Metabarcoding Reveals Temporal Patterns of Community Composition and Realized Thermal Niches of Thalassiosira Spp. (Bacillariophyceae) from the Narragansett Bay Long-Term Plankton Time Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling, DNA Extraction and Sequencing
2.2. Sequence Analysis
2.3. Comparison of HTS Data With LM Counts and Environmental Analyses
3. Results
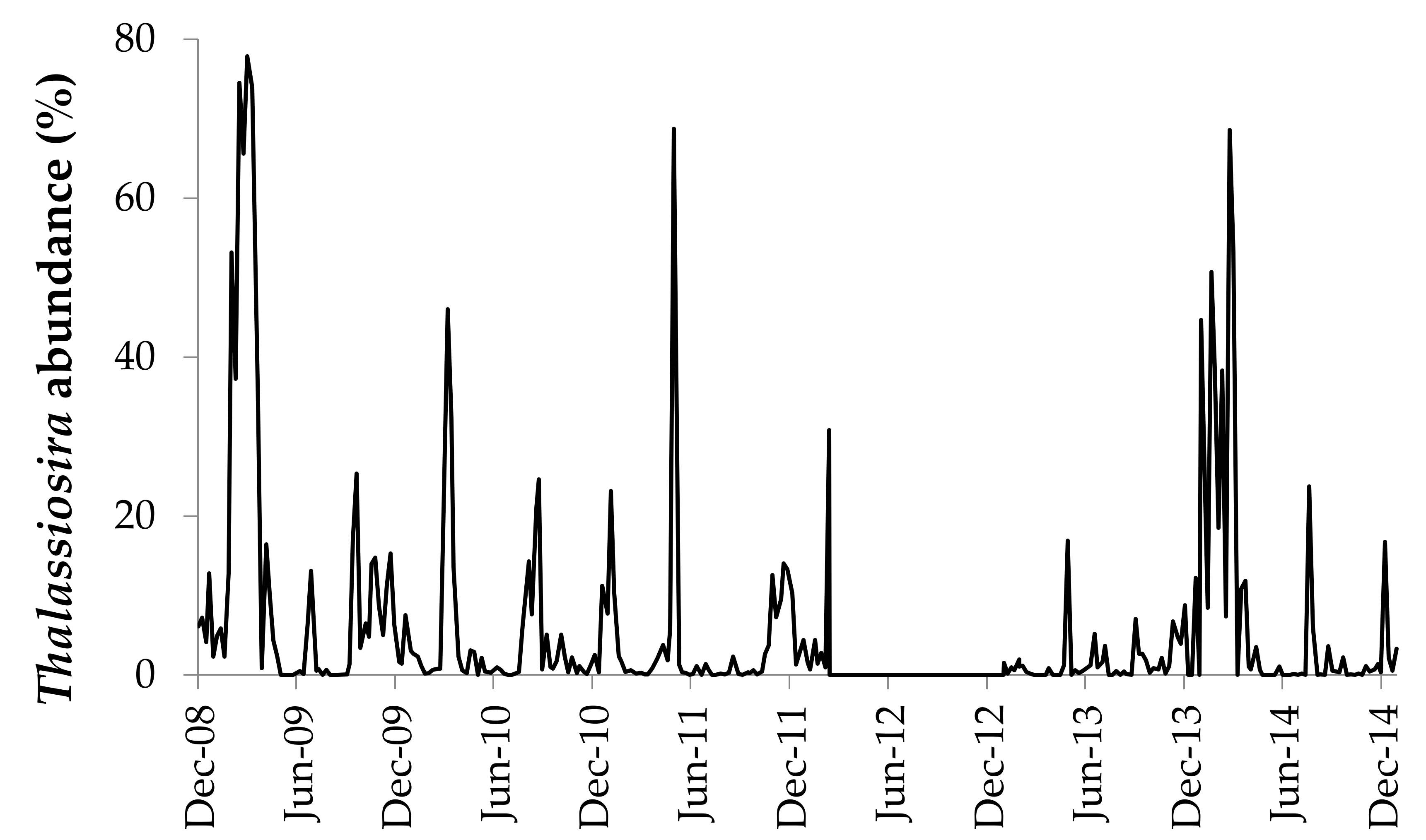
3.1. Diversity and Occurrence of Thalassiosira Based on LM
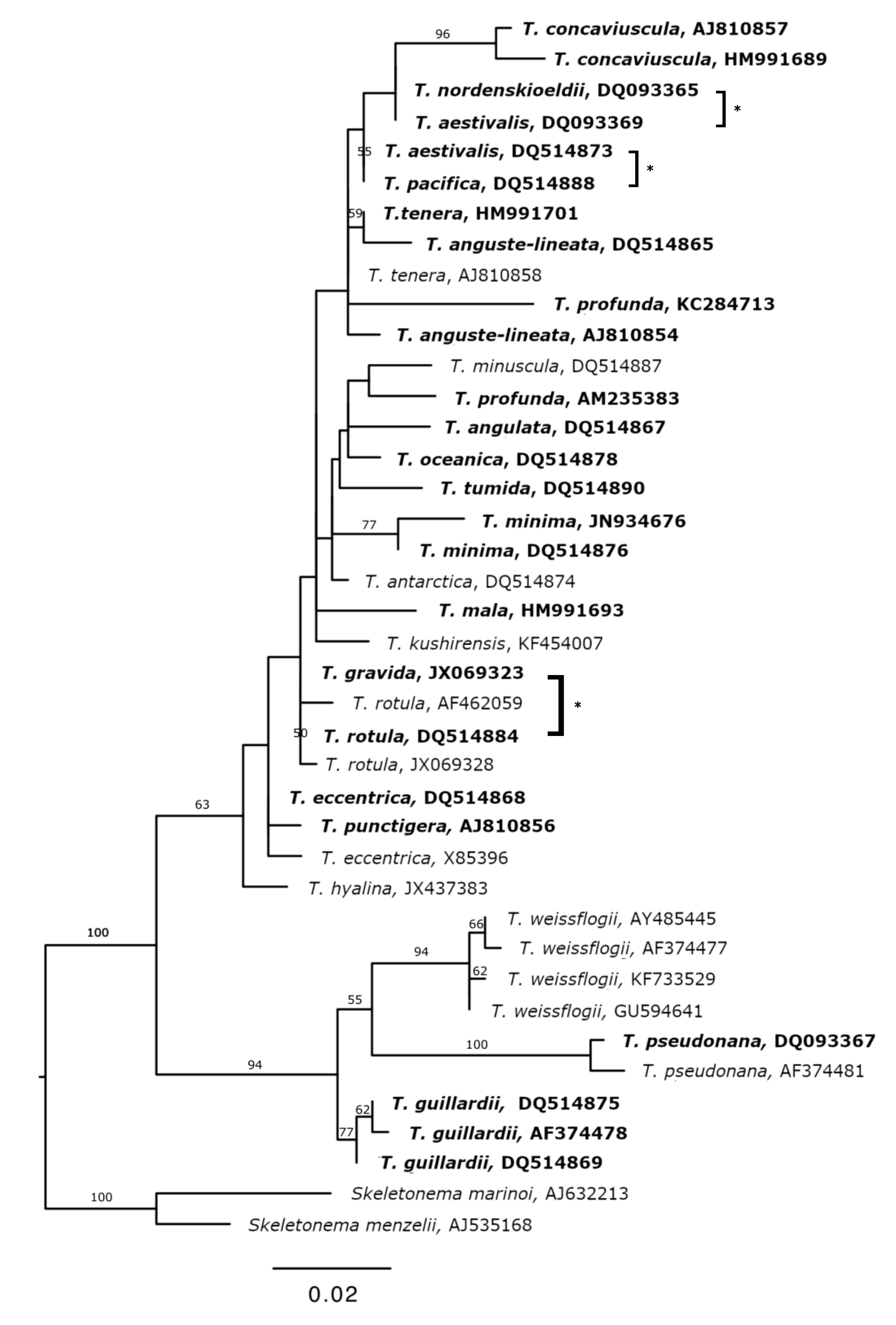
3.2. Reference Database
3.3. Sequencing Results
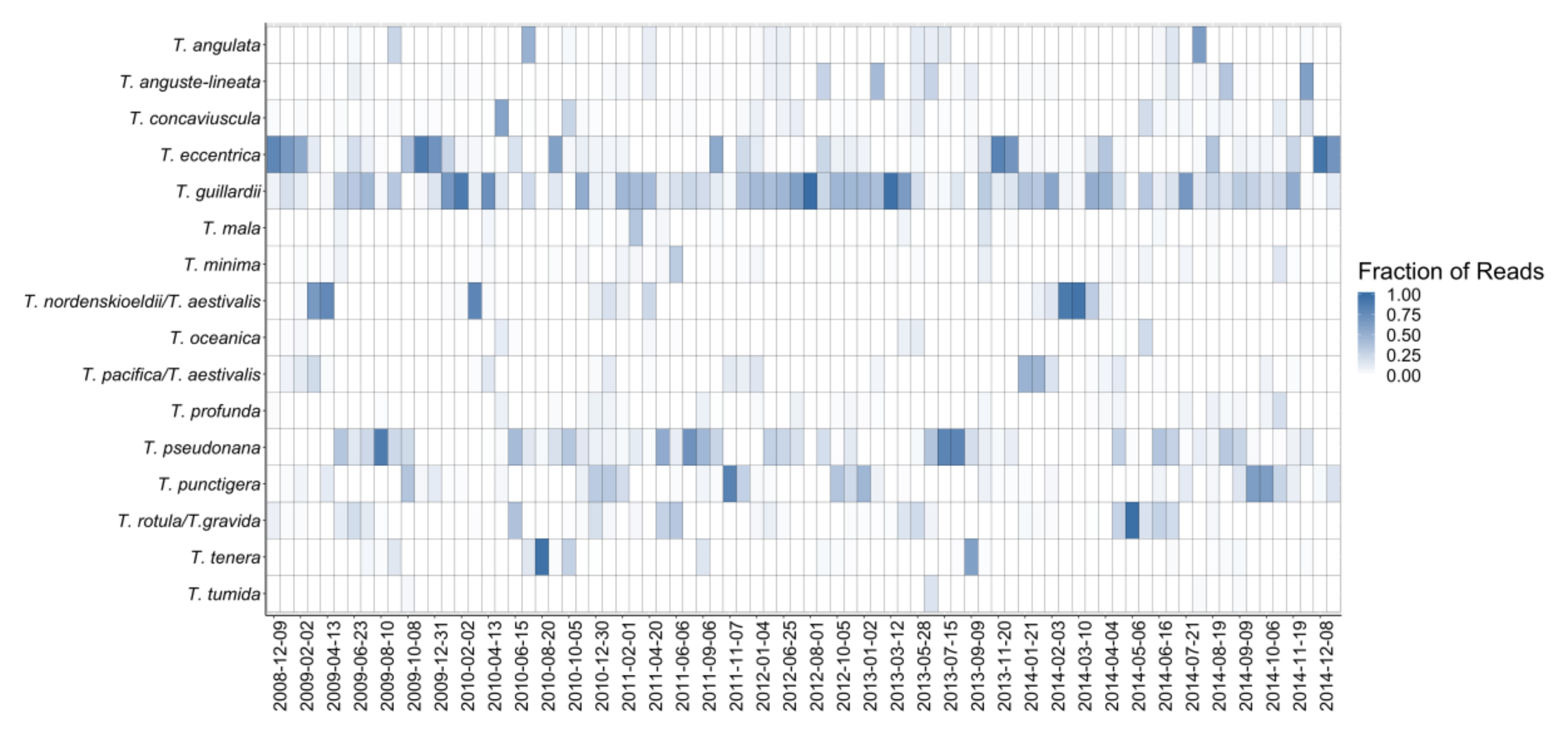
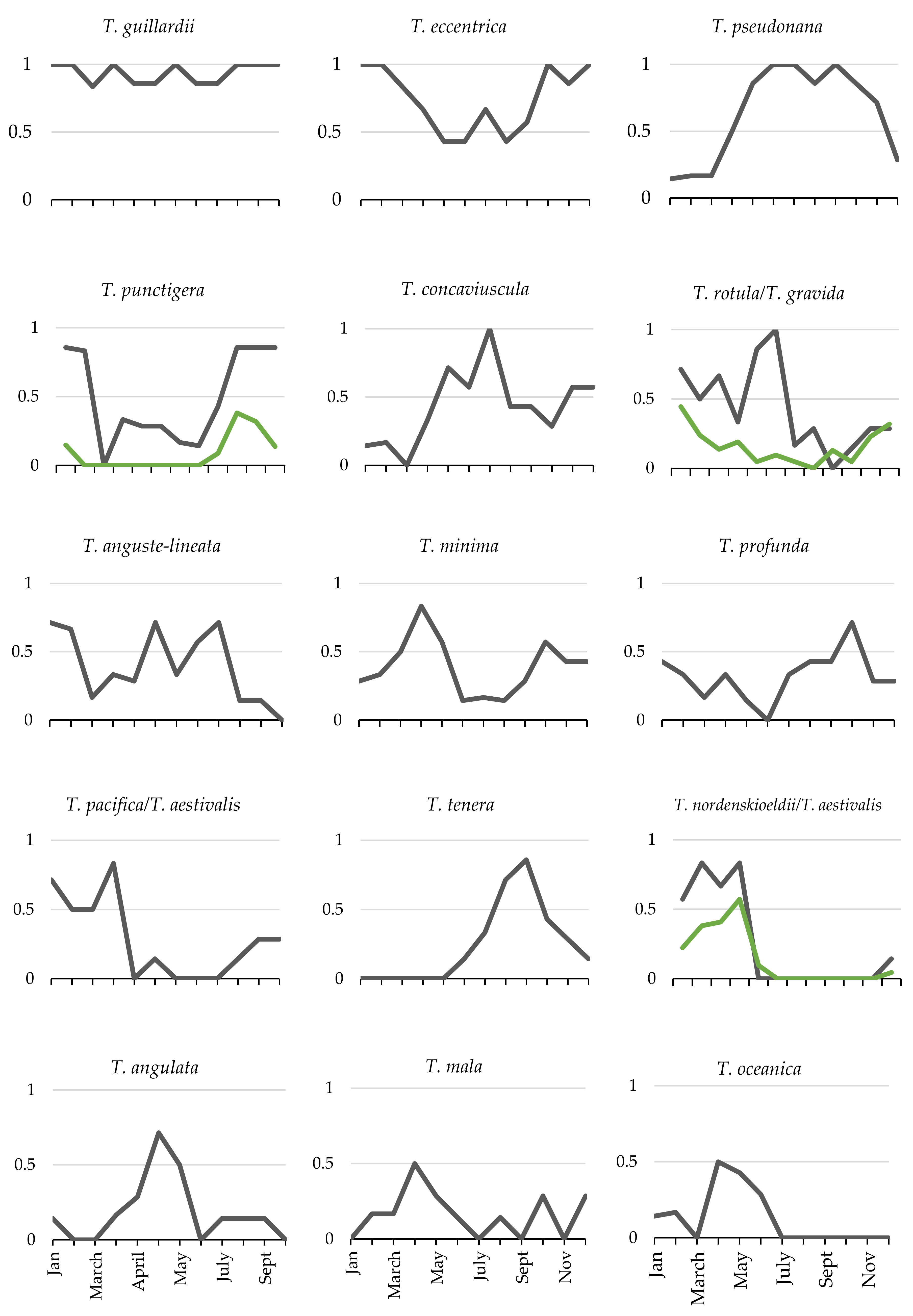
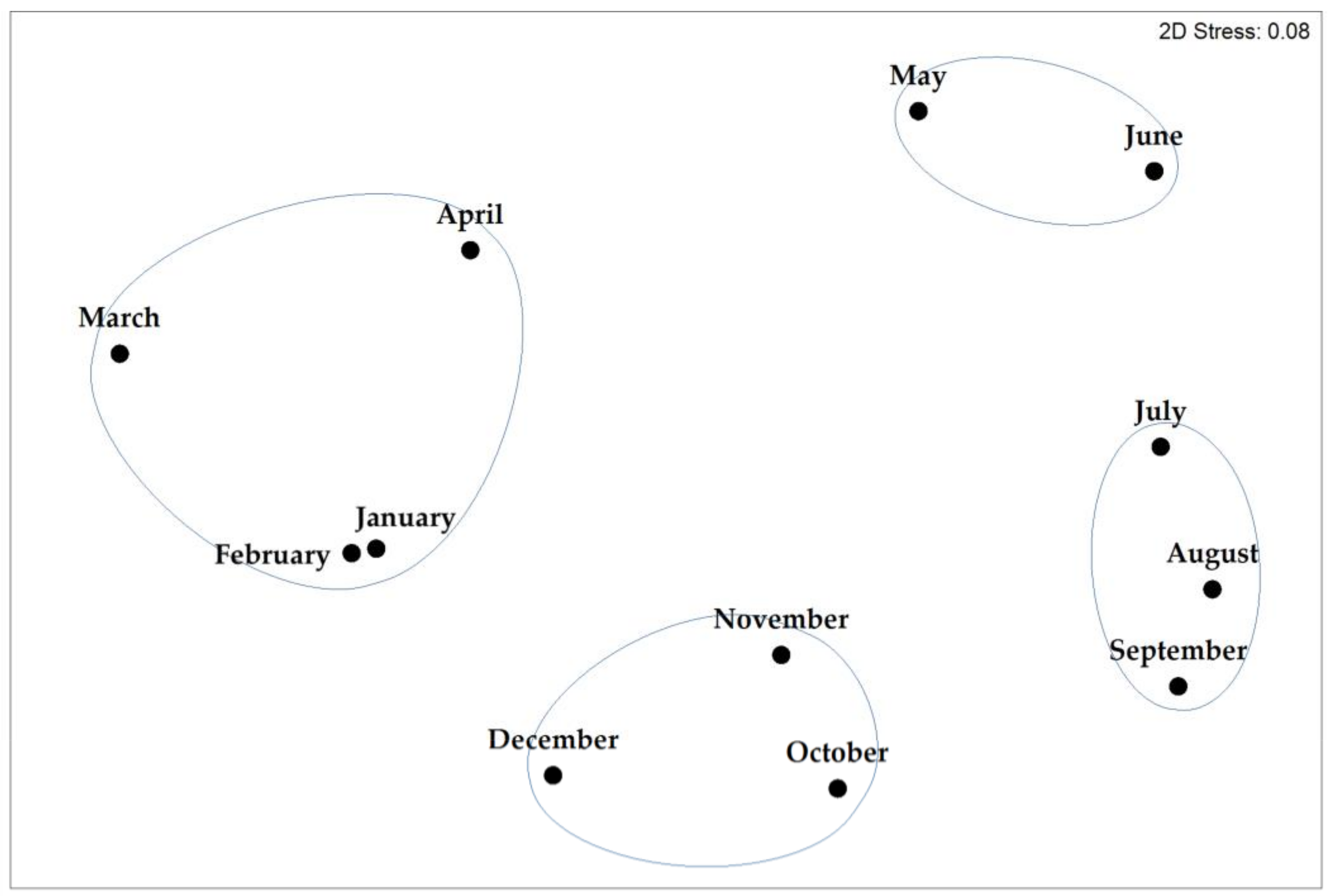
3.4. Seasonality of Thalassiosira Community Composition
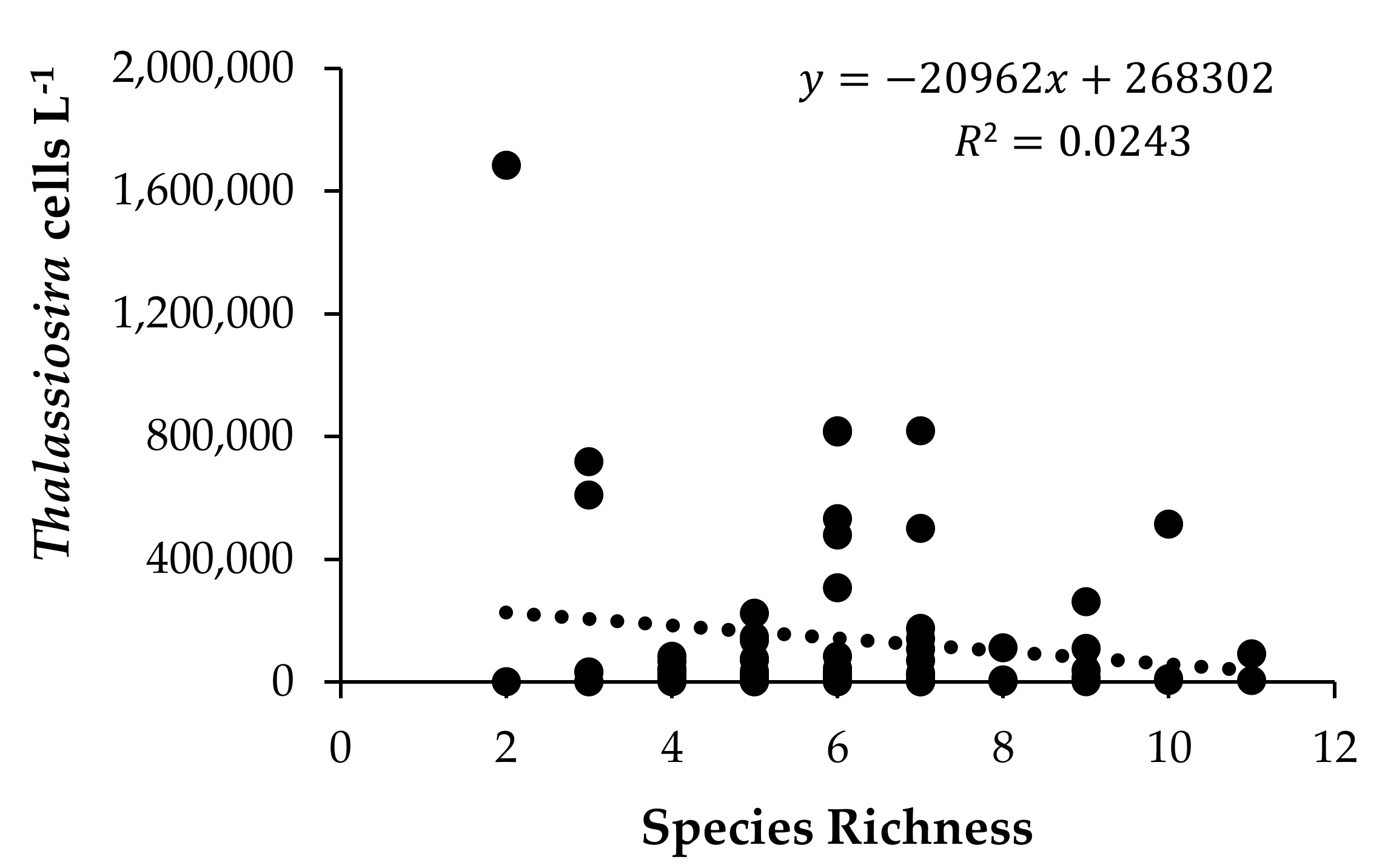
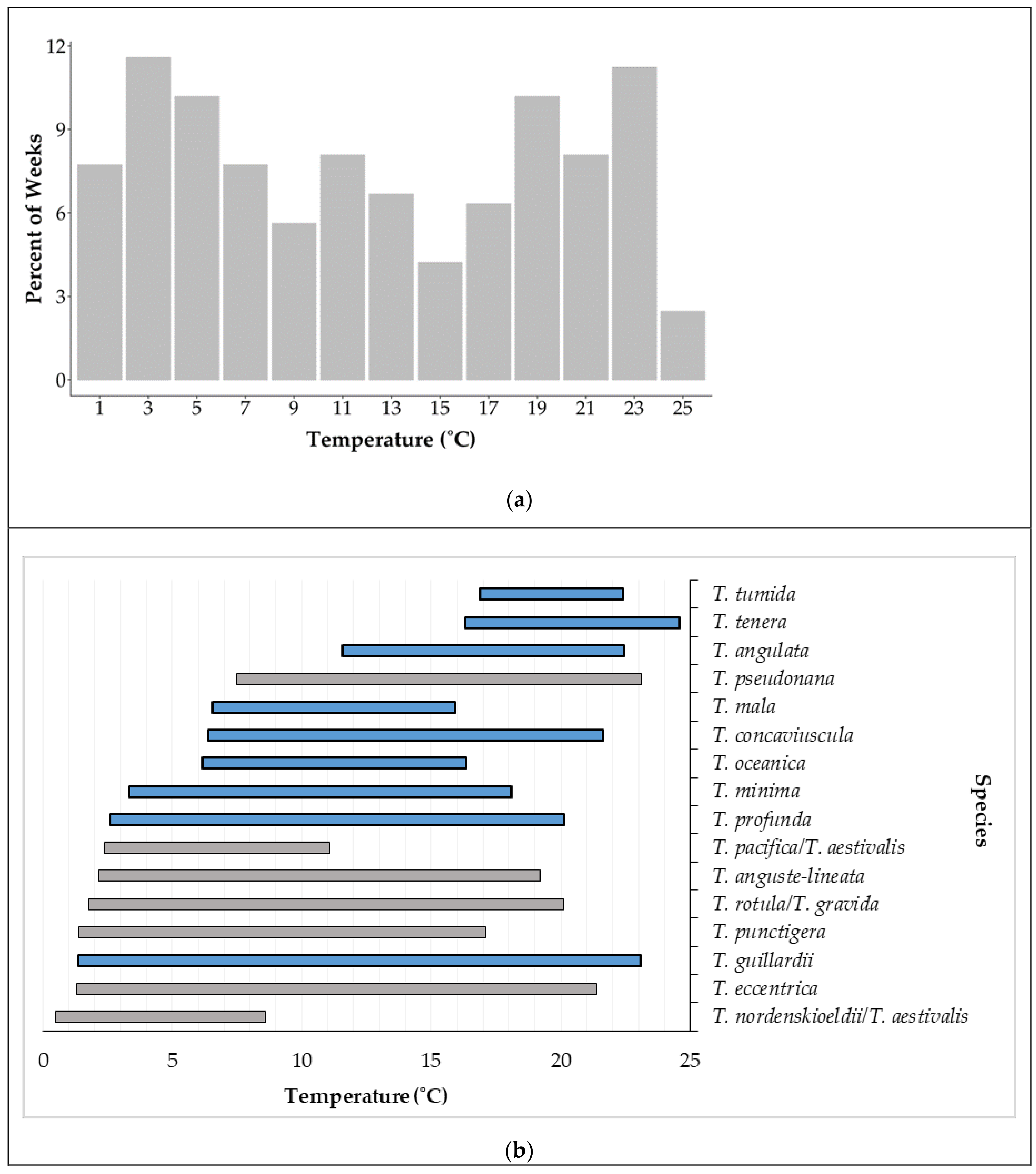
3.5. Environmental Factors Associated With Species Occurrence
4. Discussion
4.1. Utility of the 18S V4 Region for Species Identification in the Genus Thalassiosira
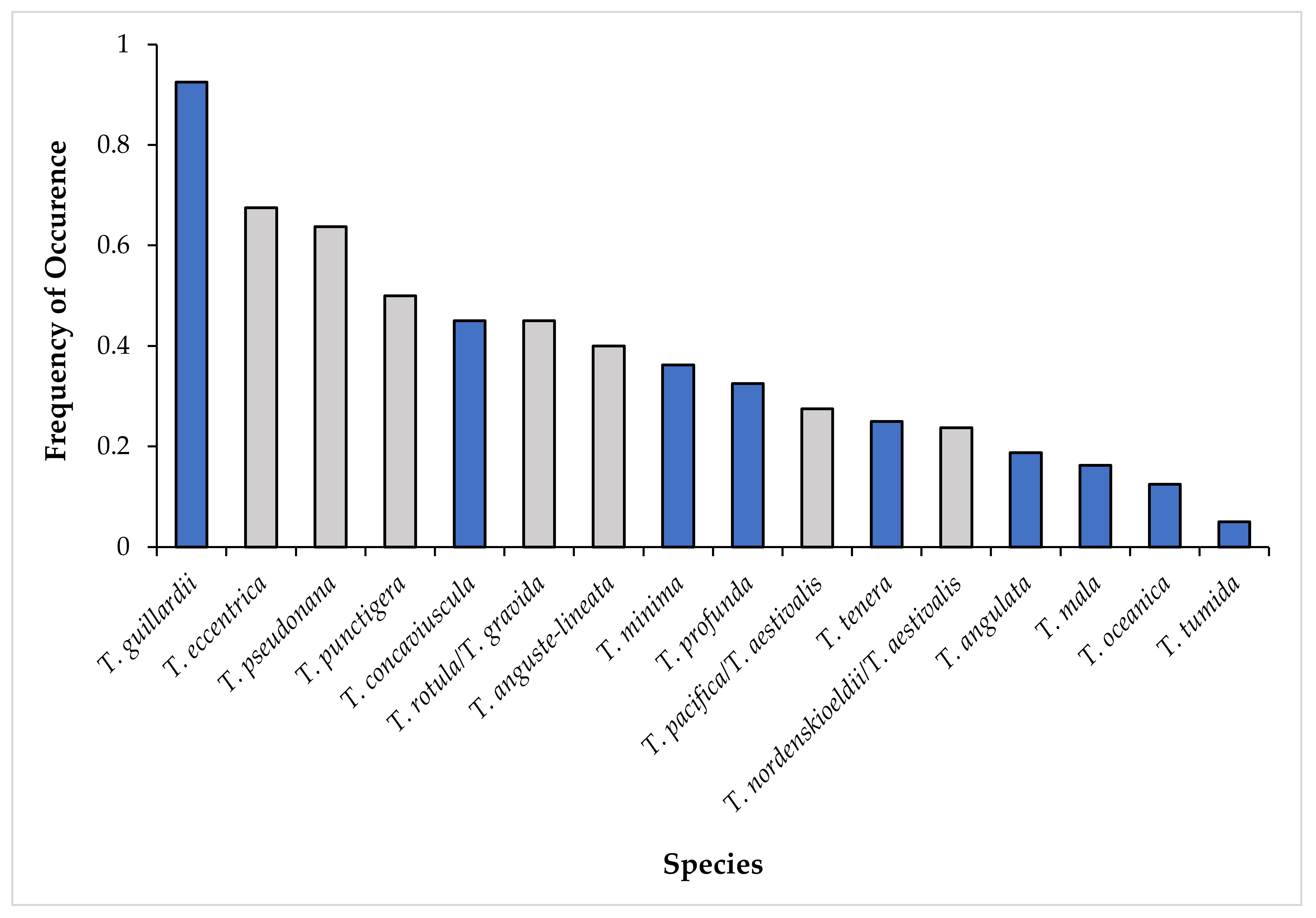
4.2. Species Diversity of Thalassiosira in Narragansett Bay
