The Effect of P4 + eCG Estrus Induction Protocol during the Deep and the Transition Anestrous Period on the Reproductive Performance of Crossbred Dairy Goats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Location, Environmental Conditions, Animals, and their Management
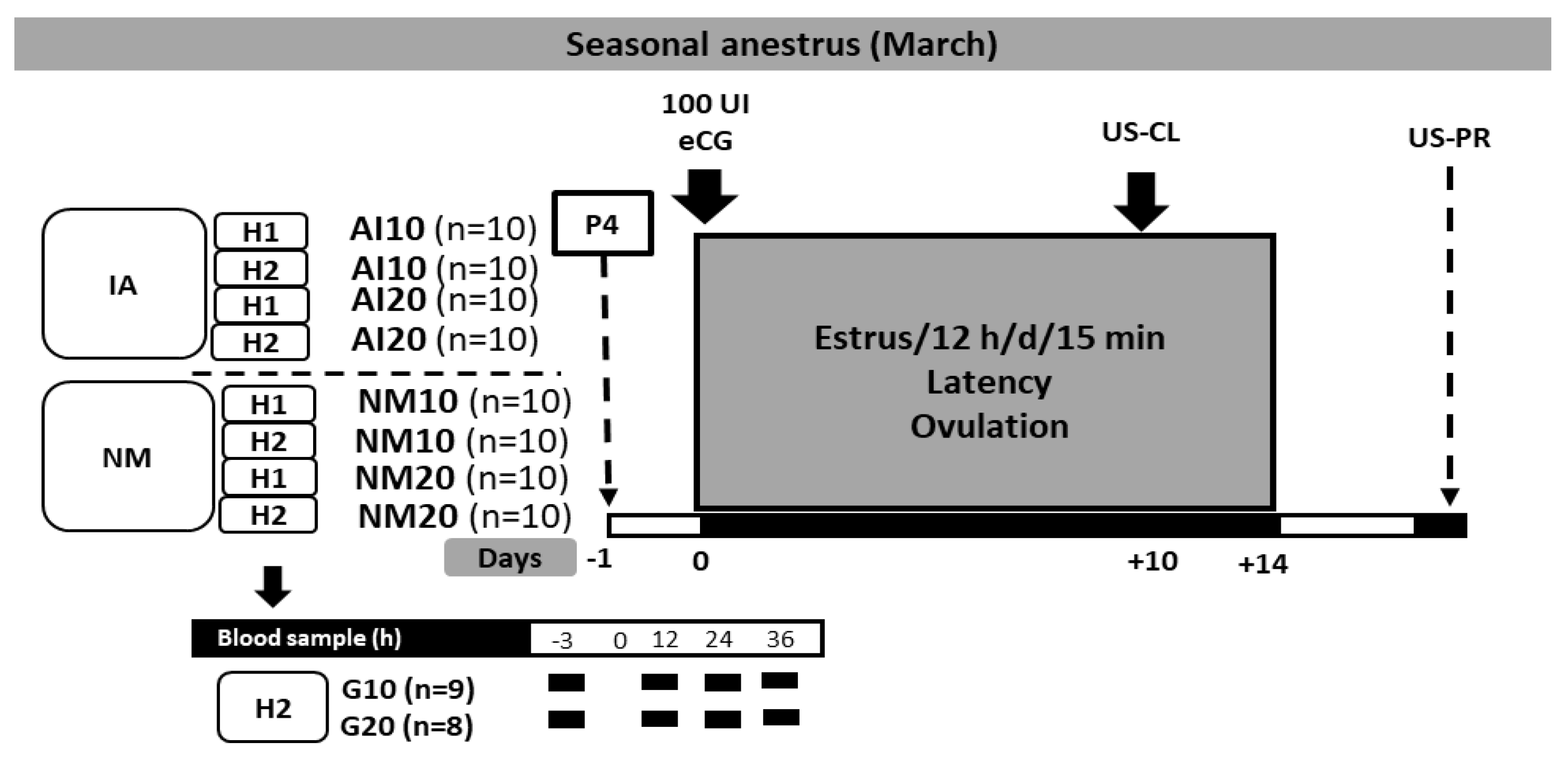
2.3. Experiment 1: Effect of Different Doses of Intramuscular Natural Progesterone (10 or 20 mg) + eCG, and Either Natural Mating or Artificial Insemination in Two Herds on Reproductive Performance of Anestrous Goats
2.4. Experiment 2: Effect of Different Doses of Intramuscular Natural Progesterone (10 or 20 mg) + eCG, Month of the Anestrous Season (March or June), and Herd (H1, H2), in Goats Exposed to Natural Matingt, on Reproductive Performance of Anestrous Goats
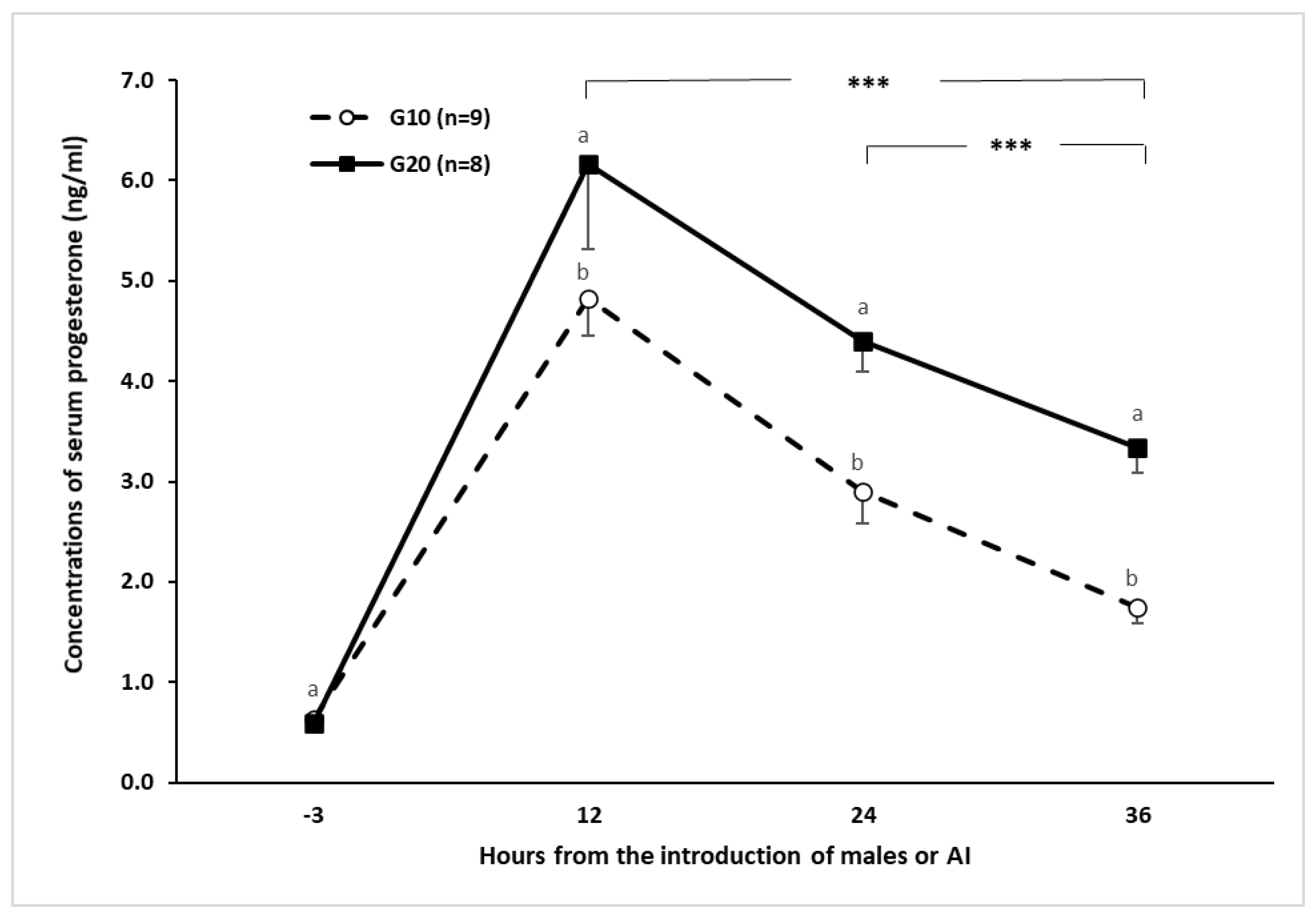
2.5. Estrus Induction, Estrus Latency, Ovulation Percentage, and Pregnancy Rate
2.6. Statistical Analyses
3. Results
3.1. Experiment 1
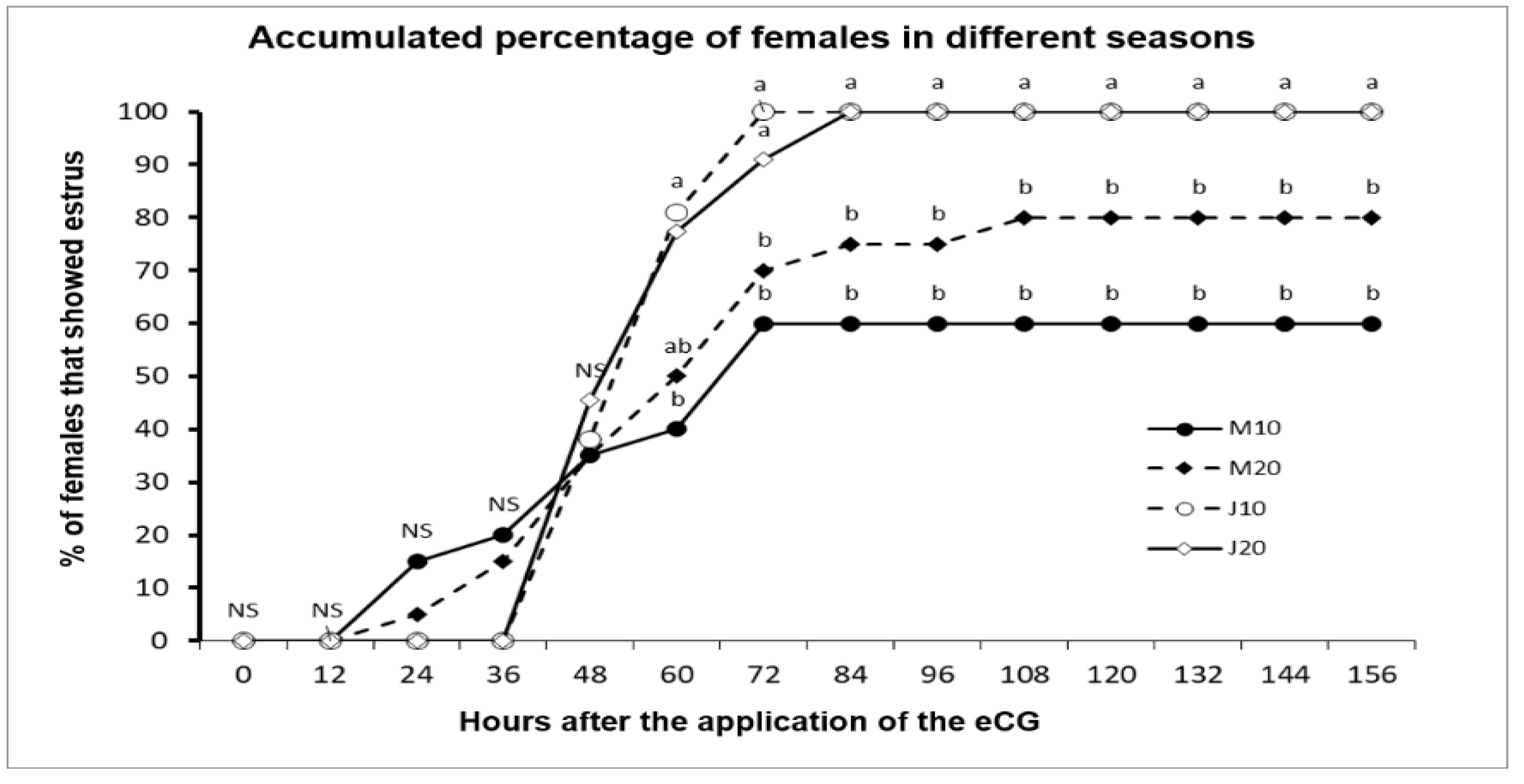
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Contreras-Villarreal, V.; Meza-Herrera, C.A.; Rivas-Muñoz, R.; Angel-Garcia, O.; Luna-Orozco, J.R.; Carrillo, E.; Mellado, M.; Véliz-Deras, F.G. Reproductive performance of seasonally anovular mixed-bred dairy goats induced to ovulate with a combination of progesterone and eCG or estradiol. Anim. Sci. J. 2016, 87, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Ros, P.; Gonzalez–Bulnes, A. Efficiency of CIDR–based protocols