Prevalence of Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease of Shrimp in Shrimp, Molluscan Shellfish and Water Samples in the Mekong Delta, Vietnam
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Isolation and Identification of V. parahaemolyticus
2.3. Detection of Pathogenic Genes
2.4. Serotyping
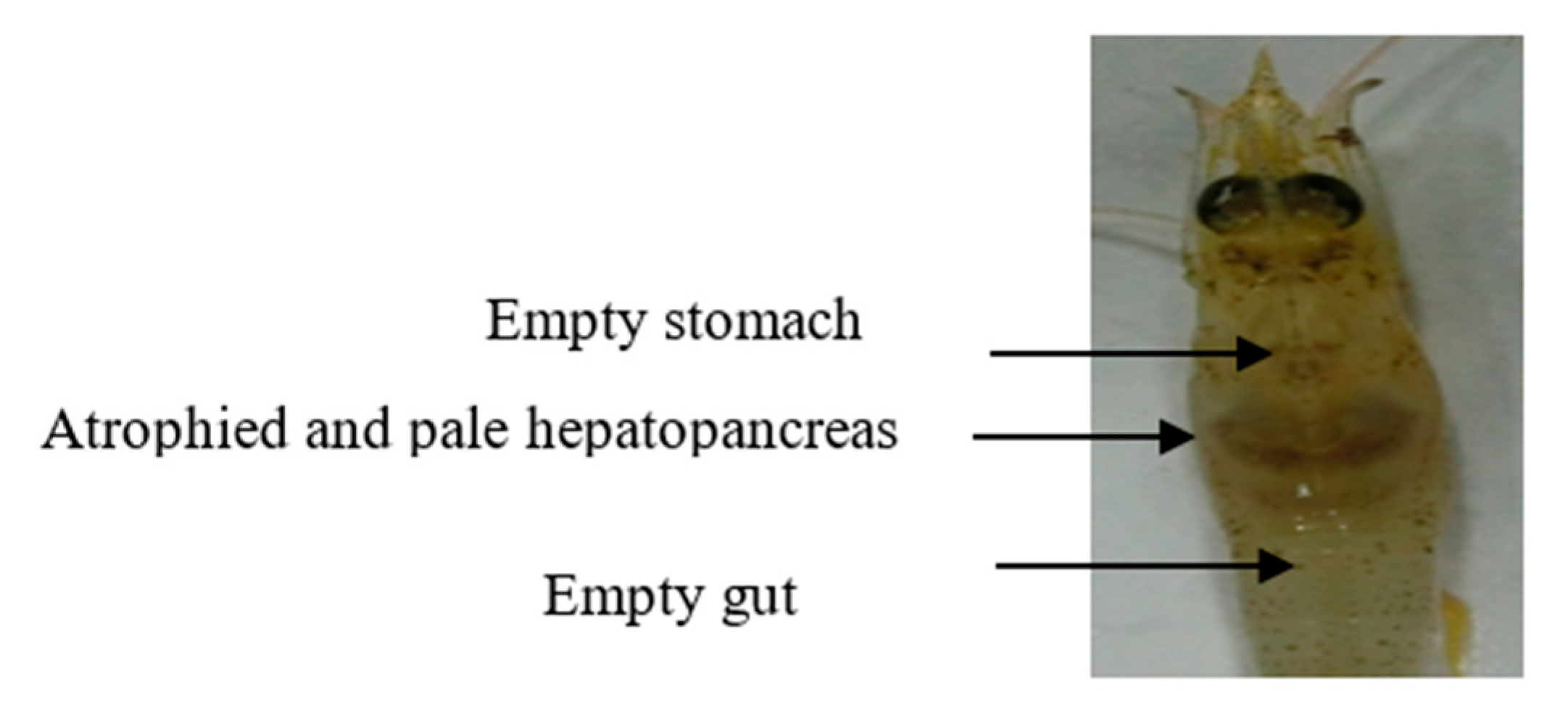
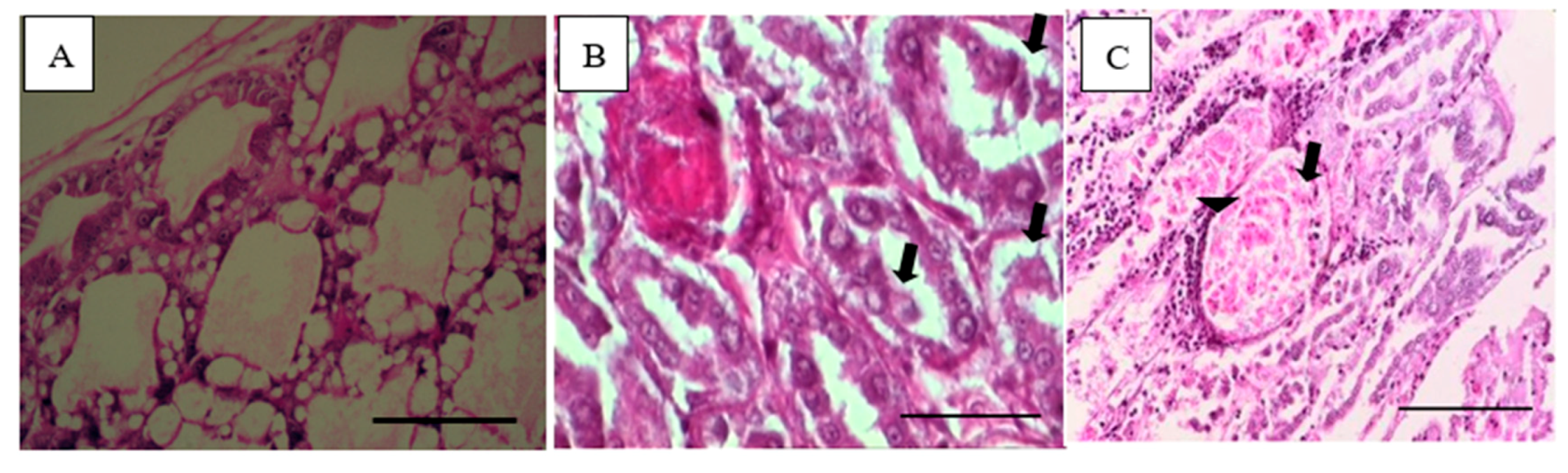
2.5. Examination of Pathogenicity of VpAHPND Strains
2.6. Examination of Antimicrobial Susceptibility
3. Results
3.1. Prevalence of VpAHPND in Shrimp, Molluscan Shellfish, and Water Samples in the Mekong Delta
3.2. Serotypes
3.3. Pathogenicity of VpAHPND
3.4. Antimicrobial Susceptibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Report of the FAO/MARD Technical Workshop on Early Mortality Syndrome (EMS) or Acute Hepatopancreatic Necrosis Syndrome (AHPNS) of Cultured Shrimp (under TCP/VIE/3304). Hanoi, Vietnam, 25–27 June 2013. FAO Fisheries and Aquaculture Report No 1053 FIRA/R1053. 2013. Available online: http://www.fao.org/3/i3422e/i3422e.pdf (accessed on 10 June 2020).
- Gomez-Gil, B.; Soto-Rodríguez, S.; Lozano, R.; BetancourtLozano, M. Draft genome sequence of Vibrio parahaemolyticus strain m0605, which causes severe mortalities of shrimps in Mexico. Genome Announc. 2014, 2, e00055-14. [Google Scholar] [CrossRef]
- Soto-Rodriguez, S.A.; Gomez-Gil, B.; Lozano-Olvera, R.; Betancourt-Lozano, M.; Morales-Covarrubias, M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in northwestern Mexico. Appl. Environ. Microbiol. 2015, 81, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, L.; Bayot, B.; Betancourt, I.; Pinzón, A. Draft genome sequence of pathogenic bacteria Vibrio parahaemolyticus strain Ba94C2, associated with acute hepatopancreatic necrosis disease isolate from south America. Genom. Data 2016, 9, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Dhar, A.K.; Piamsomboon, P.; Aranguren Caro, L.F.; Kanrar, S.; Adami, R., Jr.; Juan, Y.S. First report of acute hepatopancreatic necrosis disease (AHPND) occurring in the USA. Dis. Aquat. Org. 2019, 132, 241–247. [Google Scholar] [CrossRef]
- NACA. Report of the Asia Pacific Emergency Regional Consultation on the Emerging Shrimp Disease: Early Mortality Syndrome (EMS)/ Acute Hepatopancreatic Necrosis Syndrome (AHPNS), 9–10 Aug 2012; Network of Aquaculture Centres in Asia-Pacific: Bangkok, Thailand, 2012. [Google Scholar]
- Loc, T.; Nunan, L.; Redman, R.M.; Mohney, L.L.; Pantoja, C.R.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar]
