Affinity Isolation and Mass Spectrometry Identification of Prostacyclin Synthase (PTGIS) Subinteractome
Abstract
1. Introduction
2. Materials and Methods
2.1. Recombinant Proteins
2.1.1. Prostacyclin Synthase (PTGIS)
2.1.2. Cytochrome P450 2J2 (CYP2J2)
2.2. Tissue Lysate Preparation
2.3. Reagents and Buffers
2.4. Surface Plasmon Resonance (SPR)
2.4.1. Immobilization of the PTGIS Protein on the Optical Chip
2.4.2. Comparative Estimation of Probable Fished Protein Material in the Rat Tissue Lysates
2.4.3. Sample Preparation for Model SPR Experiments
2.5. Preparation of Control and Affine Sorbent
2.6. Estimation of Specific and Non-Specific Binding for Control and Affine Sorbent
2.7. Affinity Isolation of Protein Partners of PTGIS from Testis Tissue Lysate
2.8. Size-Exclusion Chromatography (SEC) Fractionation of Tissue Lysate
2.9. LC/MS-MS Analysis
2.10. Liquid Chromatography
2.11. Mass Spectrometry
2.12. Data Analysis
2.13. Design and Synthesis of Peptides
3. Results and Discussion
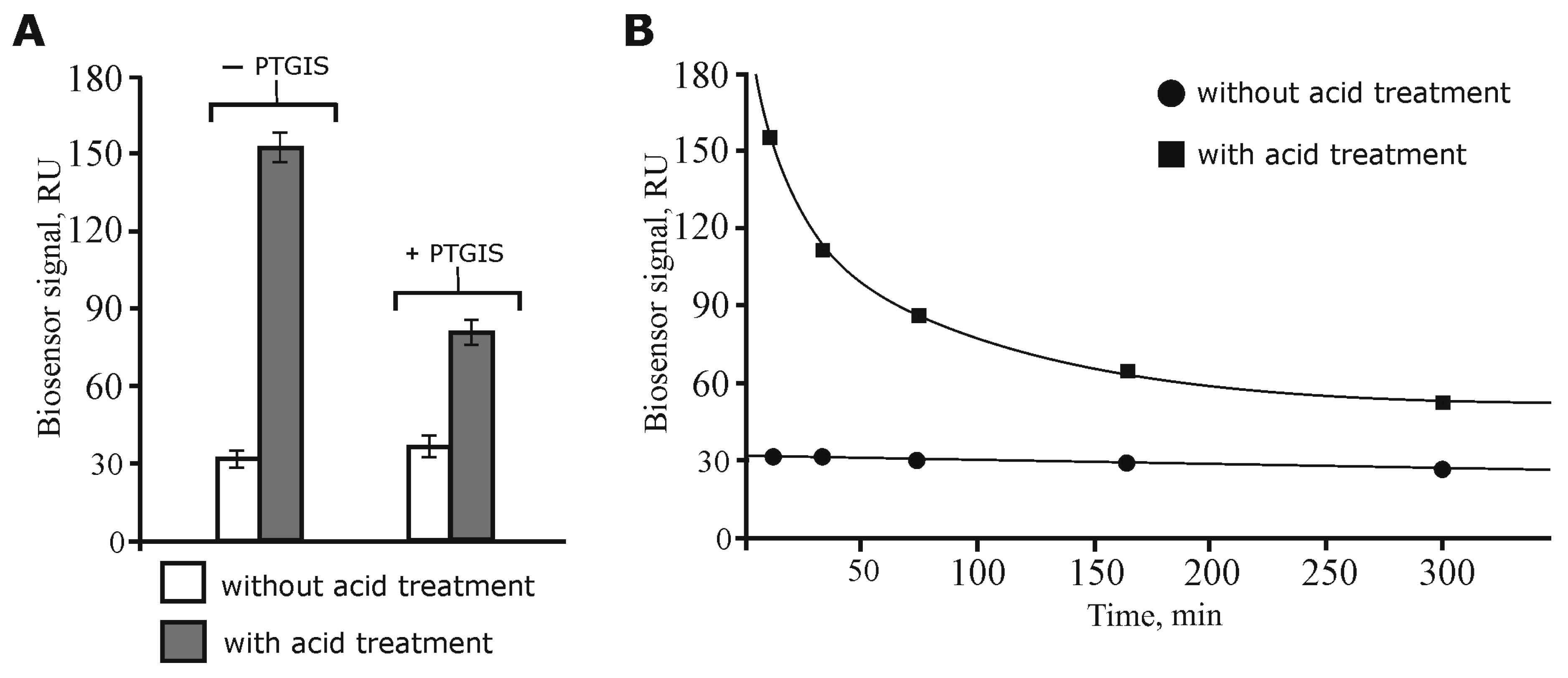
3.1. Model Experiments for Optimization of the Direct Molecular Fishing Procedure
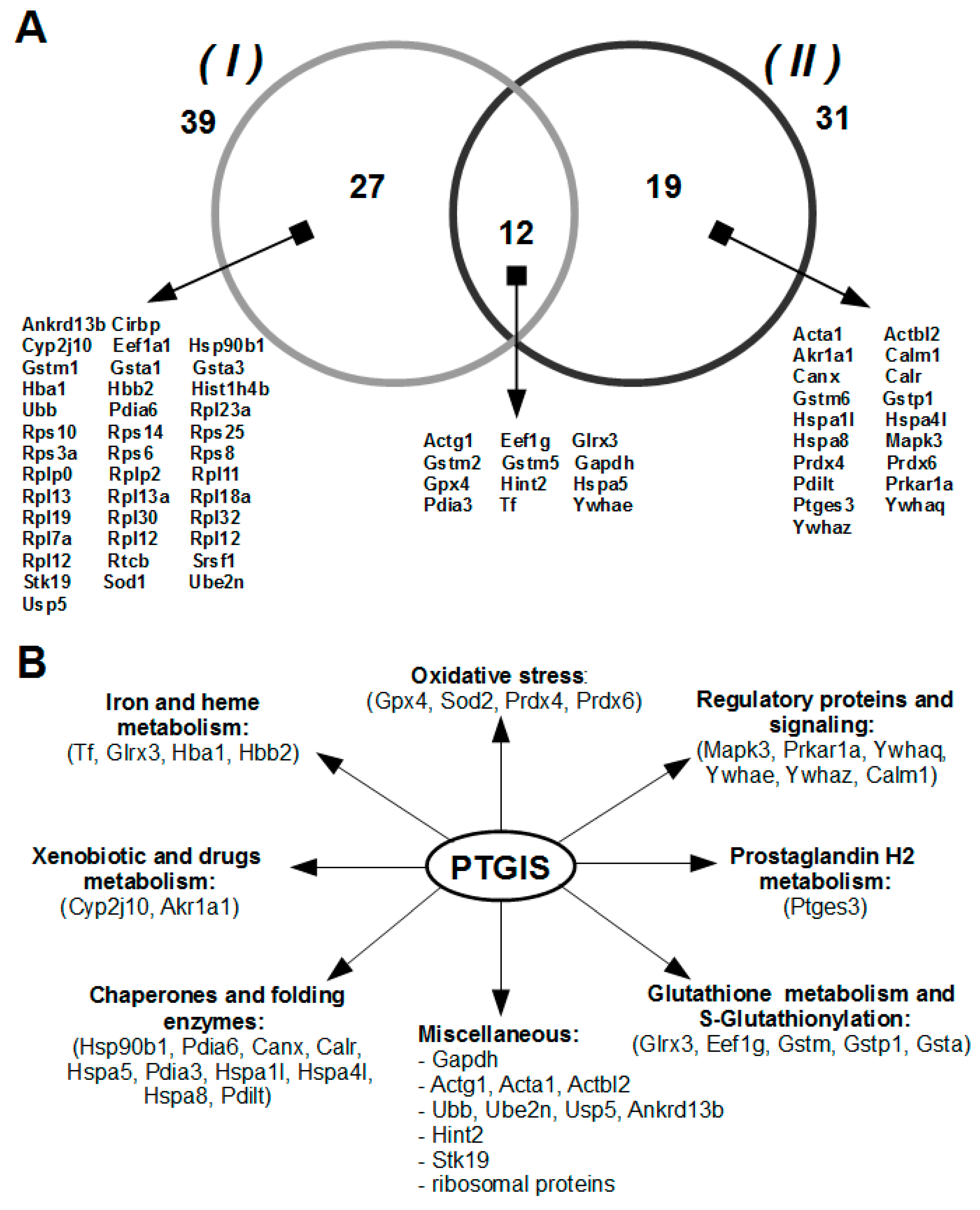
3.2. Isolation and Identification of Potential Protein Partners for PTGIS from Testis Tissue Lysate by Affinity Chromatography and LC-MS/MS
3.3. Functional Intersection between PTGIS and Identified Protein Partners
3.4. SEC Profiling of Tissue Lysate Proteins
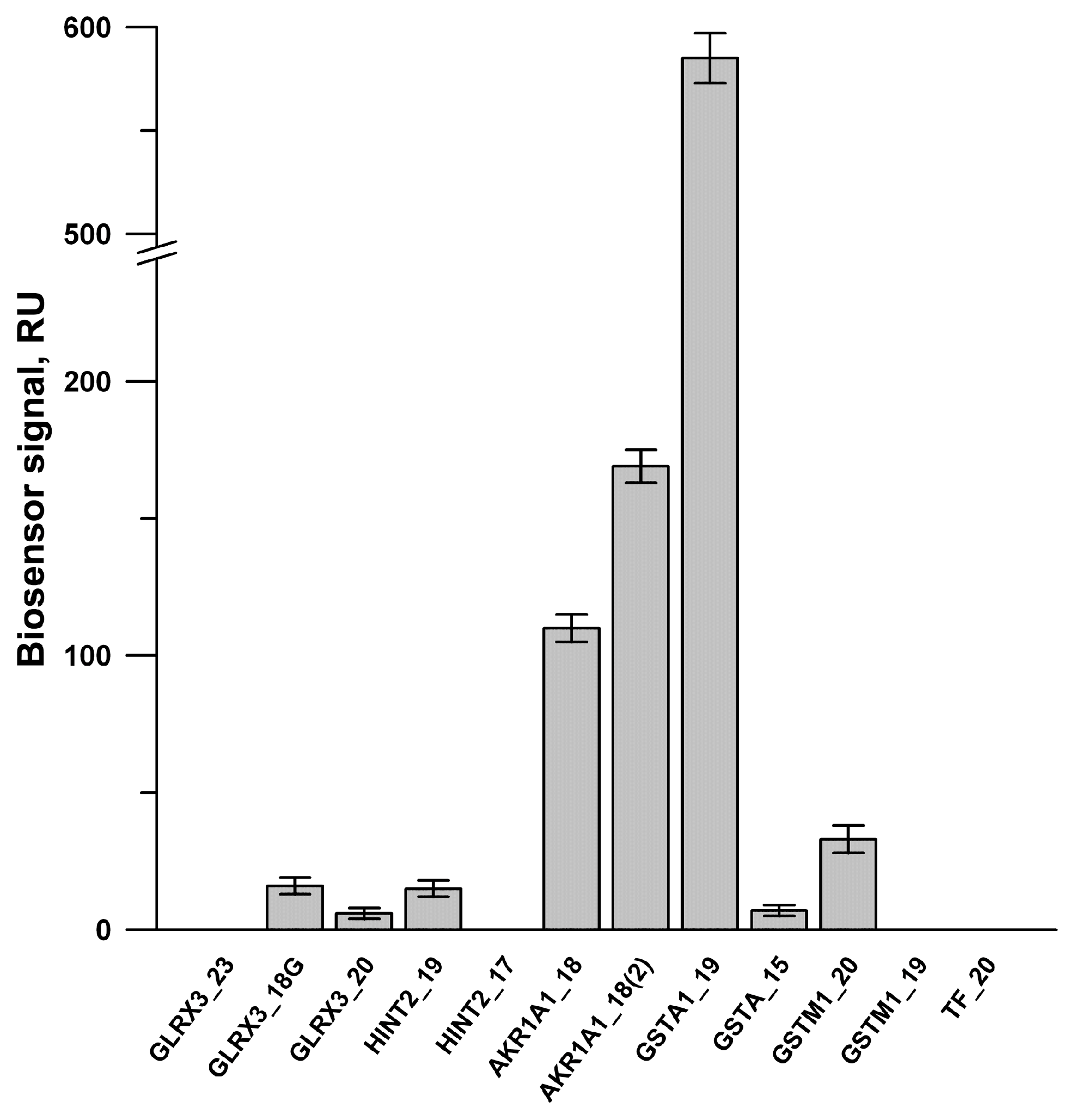
3.5. Partial SPR Validation of Protein–Protein and Protein–Peptide Interactions between PTGIS and Its Potential Protein Partners
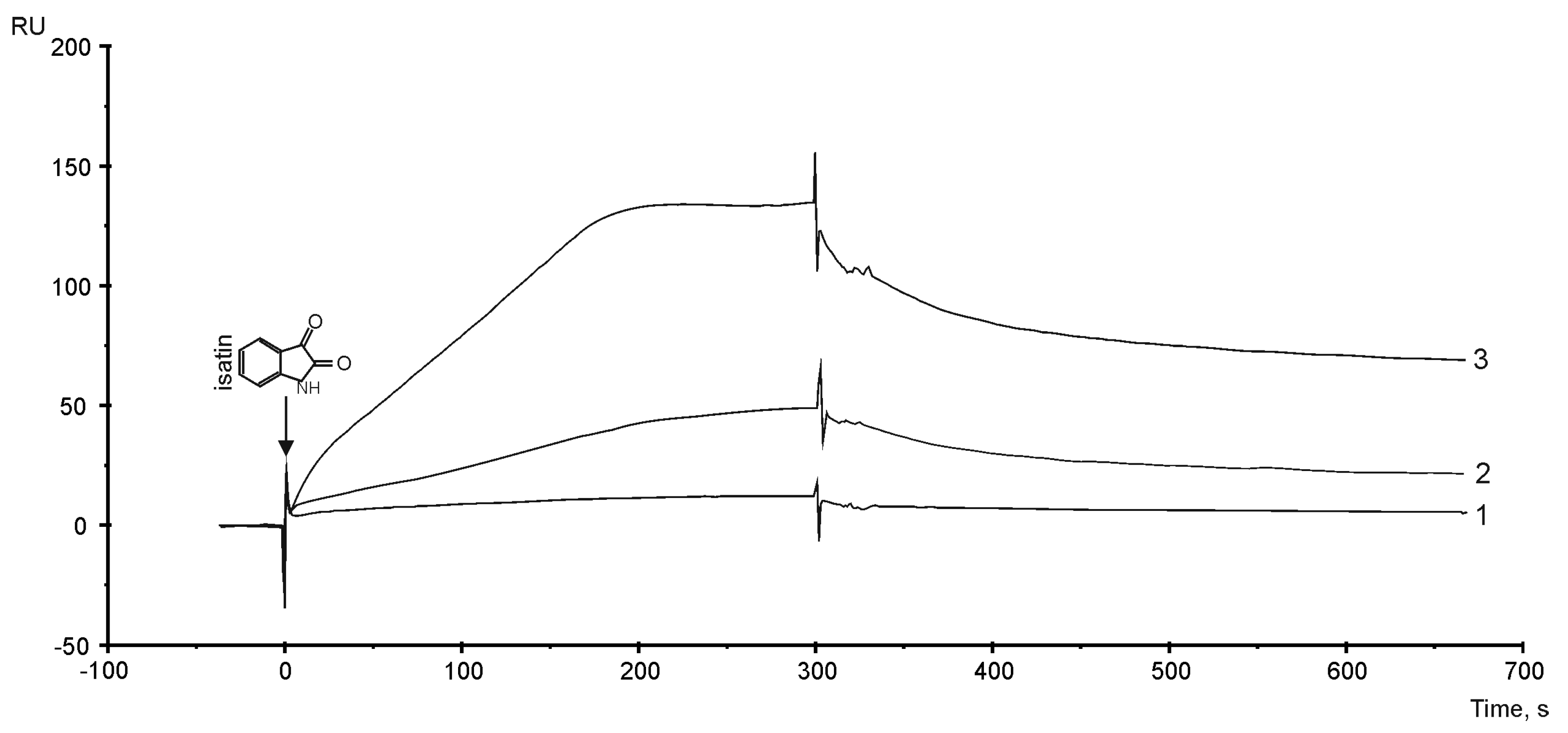
3.6. The Sensitivity of PTGIS to Small Compound Isatin (indole 2,3-dion)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PTGIS | Prostacyclin synthase |
| TBXAS1 | thromboxane synthase |
| PGH2 | prostaglandin endoperoxide H2 |
| PGE2 | prostaglandin E2 |
| Kd | dissociation constant of molecular complex |
| ACN | acetonitrile |
| DCM | dichloromethane |
| DMF | N,N-dimethylformamide |
| DTT | dithiothreitol |
| HBTU | 3-[bis(dimethylamino)methyliumyl]-3H-benzotriazol-1-oxide hexafluorophosphate |
| MTBE | methyl tert-butyl ether |
| NMM | N-methylmorpholine |
| NMP | 1-Methylpyrrolidin-2-one |
| TES | triethylsilane |
| TFA | 2,2,2-trifluoroacetic acid |
| TIPS | triisopropylsilane |
References
- Smith, W.L.; DeWitt, D.L.; Allen, M.L. Bimodal distribution of the prostaglandin I2 synthase antigen in smooth muscle cells. J. Biol. Chem. 1983, 258, 5922–5926. [Google Scholar] [PubMed]
