The Gametic Non-Lethal Gene Gal on Chromosome 5 Is Indispensable for the Transmission of the Co-Induced Semidwarfing Gene d60 in Rice
Abstract
1. Introduction
2. Materials and Methods
2.1. Genetic Analysis of d60 and Gal
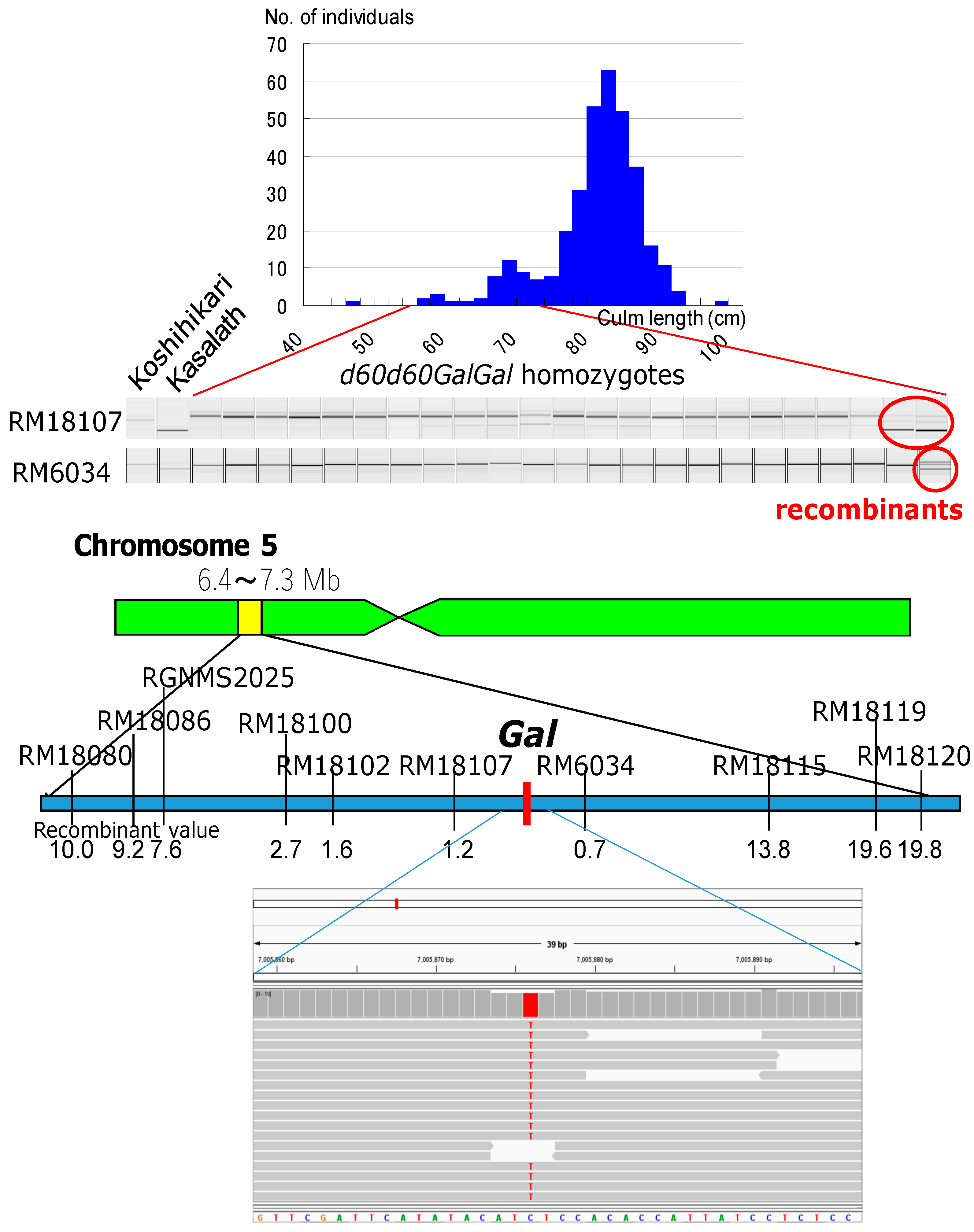
2.2. Genetic Mapping
2.3. NGS Analysis
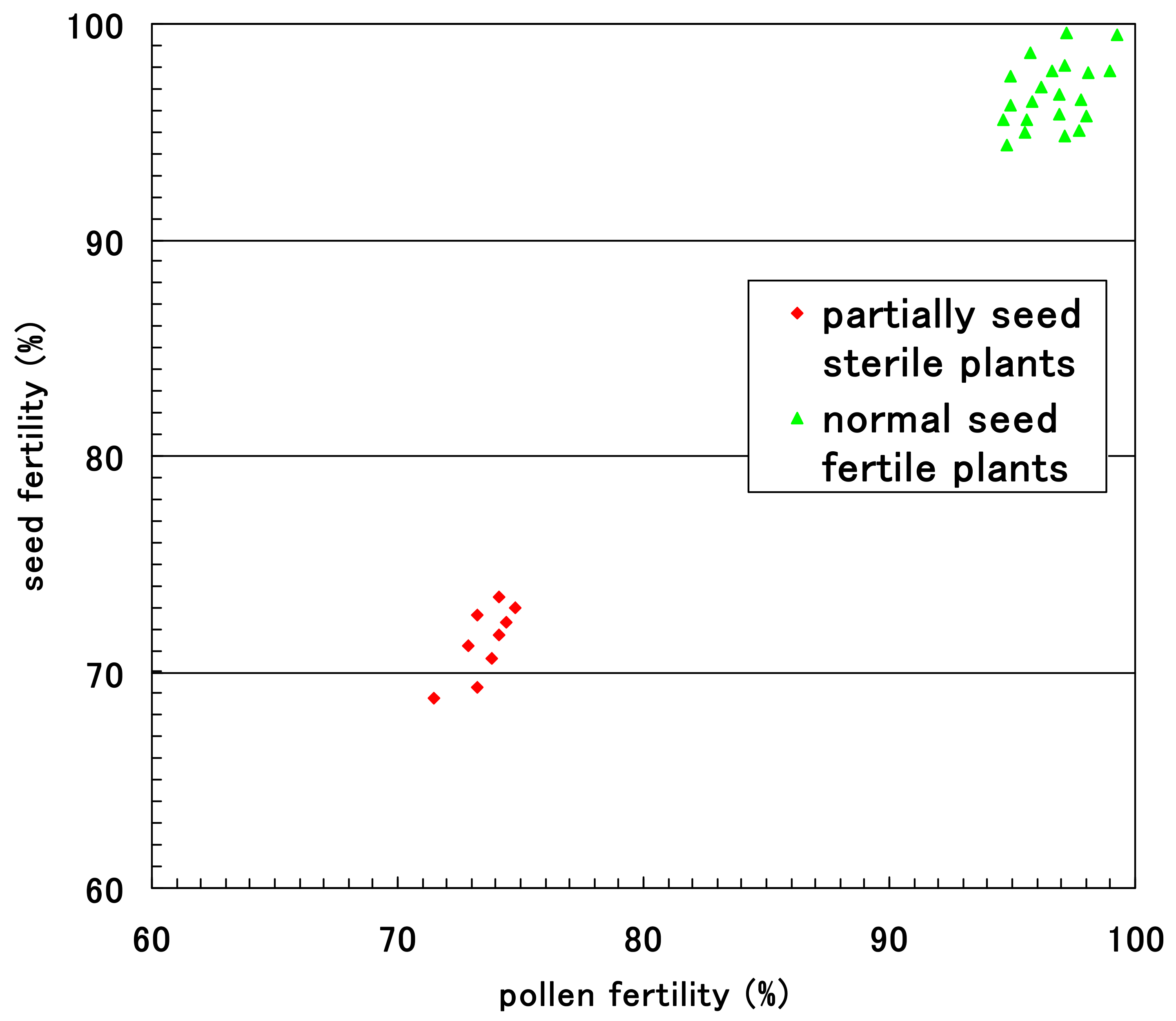
2.4. Pollen Fertility
3. Results
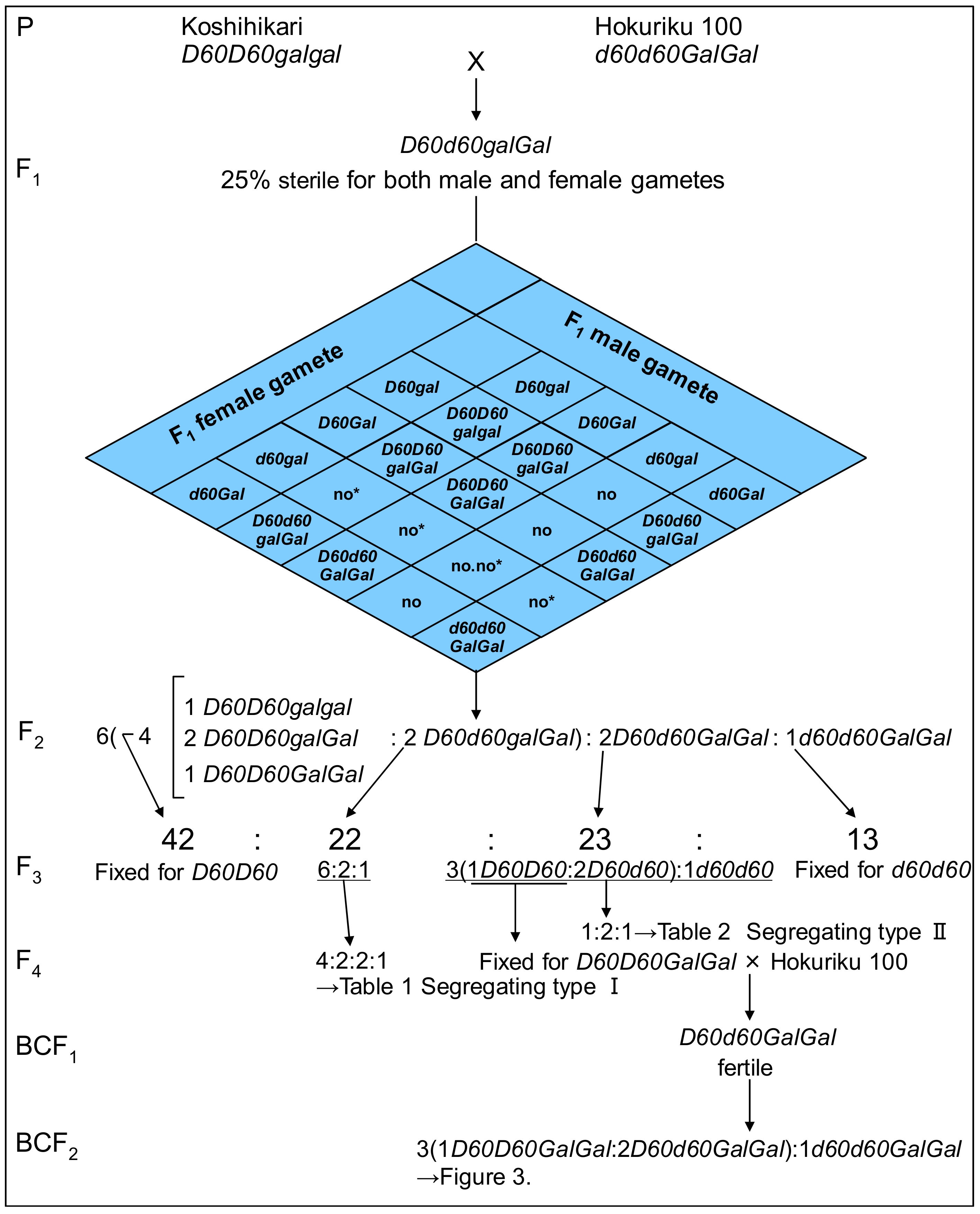
3.1. Genotyping of d60 and Gal Loci through F1 to F4
3.2. d60 is Inherited as a Single Recessive Gene in the Non-Gamete Lethal Gal-Homozygous Background
3.3. Genetic Mapping of Gal Loci
3.4. Identification of Gal Responsible SNP by NGS Analysis
3.5. Coexistence of d60 and Gal Lose Vegetative Nuclei but Two Generative Nuclei
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khush, G.S. Green revolution: Preparing for the 21st century. Genome 1999, 42, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Hergrove, T.; Coffman, W.R. Breeding History. In Rice that Changed the World: Cerebrating 50 Years of IR8; Rice Today: Laguna, Philippines, 2016; pp. 6–10. [Google Scholar]
- Jennings, P. Rice revolutions in Latin America. In Rice that Changed the World: Cerebrating 50 Years of IR8; Rice Today: Laguna, Philippines, 2016; p. 19. [Google Scholar]
- Cho, Y.G.; Eun, M.Y.; Kim, Y.K.; Chung, T.Y.; Chae, Y.A. The semidwarf gene, sd-1, of rice (Oryza sativa L). 1. Linkage with the esterase locus, EstI-2. Theor. Appl. Genet. 1994, 89, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.G.; Eun, M.Y.; McCouch, S.R.; Chae, Y.A. The semidwarf gene, sd-1, of rice (Oryza sativa L.) 2 Molecular mapping and marker-assisted selection. Theor. Appl. Genet. 1994, 89, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Ishii, T.; Mori, H.; Kuroda, J.; Horimoto, M.; Takamure, I.; Kinoshita, T.; Kamijima, O. High density molecular map of semidwarfing gene, sd-1, in rice (Oryza sativa L.). Breed. Sci. 1997, 47, 317–320. [Google Scholar] [CrossRef]
- Sasaki, A.; Ashikari, M.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Swapan, D.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Green revolution: A mutant gibberellin-synthesis gene in rice. Nature 2002, 416, 701–702. [Google Scholar] [CrossRef]
- Monna, L.; Kitazawa, N.; Yoshino, R.; Suzuki, J.; Masuda, H.; Maehara, Y.; Tanji, M.; Sato, M.; Nasu, S.; Minobe, Y. Positional cloning of rice semidwarfing gene, sd-1: Rice “Green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Res. 2002, 9, 11–17. [Google Scholar] [CrossRef]
- Spielmeyer, W.; Ellis, M.H.; Chandler, P.M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 2002, 99, 9043–9048. [Google Scholar] [CrossRef]
- Ashikari, M.; Sasaki, A.; Ueguchi-Tanaka, M.; Itoh, H.; Nishimura, A.; Datta, S.; Ishiyama, K.; Saito, T.; Kobayashi, M.; Khush, G.S.; et al. Loss-of-function of a rice gibberellin biosynthetic gene, GA20 oxidase (GA20ox-2), led to the rice ‘green revolution’. Breed. Sci. 2002, 52, 143–150. [Google Scholar] [CrossRef]
