Harvesting Environmental Microalgal Blooms for Remediation and Resource Recovery: A Laboratory Scale Investigation with Economic and Microbial Community Impact Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Measurement of Water Retention Pond Abiotic and Biotic Variables
2.3. Pure Microalgae Cultivation and Preliminary Sedimentation Tests
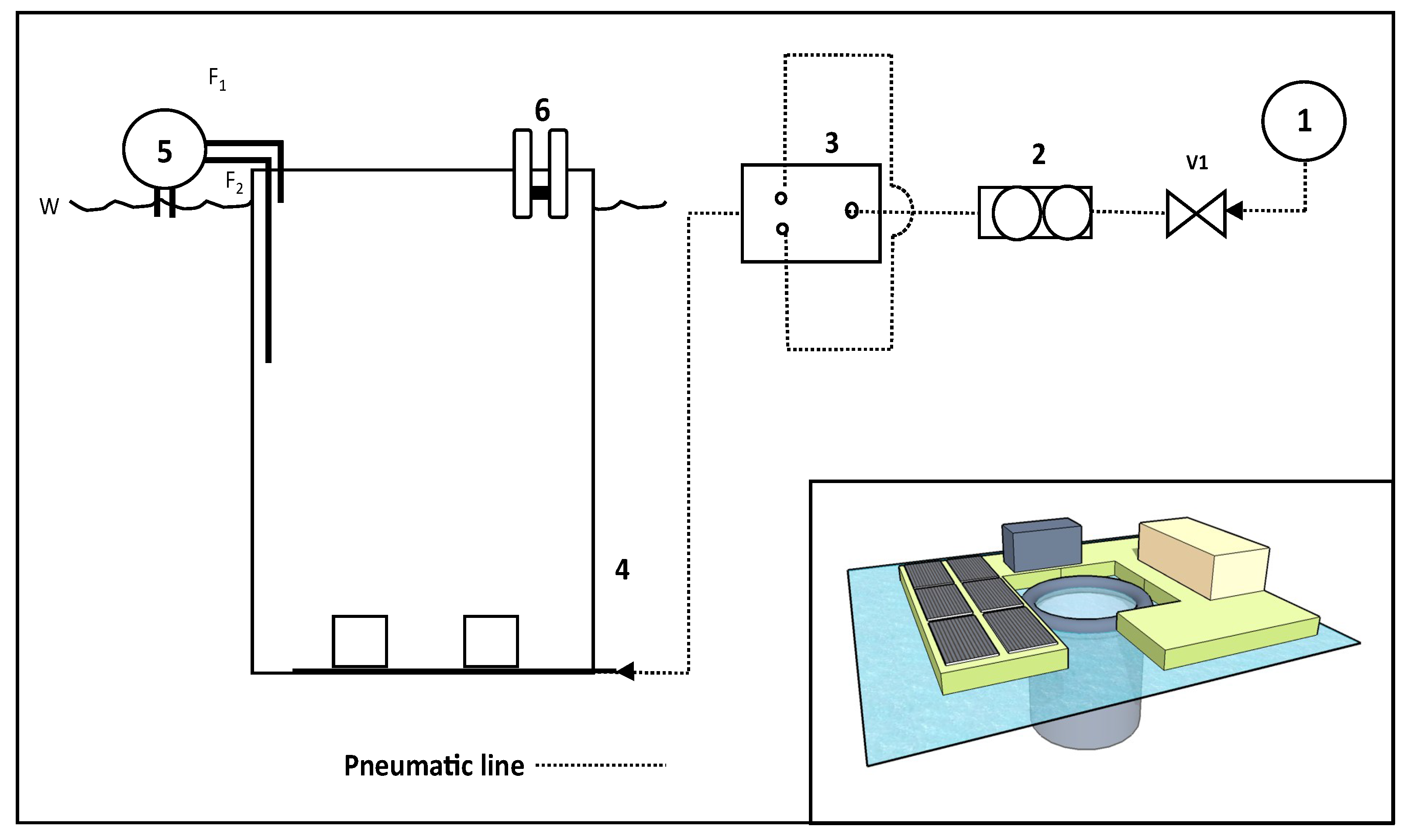
2.4. WRP Sample Sedimentation and eFLOAT
2.5. Biochemical Composition
2.6. Fatty Acid Methyl Ester Composition
2.7. Microbial Diversity Analysis (16S and 18S rDNA Gene Sequencing)
3. Results
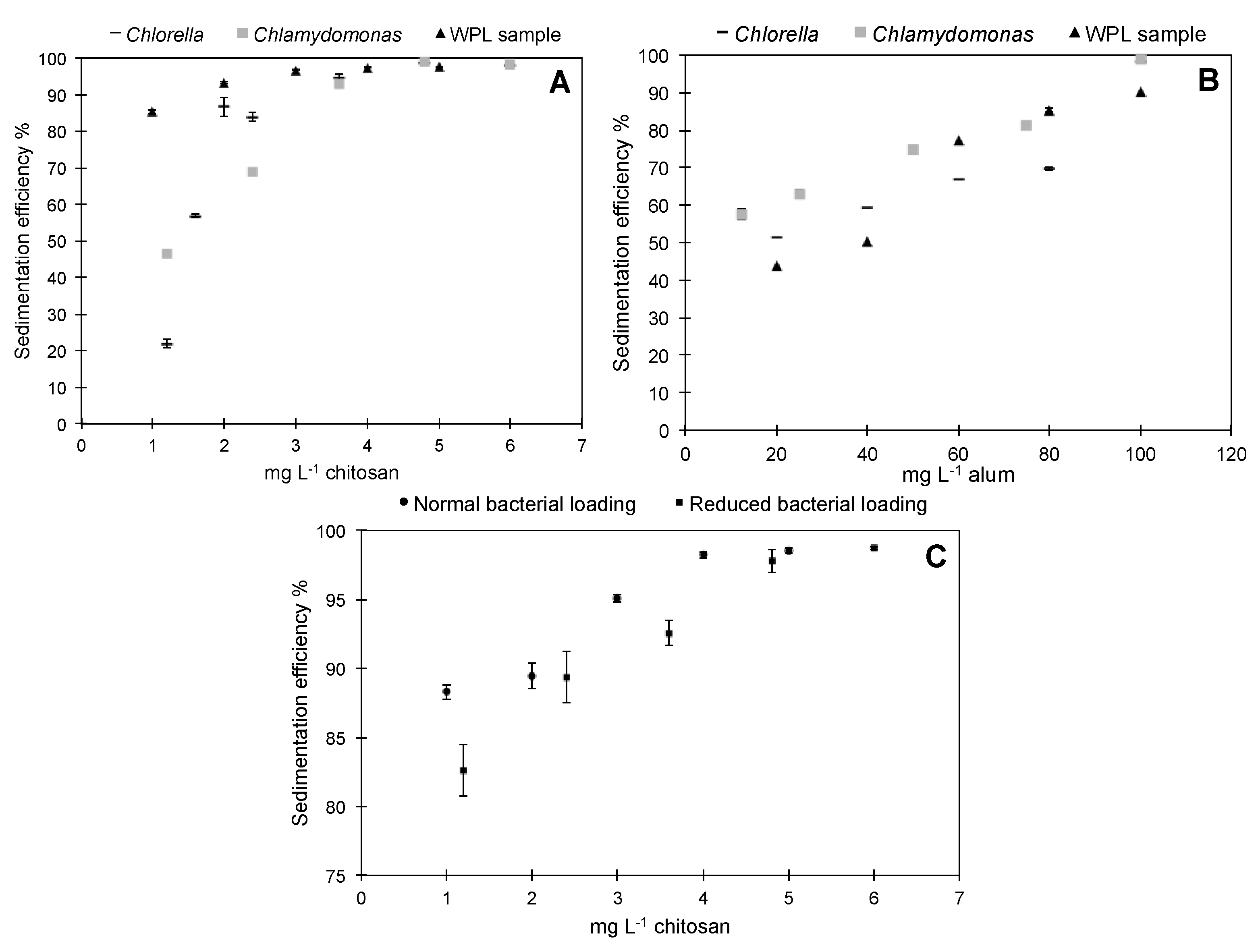
3.1. Preliminary Sedimentation Tests with Pure Microalgae and WPL Sample
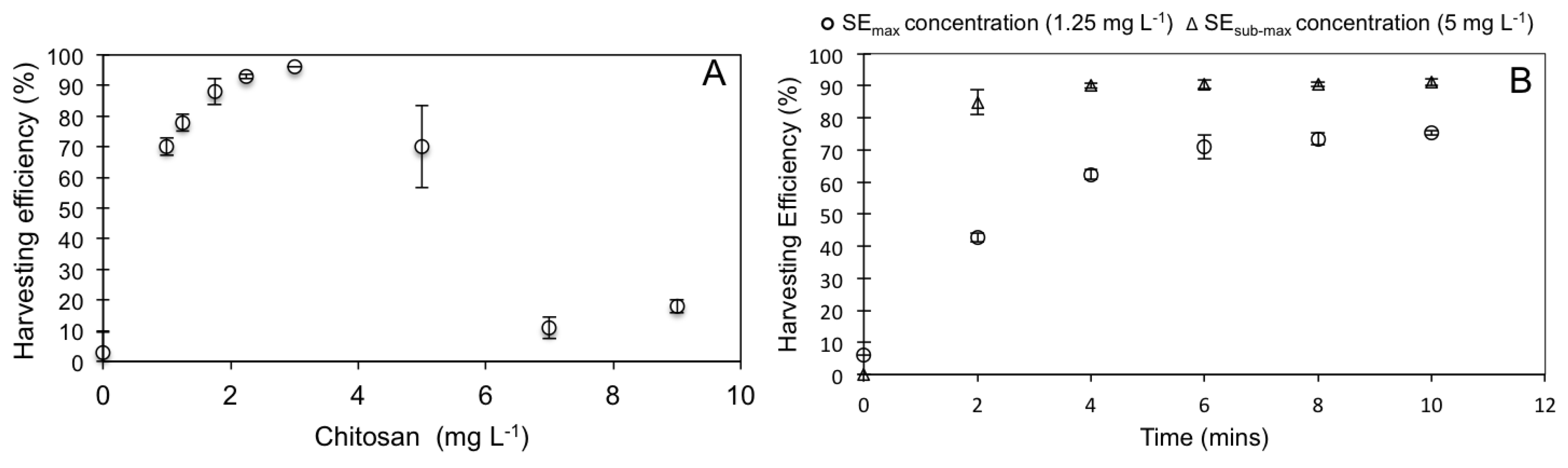