including GnRH instead of eCG for estrus synchronization in sheep. Animals 2019, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bulnes, A.; Meza–Herrera, C.A.; Rekik, M.; Ben Salem, H.; Kridli, R.T. Limiting factors and strategies for improving reproductive outputs of small ruminants reared in semi–arid environments. In Semi–Arid Environments: Agriculture, Water Supply and Vegetation; Degenovine, K.M., Ed.; Chapter 2; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; pp. 41–60. [Google Scholar]
- Simões, J. Recent advances on synchronization of ovulation in goats, out of season, for a more sustainable production. Asian Pac. J. Reprod. 2015, 4, 157–165. [Google Scholar] [CrossRef]
- Guillen-Muñoz, J.M.; Meza-Herrera, C.A.; Rivas-Muñoz, R.; Zuñiga-Garcia, Z.; Calderon-Leyva, G.; Mellado, M.; Veliz-Deras, F.G. The use of female estrogenized goats as sexual stimulator of crossbred dairy males subsequently exposed to acyclic goats during two phases of the anestrous season. Theriogenology 2018, 119, 175–182. [Google Scholar] [CrossRef]
- Menchaca, A.; Miller, V.; Salveraglio, V.; Rubianes, E. Endocrine, luteal and follicular responses after the use of the short–term protocol to synchronize ovulation in goats. Anim. Reprod. Sci. 2007, 102, 76–87. [Google Scholar] [CrossRef]
- Gonzalez-Bulnes, A.; Veiga-Lopez, A.; Garcia, P.; Garcia-Garcia, R.M.; Ariznavarreta, C.; Sanchez, M.A.; Tresguerres, J.A.F.; Cocero, J.M.; Flores, J.M. Effects of progestagens and prostaglandin analogues on ovarian function and embryo viability in sheep. Theriogenology 2005, 63, 2523–2534. [Google Scholar] [CrossRef]