- Hedden, P. Constructing dwarf rice. Nat. Biotechnol. 2003, 21, 873–874. [Google Scholar] [CrossRef]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Tomita, M.; Ishii, K. Genetic performance of the semidwarfing allele sd1 derived from a Japonica rice cultivar and minimum requirements to detect its single-nucleotide polymorphism by MiSeq whole-genome sequencing. BioMed. Res. Int. 2018, 2018, 4241725. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.W.; Rutger, J.N. Inheritance of semidwarfism in rice, Oryza sativa L. Genetics 1978, 88, 559–574. [Google Scholar] [PubMed]
- Kikuchi, F.; Itakura, N.; Ikehashi, H.; Yokoo, M.; Nakane, A.; Maruyama, K. Genetic analysis of semidwarfism in high-yielding rice varieties in Japan. Bull. Natl. Inst. Agric. Sci. 1985, 36, 125–145. [Google Scholar]
- Kikuchi, F.; Futsuhara, Y. Inheritance of Morphological Characters 2, Inheritance of Semidwarf. In Science of the Rice Plant; Matsuo, T., Shimizu, S., Tsunoda, S., Murata, Y., Kumazawa, K., Futsuhara, Y., Hoshikawa, K., Yamaguchi, H., Kikuchi, F., Eds.; Tokyo Food and Agricultural Policy Research Center: Tokyo, Japan, 1997; Volume 3, pp. 309–317. [Google Scholar]
- Itoh, H.; Tatsumi, T.; Sakamoto, T.; Otomo, K.; Toyomasu, T.; Kitano, H.; Ashikari, M.; Ichihara, S.; Matsuoka, M. A rice semi-dwarf gene, Tan-Ginbozu (D35), encodes the gibberellin biosynthesis enzyme, ent-kaurene oxidase. Plant Mol. Biol. 2004, 54, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Jin, Y.; Liu, W.; Li, F.; Fang, J.; Yin, Y.; Qian, Q.; Zhu, L.; Chu, C. DWARF AND LOW-TILLERING, a new member of the GRAS family, plays positive roles in brassinosteroid signaling in rice. Plant J. 2009, 58, 803–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Li, S.; Cheng, Z.; Li, C. The rice semi-dwarf mutant sd37, caused by a mutation in CYP96B4, plays an important role in the fine-tuning of plant growth. PLoS ONE 2014, 9, e88068. [Google Scholar] [CrossRef]
- Samoto, S.; Kanai, D. Studies on the mutation breeding in rice. I. Short stiff mutations induced by gamma-ray irradiation to the rice variety Koshihikari. Jpn. J. Breed. 1975, 12, 1–7. [Google Scholar] [CrossRef][Green Version]
- Tomita, M.; Tanisaka, T.Y.; Yamagata, H. Linkage Analysis for the Gametic Lethal Gene of Rice Variety Koshihikari and the Semidwarf Gene Induced in Koshihikari. In The Key to the Survival of the Earth, Proceedings of the 6th International Congress of the Society for the Advancement of Breeding Researches in Asia and Oceania (SABRAO), Tsukuba, Japan, 21–25 August 1989; Iyama, S., Takeda, G., Eds.; FAO/IAEA Division of Nuclear Techniques in Food and Agriculture: Vienna, Austria, 1990; pp. 345–348. [Google Scholar]
- Tanisaka, T.; Tomita, M.; Yamagata, H. Gene analysis for the semidwarfism of two mutant strains, Hokuriku 100 and Kanto 79, induced from a rice variety Koshihikari. Studies on on the utility of artificial mutations in plant breeding XVIII. Jpn. J. Breed. 1990, 40, 103–117. [Google Scholar] [CrossRef]
- Tomita, M. Combining two semidwarfing genes d60 and sd1 for reduced height in ‘Minihikari’, a new rice germplasm in the ‘Koshihikari’ genetic background. Genet. Res. 2012, 94, 235–244. [Google Scholar] [CrossRef]
- Kihara, H.; Hirayoshi, I. Development of pollens in Oryza sativa L. Agric. Hortic. 1942, 17, 685–689. [Google Scholar]
