérez-López, U.; Sgherri, C.; Miranda-Apodaca, J.; Micaelli, F.; Lacuesta, M.; Mena-Petite, A.; Quartacci, M.F.; Muñoz-Rueda, A. Concentration of phenolic compounds is increased in lettuce grown under high light intensity and elevated CO2. Plant Physiol. Biochem. 2018, 123, 233–241. [Google Scholar] [CrossRef]
- Muradov, N.; Taha, M.; Miranda, A.F.; Kadali, K.; Gujar, A.; Rochfort, S.; Stevenson, T.; Ball, A.S.; Mouradov, A. Dual application of duckweed and azolla plants for wastewater treatment and renewable fuels and petrochemicals production. Biotechnol. Biofuels 2014, 7, 30. [Google Scholar] [CrossRef]
- Moretti, A.; Siniscalco Gigliano, G. Influence of light and pH on growth and nitrogenase activity on temperate-grown Azolla. Biol. Fertil. Soils 1988, 6, 131–136. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Podpora, B.; Świderski, F.; Sadowska, A.; Rakowska, R.; Wasiak-Zys, G. Spent brewer’s yeast extracts as a new component of functional food. Czech J. Food Sci. 2016, 34, 554–563. [Google Scholar] [CrossRef]
- Makkar, H.P. Quantification of Tannins in Tree and Shrub Foliage: A Laboratory Manual; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Ray, H.; Yu, M.; Auser, P.; Blahut-Beatty, L.; McKersie, B.; Bowley, S.; Westcott, N.; Coulman, B.; Lloyd, A.; Gruber, M.Y. Expression of anthocyanins and proanthocyanidins after transformation of alfalfa with maize Lc. Plant Physiol. 2003, 132, 1448–1463. [Google Scholar] [CrossRef] [PubMed]
- Abeynayake, S.W.; Panter, S.; Mouradov, A.; Spangenberg, G. A high-resolution method for the localization of proanthocyanidins in plant tissues. Plant Meth. 2011, 7, 13. [Google Scholar] [CrossRef]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA–HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Neff, M.M.; Chory, J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998, 118, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Braglia, R.; Harren, F.; Cristescu, S. Stress responses of duckweed (Lemna minor L.) and water velvet (Azolla filiculoides Lam.) to anionic surfactant sodium-dodecyl-sulphate (SDS). Aquat. Toxicol. 2012, 110, 107–113. [Google Scholar] [CrossRef]
- Sánchez-Viveros, G.; Ferrera-Cerrato, R.; Alarcón, A. Short-term effects of arsenate-induced toxicity on growth, chlorophyll and carotenoid contents, and total content of phenolic compounds of Azolla filiculoides. Water Air Soil Pollut. 2011, 217, 455–462. [Google Scholar] [CrossRef]
- Sánchez-Viveros, G.; Gonzalez-Mendoza, D.; Alarcon, A.; Ferrera-Cerrato, R. Copper effects on photosynthetic activity and membrane leakage of Azolla filiculoides and A. caroliniana. Int. J. Agric. Biol. 2010, 12, 365–368. [Google Scholar]
- Dai, L.P.; Xiong, Z.T.; Huang, Y.; Li, M.J. Cadmium-induced changes in pigments, total phenolics, and phenylalanine ammonia-lyase activity in fronds of Azolla imbricata. Environ. Toxicol. 2006, 21, 505–512. [Google Scholar] [CrossRef]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Fernie, A.R. Leveraging Natural Variance towards Enhanced Understanding of Phytochemical Sunscreens. Trends Plant Sci. 2017, 22, 308–315. [Google Scholar] [CrossRef]
- Ishikura, N. 3-Desoxyanthocyanin and other phenolics in the water fernAzolla. Bot. Mag. Shokubutsu–Gaku–Zasshi 1982, 95, 303–308. [Google Scholar] [CrossRef]