- Hara, S.; Morishita, R.; Tone, Y.; Yokoyama, C.; Inoue, H.; Kaneda, Y.; Ogihara, T.; Tanabe, T. Overexpression of prostacyclin synthase inhibits growth of vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1995, 216, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Todaka, T.; Yokoyama, C.; Yanamoto, H.; Hashimoto, N.; Nagata, I.; Tsukahara, T.; Hara, S.; Hatae, T.; Morishita, R.; Aoki, M.; et al. Gene Transfer of Human Prostacyclin Synthase Prevents Neointimal Formation After Carotid Balloon Injury in Rats. Stroke 1999, 30, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Yabuki, T.; Shimonishi, M.; Wada, M.; Hatae, T.; Ohkawara, S.; Takeda, J.; Kinoshita, T.; Okabe, M.; Tanabe, T. Prostacyclin-deficient mice develop ischemic renal disorders, including nephrosclerosis and renal infarction. Circulation 2002, 106, 2397–2403. [Google Scholar] [CrossRef]
- Clapp, L.H.; Gurung, R. The mechanistic basis of prostacyclin and its stable analogues in pulmonary arterial hypertension: Role of membrane versus nuclear receptors. Prostaglandins Other Lipid Mediat. 2015, 120, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, M.-C.; Reynolds, J.V.; O’Byrne, K.J.; Pidgeon, G.P. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim. Biophys. Acta 2010, 1805, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Ochiai, T.; Takamura, M.; Kondo, Y.; Yokoyama, C.; Hara, S. Role of prostacyclin synthase in carcinogenesis. Prostaglandins Other Lipid Mediat. 2017, 133, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.K. Prostaglandins, prostacyclin and thromboxanes. Biochem. Educ. 1982, 10, 82–87. [Google Scholar] [CrossRef]
- Ueno, N.; Murakami, M.; Tanioka, T.; Fujimori, K.; Tanabe, T.; Urade, Y.; Kudo, I. Coupling between cyclooxygenase, terminal prostanoid synthase, and phospholipase A2. J. Biol. Chem. 2001, 276, 34918–34927. [Google Scholar] [CrossRef]
- Hamberg, M.; Samuelsson, B. On the Metabolism of Prostaglandins E1 and E2 in Man. J. Biol. Chem. 1971, 246, 6713–6721. [Google Scholar] [PubMed]
- Cawello, W.; Schweer, H.; Müller, R.; Bonn, R.; Seyberth, H.W. Metabolism and pharmacokinetics of prostaglandin E1 administered by intravenous infusion in human subjects. Eur. J. Clin. Pharmacol. 1994, 46, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.S.; Ershov, P.V.; Molnar, A.A.; Mezentsev, Y.V.; Kaluzhskiy, L.A.; Yablokov, E.O.; Florinskaya, A.V.; Gnedenko, O.V.; Medvedev, A.E.; Kozin, S.A.; et al. Direct molecular fishing in molecular partners investigation in protein–protein and protein–peptide interactions. Russ. J. Bioorg. Chem. 2016, 42, 14–21. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Ershov, P.V.; Mezentsev, Y.V.; Poverennaya, E.V.; Lisitsa, A.V.; Archakov, A.I. Protocols of protein interactomics: Molecular fishing on optical chips and magnetic nanoparticles. Biochem. Mosc. Suppl. Ser. B 2012, 6, 99–106. [Google Scholar] [CrossRef]