- Tomita, M.; Yamagata, H.; Tanisaka, T. Developmental Cytology on Gametic Abortion Caused by Induced Complementary Genes gal and d60 in Japonica Rice. In Advances in Rice Genetics, Proceedings of the International Rice Genetics Symposium, Manila, Philippines, 27–31 May 1985; International Rice Research Institute: Manila, Philippines, 2003; pp. 178–181. [Google Scholar]
- Kato, S.; Kosaka, H.; Hara, S. Classification of improved rice varieties. J. Kyushu Imp. Univ. Agric. 1928, 3, 132–147. [Google Scholar]
- Kato, S. On the affinity of rice varieties as shown by the fertility of rice plants. Cent. Agric. Inst. Kyushu Imp. Univ. 1930, 2, 241–276. [Google Scholar]
- Oka, H.I. Genic analysis for the sterility of hybrids between distantly related varieties of cultivated rice. J. Genet. 1957, 55, 397–409. [Google Scholar] [CrossRef]
- Oka, H.I. Origin of Cultivated Rice; Japan Science Social Press/Elsevier: Tokyo, Japan, 1988. [Google Scholar]
- Oka, H.I. The mechanism of sterility in the intervarietal hybrid. (Phylogenetic differentiation of the cultivated rice plant. VI.). Jpn. J. Breed. 1953, 2, 217–224. [Google Scholar] [CrossRef][Green Version]
- Oka, H.I. Analysis of genes controlling F1 sterility in rice by the use of isogenic lines. Genetics 1974, 77, 521–534. [Google Scholar]
- Kitamura, E. Genetic studies on sterility observed in hybrids between distantly related varieties of rice, Oryza sativa L. Bull. Chugoku Agric. Exp. Sta. Ser. A 1962, 8, 141–205. [Google Scholar]
- Oka, H.I. Considerations on the Genetic Basis of Intervarietal Sterility in Oryza sativa. In Rice Genetics and Cytogenetics; Elsevier: Amsterdam, The Netherlands, 1964; pp. 158–174. [Google Scholar]
- Ikehashi, H.; Araki, H. Varietal screening of compatibility types revealed in F1 fertility of distant crosses in rice. Jpn. J. Breed. 1984, 34, 304–313. [Google Scholar] [CrossRef]
- Ikehashi, H.; Araki, H. Genetics of F1 Sterility in Remote Crosses of Rice. In Rice Genetics, Proceedings of the International Rice Genetics Symposium, Manila, Philippines, 27–31 May 1985; International Rice Research Institute: Manila, Philippines, 1986; pp. 119–130. [Google Scholar]
- Ikehashi, H.; Araki, H. Multiple alleles controlling F1 sterility in remote crosses of rice (Oryza sativa). Jpn. J. Breed. 1988, 38, 283–291. [Google Scholar] [CrossRef]
- Ikehashi, H.; Araki, H.; Yanagihara, S. Screening and Analysis of Wide Compatibility Loci in Wide Crosses of Rice. In Rice Genetics II, Proceedings of the Second International Rice Genetics Symposium, Manila, Philippines, 14–18 May 1990; International Rice Research Institute: Manila, Philippines, 1991; pp. 33–43. [Google Scholar]
- Ikehashi, H.; Wan, J. Differentiation of Alleles at Seven Loci for Hybrid Sterility in Cultivated Rice (Oryza sativa L.). In Rice Genetics III, Proceedings of the Third International Rice Genetics Symposium, Manila, Philippines, 16–20 October 1995; Khush, G.S., Ed.; International Rice Research Institute: Manila, Philippines, 1996; pp. 404–408. [Google Scholar]
- Wan, J.; Yamaguchi, Y.; Kato, H.; Ikehashi, H. Two new loci for hybrid sterility in cultivated rice (Oryza sativa L.). Theor. Appl. Genet. 1996, 92, 183–190. [Google Scholar] [CrossRef]
- Sano, Y.; Sano, R.; Eiguchi, M.; Hirano, H.Y. Gamete eliminator adjacent to the wx locus as revealed by pollen analysis in rice. J. Hered. 1994, 85, 310–312. [Google Scholar] [CrossRef]