3.2. WRP Pond Sedimentation Test and eFLOAT
3.3. Resource Analysis
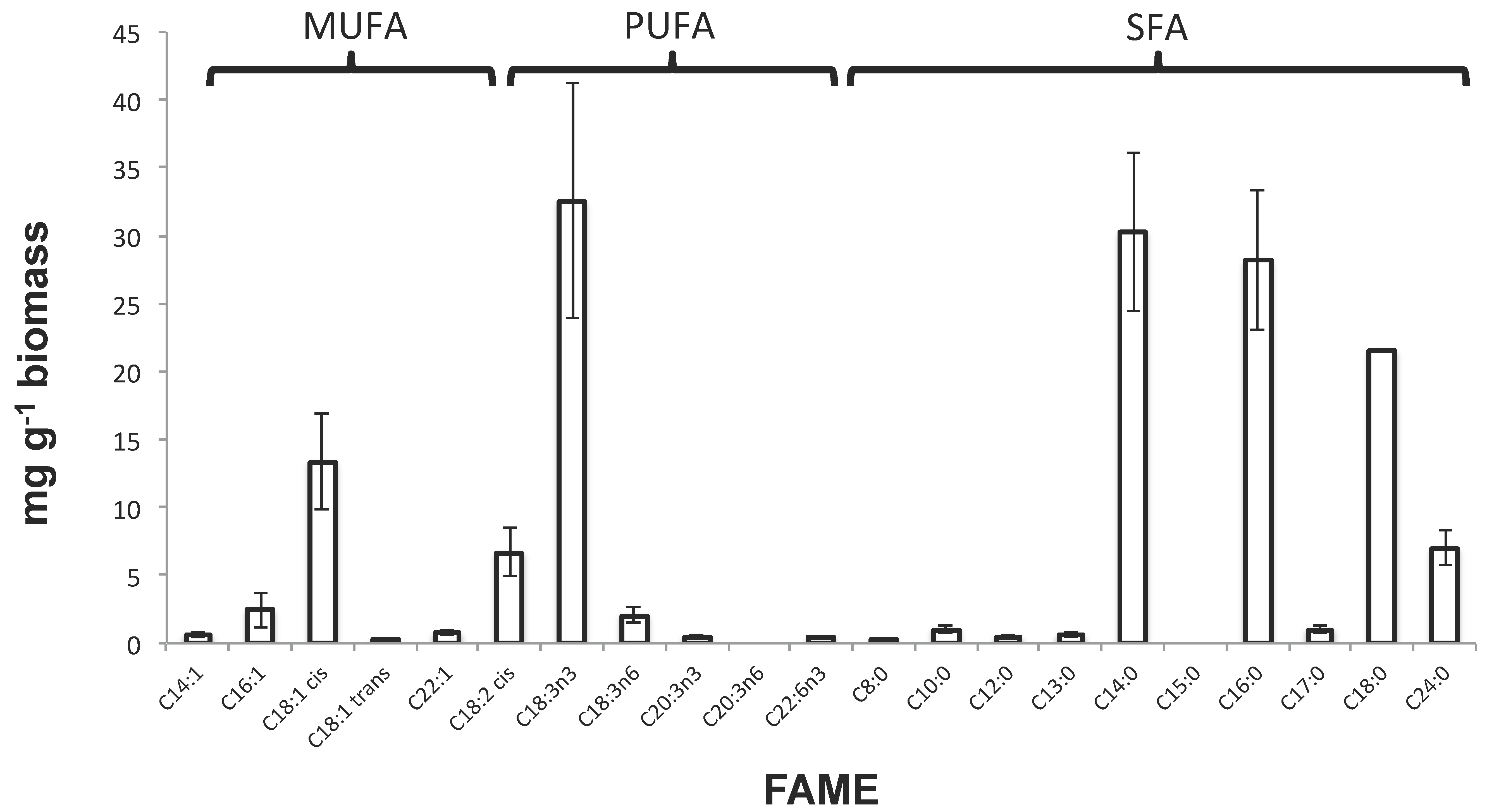
3.3.1. Total Lipids and FAME Analyses
3.3.2. Phosphorus Content Analyses
3.3.3. Protein Content Analyses
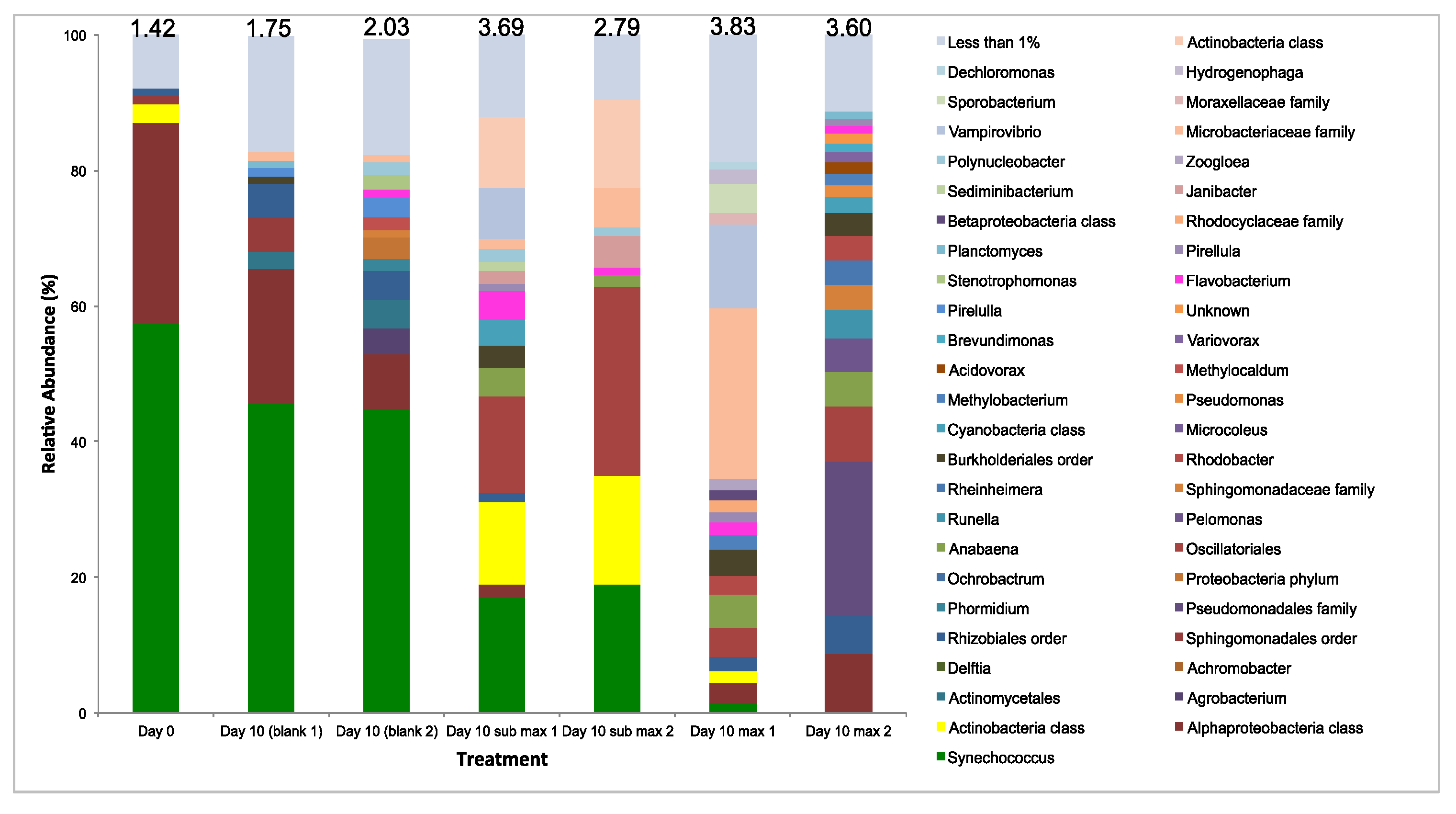
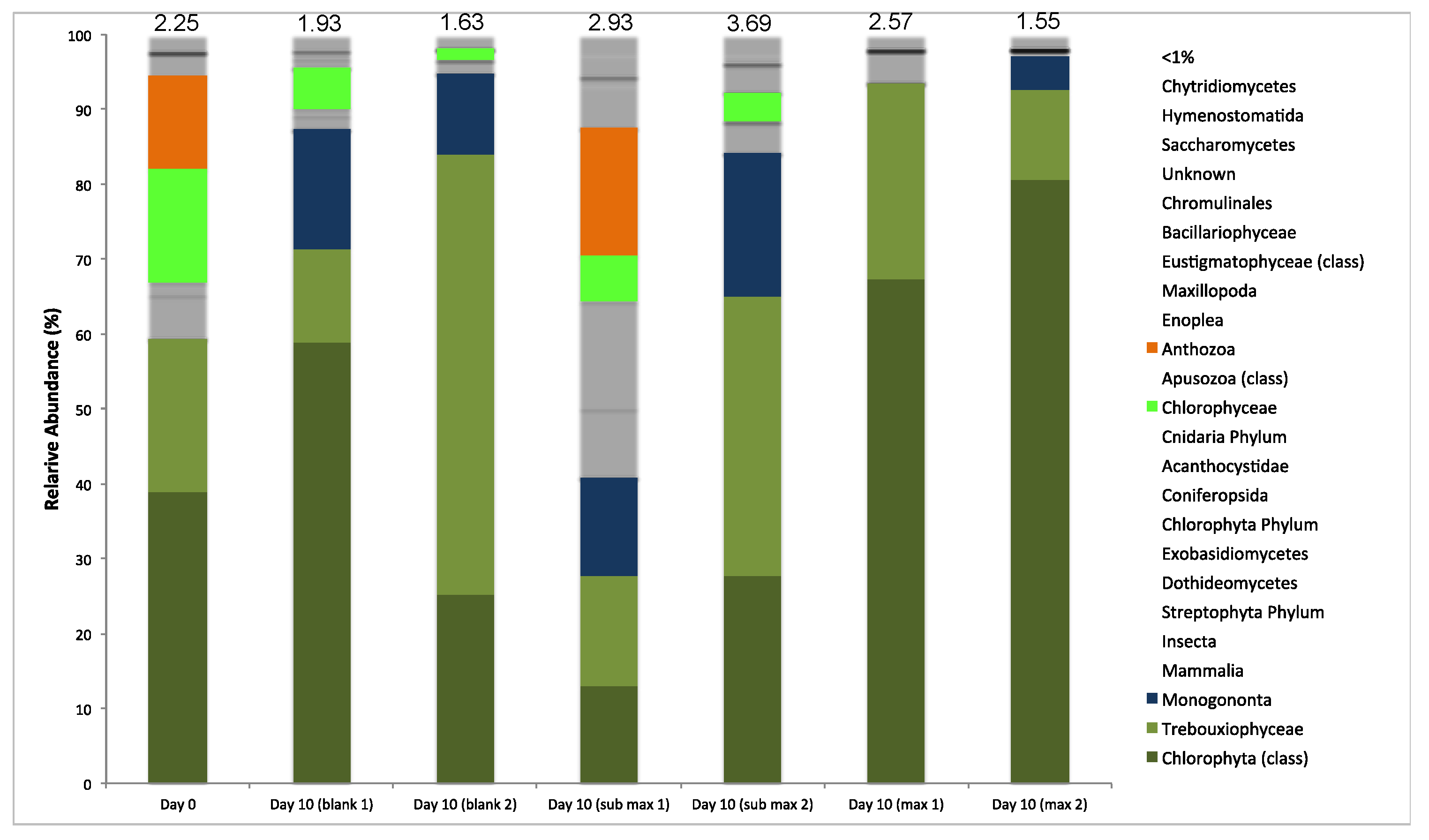
3.3.4. Microbial Diversity Analysis
3.4. A Full Size Modular eFLOAT System
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sutton, M.A. Our Nutrient World: The Challenge to Produce More Food and Energy with Less Pollution; Centre of Ecology and Hydrology: Bailrigg, UK, 2013; p. 114. [Google Scholar]
- Yang, X.E.; Wu, X.; Hao, H.L.; He, Z.L. Mechanisms and assessment of water eutrophication. J. Zhejiang Univ.-Sci. B 2008, 9, 197–209. [Google Scholar] [CrossRef] [PubMed]