- Ershov, P.V.; Mezentsev, Y.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Florinskaya, A.V.; Gnedenko, O.V.; Zgoda, V.G.; Vakhrushev, I.V.; Raeva, O.S.; Yarygin, K.N.; et al. Direct Molecular Fishing of Protein Partners for Proteins Encoded by Genes of Human Chromosome 18 in HepG2 Cell Lysate. Russ. J. Bioorg. Chem. 2018, 44, 759–768. [Google Scholar] [CrossRef]
- Ivanov, A.S.; Medvedev, A.; Ershov, P.; Molnar, A.; Mezentsev, Y.; Yablokov, E.; Kaluzhsky, L.; Gnedenko, O.; Buneeva, O.; Haidukevich, I.; et al. Protein interactomics based on direct molecular fishing on paramagnetic particles: Practical realization and further SPR validation. Proteomics 2014, 14, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Svirid, A.V.; Ershov, P.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Mezentsev, Y.V.; Florinskaya, A.V.; Sushko, T.A.; Strushkevich, N.V.; Gilep, A.A.; Usanov, S.A.; et al. Direct Molecular Fishing of New Protein Partners for Human Thromboxane Synthase. Acta Nat. 2017, 9, 92–100. [Google Scholar] [CrossRef]
- Ershov, P.; Mezentsev, Y.; Gnedenko, O.; Mukha, D.; Yantsevich, A.; Britikov, V.; Kaluzhskiy, L.; Yablokov, E.; Molnar, A.; Ivanov, A.; et al. Protein interactomics based on direct molecular fishing on paramagnetic particles: Experimental simulation and SPR validation. Proteomics 2012, 12, 3295–3298. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.-H.; Vreven, T.; Weng, Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Kim, D.E.; Chivian, D.; Baker, D. Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res. 2004, 32, W526–W531. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Florinskaya, A.V.; Ershov, P.V.; Mezentsev, Y.V.; Kaluzhskiy, L.A.; Yablokov, E.O.; Buneeva, O.A.; Zgoda, V.G.; Medvedev, A.E.; Ivanov, A.S. The Analysis of Participation of Individual Proteins in the Protein Interactome Formation. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2018, 12, 241–246. [Google Scholar]
- Florinskaya, A.; Ershov, P.; Mezentsev, Y.; Kaluzhskiy, L.; Yablokov, E.; Medvedev, A.; Ivanov, A. SPR biosensors in direct molecular fishing: Implications for protein interactomics. Sensors (Switzerland) 2018, 18, 1616. [Google Scholar] [CrossRef] [PubMed]