- Ikehashi, H. Theory and application for F1 fertility in remote cross of rice. Agric. Hortic. 1985, 60, 1099–1104. [Google Scholar]
- Ikehashi, H. Genetics of Hybrid Sterility in Wide Hybridization in Rice. In Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1991; Volume 14, pp. 113–127. [Google Scholar]
- Yuan, L.P. The Strategy of the Development of Hybrid Rice Breeding. In Current Status of Two Line Hybrid Rice Research; Yuan, L.P., Ed.; Agricultural Publishing Ltd.: Beijing, China, 1992; pp. 1–5. [Google Scholar]
- Yanagihara, S.; McCouch, S.R.; Ishikawa, K.; Ogi, Y.; Maruyama, K.; Ikehashi, H. Molecular analysis of the inheritance of the S-5 locus, conferring wide compatibility in Indica/Japonica hybrids of rice (Oryza sativa L.). Theor. Appl. Genet. 1995, 90, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.Q.; Liu, K.; Jiang, J.X.; Song, X.; Xu, C.G.; Li, X.H.; Zhang, Q. Delimitation of the rice wide compatibility gene S5n to a 40-kb DNA fragment. Theor. Appl. Genet. 2005, 111, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ding, J.; Ouyang, Y.; Du, H.; Yang, J.; Cheng, K.; Zhao, J.; Qiu, S.; Zhang, X.; Yao, J.; et al. A triallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 11436–11441. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, S.; Kato, H.; Ikehashi, H. A new locus for multiple alleles causing hybrid sterility between an Aus variety and Javanica varieties in rice (Oryza sativa L.). Jpn. J. Breed. 1992, 42, 793–801. [Google Scholar] [CrossRef]
- Wan, J.; Yanagihara, S.; Kato, H.; Ikehashi, H. Multiple alleles at a new locus hybrid sterility between a Korean Indica variety and a Javanica variety in rice (Oryza sativa L.). Jpn. J. Breed. 1993, 43, 507–516. [Google Scholar]
- Wan, J.; Ikehashi, H. Identification of a new locus S-16 causing hybrid sterility in native rice varieties (Oryza sativa L.) from Tai-hu Lake region and Yunnan Province, China. Breed. Sci. 1995, 45, 461–470. [Google Scholar] [CrossRef]
- Liu, K.D.; Wang, J.; Li, H.B.; Xu, C.G.; Liu, A.M.; Li, X.H.; Zhang, Q. A genome-wide analysis of wide compatibility in rice and the precise location of the S5 locus in the molecular map. Theor. Appl. Genet. 1997, 95, 809–814. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.D.; Xu, C.G.; Li, X.H.; Zhang, Q.F. The high level of wide compatibility of variety ‘Dular’ has a complex genetic basis. Theor. Appl. Genet. 1998, 97, 407–412. [Google Scholar] [CrossRef]
- Liu, Y.S.; Zhu, L.H.; Sun, J.S.; Chen, Y. Mapping QTLs for defective female gametophyte development in an inter-subspecific cross in Oryza sativa L. Theor. Appl. Genet. 2001, 102, 1243–1251. [Google Scholar] [CrossRef]
- Song, X.; Qiu, S.Q.; Xu, C.G.; Li, X.H.; Zhang, Q. Genetic dissection of embryo sac fertility, pollen fertility, and their contributions to spikelet fertility of intersubspecific hybrids in rice. Theor. Appl. Genet. 2005, 110, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Wang, C.; Zheng, T.; Zhao, Z.; Ikehashi, H.; Wan, J. A new gene located on chromosome 2 causing hybrid sterility in a remote cross of rice. Plant Breed. 2005, 124, 440–445. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Jiang, L.; Zhu, S.; Ikehashi, H.; Wan, J. Identification of a new hybrid sterility gene in rice (Oryza sativa L.). Euphytica 2006, 151, 331–337. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Lu, Y.G. Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa). I. Diallel analysis of the hybrid sterility among isogenic F1 sterile lines. Chin. J. Rice Sci. 1989, 3, 97–101. [Google Scholar]