- Lee, C.-T.; Chen, I.-T.; Yang, Y.-T.; Kod, T.-P.; Huang, Y.-T.; Huang, J.-Y.; Huang, M.-F.; Lin, S.-J.; Chen, C.-Y.; Lin, S.-S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef]
- Hien, N.T.; Huong, N.T.L.; Chuong, V.D.; Nga, N.T.V.; Quang, P.H.; Hang, B.T.V.; Long, N.V. Status of acute hepatopancreatic necrosis disease (AHPND) and other emerging diseases of penaeid shrimps in Viet Nam. In Addressing Acute Hepatopancreatic Necrosis Disease (AHPND) and Other Transboundary Diseases for Improved Aquatic Animal Health in Southeast Asia: Proceedings of the ASEAN Regional Technical Consultation on EMS/AHPND and Other Transboundary Diseases for Improved Aquatic Animal Health in Southeast Asia, Makati City, Philippines, 22–24 February 2016; Pakingking, R.V., Jr., De Jesus-Ayson, E.G.T., Acosta, B.O., Eds.; Aquaculture Department, Southeast Asian Fisheries Development Center: Iloilo, Philippines, 2016; pp. 88–95. [Google Scholar]
- Oanh, D.T.; Phuong, N.T. Dangerous diseases in shrimp culture in the Mekong Delta. Sci. J. Can Tho Univ. Vietnam 2012, 22, 106–118. [Google Scholar]
- Kim, Y.B.; Okuda, J.; Matsumoto, C.; Takahashi, N.; Hashimoto, S.; Nishibuchi, M. Identification of Vibrio parahaemolyticus strains at the species level by PCR targeted to the toxR gene. J. Clin. Microbiol. 1999, 37, 1173–1177. [Google Scholar] [CrossRef]
- Han, J.E.; Tang, K.F.J.; Tran, L.H.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Organ. 2015, 113, 33–40. [Google Scholar] [CrossRef]
- Lightner, D.V. A Handbook of Shrimp Pathology and Diagnostic Procedures for Diseases of Cultured Penaeid Shrimp; World Aquaculture Society: Baton Rouge, LA, USA, 1996. [Google Scholar]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement; CLSI Document M100-S24; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Galani, I.; Kontopidou, F.; Souli, M.; Rekatsina, P.-D.; Koratzanis, E.; Deliolanis, J.; Giamarellou, H. Colistin susceptibility testing by Etest and disk diffusion methods. Int. J. Antimicrob. Agents. 2008, 31, 434–439. [Google Scholar] [CrossRef]
- Fujisaki, M.; Sadamoto, S.; Ikedo, M.; Totsuka, K.; Kaku, M.; Tateda, K.; Hirakata, Y.; Yamaguchi, K. Development of interpretive criteria for tebipenem disk diffusion susceptibility testing with Staphylococcus spp. and Haemophilus influenzae. J. Infect. Chemother. 2011, 17, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Daniels, N.A.; Ray, B.; Easton, A.; Marano, N.; Kahn, E.; McShan, A.L.; Rosario, L.D.; Baldwin, T.; Kingsley, M.A.; Puhr, N.D.; et al. Emergence of a new Vibrio parahaemolyticus serotype in raw oysters. A prevention quandary. JAMA 2000, 284, 1541–1545. [Google Scholar] [CrossRef] [PubMed]