- Leboeuf, B.; Forgerit, Y.; Bernelas, D.; Pougnard, J.L.; Senty, E.; Driancourt, M.A. Efficacy of two types of vaginal sponges to control onset of oestrus, time of preovulatory LH peak and kidding rate in goats inseminated with variable numbers of spermatozoa. Theriogenology 2003, 60, 1371–1378. [Google Scholar] [CrossRef]
- Viñoles, C.; Meikle, A.; Martin, G.B. Short–term nutritional treatments grazing legumes or feeding concentrates increase prolificacy in Corriedale ewes. Anim. Reprod. Sci. 2009, 113, 82–92. [Google Scholar] [CrossRef]
- Vilariño, M.; Rubinaes, E.; Menchaca, A. Ovarian response and pregnancy rate with previously used intravaginal progesterone releasing devices for fixed–time artificial insemination in sheep. Theriogenology 2013, 79, 206–210. [Google Scholar] [CrossRef]
- Martemucci, G.; D’Alessandro, A.G. Synchronization of oestrus and ovulation by short time combined FGA, PGF2α, GnRH, eCG treatments for natural service or AI fixed–time. Anim. Reprod. Sci. 2011, 123, 32–39. [Google Scholar] [CrossRef]
- Bartlewski, M.P.; Beard, P.A.; Cook, J.S.; Honaramooza, A.; Rawling, C.N. Ovarian antral follicular dynamics and their relationship ëith endocrine variables throughout the oestrous cycle in breeds of sheep differing in profilificacy. J. Reprod. Fertil. 