- O’neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The rise of harmful cyanobacteria blooms: The potential roles of eutrophication and climate change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloglu, I.; et al. Record-setting algal bloom in lake erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Dodds, W.K.; Bouska, W.W.; Eitzmann, J.L.; Pilger, T.J.; Pitts, K.L.; Riley, A.J.; Schloesser, J.T.; Thornbrugh, D.J. Eutrophication of U.S. Freshwaters: Analysis of potential economic damages. Environ. Sci. Technol. 2009, 43, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Le, C.; Zha, Y.; Li, Y.; Sun, D.; Lu, H.; Yin, B. Eutrophication of lake waters in china: Cost, causes, and control. Environ. Manag. 2010, 45, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Wentworth, J. Diffuse Pollution of Water by Agriculture; Houses of Parliament: London, UK, 2014. [Google Scholar]
- Spears, B.M.; Carvalho, L.; Perkins, R.; Kirika, A.; Paterson, D.M. Long-term variation and regulation of internal phosphorus loading in loch leven. Hydrobiologia 2012, 681, 23–33. [Google Scholar] [CrossRef]
- Spears, B.M.; Dudley, B.; Reitzel, K.; Rydin, E. Geo-engineering in lakes-a call for consensus. Environ. Sci. Technol. 2013, 47, 3953–3954. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Chow, S.; Ketter, B.; Fung