In Vitro Propagation of Rheophytic Orchid, Epipactis flava Seidenf.—A Comparison of Semi-Solid, Continuous Immersion and Temporary Immersion Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of Different In Vitro Culture Systems
2.2. Greenhouse Acclimatization and Ex Vitro Culture
2.3. Statistical Analysis
3. Results
3.1. Growth and Development of E. flava Plantlets Under Different In Vitro Culture Systems
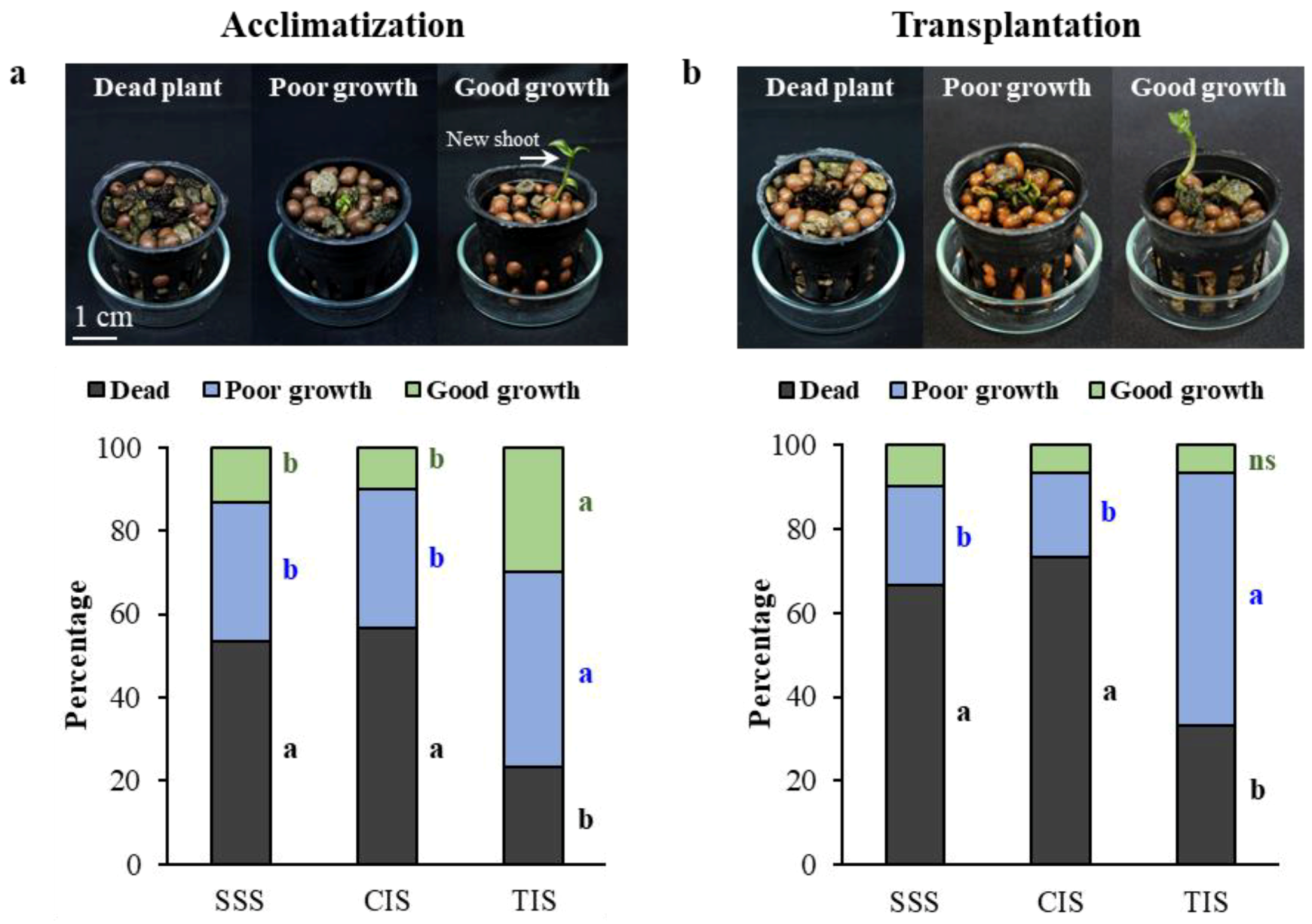
3.2. Acclimatization and Ex Vitro Culture of Plantlets Derived From Different In Vitro Culture Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedersen, H.; Srimuang, K.; Banziger, H.; Watthana, S. Pollination-system diversity in Epipactis (Orchidaceae): New insights from studies of E. flava in Thailand. Plant Syst. Evol. 2018. [Google Scholar] [CrossRef]
- Pedersen, H.; Watthana, S.; Srimuang, K. Orchids in the torrent: On the circumscription, conservation and rheophytic habit of Epipactis flava. Bot. J. Linn. Soc. 2013, 172, 358–370. [Google Scholar] [CrossRef]
- Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. In vitro propagation of Epipactis flava Seidenf., an endangered rheophytic orchid: A first study on factors affecting asymbiotic seed germination, seedling development and greenhouse acclimatization. Plant Cell Tiss. Organ Cult. 2018, 135, 419–432. [Google Scholar] [CrossRef]
- Santisuk, T.; Chayamarit, K.; Pooma, R.; Suddee, S. Thailand Red Data: Plants; Integrated Promotion Technology: Bangkok, Thailand, 2006. [Google Scholar]
- Chamchumroon, V.; Nanthawan, S.; Naiyana, T.; Manop, P.; Sommanussa, T. Threatened Plants in Thailand; Department of National Parks, Wildlife and Plant Conservation: Bangkok, Thailand, 2017; p. 224. [Google Scholar]
- Aggarwal, S.; Zettler, L.W. Reintroduction of an endangered terrestrial orchid, Dactylorhiza hatagirea (D. Don) Soo, assisted by symbiotic seed germination: First report from the Indian subcontinent. Nat. Sci. 2010, 8, 139–145. [Google Scholar]
- Wu, R.Z.; Baque, M.A.; Paek, K.Y. Establishment of a large-scale micropropagation system for Anoectochilus formosanus in bioreactors. Acta Hortic. 2014, 878, 167–174. [Google Scholar] [CrossRef]