- Zhang, G.Q.; Lu, Y.G. Genetic studies of the hybrid sterility in cultivated rice (Oryza sativa) II. A genic model for F1 pollen sterility. Acta Genet. Sin. 1993, 20, 222–228. [Google Scholar]
- Zhang, Q.F.; Shen, B.Z.; Dai, X.K.; Mei, M.H.; Maroof, M.A.S.; Li, Z.B. Using bulked extremes and recessive class to map genes for photoperiod-sensitive genic male-sterility in rice. Proc. Natl. Acad. Sci. USA 1994, 91, 8675–8679. [Google Scholar] [CrossRef]
- Zhang, R.I.; Xue, G.X.; Song, J.X.; Jiang, X.W. Abnormality in male organ development and fertility of photoperiod-sensitive genic male sterile rice plants under short day condition. Acta Bot. Sin. 1999, 41, 1317–1323. [Google Scholar]
- Li, W.T.; Zeng, R.Z.; Zhang, Z.M.; Zhang, G.Q. Mapping of S-b locus for F1 pollen sterility in cultivated rice using PCR based markers. Acta Bot. Sin. 2002, 44, 463–467. [Google Scholar]
- Wang, G.W.; He, Y.Q.; Xu, C.G.; Zhang, Q.F. Fine mapping of f5-Du, a gene conferring wide-compatibility for pollen fertility in inter-subspecific hybrids of rice (Oryza sativa L.). Theor. Appl. Genet. 2006, 112, 382–387. [Google Scholar] [CrossRef]
- Long, Y.; Zhao, L.; Niu, B.; Su, J.; Wu, H.; Chen, Y.; Zhang, Q.; Guo, J.; Zhuang, C.; Mei, M.; et al. Hybrid male sterility in rice controlled by interaction between divergent alleles of two adjacent genes. Proc. Natl. Acad. Sci. USA 2008, 105, 18871–18876. [Google Scholar] [CrossRef]
- Sano, Y.; Chu, Y.E.; Oka, H.I. Genetic studies of speciation in cultivated rice. 1. Genic analysis for the F1 sterility between Oryza sativa L. and Oryza glaberrima Steud. Jpn. J. Genet. 1979, 54, 121–132. [Google Scholar] [CrossRef]
- Sano, Y. A new gene controlling sterility in F1 hybrids of two cultivated rice species. J. Hered. 1983, 74, 435–439. [Google Scholar] [CrossRef]
- Sano, Y. The genetic nature of gamete eliminator in rice. Genetics 1990, 125, 183–191. [Google Scholar] [PubMed]
- Sano, Y. Genetic comparisons of chromosome 6 between wild and cultivated rice. Jpn. J. Breed. 1992, 42, 561–572. [Google Scholar] [CrossRef]
- Sakata, M.; Yamagata, Y.; Doi, K.; Yoshimura, A. Two linked genes on rice chromosome 2 for F1 pollen sterility in a hybrid between Oryza sativa and O. glumaepatula. Breed. Sci. 2014, 64, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Yoshimura, A.; Iwata, N. RFLP mapping and QTL analysis of heading date and pollen sterility using backcross populations between Oryza sativa L. and Oryza glaberrima Steud. Breed. Sci. 1998, 48, 395–399. [Google Scholar] [CrossRef]
- Sano, Y. Pollen-killers in rice. Jpn. J. Breed. 1994, 44, 298. [Google Scholar]
- Chang, Z.; Chen, Z.; Wang, N.; Xie, G.; Lu, J.; Yan, W.; Zhou, J.; Tang, X.; Deng, X.W. Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc. Natl. Acad. Sci. USA 2016, 113, 14145–14150. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, Q.; Zhang, L.; Mao, B.; Yan, D.; Jin, Q.; He, Z. Fine mapping and candidate gene analysis of the novel thermo-sensitive genic male sterility tms9-1 gene in rice. Theor. Appl. Genet. 2014, 127, 1173–1182. [Google Scholar] [CrossRef]