Shek, C.; Yacar, D.; Tang, Y.; Zivojnovich, M.; Betenbaugh, M.J.; Bouwer, E.J. Phytoremediation of agriculture runoff by filamentous algae poly-culture for biomethane production, and nutrient recovery for secondary cultivation of lipid generating microalgae. Bioresour. Technol. 2016, 222, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Srinivasan, A.; Viraraghavan, T. Dissolved air flotation in industrial wastewater treatment. In Water and Wastewater Treatment Technologies; Eolss Publishers: Oxford, UK, 2008. [Google Scholar]
- Makuta, T.; Takemura, F. Simulation of micro gas bubble generation of uniform diameter in an ultrasonic field by a boundary element method. Phys. Fluids 2006, 18. [Google Scholar] [CrossRef]
- Eddy, M.; Tchobanoglous, G.; Burton, F.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill Education: New York, NY, USA, 2002. [Google Scholar]
- Zimmerman, W.B.; Tesar, V.; Butler, S.; Bandulasena, H. Microbubble generation. Recent Pat. Eng. 2008, 2, 1–8. [Google Scholar] [CrossRef]
- Hanotu, J.; Bandulasena, H.C.H.; Zimmerman, W.B. Microflotation performance for algal separation. Biotechnol. Bioeng. 2011, 109, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, W.B.; Tesar, V.; Bandulasena, H.; Omotowa, O. Efficiency of an Aerator Driven by Fluidic Oscillation. Part ii: Pilot Scale Trials with Flexible Membrane Diffusers. Available online: http://eyrie.shef.ac.uk/steelCO2/open/aerationefficiencypart2.pdf (accessed on 24 December 2017).