1999, 115, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Swelum, A.A.; Alowaimer, A.N.; Abouheif, M.A. Use of fluorogestone acetate sponges or controlled internal drug release for estrus synchronization in ewes: Effects of hormonal profiles and reproductive performance. Theriogenology 2015, 84, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Menchaca, A.; Rubianes, E. New treatments associated with timed artificial insemination in small ruminants. Reprod. Fert. Develop. 2004, 16, 403–413. [Google Scholar] [CrossRef]
- Rubianes, E.; De Castro, T.; Kmaid, S. Estrous response after a short progesterone priming in seasonally anestrous goats. Theriogenology 1998, 1, 356. [Google Scholar] [CrossRef]
- Gatti, M.; Zunino, P.; Ungerfeld, R. Changes in the aerobic vaginal bacterial mucous load after treatment with intravaginal sponges in anoestrous ewes: Effect of medroxiprogesterone acetate and antibiotic treatment use. Reprod. Domest. Anim. 2011, 46, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Espino, A.S.; Meza-Herrera, C.A.; Carrillo, E.; González-Álvarez, V.H.; Guillen-Muñoz, J.M.; Ángel-García, O.; Mellado, M.; Véliz–Deras, F.G. Reproductive outcomes of Alpine goats primed with progesterone and treated with human chorionic gonadotropin during the anestrous–to–estrus transition season. Anim. Reprod. Sci. 2016, 167, 133–138. [Google Scholar] [CrossRef]
- FASS. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- Guide for the Care and Use of Laboratory Animals, 1st ed.; National Academy of Medicine: Harlan, Mexico City, Mexico, 2010.