- Zhang, B.; Sarsaiya, S.; Pan, X.; Jin, L.; Xu, D.; Zhang, B. Optimization of nutritional conditions using a temporary immersion bioreactor system for the growth of Bletilla striata pseudobulbs and accumulation of polysaccharides. Sci. Hortic. 2019, 240, 155–161. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, R.; Khajuria, R.K.; Mallubhotla, S.; Ahuja, A. Bacoside biosynthesis during in vitro shoot multiplication in Bacopa monnieri (L.) Wettst. grown in Growtek and air lift bioreactor. Indian J. Biotechnol. 2015, 14, 547–551. [Google Scholar]
- Snyman, S.J.; Nkwanyana, P.D.; Watt, M.P. Alleviation of hyperhydricity of sugarcane plantlets produced in RITA® vessels and genotypic and phenotypic characterization of acclimated plants. S. Afr. J. Bot. 2011, 77, 685–692. [Google Scholar] [CrossRef]
- Moreira, A.L.; Silva, A.B.; Santos, A.; Reis, C.O.D.; Landyraf, P.R.C. Cattleya walkeriana growth in different micropropagation systems. Ciênc. Rural 2013, 43, 1804–1810. [Google Scholar] [CrossRef]
- Frometa, O.M.; Morgado, M.M.E.; Silva, J.A.T.; Morgado, D.T.P.; Gradaille, M.A.D. In vitro propagation of Gerbera jamesonii Bolus ex Hooker f. in a temporary immersion bioreactor. Plant Cell Tiss. Organ Cult. 2017, 129, 543–551. [Google Scholar] [CrossRef]
- Ibrahim, R. The potential of bioreactor technology for large-scale plant micropropagation. Acta Hortic. 2017, 1155, 573–584. [Google Scholar] [CrossRef]
- Vives, K.; Andujar, I.; Lorenzo, J.C.; Concepcion, O.; Hernandez, M.; Escalona, M. Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tiss. Organ Cult 2017. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Escalona, M.; Lorenzo, J.C.; González, B.; Daquinta, M.; González, J.L.; Desjardins, Y.; Borroto, C.G. Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep. 1999, 18, 743–748. [Google Scholar] [CrossRef]
- Godoy, S.; Tapia, E.; Seit, P.; Andrade, D.; Sanchez, E.; Andrade, P.; Almeida, A.M.; Prieto, H. Temporary immersion systems for the mass propagation of sweet cherry cultivars and cherry rootstocks: Development of a micropropagation procedure and effect of culture conditions on plant quality. In Vitro Cell. Dev. Biol. Plant 2017, 53, 494–504. [Google Scholar] [CrossRef]