4.3. Seasonal Patterns of Species Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef]
- Tréguer, P.J.; De La Rocha, C.L. The world ocean silica cycle. Annu. Rev. Mar. Sci. 2013, 5, 477–501. [Google Scholar] [CrossRef]
- Rousseaux, C.; Gregg, W. Interannual variation in phytoplankton primary production at a global scale. Remote Sens. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Mann, D.G.; Droop, S.J.M. Biodiversity, biogeography and conservation of diatoms. Hydrobiologia 1996, 336, 19–32. [Google Scholar] [CrossRef]
- Mann, D.G.; Vanormelingen, P. An inordinate fondness? The number, distributions, and origins of diatom species. J. Eukaryot. Microbiol. 2013, 60, 414–420. [Google Scholar] [CrossRef]
- Alexander, H.; Jenkins, B.D.; Rynearson, T.A.; Dyhrman, S.T. Metatranscriptome analyses indicate resource partitioning between diatoms in the field. Proc. Natl. Acad. Sci. USA 2015, 112, E2182–E2190. [Google Scholar] [CrossRef]
- Amato, A.; Kooistra, W.H.C.F.; Levialdi Ghiron, J.H.; Mann, D.G.; Proschold, T.; Montresor, M. Reproductive isolation among sympatric cryptic species in marine diatoms. Protist 2007, 158, 193–207. [Google Scholar] [CrossRef]
- Beszteri, B.; Acs, E.; Medlin, L.K. Ribosomal DNA sequence variation among sympatric strains of the Cyclotella meneghiniana complex (Bacillariophyceae) reveals cryptic diversity. Protist 2005, 156, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, K.; Rignanese, D.; Olson, R.; Rynearson, T. Molecular subdivision of the marine diatom Thalassiosira rotula in relation to geographic distribution, genome size, and physiology. BMC Evol. Biol. 2012, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Hamsher, S.; LeGresley, M.; Martin, J.; Saunders, G. A comparison of morphological and molecular-based surveys to estimate the species richness of Chaetoceros and Thalassiosira (Bacillariophyta), in the Bay of Fundy. PLoS ONE 2013, 8, e73521. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, K.; Aristegui, J.; Armand, L.; Assmy, P.; Beker, B.; Bode, A.; Breton, E.; Cornet, V.; Gibson, J.; Gosselin, M.P.; et al. A global diatom database-abundance, biovolume and biomass in the world ocean. Earth Syst. Sci. Data 2012, 4, 149–165. [Google Scholar] [CrossRef]