- Gallego–Calvo, L.; Gatica, M.C.; Guzmán, J.L.; Zarazaga, L.A. Role of body condition score and body weight in the control of seasonal reproduction in Blanca Andaluza goats. Anim. Reprod Sci. 2014, 151, 157–163. [Google Scholar] [CrossRef]
- Fabre–Nys, C.; Gelez, H. Sexual behavior in ewes and other domestic ruminants. Horm Behav. 2007, 52, 18–25. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, C.; Totton, S.C.; Cullen, J.N.; O’Connor, A.M. Reporting and analysis of repeated measurements in preclinical animal experiments. PLoS ONE 2019, 14, e0220879. [Google Scholar] [CrossRef]
- Hauke, J.; Kossowki, T. Comparisons of values of Pearson´s and Spearman´s correlation coefficients on the same sets of data. Quest. Geogr. 2011, 30, 87–93. [Google Scholar]
- Menchaca, A.; Rubianes, E. Relation between progesterone concentrations during the early luteal phase and follicular dynamics in goats. Theriogenology 2002, 57, 1411–1419. [Google Scholar] [CrossRef]
- Véliz, F.G.; Meza-Herrera, C.A.; De Santiago-Miramontes, M.A.; Arellano-Rodriguez, G.; Leyva, C.; Rivas-Muñoz, R.; Mellado, M. Effect of parity and progesterone priming on induction of reproductive function in Saanen goats by buck exposure. Liv. Sci. 2009, 125, 261–265. [Google Scholar] [CrossRef]
- Adams, G.P. Comparative patterns of follicle development and selection in ruminants. J. Reprod Fertil Suppl. 1999, 54, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.P.; Matteri, R.L.; Ginther, O.J. Effect of progesterone on ovarian follicles, emergence of follicular waves and circulating follicle–stimulating hormone in heifers. J. Reprod. Fertil. 1992, 96, 627–640. [Google Scholar] [CrossRef]
- Savio, J.D.; Thatcher, W.W.; Badinga, L.; de la Sota, R.L.; Wolfenson, D. Regulation of dominant follicle turnover during the oestrus cycle in cows. J. Reprod. Fertil. 1993, 97, 197–203. [Google Scholar] [CrossRef]
- Meza–Herrera, C.A. Puberty, kisspeptin and glutamate: A ceaseless golden braid. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 97–124. [Google Scholar]
- Meza–Herrera, C.A.; Tena–Sempere, M. Interface between Nutrition and Reproduction: The very basis of production. In Animal Reproduction in Livestock—Encyclopedia of Life Support Systems; Astiz, S., Gonzalez, A., Eds.; Chapter 3; Eolss Publishers: Oxford, UK, 2012. [Google Scholar]
- Johnson, S.K.; Dailey, R.A.; Inskeep, E.K.; Lewis, P.E. Effect of peripheral concentrations of progesterone on follicular growth and fertility in ewes. Dom. Anim. Endocr. 1996, 13, 69–79. [Google Scholar] [CrossRef]
- Arroyo-Ledezma, J.; Gallegos-Sánchez, J.; Villa Godoy, A.; Valencia Méndez, J. Sistemas neurales de retroalimentación durante el ciclo reproductivo anual de la oveja: Una revisión. Interciencia 2006, 31, 8–15. [Google Scholar]