- Ershov, P.V.; Mezentsev, Y.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Vakhrushev, I.V.; Gnedenko, O.V.; Florinskaya, A.V.; Gilep, A.A.; Usanov, S.A.; Yarygin, K.N.; et al. A Method of Lysate Preparation to Improve the Isolation Efficiency of Protein Partners for Target Proteins Encoded by the Genes of Human Chromosome 18. Biomed. Chem. Res. Methods 2019, 2, e00090. [Google Scholar] [CrossRef]
- Ershov, P.V.; Mezentsev, Y.V.; Yablokov, E.O.; Kaluzhsky, L.A.; Florinskaya, A.V.; Buneeva, O.A.; Medvedev, A.E.; Ivanov, A.S. Effect of Bioregulator Isatin on Protein–Protein Interactions Involving Isatin-Binding Proteins. Russ. J. Bioorg. Chem. 2018, 44, 193–198. [Google Scholar] [CrossRef]
- Barski, O.A.; Tipparaju, S.M.; Bhatnagar, A. The Aldo-Keto Reductase Superfamily and its Role in Drug Metabolism and Detoxification. Drug Metab. Rev. 2008, 40, 553–624. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, P.J.; Morgenstern, R.; Mancini, J.; Ford-Hutchinson, A.; Persson, B. Common structural features of MAPEG—A widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Protein Sci. 1999, 8, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, P.-J.; Thorén, S.; Morgenstern, R.; Samuelsson, B. Identification of human prostaglandin E synthase: A microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA 1999, 96, 7220–7225. [Google Scholar] [CrossRef]
- Buckley, B.J.; Kent, R.S.; Whorton, A.R. Regulation of endothelial cell prostaglandin synthesis by glutathione. J. Biol. Chem. 1991, 266, 16659–16666. [Google Scholar]
- Walsh, S.W.; Wang, Y. Deficient glutathione peroxidase activity in preeclampsia is associated with increased placental production of thromboxane and lipid peroxides. Am. J. Obstet. Gynecol. 1993, 169, 1456–1461. [Google Scholar] [CrossRef]
- Bogaards, J.J.; Venekamp, J.C.; van Bladeren, P.J. Stereoselective conjugation of prostaglandin A2 and prostaglandin J2 with glutathione, catalyzed by the human glutathione S-transferases A1-1, A2-2, M1a-1a, and P1-1. Chem. Res. Toxicol. 1997, 10, 310–317. [Google Scholar] [CrossRef]
- Ellgaard, L.; Ruddock, L.W. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005, 6, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, K.; Wang, W.; Niu, Y.; Li, J.; Dong, Y.; Liu, Y.; Wang, C.-C.; Wang, L.; Liang, H. Crystal and solution structures of human protein-disulfide isomerase-like protein of the testis (PDILT) provide insight into its chaperone activity. J. Biol. Chem. 2018, 293, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Takase, K.; Kishino, J.; Fujita, M.; Okamura, N.; Sakaeda, T.; Fujimoto, M.; Yagami, T. Proteomic Identification of Protein Targets for 15-Deoxy-Δ12,14-Prostaglandin J2 in Neuronal Plasma Membrane. PLoS ONE 2011, 6, e17552. [Google Scholar] [CrossRef]