- Cohen, M.F.; Sakihama, Y.; Takagi, Y.C.; Ichiba, T.; Yamasaki, H. Synergistic effect of deoxyanthocyanins from symbiotic fern Azolla spp. on hrmA gene induction in the cyanobacterium Nostoc punctiforme. Mol. Plant Microbe Interact. 2002, 15, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.F.; Meziane, T.; Tsuchiya, M.; Yamasaki, H. Feeding deterrence of Azolla in relation to deoxyanthocyanin and fatty acid composition. Aquat. Bot. 2002, 74, 181–187. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Chowdhury, R.; Bhattacharjee, C. Isolation and structural elucidation of flavonoids from aquatic fern Azolla microphylla and evaluation of free radical scavenging activity. Int. J. Pharm. Pharm. Sci. 2013, 5, 743–749. [Google Scholar]
- Tao, X.; Fang, Y.; Huang, M.J.; Xiao, Y.; Liu, Y.; Ma, X.R.; Zhao, H. High flavonoid accompanied with high starch accumulation triggered by nutrient starvation in bioenergy crop duckweed (Landoltia punctata). BMC Genom. 2017, 18, 166. [Google Scholar] [CrossRef]
- Pereira, A.L.; Carrapiço, F. Histochemistry of simple hairs from the foliar cavities of Azolla filiculoides. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2007, 141, 323–328. [Google Scholar] [CrossRef]
- Abeynayake, S.W.; Panter, S.; Chapman, R.; Webster, T.; Rochfort, S.; Mouradov, A.; Spangenberg, G. Biosynthesis of proanthocyanidins in white clover flowers: Cross talk within the flavonoid pathway. Plant Physiol. 2012, 158, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Ladha, J.K.; Simpson, I.C.; Ottow, J.C.G. Parameters Affecting Residue Nitrogen Mineralization in Flooded Soils. Soil Sci. Soc. Am. J. 1994, 58, 1666–1671. [Google Scholar] [CrossRef]
- Costa, M.L.; Santos, M.C.; Carrapiço, F. Biomass characterization of Azolla filiculoides grown in natural ecosystems and wastewater. Hydrobiologia 1999, 415, 323–327. [Google Scholar] [CrossRef]
- Wang, Y.; Douglas, G.; Waghorn, G.; Barry, T.; Foote, A. Effect of condensed tannins in Lotus corniculatus upon lactation performance in ewes. J. Agric. Sci. 1996, 126, 353–362. [Google Scholar] [CrossRef]
- Mueller-Harvey, I.; Bee, G.; Dohme-Meier, F.; Hoste, H.; Karonen, M.; Kölliker, R.; Lüscher, A.; Niderkorn, V.; Pellikaan, W.F.; Salminen, J.-P.; et al. Benefits of Condensed Tannins in Forage Legumes Fed to Ruminants: Importance of Structure, Concentration, and Diet Composition. Crop Sci. 2019, 59, 861–885. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in plant-herbivore interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Mariotti, F.; Tomé, D.; Mirand, P.P. Converting nitrogen into protein—Beyond 6.25 and Jones’ factors. Crit. Rev. Food Sci. Nutr. 2008, 48, 177–184. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chen, F.; Cheng, Z.; Wang, Y.; Yang, P.; Zhang, Y.; Chan, Z. Manipulation of arginase expression modulates abiotic stress tolerance in Arabidopsis: Effect on arginine metabolism and ROS accumulation. J. Exp. Bot. 2013, 64, 1367–1379. [Google Scholar] [CrossRef]
- Winter, G.; Todd, C.D.; Trovato, M.; Forlani, G.; Funck, D. Physiological implications of arginine metabolism in plants. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Joshi, V.; Joung, J.-G.; Fei, Z.; Jander, G. Interdependence of threonine, methionine and isoleucine metabolism in plants: Accumulation and transcriptional regulation under abiotic stress. Amino Acids 2010, 39, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Savka, M.A.; Hudson, A.O. The Synthesis and Role of β-Alanine in Plants. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Yuan, S.; Li, Z.; Zhou, M.; Wu, P.; Hu, Q.; Mendu, V.; Wang, L.; Luo, H. STRESS INDUCED FACTOR 2, a leucine-rich repeat kinase regulates basal plant pathogen defense. Plant Physiol. 2018, 176, 3062–3080. [Google Scholar] [CrossRef]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.-G. Signaling role of glutamate in plants. Front. Plant. Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).