- Abecia, J.A.; Forcada, F.; González-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef]
- McNeilly, A.S.; Picton, H.M.; Campbell, B.K.; Baird, D.T. Gonadotrophic control of follicle growth in the ewe. J. Reprod. Fertil. 1991, 43, 177–186. [Google Scholar] [CrossRef]
- Bartlewski, P.M.; Seaton, P.; Oliveira, M.E.F.; Kridli, R.T.; Murawski, M.; Schwarz, T. Intrinsic determinants and predictors of superovulatory yields in sheep: Circulating concentrations of reproductive hormones, ovarian status, and antral follicular blood flow. Theriogenology 2016, 86, 130–143. [Google Scholar] [CrossRef]
- Evans, G.; Maxwell, W. Conservación de semen durante corto tiempo. In Inseminación Artificial en Ovejas y Cabras; Acribia: Zaragoza, Spain, 1990; pp. 119–122. [Google Scholar]
- Ángel-García, O.; Meza-Herrera, C.A.; Guillen-Muñoz, J.M.; Carrillo-Castellanos, E.; Luna-Orozco, J.R.; Mellado, M.; Véliz-Deras, F.G. Seminal characteristics, libido and serum testosterone concentrations in mixed–breed goat bucks receiving testosterone during the non–breeding period. J. Appl. 2015, 43, 457–461. [Google Scholar] [CrossRef]
- Arroyo-Ledezma, J. Estacionalidad reproductiva de la oveja en México. Trop. Subtrop. Agroecosystems 2011, 14, 829–845. [Google Scholar]
- Gonzalez-Bulnes, A.; Pallares, P.; Ovilo, C. Ovulation, implantation and placentation in females with obesity and metabolic disorders: Life in the balance. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Ross, T.; Hawkins, D.; Hallford, D. Interactions between metabolic status, pre–breeding protein supplementation, uterine pH, and embryonic mortality in ewes: Preliminary observations. Trop. Anim. Health Prod. 2006, 38, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Meza-Herrera, C.A.; Ross, T.; Hallford, D.; Hawkins, D. Gonzalez–Bulnes, A. High periconceptional protein intake modifies uterine and embryonic relationships increasing early pregnancy losses and embryo growth retardation in sheep. Reprod. Domest. Anim. 2010, 45, 723–728. [Google Scholar]

| Response Variables | Herd | Interaction Effect 1 Herd × Type of Breeding × P4 Dose | P4 Dose | |||||
|---|---|---|---|---|---|---|---|---|
| AI + 10 | AI + 20 | NM + 10 | NM + 20 | 10 mg | 20 mg | |||
| Estrus Induction (%) | H1 | 92.5 ± 0.05 A | 100 ± 0.08 a | 90 ± 0.08 a | 100 ± 0.08 a | 80 ± 0.08 a | 60 ± 0.05 B | 82.5 ± 0.05 A |
| H2 | 50 ± 0.05 B | 20 ± 0.08 b | 80 ± 0.08 a | 20 ± 0.08 b | 80 ± 0.08 a | |||
| Estrus Latency (h) | H1 | 49.6 ± 2.5 A | 46 ± 2.1 a | 46.6 ± 2.8 a | 49.4 ± 3.7 a | 55.5 ± 4.0 a | 43.5 ± 4.1 B | 56.3 ± 2.6 A |
| H2 | 50.2 ± 4.2 A | 54 ± 2.6 a | 60 ± 6.4 a | 24 ± 0.0 b | 63 ± 8.5 a | |||
| Ovulation (%) | H1 | 80 ± 0.0 A | 100 ± 0.14 a | 40 ± 0.14 ab | 90 ± 0.14 a | 70 ± 0.14 a | 85 ± 0.1 A | 70.2 ± 0.0 A |