- Aguiar, M.; Masse, R.; Gibbs, B.F. Regulation of Cytochrome P450 by Posttranslational Modification. Drug Metab. Rev. 2005, 37, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Tinti, M.; Murugesan, G.; Berrett, E.; Stafford, M.; Toth, R.; Cole, C.; MacKintosh, C.; Barton, G.J. 14-3-3-Pred: Improved methods to predict 14-3-3-binding phosphopeptides. Bioinformatics 2015, 31, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-N.; Lu, J.-X.; Wang, X.-Q.; Shan, M.-R.; Miao, Z.; Pan, G.-P.; Jian, X.; Li, P.; Ping, S.; Pang, X.-Y.; et al. S-Nitrosylation of Prostacyclin Synthase Instigates Nitrate Cross-Tolerance In Vivo. Clin. Pharmacol. Ther. 2019, 105, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Dutka, T.L.; Mollica, J.P.; Lamboley, C.R.; Weerakkody, V.C.; Greening, D.W.; Posterino, G.S.; Murphy, R.M.; Lamb, G.D. S-nitrosylation and S-glutathionylation of Cys134 on troponin I have opposing competitive actions on Ca2+ sensitivity in rat fast-twitch muscle fibers. Am. J. Physiol. Cell Physiol. 2017, 312, C316–C327. [Google Scholar] [CrossRef]
- Dietz, K.-J. Redox control, redox signaling, and redox homeostasis in plant cells. Int. Rev. Cytol. 2003, 228, 141–193. [Google Scholar]
- Haunhorst, P.; Hanschmann, E.-M.; Bräutigam, L.; Stehling, O.; Hoffmann, B.; Mühlenhoff, U.; Lill, R.; Berndt, C.; Lillig, C.H. Crucial function of vertebrate glutaredoxin 3 (PICOT) in iron homeostasis and hemoglobin maturation. Mol. Biol. Cell 2013, 24, 1895–1903. [Google Scholar] [CrossRef]
- Li, H.; Mapolelo, D.T.; Randeniya, S.; Johnson, M.K.; Outten, C.E. Human Glutaredoxin 3 Forms [2Fe-2S]-Bridged Complexes with Human BolA2. Biochemistry 2012, 51, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.L.; Pinto, J.T.; Callery, P.S. Reversible and irreversible protein glutathionylation: Biological and clinical aspects. Expert Opin. Drug Metab. Toxicol. 2011, 7, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Meunier, L.; Usherwood, Y.-K.; Chung, K.T.; Hendershot, L.M. A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol. Biol. Cell 2002, 13, 4456–4469. [Google Scholar] [CrossRef] [PubMed]
- Askari, A.; Thomson, S.J.; Edin, M.L.; Zeldin, D.C.; Bishop-Bailey, D. Roles of the epoxygenase CYP2J2 in the endothelium. Prostaglandins Other Lipid Mediat. 2013, 107, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.Y. Transformation of prostacyclin (PGI2) to a biologically active metabolite: 5(6)-oxido-PGI1 by cytochrome P450-dependent epoxygenase. Adv. Exp. Med. Biol. 1990, 281, 245–250. [Google Scholar] [PubMed]