- An, C.H.; Kim, Y.W.; Moon, H.K.; Yi, J.S. Effects of in vitro culture types on regeneration and acclimatization of yellow poplar (Liriodendron tulipifera L.) from somatic embryos. J. Plant Biotechnol. 2016, 43, 110–118. [Google Scholar] [CrossRef]
- Jesionek, A.; Kokotkiewicz, A.; Wlodarska, P.; Zabiegala, B.; Bucinski, A.; Luczkiewicz, M. Bioreactor shoot cultures of Rhododendron tomentosum (Ledum palustre) for a large-scale production of bioactive volatile compounds. Plant Cell Tiss. Organ Cult. 2017, 131, 51–64. [Google Scholar] [CrossRef]
- Ramirez-Mosqueda, M.A.; Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. In Vitro Cell. Dev. Biol. Plant 2016. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wu, K.L.; Zhang, J.X.; Deng, R.F.; Duan, J.; Silva, J.A.T.; Huang, W.C.; Zeng, S.J. Embryo development in association with asymbiotic seed germination in vitro of Paphiopedilum armeniacum S.C. Chen et F.Y. Liu. Sci. Rep. 2015, 12, 16356. [Google Scholar] [CrossRef]
- Yan, H.; Liang, C.; Li, Y. Improved growth and quality of Siraitia grosvenorii plantlets using a temporary immersion system. Plant Cell Tiss. Organ Cult. 2010, 103, 131–135. [Google Scholar] [CrossRef]
- Etienne, H.; Berthouly, M. Temporary immersion systems in plant micropropagation. Plant Cell Tiss. Organ Cult. 2002, 69, 215–231. [Google Scholar] [CrossRef]
- Sreedhar, R.V.; Venkatachalam, L.; Neelwarne, B. Hyperhydricity-Related Morphologic and Biochemical Changes in Vanilla (Vanilla planifolia). J. Plant Growth Reg. 2009, 28, 46–57. [Google Scholar] [CrossRef]
- Ramos-Castellá, A.; Iglesias-Andreu, L.G.; Bello-Bello, J.; Lee-Espinosa, H. Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. In Vitro Cell. Dev. Biol. Plant 2014, 50, 576–581. [Google Scholar] [CrossRef]
- Arencibia, A.D.; Vergara, C.; Quiroz, K.; Carrasco, B.; Bravo, C.; Garcia-Gonzales, R. An approach for micropropagation of blueberry (Vaccinium corymbosum L.) plants mediated by temporary immersion bioreactors (TIBs). Am. J. Plant Sci. 2013, 4, 1022–1028. [Google Scholar] [CrossRef]