- Pratt, D.M. The winter-spring diatom flowering in Narragansett Bay. Limnol. Oceanogr. 1965, 10, 173–184. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Beszteri, B.; Drebes, G.; Halliger, H.; Van Beusekom, J.E.E.; Janisch, S.; Wiltshire, K.H. Thalassiosira species (Bacillariophyceae, Thalassiosirales) in the North Sea at Helgoland (German Bight) and Sylt (North Frisian Wadden Sea) – a first approach to assessing diversity. Eur. J. Phycol. 2007, 42, 271–288. [Google Scholar] [CrossRef]
- Yoshie, N.; Suzuki, K.; Kuwata, A.; Nishioka, J.; Saito, H. Temporal and spatial variations in photosynthetic physiology of diatoms during the spring bloom in the western subarctic Pacific. Mar. Ecol. Prog. Ser. 2010, 399, 39–52. [Google Scholar] [CrossRef]
- Muylaert, K.; Sabbe, K. The diatom genus Thalassiosira (Bacillariophyta) in the estuaries of the Schelde (Belgium/The Netherlands) and the Elbe (Germany). Bot. Mar. 1996, 36, 103–115. [Google Scholar] [CrossRef]
- Harris, A.; Medlin, L.; Lewis, J.; Jones, K. Thalassiosira species (Bacillariophyceae) from a Scottish sea-loch. Eur. J. Phycol. 1995, 30, 117–131. [Google Scholar] [CrossRef]
- Aizawa, C.; Tanimoto, M.; Jordan, R. Living diatom assemblages from North Pacific and Bering Sea surface waters during summer 1999. Deep Sea Res. II 2005, 52, 2186–2205. [Google Scholar] [CrossRef]
- Kaczmarska, I.; Beaton, M.; Benoit, A.C.; Medlin, L.K. Molecular phylogeny of selected members of the order Thalassiosirales (Bacillariophyta) and evolution of the fultoportula. J. Phycol. 2006, 42, 121–138. [Google Scholar] [CrossRef]
- Hasle, G.R. Some freshwater and brackish water species of the diatom genus Thalassiosira Cleve. Phycologia 1978, 17, 263–292. [Google Scholar] [CrossRef]
- Rodriguez-Ramos, T.; Dornelas, M.; Maranon, E.; Cermeno, P. Conventional sampling methods severely underestimate phytoplankton species richness. J. Plankton Res. 2013, 36, 334–343. [Google Scholar] [CrossRef]
- Canesi, K.; Rynearson, T.A. Temporal variation of Skeletonema community composition from a long-term time series in Narragansett Bay identified using high-throughput sequencing. Mar. Ecol. Prog. Ser. 2016, 556, 1–16. [Google Scholar] [CrossRef]
- De Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahé, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348. [Google Scholar] [CrossRef] [PubMed]
- Endo, H.; Ogata, H.; Suzuki, K. Contrasting biogeography and diversity patterns between diatoms and haptophytes in the central Pacific Ocean. Sci. Rep. UK 2018, 8, 10916. [Google Scholar] [CrossRef] [PubMed]
- Piredda, R.; Claverie, J.-M.; Decelle, J.; de Vargas, C.; Dunthorn, M.; Edvardsen, B.; Eikrem, W.; Forster, D.; Kooistra, W.H.C.F.; Logares, R.; et al. Diatom diversity through HTS-metabarcoding in coastal European seas. Sci. Rep. 2018, 8, 18059. [Google Scholar] [CrossRef] [PubMed]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; de Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef] [PubMed]
- Narragansett Bay Long-Term Plankton Time Series. Available online: https://web.uri.edu/gso/research/plankton/ (accessed on 29 May 2016).