- Changchai, N.; Saunjit, S. Occurrence of Vibrio parahaemolyticus and Vibrio vulnificus in retail raw oysters from the eastern coast of Thailand. Trop. Med. Public Health 2014, 45, 662–669. [Google Scholar]
- Malcolm, T.T.H.; Cheah, Y.K.; Radzi, C.W.J.W.M.; Kasim, F.A.; Kantilal, H.K.; John, T.Y.H.; Martinez-Urtaza, J.; Nakaguchi, Y.; Nishibuchi, M.; Son, R. Detection and quantification of pathogenic Vibrio parahaemolyticus in shellfish by using multiplex PCR and loop-mediated isothermal amplification assay. Food Control 2015, 47, 664–671. [Google Scholar] [CrossRef]
- Letchumanan, V.; Chan, K.-G.; Lee, L.-H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014, 5, 1–13. [Google Scholar] [CrossRef]
- Chonsin, K.; Matsuda, S.; Theethakaew, C.; Kodam, T.; Junjhon, J.; Suzuki, Y.; Suthienkul, O.; Iida, T. Genetic diversity of Vibrio parahaemolyticus strains isolated from farmed Pacific white shrimp and ambient pond water affected by acute hepatopancreatic necrosis disease outbreak in Thailand. FEMS Microbiol. Lett. 2016, 362, 1–5. [Google Scholar]
- Kongrueng, J.; Yingkajorn, M.; Bunpa, S.; Sermwittayawong, N.; Singkhamanan, K.; Vuddhakul, V. Characterization of Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease in southern Thailand. J. Fish. Dis. 2015, 38, 957–966. [Google Scholar] [CrossRef]
- Schroeder, M.; Brooks, B.D.; Brooks, A.E. The Complex relationship between virulence and antibiotic resistance. Genes 2017, 8, 39. [Google Scholar] [CrossRef]
- Boonyawiwat, V.; Nga, N.T.V.; Bondadreantaso, M.G. Risk factors associated with acute hepatopancreatic necrosis disease (AHPND) outbreak in the Mekong Delta, Viet Nam. Asian Fish. Sci. 2018, 31S, 226–241. [Google Scholar]
- Kondo, H.; Van, P.T.; Lua, D.T.; Hirono, I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc. 2015, 3, e00978-15. [Google Scholar] [CrossRef]
- Dong, X.; Wang, H.; Xie, G.; Zou, P.; Guo, C.; Liang, Y.; Huang, J. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg. Microbes Infect. 2017, 6, e2. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, M.M.; Salman, A.E.B.; Roess, A.A. Virulence and antibiotic resistance of Vibrio parahaemolyticus isolates from seafood from three developing countries and of worldwide environmental, seafood, and clinical isolates from 2000 to 2017. J. Food Prot. 2017, 80, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Mohney, L.L.; Tang, K.F.J.; Pantoja, C.R.; Lightner, D.V. Plasmid mediated tetracycline resistance of Vibrio parahaemolyticusassociated with acute hepatopancreatic necrosis disease (AHPND) in shrimp. Aquacul. Rep. 2015, 2, 17–21. [Google Scholar] [CrossRef]