- Frouin, J.; Filloux, D.; Taillebois, J.; Grenier, C.; Montes, F.; De Lamotte, F.; Jean-Luc, V.; Brigitte, C.; Nourollah, A. Positional cloning of the rice male sterility gene ms-IR36, widely used in the inter-crossing phase of recurrent selection schemes. Mol. Breed. 2014, 33, 555–567. [Google Scholar] [CrossRef]
- Charleswotth, D. Origins of rice cytoplasmic male sterility genes. Cell Res. 2017, 27, 3–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rick, C.M. Abortion of male and female gametes in the tomato determined by allelic interaction. Genetics 1966, 53, 85–96. [Google Scholar] [PubMed]
- Sano, Y. Is an egg-killer present in rice. Theor. Appl. Genet. 1993, 86, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Loegerlng, W.G.; Sears, E.R. Distorted inheritance of stem rust resistance of Timstein wheat caused by a pollen-killing gene. Can. J. Genet. Cytol. 1963, 5, 67–72. [Google Scholar]
- Cameron, D.R.; Moav, R. Inheritance in Nicotiana tubucum XXVII: Pollen Killer, an alien genetic locus inducing abortion of microspores not carrying it. Genetics 1957, 42, 326–335. [Google Scholar]
- Maan, S.S. Cytoplasmic Variability and Speciation in Triticinae. In Prairie: A Multiple View; Wall, M.K., Ed.; University North Dakoda Press: Grand Forks, ND, USA, 1975; pp. 255–281. [Google Scholar]
- Endo, T.R.; Tsunewaki, K. Sterility of common wheat with Aegilops triuncialis cytoplasm. J. Hered. 1975, 66, 13–18. [Google Scholar] [CrossRef]
- Kibirige-Sebunya, I.; Knott, D.R. Transfer of stem rust resistance to wheat from an Agropyron chromosome having a gametocidal effect. Can. J. Genet. Cytol. 1983, 25, 215–221. [Google Scholar] [CrossRef]
- Maan, S.S. Cytoplasmic homology between Aegilops squarrosa L. and Ae. cylindrica HOST. Crop. Sci. 1976, 16, 757–761. [Google Scholar] [CrossRef]
- Scoles, G.J.; Kibirige-Sebunya, I.N. Preferential abortion of gametes in wheat induced by an Agropyron chromosome. Can. J. Genet. Cytol. 1983, 25, 1–6. [Google Scholar] [CrossRef]
- Iwata, N.; Nagamatsu, T.; Omura, T. Abnormal segregation of waxy and apiculus coloration by a gametophyte gene belonging to the first linkage group in rice. Jpn. J. Breed. 1964, 14, 33–39. [Google Scholar] [CrossRef]
- Nakagahra, M. Genetic mechanism on the distorted segregation of marker genes belonging to the eleventh linkage group in cultivated rice. Jpn. J. Breed. 1972, 22, 232–238. [Google Scholar] [CrossRef]
- Nakagahra, M.; Omura, T.; Iwata, N. Gametophyte genes and their loci on the eleventh linkage group of cultivated rice. Jpn. J. Breed. 1972, 22, 305–312. [Google Scholar] [CrossRef]
- Nakagahra, M.; Omura, T.; Iwata, N. New certation gene on the first linkage group found by inter-subspecific hybridization of cultivated rice. J. Fac. Agric. Kyushu Univ. 1974, 19, 157–167. [Google Scholar]
- Mori, K.; Kinoshita, T.; Takahashi, M. Segregation distortion and its causation of an endosperm character in crosses of distantly related rice varieties. Genetical studies on rice plant, LVIII. Mem. Fac. Agric. Hokkaido Univ. 1973, 9, 74–86. [Google Scholar]