- Smayda, T.J.; The Bunker C Community. Narragansett Bay Plankton Time Series (1959–1997). Available online: http://www.nabats.org/nabats-phytoplankton-data.html (accessed on 1 November 2019).
- Karentz, D.; Smayda, T.J. Temperature and seasonal occurrence patterns of 30 dominant phytoplankton species in Narragansett Bay over a 22-year period (1959—1980). Mar. Ecol. Prog. Ser. 1984, 18, 277–293. [Google Scholar] [CrossRef]
- Tomas, C.R. Identifying Marine Phytoplankton; Academic Press: New York, NY, USA, 1997; p. 858. [Google Scholar]
- Belcher, J.; Swale, E. Notes on some small Thalassiosira species (Bacillariophyceae) from the plankton of the lower Thames and other British Estuaries (identified by transmission electron microscopy). Br. Phycol. J. 1986, 21, 139–145. [Google Scholar] [CrossRef]
- Hasle, G.R.; Fryxell, G. The genus Thalassiosira: Some species with a linear areola array. Beih. Noca Hedwig. 1977, 54, 15–66. [Google Scholar]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Eren, A.M.; Morrison, H.G.; Lescault, P.J.; Reveillaud, J.; Vineis, J.H.; Sogin, M.L. Minimum entropy decomposition: Unsupervised oligotyping for sensitive partitioning of high-throughput marker gene sequences. ISME J. 2015, 9, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Meth. 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of phyml 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- University of Rhode Island Marine Ecosystems Research laboratory. Available online: http://www.gso.uri.edu/merl/data.htm (accessed on 29 May 2016).
- National Estuarine Research Reserve Centralized Data Management Office. Available online: http://cdmo.baruch.sc.edu/ (accessed on 29 May 2016).
- Zhu, F.; Massana, R.; Not, F.; Marie, D.; Vaulot, D. Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol. Ecol. 2005, 52, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Mäki, A.; Salmi, P.; Mikkonen, A.; Kremp, A.; Tiirola, M. Sample preservation, DNA or RNA extraction and data analysis for high-throughput phytoplankton community sequencing. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Maria Trigueros, J.; Orive, E. Seasonal variations of diatoms and dinoflagellates in a shallow, temperate estuary, with emphasis on neritic assemblages. Hydrobiologia 2001, 444, 119–133. [Google Scholar] [CrossRef]
- Hasle, G.R. The biogeography of some marine planktonic diatoms. Deep Sea Res. 1976, 23, 319–338. [Google Scholar]
- Leblanc, K.; Quéguiner, B.; Diaz, F.; Cornet, V.; Michel-Rodriguez, M.; Durrieu de Madron, X.; Bowler, C.; Malviya, S.; Thyssen, M.; Grégori, G.; et al. Nanoplanktonic diatoms are globally overlooked but play a role in spring blooms and carbon export. Nat. Commun. 2018, 9, 953. [Google Scholar] [CrossRef]
- Marshall, H.G.; Lacouture, R.V.; Buchanan, C.; Johnson, J.M. Phytoplankton assemblages associated with water quality and salinity regions in Chesapeake Bay, USA. Estuar. Coast. Shelf Sci. 2006, 69, 10–18. [Google Scholar] [CrossRef]
- Hevia-Orube, J.; Orive, E.; David, H.; Díez, A.; Laza-Martínez, A.; Miguel, I.; Seoane, S. Molecular and morphological analyses of solitary forms of brackish Thalassiosiroid diatoms (Coscinodiscophyceae), with emphasis on their phenotypic plasticity. Eur. J. Phycol. 2016, 51, 11–30. [Google Scholar] [CrossRef]
- Armbrust, E.V.; Berges, J.A.; Bowler, C.; Green, B.R.; Martinez, D.; Putnam, N.H.; Zhou, S.G.; Allen, A.E.; Apt, K.E.; Bechner, M.; et al. The genome of the diatom Thalassiosira pseudonana: Ecology, evolution, and metabolism. Science 2004, 306, 79–86. [Google Scholar] [CrossRef]
- Thomas, M.K.; Kremer, C.T.; Klausmeier, C.A.; Litchman, E. A global pattern of thermal adaptation in marine phytoplankton. Science 2012, 338, 1085–1088. [Google Scholar] [CrossRef]
- Durbin, E.G. Studies on the autoecology of the marine diatom Thalassiosira nordenskioeldii Cleve. 1. The influence of daylength, light intensity, and temperature on growth. J. Phycol. 1974, 10, 220–225. [Google Scholar] [CrossRef]
- Fulweiler, R.W.; Oczkowski, A.J.; Miller, K.M.; Oviatt, C.A.; Pilson, M.E.Q. Whole truths vs. half truths – And a search for clarity in long-term water temperature records. Estuar. Coast. Shelf Sci. 2015, 157, A1–A6. [Google Scholar] [CrossRef]