- Berthouly, M.; Etienne, H. Temporary immersion system: A new concept for use liquid medium in mass propagation. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer (Pays-Bas): Dordrecht, The Netherlands, 2005; pp. 165–195. [Google Scholar]
- Zhao, Y.; Sun, W.; Wang, Y.; Saxena, P.K.; Liu, C.Z. Improving mass multiplication of Rhodiola cremulata shoots using temporary immersion bioreactor with forced ventilation. Appl. Biochem. Biotechnol. 2012, 166, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Aragón, C.E.; Escalona, M.; Rodriguez, R.; Cañal, M.J.; Capote, I.; Pina, D.; González-Olmedo, J. Effect of sucrose, light, and carbon dioxide on plantain micropropagation in temporary immersion bioreactors. In Vitro Cell. Dev. Biol. Plant 2010, 46, 89–94. [Google Scholar] [CrossRef]
- Georgiev, V.; Schumann, A.; Pavlov, A.; Bley, T. Temporary immersion systems in plant biotechnology. Eng. Life Sci. 2014, 14, 607–621. [Google Scholar] [CrossRef]
- Hazarika, B.N. Acclimatization of tissue-cultured plants. Curr. Sci. 2003, 85, 1704–1712. [Google Scholar]
- Dohling, S.; Kumaria, S.; Tandon, P. Multiple shoot induction from axillary bud culture of the medicinal orchid, Dendrobium longicornu. AoB Plants 2012, 2012. [Google Scholar] [CrossRef]
- Yang, S.H.; Yeh, D.M. In vitro leaf anatomy, ex vitro photosynthetic behaviors and growth of Calathea orbifolia (Linden) Kennedy plants obtained from semi-solid medium and temporary immersion systems. Plant Cell Tiss. Organ Cult. 2008, 93, 201–207. [Google Scholar] [CrossRef]
- Aragón, C.E.; Sánchez, C.; González-Olmedo, J.; Escalona, M.; Carvalho, L. Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biol. Plant 2014, 58, 29–38. [Google Scholar] [CrossRef]
- Decruse, S.W.; Reny, N.; Shyylajakumari, S.; Krishnan, P.N. In vitro propagation and field establishment of Eulophia cullenii (Wight) Bl., a critically endangered orchid of Western Ghats, India through culture of seeds and axenic seedling-derived rhizomes. In Vitro Cell. Dev. Biol. Plant 2013, 49, 520–528. [Google Scholar] [CrossRef]