| H2 | 75 ± 0.1 A | 10 ± 0.14 b | 60 ± 0.14 a | 20 ± 0.14 b | 60 ± 0.14 a | |||
| Ovulation rate (n) | H1 | 2.2 ± 0.2 A | 3.3 ± 0.4 a | 3.7 ± 0.1 a | 2.5 ± 0.2 a | 2.8 ± 0.2 a | 1.7 ± 0.34 A | 1.4 ± 0.2 A |
| H2 | 0.8 ± 0.3 B | 1 ± 0.0 b | 1.6 ± 0.1 ab | 1 ± 0.0 b | 1 ± 0.0 b | |||
| Fertility (%) | H1 | 42 ± 0.0 A | 50 ± 0.15 ab | 0 ± 0.0 b | 70 ± 0.15 a | 50 ± 0.15 ab | 42 ± 0.12 A | 37 ± 0.0 A |
| H2 | 37.5 ± 0.1 A | 0 ± 0.0 b | 37.5 ± 0.15 b | 100 ± 0.15 a | 50 ± 0.15 ab | |||
| Breeding Type | 25 ± 0.1 B | 54 ± 0.1 A | ||||||
| Pregnancy (%) | H1 | 40 ± 0.0 A | 50 ± 0.15 ab | 0 ± 0.0 b | 70 ± 0.15 a | 40 ± 0.15 b | 32 ± 0.06 A | 30 ± 0.06 A |
| H2 | 22 ± 0.0 A | 0 ± 0.0 b | 30 ± 0.15 b | 10 ± 0.15 b | 50 ± 0.15 ab | |||
| Breeding Type | 20 ± 0.06 B | 42.5 ± 0.06 A | ||||||
| Response Variables | Herd | Interaction Effect 1 | P4 Dose | |||||
|---|---|---|---|---|---|---|---|---|
| Herd × Month of Breeding × P4 Dose | ||||||||
| March | June | |||||||
| M10 | M20 | J10 | J20 | 10 mg | 20 mg | |||
| Estrus Induction (%) | H1 | 93.8 ± 0.04 A | 100 ± 0.08 a | 80 ± 0.08 a | 100 ± 0.07 a | 100 ± 0.07 a | 80.2 ± 0.04 A | 89.8 ± 0.04 A |
| H2 | 74.2 ± 0.05 B | 20 ± 0.08 b | 80 ± 0.08 a | 100 ± 0.08 a | 100 ± 0.08 a | |||
| Month | 70.0 ± 0.05 B | 100 ± 0.04 A | ||||||
| Estrus Latency (h) | H1 | 51.9 ± 3.1 A | 49.4 ± 5.9 a | 44.4 ± 5.6 a | 59.1 ± 4.9 a | 57.2 ± 4.9 a | 42.9 ± 3.1 A | 52.4 ± 3.23 B |
| H2 | 42.4 ± 3.4 B | 4.8 ± 5.6 b | 50.4 ± 5.6 a | 57.3 ± 5.9 a | 58.5 ± 6.3 a | |||
| Month | 37.2 ± 3.44 B | 58.0 ± 3.32 B | ||||||
| Ovulation (%) | H1 | 71.5 ± 0.07 A | 90 ± 0.14 a | 70 ± 0.14 a | 69.2 ± 0.13 a | 61.5 ± 0.13 ab | 59.3 ± 0.07 A | 65.7 ± 0.07 A |
| H2 | 51.5 ± 0.07 B | 20 ± 0.14 b | 60 ± 0.14 ab | 55.6 ± 0.15 ab | 75 ± 0.16 a | |||
| Ovulation rate (n) | H1 | 1.4 ± 0.15 A | 2.3 ± 0.30 a | 1.6 ± 0.30 ab | 1.1 ± 0.26 bc | 0.8 ± 0.26 b cd | 1.2 ± 0.15 A | 1.0 ± 0.15 A |
| H2 | 0.7 ± 0.17 B | 0.2 ± 0.30 d | 0.6 ± 0.30 cd | 1.1 ± 0.32 cb | 1.2 ± 0.34 bc | |||
| Fertility (%) | H1 | 61.3 ± 0.07 B | 100 ± 0.15 a | 50 ± 0.15 b | 100 ± 0.13 a | 100 ± 0.13 a | 55.9 ± 0.05 A | 56.8 ± 0.05 A |
| H2 | 100 ± 0.0 A | 50 ± 0.15 b | 62.5 ± 0.15 b | 100 ± 0.16 a | 100 ± 0.17 a | |||
| Pregnancy (%) | H1 | 58.1 ± 0.07 A | 70 ± 0.15 a | 40 ± 0.15 ab | 61.5 ± 0.13 a | 61.5 ± 0.13 a | 44 ± 0.07 A | 51 ± 0.07 A |
| H2 | 35.5 ± 0.08 B | 10 ± 0.15 b | 50 ± 0.15 ab | 33.3 ± 0.16 ab | 50 ± 0.17 ab | |||
| Interaction Effect Month of Breeding × Herd 1 or Month of Breeding × P4 Dose 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| March | June | |||||||
| Herd | M10 | M20 | Herd | J10 | J20 | |||
| H1 | H2 | H1 | H2 | |||||
| Estrus induction (%) | ||||||||
| Month × herd 1 | 90 ± 0.06 a | 50 ± 0.06 b | 100 ± 0.05 a | 100 ± 0.07 a | ||||