- Maekawa, M.; Kinoshita, T.; Takahashi, M. A new gametophyte gene in the second linkage group of rice. J. Fac. Agric. Hokkaido Univ. 1981, 60, 107–114. [Google Scholar]
- Maekawa, M.; Kita, F. New gametophyte genes located in the third linkage group (Chromosome 3) of rice. Jpn. J. Breed. 1985, 35, 25–31. [Google Scholar] [CrossRef]
- Kinoshita, T.; Takamure, I. Inheritance and linkage relationship on zebra chlorosis and zebra necrosis in rice. Genetical studies on rice plant, LXXXVIII. J. Fac. Agric. Hokkaido Univ. 1984, 61, 445–455. [Google Scholar]
- Lin, S.Y.; Ikehashi, H.; Yanagihara, S.; Kawashima, A. Segregation distortion via male gametes in hybrids between indica and japonica or wide-compatibility varieties of rice (Oryza sativa L). Theor. Appl. Genet. 1992, 84, 812–818. [Google Scholar] [CrossRef]
- Kinoshita, T. Report of committee on gene symbolization, nomenclature and linkage groups. Rice Genet. Newls. 1995, 12, 9–115. [Google Scholar]
- Lu, C.G.; Takabatake, K.; Ikehashi, H. Identification of segregation-distortion-neutral alleles to improve pollen fertility of indica-japonica hybrids in rice (Oryza sativa L.). Euphytica 2000, 113, 101–107. [Google Scholar] [CrossRef]
- Kaizuma, N.; Hirano, J.; Gotoh, T. Breeding of wheat strains with short and stiff culm by r-ray irradiation and some experimental results related to mutation breeding method. Bull. Chugoku Agric. Exp. Stn. 1967, 14, 1–21. [Google Scholar]
- Toda, M.; Nakada, T.; Miki, S.; Tsukada, T. Studies on mutation breeding in barley and wheat plants. II. Breeding of a new variety and desirable short-culm strains in wheat by gamma-ray irradiations. Jpn. J. Breed. 1972, 22, 239–245. [Google Scholar] [CrossRef]
- Tomita, M. Introgression of Green Revolution sd1 gene into isogenic genome of rice super cultivar Koshihikari to create novel semidwarf cultivar ‘Hikarishinseiki’ (Koshihikari-sd1). Field Crops Res. 2009, 114, 173–181. [Google Scholar] [CrossRef]

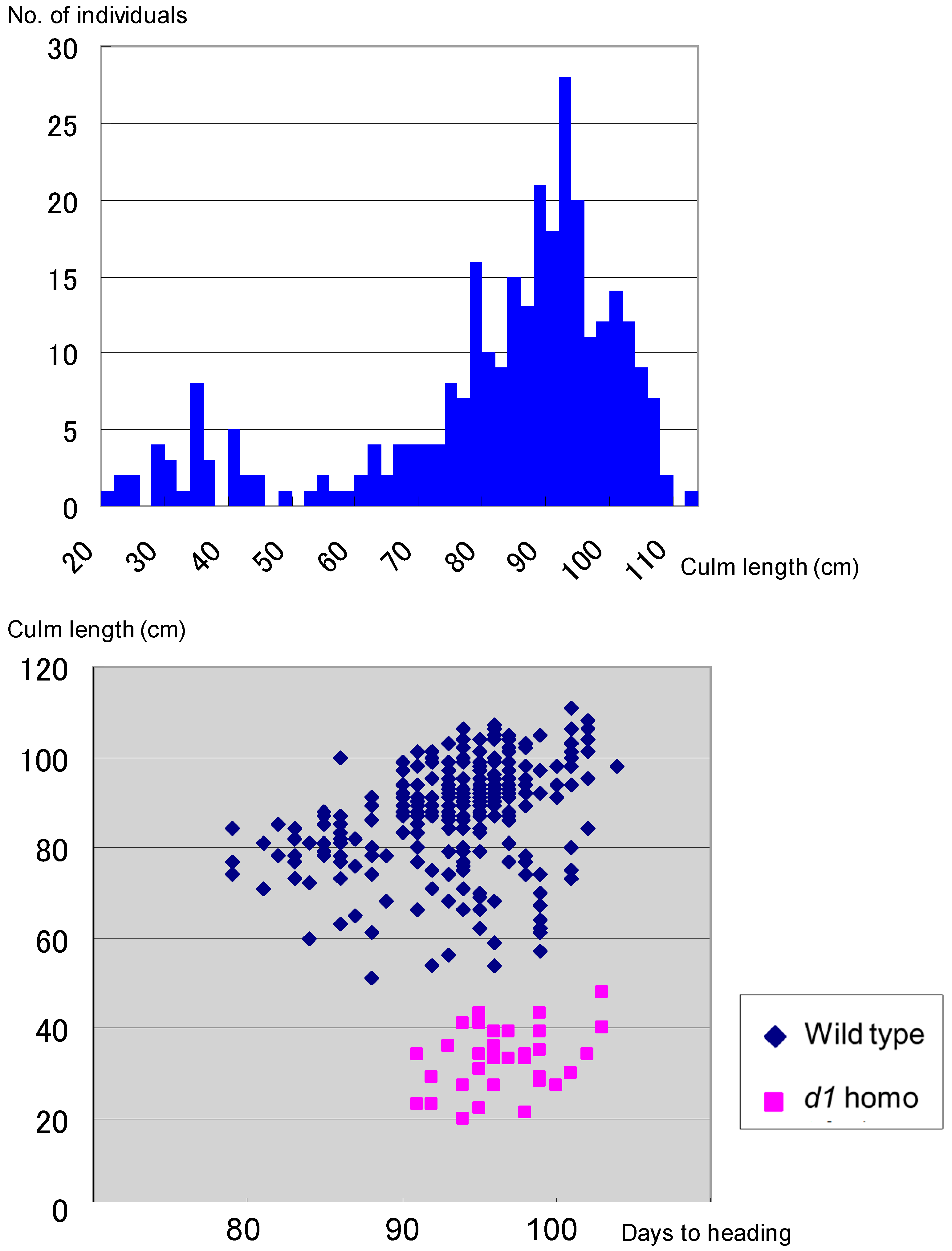

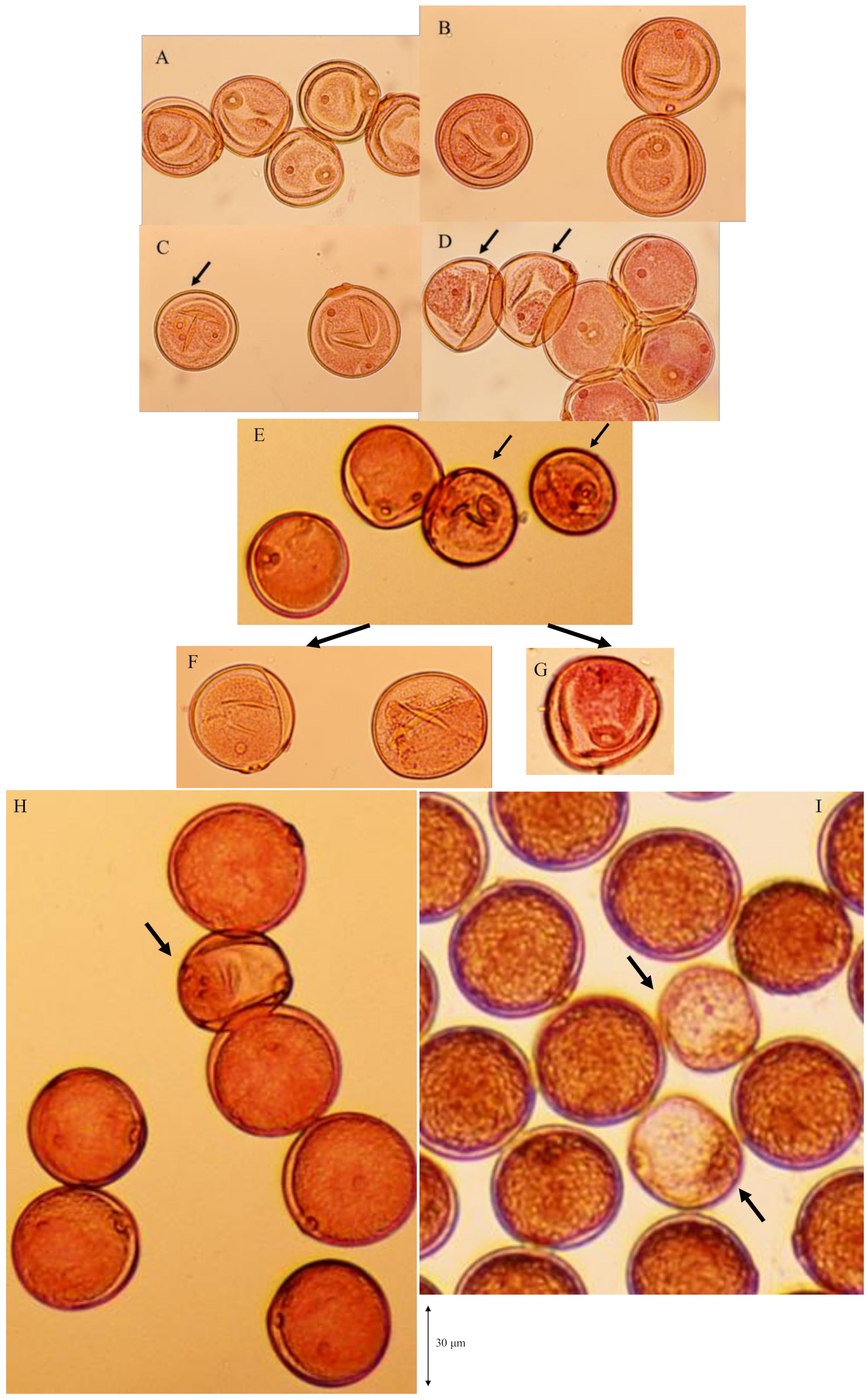
 : Empty pollen.
: Empty pollen.
 : Empty pollen.
: Empty pollen.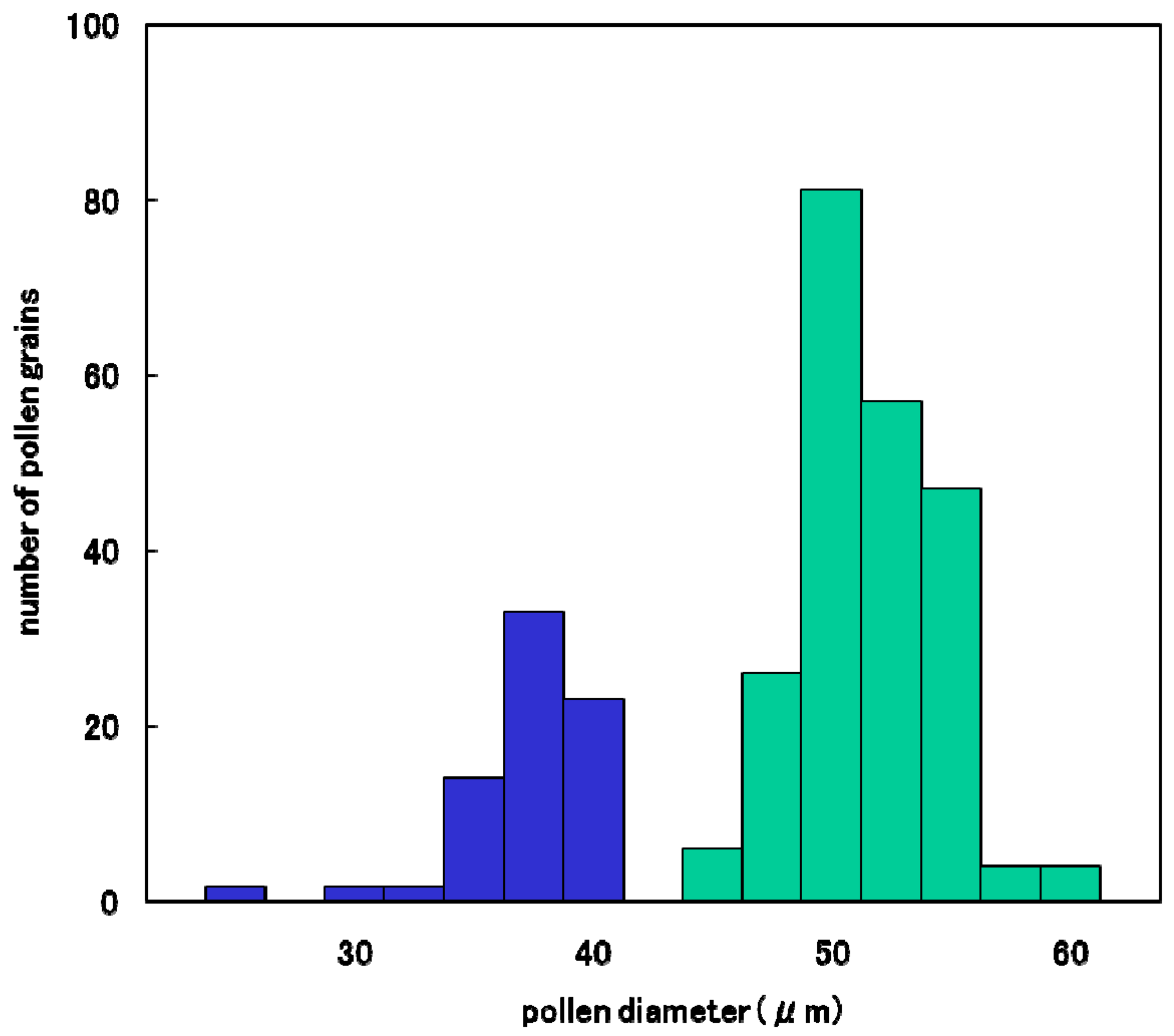
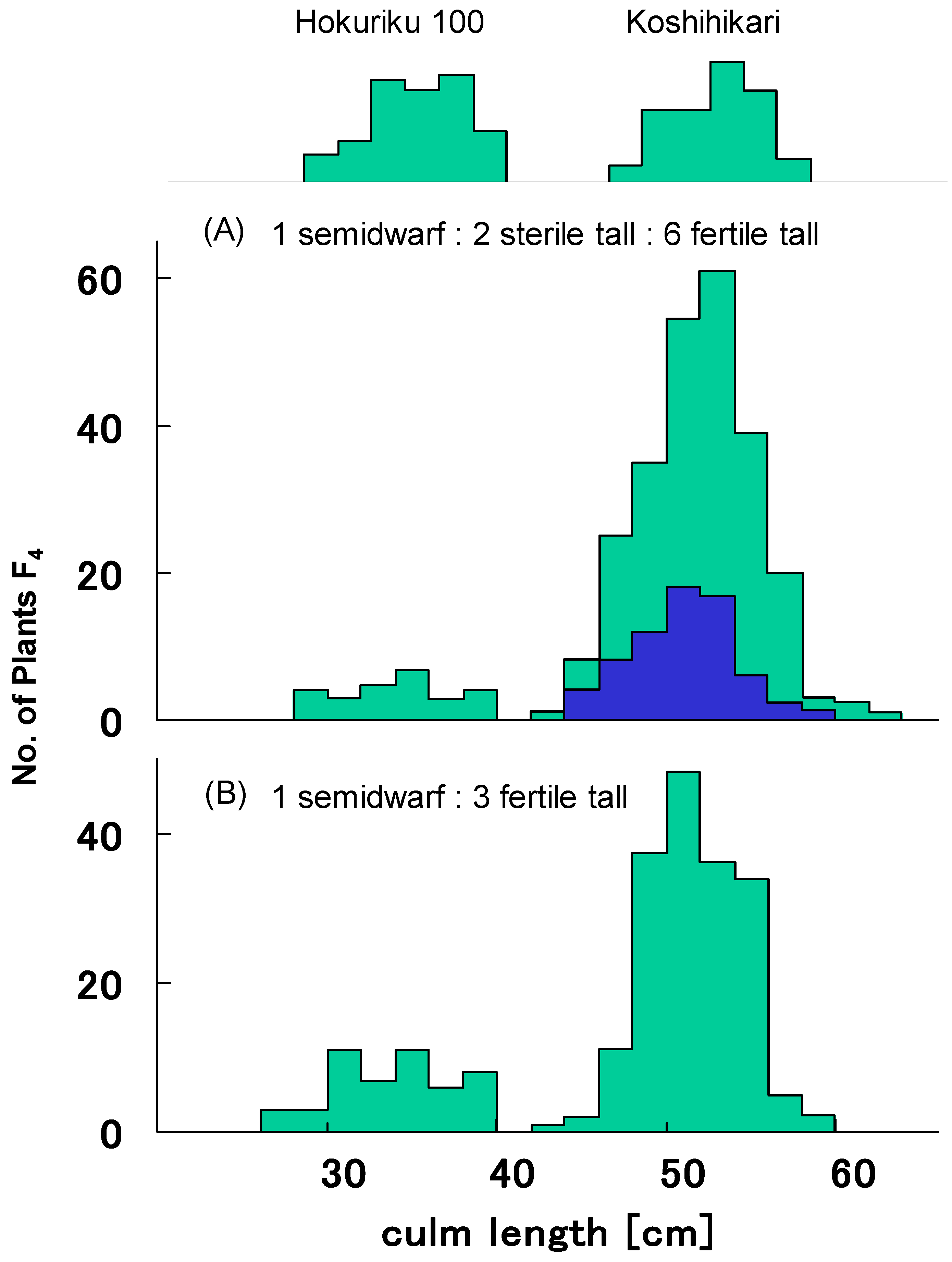
| Phenotype and Genotype of F3 | Frequency Distribution1) of Culm Length and Seed Fertility in Representative F4 Lines | F4 Lines | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Culm Length (cm) | Observed No. | Expected | |||||||||||||||||||||
| 50 | 60 | 70 | 80 | No. | Ratio | ||||||||||||||||||
| Hokuriku 100 type (semidwarf) | d60d60GalGal | 5 | 9 | 5 | 5 | 1 | 11 | 11.1 | 1 | ||||||||||||||
| 1 | 1 | 2 | 7 | 9 | 2 | 2 | 1 | ||||||||||||||||
| 4 | 5 | 6 | 5 | 3 | 2 | ||||||||||||||||||
| 1 | 4 | 6 | 7 | 5 | 1 | 1 | |||||||||||||||||
| 1 | 2 | 8 | 8 | 1 | 2 | ||||||||||||||||||
| Koshihikari type (tall and approx. 30% sterile) | D60d60Galgal | 1 | 1 | 6 | 5 | 7 | 2 | 2 | 24 | 22.2 | 2 | ||||||||||||
| (1) | (1) | (1) | 1 | ||||||||||||||||||||
| 2 | 3 | 1 | 1 | 3 | 5 | 6 | 4 | 1 | |||||||||||||||
| (1) | (2) | ||||||||||||||||||||||
| 1 | 1 | 1 | 1 | 3 | 7 | 5 | 2 | 2 | |||||||||||||||
| (2) | (3) | (1) | (1) | ||||||||||||||||||||
| 1 | 1 | 2 | 1 | 3 | 2 | 1 | 3 | 3 | 7 | 1 | 1 | ||||||||||||
| (1) | (3) | ||||||||||||||||||||||