- Borkman, D.G.; Smayda, T.J. Gulf Stream position and winter NAO as drivers of long-term variations in the bloom phenology of the diatom Skeletonema costatum “species-complex” in Narragansett Bay, RI, USA. J. Plankton. Res. 2009, 31, 1407–1425. [Google Scholar] [CrossRef]
- Lawrence, C.M.; Menden-Deuer, S. Drivers of protistan grazing pressure: Seasonal signals of plankton community composition and environmental conditions. Mar. Ecol. Prog. Ser. 2012, 459, 39–52. [Google Scholar] [CrossRef]
- Deason, E.; Smayda, T. Ctenophore-zooplankton-phytoplankton interactions in Narragansett Bay, Rhode Island, USA, during 1972-1977. J. Plankton. Res. 1981, 4, 203–217. [Google Scholar] [CrossRef]
- McQuoid, M.R.; Hobson, L.A. Diatom resting stages. J. Phycol. 1996, 32, 889–902. [Google Scholar] [CrossRef]
- McQuoid, M.R. Influence of salinity on seasonal germination of resting stages and composition of microplankton on the Swedish west coast. Mar. Ecol. Prog. Ser. 2005, 289, 151–163. [Google Scholar] [CrossRef]
| Species | Cell Diameter (µm) | Geographic Range | LM 1959–1997 | LM 1999–2014 | HTS 2008–2014 |
|---|---|---|---|---|---|
| T. aestivalis | 14–56 | Warm to temperate | 7 | * | |
| T. angulata | 12–39 | Cold to temperate 1 | x | ||
| T. anguste-lineata | 14–78 | Cosmopolitan | 1 | x | |
| T. concaviuscula | 14–56 1 | Neritic, cold to temperate 1 | x | ||
| T. decipiens | 9–40 | Cold to temperate | x | - | |
| T. eccentrica | 15–110 | Cosmopolitan, except polar zones | 2 | 1 | x |
| T. gravida | 17–62 | Cold to temperate | x | * | * |
| T. guillardii | 4–14 2 | Marine and brackish 2 | x | ||
| T. hyalina | 16–45 | Cold to temperate | 1 | ||
| T. mala | 4–10 | Warm to temperate | x | ||
| T. minima | 5–15 | Cosmopolitan, except polar zones | x | ||
| T. nordenskioeldii | 10–50 | Cold to temperate | x | x | * |
| T. oceanica | 3–12 | Mainly warm waters | x | ||
| T. pacifica | 7–46 | Cosmopolitan, except polar zones | 9 | * | |
| T. profunda | 1.8–5 3 | Cosmopolitan 2 | x | ||
| T. pseudonana | 2.3–5.5 | Cosmopolitan | x | x | |
| T. punctigera | 40–186 | Warm to temperate | 8 | x | x |
| T. rotula | 8–55 | Cosmopolitan | x | * | * |
| T. subtilis | 15–32 | Warm to temperate | 5 | - | |
| T. tenera | 10–29 | Cosmopolitan 4 | x | ||
| T. tumida | 21–137 | Southern cold water region | x | ||
| T. spp | x | x |
| Environmental Variable | Range | Median |
|---|---|---|
| Sea surface temperature (°C) | 0.5–24.6 | 13.9 |
| Sea surface salinity | 18.60–32.39 | 29.93 |
| Average daily PAR (mmol m−2) | 16.21–572.05 | 247.87 |
| Dissolved inorganic nitrogen (µM) | 0.14–15.80 | 2.61 |
| Dissolved inorganic phosphorus (µM) | 0.04–1.87 | 0.72 |
| Silicate (µM) | 0.05–38.12 | 11.46 |
| Chlorophyll a (μg L−1) | 0.23–29.80 | 5.14 |
| Correlation | Environmental Variables |
|---|---|
| 0.312 | Surface Temperature |
| 0.309 | Surface Temperature, DIP |
| 0.298 | Surface Temperature, Si |
| 0.296 | Surface Temperature, Surface Salinity |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rynearson, T.A.; Flickinger, S.A.; Fontaine, D.N. Metabarcoding Reveals Temporal Patterns of Community Composition and Realized Thermal Niches of Thalassiosira Spp. (Bacillariophyceae) from the Narragansett Bay Long-Term Plankton Time Series. Biology 2020, 9, 19. https://doi.org/10.3390/biology9010019
Rynearson TA, Flickinger SA, Fontaine DN. Metabarcoding Reveals Temporal Patterns of Community Composition and Realized Thermal Niches of Thalassiosira Spp. (Bacillariophyceae) from the Narragansett Bay Long-Term Plankton Time Series. Biology. 2020; 9(1):19. https://doi.org/10.3390/biology9010019
Chicago/Turabian StyleRynearson, Tatiana A., Sarah A. Flickinger, and Diana N. Fontaine. 2020. "Metabarcoding Reveals Temporal Patterns of Community Composition and Realized Thermal Niches of Thalassiosira Spp. (Bacillariophyceae) from the Narragansett Bay Long-Term Plankton Time Series" Biology 9, no. 1: 19. https://doi.org/10.3390/biology9010019
APA StyleRynearson, T. A., Flickinger, S. A., & Fontaine, D. N. (2020). Metabarcoding Reveals Temporal Patterns of Community Composition and Realized Thermal Niches of Thalassiosira Spp. (Bacillariophyceae) from the Narragansett Bay Long-Term Plankton Time Series. Biology, 9(1), 19. https://doi.org/10.3390/biology9010019