- Done, H.Y.; Venkatesan, A.K.; Halden, R.U. Does the recent growth of aquaculture create antibiotic resistance threats; Different from those associated with land animal production in agriculture? AAPS J. 2015, 17, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Serrano, P. Responsible Use of Antibiotics in Aquaculture. FAO Fisheries Technical Paper. 2005. No. 469. Available online: http://www.fao.org/3/a-a0282e.pdf (accessed on 10 June 2020).
- Chiou, J.; Li, R.; Chen, S. CARB-17 family of-lactamases mediates intrinsic resistance to penicillins in Vibrio parahaemolyticus. Antimicrob. Agents Chemother. 2015, 6, 3593–3595. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, V.; Yin, W.-F.; Lee, L.-H.; Chan, K.-G. Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front. Microbiol. 2015, 6, 1–11. [Google Scholar] [CrossRef]
| Origins | Sample Types | No. of Samples | No. of VpAHPND Positive Samples (%) |
|---|---|---|---|
| Seafood samples | |||
| Retail shops | Molluscan shellfish | 298 | 2 (0.7) |
| Shrimp | 32 | 0 (0.0) | |
| Subtotal | 330 | 2 (0.6) | |
| Farms | Molluscan shellfish | 16 | 0 (0.0) |
| Shrimp | 71 | 7 (9.9) | |
| Subtotal | 87 | 7 (8.0) | |
| Total | 417 | 9 (2.2) | |
| Water samples | |||
| Farms | White hard clam farms | 22 | 0 (0.0) |
| Shrimp ponds | 42 | 2 (4.8) | |
| Total | 64 | 2 (3.1) | |
| No. of Samples | Strain Names | Serotypes | Pathogenic Genes | Species-Specific Gene (toxR) | Pathogenicity to Shrimp | Antimicrobial Resistant Patterns | Origins | |
|---|---|---|---|---|---|---|---|---|
| pirAvp | pirBvp | |||||||
| 1 | VP-AHPND-1 | O1:K64 | + | + | + | + | AMP-COL c | Shellfish in shop |
| 2 | VP-AHPND-2 | O1:K68 | + | + | + | + | COL-STM | Shellfish in shop |
| 3 | VP-AHPND-3 | O1:KUT a | + | + | + | + | AMP- COL- STM | Water at shrimp pond |
| 4 | VP-AHPND-4 | O1:KUT | + | + | + | + | AMP-COL-OTT-STM | Water at shrimp pond |
| 5 | VP-AHPND-5 | O1:K68 | + | + | + | + | AMP-COL- NAL-OTT-STM | Shrimp at shrimp pond |
| 6 | VP-AHPND-6 | O1:KUT | + | + | + | NE b | AMP-COL-OTT-STM | Shrimp at shrimp pond |
| VP-AHPND-7 | O1:KUT | - | + | + | NE | AMP-COL-OTT-STM | Shrimp at shrimp pond | |
| 7 | VP-AHPND-8 | O1:K25 | + | + | + | NE | AMP-COL | Shrimp at shrimp pond |