| 1 | 1 | 1 | 3 | 1 | 3 | 2 | 6 | 5 | 3 | ||||||||||||||
| (1) | (2) | (3) | |||||||||||||||||||||
| Koshihikari type (tall) | D60d60GalGal | 5 | 1 | 1 | 1 | 2 | 5 | 6 | 3 | 1 | 19 | 22.2 | 2 | ||||||||||
| 1 | 2 | 2 | 1 | 2 | 1 | 3 | 2 | 6 | 4 | 2 | |||||||||||||
| 1 | 3 | 1 | 1 | 3 | 2 | 3 | 5 | 4 | 2 | ||||||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 4 | 3 | 3 | 3 | ||||||||||||
| 1 | 2 | 2 | 1 | 5 | 5 | 4 | 3 | 2 | |||||||||||||||
| D60D60GalGal D60D60Galgal D60D60galgal | 3 | 5 | 1 | 6 | 3 | 3 | 2 | 2 | 46 | 44.4 | 4 | ||||||||||||
| 1 | 2 | 9 | 8 | 5 | 1 | ||||||||||||||||||
| 2 | 2 | 8 | 3 | 5 | 2 | ||||||||||||||||||
| 1 | 3 | 3 | 4 | 8 | 5 | 1 | 1 | ||||||||||||||||
| 1 | 5 | 8 | 6 | 4 | 1 | ||||||||||||||||||
| Total | 100 | 100 | |||||||||||||||||||||
| Test for two-gene segregation (1:2:2:4): Χ2 = 0.67, 0.80 < p < 0.90 | |||||||||||||||||||||||
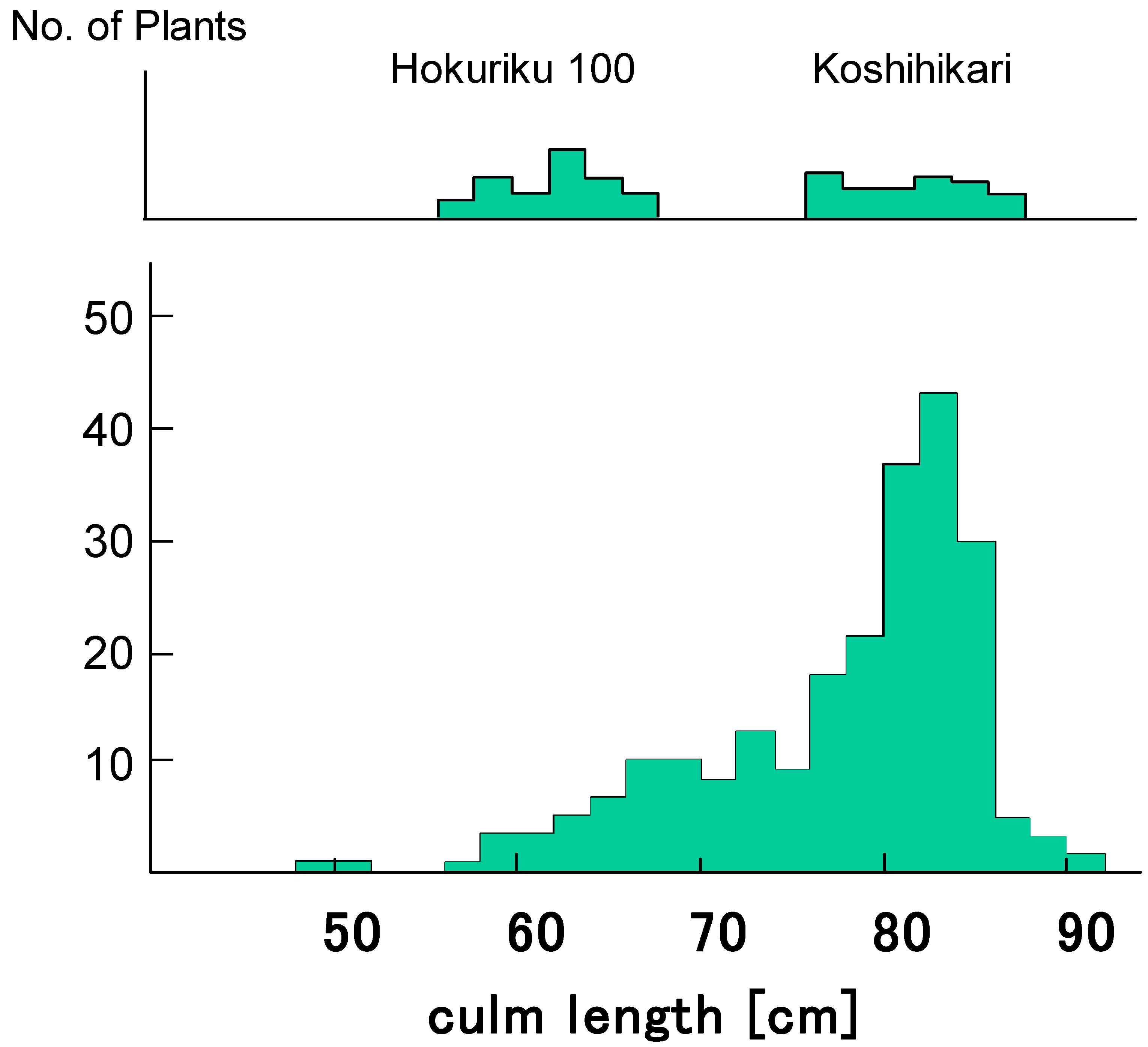
| Phenotype and Genotype of F3 | Frequency Distribution of Culm Length and Seed Fertility in F4 Lines | F4 Lines | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Culm Length (cm) | Observed No. | Expected | |||||||||||||||||||||
| 50 | 60 | 70 | 80 | No. | Ratio | ||||||||||||||||||
| Hokuriku 100 type (semidwarf) | d60d60GalGal | 1 | 1 | 1 | 13 | 4 | 3 | 1 | 1 | 24 | 25.0 | 1 | |||||||||||
| 6 | 7 | 6 | 1 | 5 | |||||||||||||||||||
| 1 | 3 | 4 | 7 | 8 | 1 | ||||||||||||||||||
| 1 | 2 | 9 | 4 | 4 | 3 | 1 | |||||||||||||||||
| 1 | 3 | 4 | 7 | 5 | 4 | 1 | |||||||||||||||||
| Koshihikari type (tall) | D60d60GalGal | 2 | 2 | 1 | 1 | 2 | 2 | 3 | 2 | 6 | 3 | 1 | 49 | 50.0 | 2 | ||||||||
| 2 | 1 | 4 | 1 | 2 | 2 | 5 | 3 | 2 | 2 | 1 | |||||||||||||
| 1 | 1 | 1 | 1 | 1 | 2 | 1 | 5 | 3 | 7 | 1 | |||||||||||||
| 1 | 2 | 1 | 1 | 6 | 6 | 3 | 2 | 1 | 2 | ||||||||||||||
| 2 | 2 | 1 | 3 | 6 | 3 | 4 | 5 | ||||||||||||||||
| D60D60GalGal | 1 | 1 | 5 | 8 | 7 | 1 | 1 | 27 | 25.0 | 1 | |||||||||||||
| 2 | 8 | 7 | 5 | 2 | 1 | ||||||||||||||||||
| 3 | 3 | 7 | 5 | 3 | 3 | 1 | |||||||||||||||||
| 6 | 5 | 6 | 4 | 2 | 2 | ||||||||||||||||||
| 1 | 2 | 5 | 8 | 5 | 4 | ||||||||||||||||||
| Total | 100 | 100 | |||||||||||||||||||||
| Test for one-gene segregation (1:2:1): Χ2 = 0.22, 0.80 < p < 0.90 | |||||||||||||||||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, M.; Tanisaka, T. The Gametic Non-Lethal Gene Gal on Chromosome 5 Is Indispensable for the Transmission of the Co-Induced Semidwarfing Gene d60 in Rice. Biology 2019, 8, 94. https://doi.org/10.3390/biology8040094
Tomita M, Tanisaka T. The Gametic Non-Lethal Gene Gal on Chromosome 5 Is Indispensable for the Transmission of the Co-Induced Semidwarfing Gene d60 in Rice. Biology. 2019; 8(4):94. https://doi.org/10.3390/biology8040094
Chicago/Turabian StyleTomita, Motonori, and Takatoshi Tanisaka. 2019. "The Gametic Non-Lethal Gene Gal on Chromosome 5 Is Indispensable for the Transmission of the Co-Induced Semidwarfing Gene d60 in Rice" Biology 8, no. 4: 94. https://doi.org/10.3390/biology8040094
APA StyleTomita, M., & Tanisaka, T. (2019). The Gametic Non-Lethal Gene Gal on Chromosome 5 Is Indispensable for the Transmission of the Co-Induced Semidwarfing Gene d60 in Rice. Biology, 8(4), 94. https://doi.org/10.3390/biology8040094