- Huang, Y.; Misquitta, S.; Blond, S.Y.; Adams, E.; Colman, R.F. Catalytically Active Monomer of Glutathione S-Transferase π and Key Residues Involved in the Electrostatic Interaction between Subunits. J. Biol. Chem. 2008, 283, 32880–32888. [Google Scholar] [CrossRef]
- Vargo, M.A.; Nguyen, L.; Colman, R.F. Subunit interface residues of glutathione S-transferase A1-1 that are important in the monomer-dimer equilibrium. Biochemistry 2004, 43, 3327–3335. [Google Scholar] [CrossRef]
- Fabrini, R.; De Luca, A.; Stella, L.; Mei, G.; Orioni, B.; Ciccone, S.; Federici, G.; Lo Bello, M.; Ricci, G. Monomer-dimer equilibrium in glutathione transferases: A critical re-examination. Biochemistry 2009, 48, 10473–10482. [Google Scholar] [CrossRef]
- Hearne, J.L.; Colman, R.F. Catalytically active monomer of class mu glutathione transferase from rat. Biochemistry 2006, 45, 5974–5984. [Google Scholar] [CrossRef]
- Adler, V.; Yin, Z.; Fuchs, S.Y.; Benezra, M.; Rosario, L.; Tew, K.D.; Pincus, M.R.; Sardana, M.; Henderson, C.J.; Wolf, C.R.; et al. Regulation of JNK signaling by GSTp. EMBO J. 1999, 18, 1321–1334. [Google Scholar] [CrossRef]
- Kura, T.; Takahashi, Y.; Takayama, T.; Ban, N.; Saito, T.; Kuga, T.; Niitsu, Y. Glutathione S-transferase-pi is secreted as a monomer into human plasma by platelets and tumor cells. Biochim. Biophys. Acta 1996, 1292, 317–323. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanisms of actions, and their biomedical implications. BioFactors 2018, 44, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Ershov, P.V.; Mezentsev, Y.V.; Yablokov, E.O.; Kaluzhskiy, L.A.; Florinskaya, A.V.; Svirid, A.V.; Gilep, A.A.; Usanov, S.A.; Medvedev, A.E.; Ivanov, A.S. Specificity of Isatin Interaction with Cytochromes P450. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2018, 12, 130–135. [Google Scholar] [CrossRef]
| No | Mw, Da | Name | Sequence | Purity by HPLC, % |
|---|---|---|---|---|
| 1 | 2640 | GLRX3_23 | K-E-L-E-A-S-E-E-L-D-T-I-C-P-K-A-A-E-N-L-Y-F-Q | >90 |
| 2 | 2597 | GSTM1_20 | D-Y-D-R-S-Q-W-L-N-E-K-F-K-L-G-L-D-F-P-N-L | >90 |
| 3 | 2437 | TF-20 | T-D-F-P-Q-L-C-Q-L-C-P-G-C-G-C-S-T-L-N-Q-Y-F | >90 |
| 4 | 2284 | GSTM1_19 | N-Q-T-M-D-N-H-M-Q-L-G-M-I-C-Y-N-P-E-F | >95 |
| 5 | 2278 | GLRX3_20 | D-I-V-K-E-L-K-E-N-G-E-L-L-P-I-L-R-G-E-N | >90 |
| 6 | 2230 | GSTA1_19 | Q-T-R-A-I-L-N-Y-I-A-S-K-Y-N-L-Y-G-K-D | >90 |
| 7 | 2141 | AkrA1_18(2) | K-R-V-P-R-D-A-G-H-P-L-Y-P-F-N-D-P-Y | >95 |
| 8 | 2042 | HINT2_17 | S-V-Y-H-L-H-I-H-V-L-G-G-R-Q-L-Q-W | >90 |
| 9 | 2202 | AkrA1_18 | K-Q-L-D-A-L-N-K-N-W-R-Y-I-V-P-M-I-T | >90 |
| 10 | 1976 | HINT2_19 | T-A-K-A-E-G-L-G-D-G-Y-R-L-V-I-N-D-G-K | >90 |
| 11 | 1961 | GLRX3_18 | E-V-G-S-A-G-Q-F-E-E-L-L-R-L-K-A-K-S | >90 |