| 8 | VP-AHPND-9 | O1:K25 | + | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond |
| VP-AHPND-10 | O1:KUT | + | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond | |
| 9 | VP-AHPND-11 | O1:KUT | + | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond |
| VP-AHPND-12 | O1:K25 | + | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond | |
| 10 | VP-AHPND-13 | O1:K25 | + | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond |
| VP-AHPND-14 | O1:KUT | + | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond | |
| 11 | VP-AHPND-15 | O3:K31 | + | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond |
| VP-AHPND-16 | O3:K31 | - | + | + | NE | AMP-COL-STM | Shrimp at shrimp pond | |
| Antimicrobial Agents | No. of Resistant Strains (%) |
|---|---|
| Colistin | 16 (100.0) |
| Ampicillin | 15 (93.8) |
| Streptomycin | 14 (87.5) |
| Oxytetracycline | 4 (25.0) |
| Nalidixic acid | 1 (6.3) |
| Chloramphenicol | 0 (0.0) |
| Gentamicin | 0 (0.0) |
| Kanamycin | 0 (0.0) |
| Ofloxacin | 0 (0.0) |
| Tebipenem | 0 (0.0) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong To, T.T.; Yanagawa, H.; Khanh Thuan, N.; Hiep, D.M.; Cuong, D.V.; Khai, L.T.L.; Taniguchi, T.; Kubo, R.; Hayashidani, H. Prevalence of Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease of Shrimp in Shrimp, Molluscan Shellfish and Water Samples in the Mekong Delta, Vietnam. Biology 2020, 9, 312. https://doi.org/10.3390/biology9100312
Hong To TT, Yanagawa H, Khanh Thuan N, Hiep DM, Cuong DV, Khai LTL, Taniguchi T, Kubo R, Hayashidani H. Prevalence of Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease of Shrimp in Shrimp, Molluscan Shellfish and Water Samples in the Mekong Delta, Vietnam. Biology. 2020; 9(10):312. https://doi.org/10.3390/biology9100312
Chicago/Turabian StyleHong To, Tran Thi, Haruka Yanagawa, Nguyen Khanh Thuan, Du Minh Hiep, Doan Van Cuong, Ly Thi Lien Khai, Takahide Taniguchi, Ryoichi Kubo, and Hideki Hayashidani. 2020. "Prevalence of Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease of Shrimp in Shrimp, Molluscan Shellfish and Water Samples in the Mekong Delta, Vietnam" Biology 9, no. 10: 312. https://doi.org/10.3390/biology9100312
APA StyleHong To, T. T., Yanagawa, H., Khanh Thuan, N., Hiep, D. M., Cuong, D. V., Khai, L. T. L., Taniguchi, T., Kubo, R., & Hayashidani, H. (2020). Prevalence of Vibrio parahaemolyticus Causing Acute Hepatopancreatic Necrosis Disease of Shrimp in Shrimp, Molluscan Shellfish and Water Samples in the Mekong Delta, Vietnam. Biology, 9(10), 312. https://doi.org/10.3390/biology9100312