- Kauth, P.J.; Vendrame, W.A.; Kane, M.E. In vitro seed culture and seedlings development of Calopogon tuberosus. Plant Cell Tiss. Organ Cult. 2006, 85, 91–102. [Google Scholar] [CrossRef]

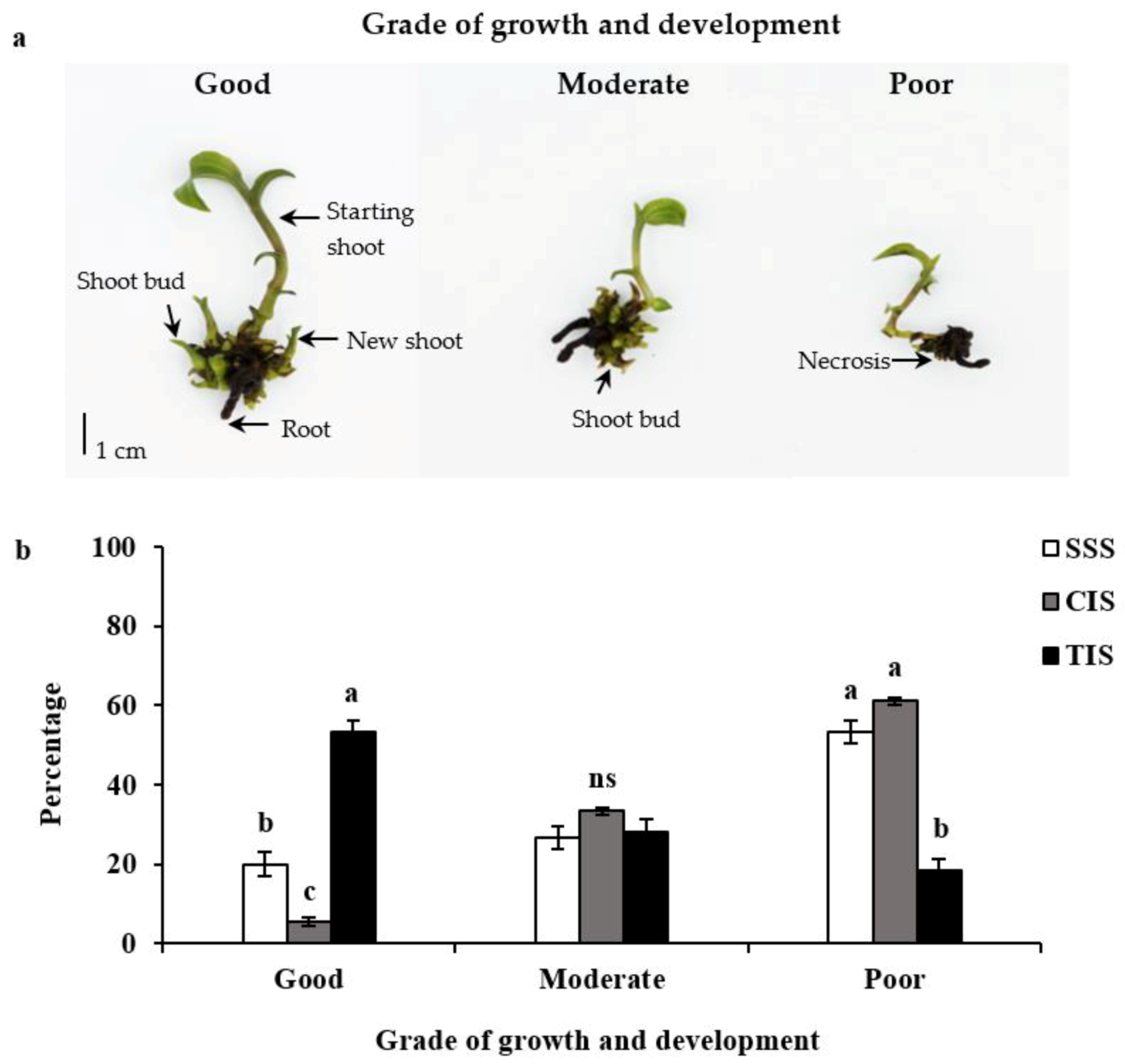
| Parameter | Culture Systems 1 | ||
|---|---|---|---|
| SSS | CIS | TIS | |
| Survival rate (%) | 100.0 ± 0.0 ns | 100.0 ± 0.0 | 100.0 ± 0.0 |
| New shoot formation (%) | 86.7 ± 0.6 b | 70.0 ± 1.1 c | 96.7 ± 1.3 a |
| Number of new shoots per explant | 1.0 ± 0.0 b | 0.8 ± 0.2 b | 1.5 ± 0.1 a |
| Number of new shoots per replication | 19.0 ± 1.0 b | 15.7 ± 1.5 b | 29.3 ± 8.6 a |
| Shoot bud formation (%) | 46.7 ± 1.3 b | 40.0 ± 1.1 b | 91.7 ± 1.7 a |
| Number of shoot buds per explant | 3.9 ± 0.1 b | 5.5 ± 0.2 b | 8.1 ± 0.4 a |
| Number of shoot buds per replication | 78.7 ± 5.5 b | 110.7 ± 14.6 b | 161.0 ± 35.6 a |
| Number of roots per explant | 3.8 ± 0.0 ab | 3.6 ± 0.0 b | 4.4 ± 0.1 a |
| Number of leaves per shoot 2 | 2.8 ± 0.1 b | 2.7 ± 0.5 b | 4.4 ± 0.1 a |
| Shoot height (mm) 2 | 14.0 ± 0.4 b | 14.3 ± 3.4 b | 29.4 ± 0.8 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunakhonnuruk, B.; Inthima, P.; Kongbangkerd, A. In Vitro Propagation of Rheophytic Orchid, Epipactis flava Seidenf.—A Comparison of Semi-Solid, Continuous Immersion and Temporary Immersion Systems. Biology 2019, 8, 72. https://doi.org/10.3390/biology8040072
Kunakhonnuruk B, Inthima P, Kongbangkerd A. In Vitro Propagation of Rheophytic Orchid, Epipactis flava Seidenf.—A Comparison of Semi-Solid, Continuous Immersion and Temporary Immersion Systems. Biology. 2019; 8(4):72. https://doi.org/10.3390/biology8040072
Chicago/Turabian StyleKunakhonnuruk, Boworn, Phithak Inthima, and Anupan Kongbangkerd. 2019. "In Vitro Propagation of Rheophytic Orchid, Epipactis flava Seidenf.—A Comparison of Semi-Solid, Continuous Immersion and Temporary Immersion Systems" Biology 8, no. 4: 72. https://doi.org/10.3390/biology8040072
APA StyleKunakhonnuruk, B., Inthima, P., & Kongbangkerd, A. (2019). In Vitro Propagation of Rheophytic Orchid, Epipactis flava Seidenf.—A Comparison of Semi-Solid, Continuous Immersion and Temporary Immersion Systems. Biology, 8(4), 72. https://doi.org/10.3390/biology8040072