| 12 | 1897 | GSTA1_15 | M-D-E-K-S-L-E-E-A-R-K-I-F-R-F | >90 |
| Tissue Lysates | Biosensor Signal after Sample Injection, RU * | Dissociation Rate, RU/min | Relative Dissociation, % | ||
|---|---|---|---|---|---|
| - | After 30 s | After 360 s | Δ300 s | - | - |
| Testis | 126 ± 4 ** | 88 ± 4 | 38 | 8 | 30% |
| Lung | 74 ± 3 | 56 ± 3 | 18 | 4 | 24% |
| Liver | 65 ± 3 | 52 ± 3 | 13 | 3 | 20% |
| Heart | 51 ± 3 | 38 ± 2 | 13 | 3 | 25% |
| Aorta | 48 ± 2 | 26 ± 2 | 22 | 5 | 46% |
| Brain | 32 ± 2 | 19 ± 2 | 13 | 3 | 41% |
| Buffer Type | Volume, mL | Volume (on a Cumulative Basis), mL | Empty Sorbent (without PTGIS Immobilization) | Affine Sorbent (with PTGIS) |
|---|---|---|---|---|
| Wash buffer | Average binding levels (n = 2) with PTGIS on the chip, RU * | |||
| 1X HBS-EP+ | 1 | 1.0 | 67 | 73 |
| 1X HBS-EP+ | 1 | 2.0 | 58 | 48 |
| 1X HBS-EP+ | 1 | 3.0 | 17 | 23 |
| 1X HBS-EP+ | 1 | 4.0 | 3 | 8 |
| 1X HBS-EP+ | 1 | 5.0 | 0 | 5 |
| Total | 145 | 157 | ||
| Elution buffer | ||||
| 0.25 M NaCl and 0.4% CHAPS | 0.5 | 5.5 | 0 | 0 |
| 0.5 M NaCl and 0.4% CHAPS | 0.5 | 6.0 | 0 | 7 |
| 0.75 M NaCl and 0.4% CHAPS | 0.5 | 6.5 | 0 | 8 |
| 1.0 M NaCl and 0.4% CHAPS | 0.5 | 7.0 | 1 | 13 |
| 1.5 M NaCl and 0.4% CHAPS | 0.5 | 7.5 | 1 | 10 |
| 2.0 M NaCl and 0.4% CHAPS | 0.5 | 8.0 | 8 | 26 |
| Total | 10 | 54 | ||
| Protein Name | Gene Name | Subunit Structure * | Groups of Proteins ** (According to SEC Profiling of Lysate) | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | |||
| PROTEIN PARTNERS ISOLATED FROM INTACT LYSATE ONLY | |||||||
| Cold-inducible RNA-binding protein | Cirbp | ■ | |||||
| Cytochrome P450, family 2, subfamily j, polypeptide 10 | Cyp2j10 | ■ | |||||
| Elongation factor 1-alpha 1 | Eef1a1 | ■ | |||||
| Endoplasmin | Hsp90b1 | ■ | |||||
| Glutathione S-transferase | Gstm1 | Homodimer | ■ | ||||
| Glutathione S-transferase alpha-1 | Gsta1 | Homodimer | ■ | ||||
| Glutathione S-transferase alpha-3 | Gsta3 | Homodimer | ■ | ||||
| Hemoglobin subunit alpha-1/2 | Hba1 | ■ | |||||
| Hemoglobin subunit beta-2 | Hbb2 | ■ | |||||
| Histone H4 | Hist1h4b | Heterooligomer | ■ | ||||
| Protein disulfide-isomerase A6 | Pdia6 | ■ | |||||
| Superoxide dismutase [Cu-Zn] | Sod1 | Homodimer | ■ | ||||
| tRNA-splicing ligase RtcB homolog | Rtcb | ■ | |||||
| Ubiquitin-conjugating enzyme E2 N | Ube2n | Heterodimer | ■ | ||||
| PROTEIN PARTNERS ISOLATED FROM ACID TREATED LYSATE ONLY | |||||||
| 14-3-3 protein theta | Ywhaq | Homodimer | ■ | ■ | |||
| 14-3-3 protein zeta/delta | Ywhaz | Homodimer | ■ | ■ | |||
| Actin, alpha skeletal muscle | Acta1 | ■ | |||||