| Month × dose 2 | 60 ± 0.07 c | 80 ± 0.07 b | 100 ± 0.06 a | 100 ± 0.06 a | ||||
| Estrus Latency (h) | ||||||||
| Month × herd 1 | 47 ± 4.4 a | 27 ± 7.7 b | 58 ± 2.1 a | 58 ± 2.6 a | ||||
| Month × dose 2 | 27 ± 4.6 b | 47 ± 4.6 a | 58.3 ± 4.4 a | 57.7 ± 4.5 a | ||||
| Ovulation (%) | ||||||||
| Month × herd 1 | 80 ± 0.10 a | 40 ± 0.10 b | 65 ± 0.09 ab | 64 ± 0.11 ab | ||||
| Ovulation rate (n) | ||||||||
| Month × herd 1 | 1.9 ± 0.21 a | 0.4 ± 0.21 c | 1.0 ± 0.19 bc | 1.1 ± 0.23 b | ||||
| Fertility (%) | ||||||||
| Month × herd 1 | 61.1 ± 0.11 a | 60 ± 0.11 a | 61.5 ± 0.09 a | 41.2 ± 0.12 b | ||||
| Pregnancy (%) | ||||||||
| Month × herd 1 | 55 ± 0.11 ab | 30 ± 0.11 b | 61.5 ± 0.09 a | 41.10.12 ab | ||||
| Variables | LW (kg) | BCS (units) | EL (h) | EI (%) | OVP (%) | OR (units) | PREG (%) |
|---|---|---|---|---|---|---|---|
| LW (kg) | 1 | 0.256 0.110 | 0.057 0.724 | 0.035 0.825 | 0.097 0.548 | 0.137 0.398 | −0.02 0.897 |
| BCS (units) | 1 | 0.172 0.288 | 0.346 0.028 | 0.446 0.003 | 0.581 0.001 | 0.201 0.217 | |
| EL (h) | 1 | 0.836 0.001 | 0.581 0.001 | 0.440 0.004 | 0.421 0.006 | ||
| EI (%) | 1 | 0.801 0.001 | 0.640 0.001 | 0.562 0.001 | |||
| OVP (%) | 1 | 0.798 0.001 | 0.701 0.001 | ||||
| OR (units) | 1 | 0.632 0.001 | |||||
| PREG (%) | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Véliz-Deras, F.G.; Meza-Herrera, C.A.; Mellado, M.; Contreras-Villarreal, V.; Gaytán-Alemán, L.R.; Guillén-Muñoz, J.M. The Effect of P4 + eCG Estrus Induction Protocol during the Deep and the Transition Anestrous Period on the Reproductive Performance of Crossbred Dairy Goats. Biology 2020, 9, 311. https://doi.org/10.3390/biology9100311
Véliz-Deras FG, Meza-Herrera CA, Mellado M, Contreras-Villarreal V, Gaytán-Alemán LR, Guillén-Muñoz JM. The Effect of P4 + eCG Estrus Induction Protocol during the Deep and the Transition Anestrous Period on the Reproductive Performance of Crossbred Dairy Goats. Biology. 2020; 9(10):311. https://doi.org/10.3390/biology9100311
Chicago/Turabian StyleVéliz-Deras, Francisco G., César A. Meza-Herrera, Miguel Mellado, Viridiana Contreras-Villarreal, Leticia R. Gaytán-Alemán, and Juan M. Guillén-Muñoz. 2020. "The Effect of P4 + eCG Estrus Induction Protocol during the Deep and the Transition Anestrous Period on the Reproductive Performance of Crossbred Dairy Goats" Biology 9, no. 10: 311. https://doi.org/10.3390/biology9100311
APA StyleVéliz-Deras, F. G., Meza-Herrera, C. A., Mellado, M., Contreras-Villarreal, V., Gaytán-Alemán, L. R., & Guillén-Muñoz, J. M. (2020). The Effect of P4 + eCG Estrus Induction Protocol during the Deep and the Transition Anestrous Period on the Reproductive Performance of Crossbred Dairy Goats. Biology, 9(10), 311. https://doi.org/10.3390/biology9100311