| Alcohol dehydrogenase [NADP(+)] | Akr1a1 | ■ | |||||
| Calmodulin-1 | Calm1 | ■ | |||||
| Calnexin | Canx | ■ | |||||
| Calreticulin | Calr | ■ | |||||
| cAMP-dependent protein kinase type I-alpha regulatory subunit | Prkar1a | ■ | |||||
| Heat shock 70 kDa protein 1-like | Hspa1l | ■ | |||||
| Heat shock cognate 71 kDa protein | Hspa8 | ■ | |||||
| Peroxiredoxin-4 | Prdx4 | Homodimer | ■ | ||||
| Peroxiredoxin-6 | Prdx6 | Homodimer | ■ | ||||
| Protein disulfide-isomerase-like protein of the testis | Pdilt | Homodimer | ■ | ||||
| Ptges3 protein | Ptges3 | ■ | |||||
| PROTEIN PARTNERS ISOLATED FROM BOTH INTACT AND ACID TREATED LYSATES | |||||||
| 14-3-3 protein epsilon | Ywhae | Homodimer | ■ | ■ | |||
| 78 kDa glucose-regulated protein | Hspa5 | ■ | |||||
| Actin, cytoplasmic 2 | Actg1 | ■ | |||||
| Elongation factor 1-gamma | Eef1g | ■ | |||||
| Glutathione peroxidase | Gpx4 | ■ | |||||
| Glutathione S-transferase | Gstm2 | Homodimer | ■ | ||||
| Glutathione S-transferase Mu 5 | Gstm5 | Homodimer | ■ | ||||
| Glyceraldehyde-3-phosphate dehydrogenase | Gapdh | Homotetramer | ■ | ||||
| Protein disulfide-isomerase | Pdia3 | ■ | |||||
| Serotransferrin | Tf | ■ | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ershov, P.V.; Mezentsev, Y.V.; Kopylov, A.T.; Yablokov, E.O.; Svirid, A.V.; Lushchyk, A.Y.; Kaluzhskiy, L.A.; Gilep, A.A.; Usanov, S.A.; Medvedev, A.E.; et al. Affinity Isolation and Mass Spectrometry Identification of Prostacyclin Synthase (PTGIS) Subinteractome. Biology 2019, 8, 49. https://doi.org/10.3390/biology8020049
Ershov PV, Mezentsev YV, Kopylov AT, Yablokov EO, Svirid AV, Lushchyk AY, Kaluzhskiy LA, Gilep AA, Usanov SA, Medvedev AE, et al. Affinity Isolation and Mass Spectrometry Identification of Prostacyclin Synthase (PTGIS) Subinteractome. Biology. 2019; 8(2):49. https://doi.org/10.3390/biology8020049
Chicago/Turabian StyleErshov, Pavel V., Yuri V. Mezentsev, Arthur T. Kopylov, Evgeniy O. Yablokov, Andrey V. Svirid, Aliaksandr Ya. Lushchyk, Leonid A. Kaluzhskiy, Andrei A. Gilep, Sergey A. Usanov, Alexey E. Medvedev, and et al. 2019. "Affinity Isolation and Mass Spectrometry Identification of Prostacyclin Synthase (PTGIS) Subinteractome" Biology 8, no. 2: 49. https://doi.org/10.3390/biology8020049
APA StyleErshov, P. V., Mezentsev, Y. V., Kopylov, A. T., Yablokov, E. O., Svirid, A. V., Lushchyk, A. Y., Kaluzhskiy, L. A., Gilep, A. A., Usanov, S. A., Medvedev, A. E., & Ivanov, A. S. (2019). Affinity Isolation and Mass Spectrometry Identification of Prostacyclin Synthase (PTGIS) Subinteractome. Biology, 8(2), 49. https://doi.org/10.3390/biology8020049