- Hanotu, J.; Karunakaran, E.; Bandulasena, H.; Biggs, C.; Zimmerman, W.B. Harvesting and dewatering yeast by microflotation. Biochem. Eng. J. 2014, 82, 174–182. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Stanley, M.; Jenkins, T. A UK Roadmap for Algal Technologies. NERC-TSB: 2013. Available online: http://discovery.ucl.ac.uk/id/eprint/1462835 (accessed on 24 Decemebr 2017).
- Wellburn, A.R. The spectral determination of chlorophyll-a and chlorophhyll-b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Teeling, H.; Fuchs, B.M.; Becher, D.; Klockow, C.; Gardebrecht, A.; Bennke, C.M.; Kassabgy, M.; Huang, S.; Mann, A.J.; Waldmann, J.; et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 2012, 336, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Brittle, S.; Desai, P.; Ng, W.C.; Dunbar, A.; Howell, R.; Tesar, V.; Zimmerman, W.B. Minimising microbubble size through oscillation frequency control. Chem. Eng. Res. Des. 2015, 104, 357–366. [Google Scholar] [CrossRef]
- Mayers, J.J.; Flynn, K.J.; Shields, R.J. Rapid determination of bulk microalgal biochemical composition by fourier-transform infrared spectroscopy. Bioresour. Technol. 2013, 148, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, S.O.; Barbarino, E.; Lavin, P.L.; Marque, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. Citation-classic—A modified single solution method for the determination of phosphate in natural-waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Vandenbrouck, T.; Jones, O.A.H.; Dom, N.; Griffin, J.L.; De Coen, W. Mixtures of similarly acting compounds in daphnia magna: From gene to metabolite and beyond. Environ. Int. 2010, 36, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.A.; Couto, N.; Beckerman, A.P.; Pandhal, J. A metaproteomic analysis of the response of a freshwater microbial community under nutrient enrichment. Front. Microbiol. 2016, 7, 1172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kobert, K.; Flouri, T.; Stamatakis, A. Pear: A fast and accurate illumina paired-end read merger. Bioinformatics 2014, 30, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than blast. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.; Rideout, J.; Kopylova, E.; Bolyen, E.; Patnode, J.; Ellett, Z.; McDonald, D.; Wolfe, B.; Maurice, C.; Dutton, R.; et al. A standardized, extensible framework for optimizing classification improves marker-gene taxonomic assignments. PeerJ PrePrints 2015, 3. [Google Scholar] [CrossRef]
- Bao, E.; Jiang, T.; Kaloshian, I.; Girke, T. Seed: Efficient clustering of next-generation sequences. Bioinformatics 2011, 27, 2502–2509. [Google Scholar] [CrossRef] [PubMed]
- Shannon, C.E. The mathematical theory of communication (reprinted). MDComputers 1997, 14, 306–317. [Google Scholar]
- Divakaran, R.; Pillai, V.N.S. Flocculation of algae using chitosan. J. Appl. Phycol. 2002, 14, 419–422. [Google Scholar] [CrossRef]
- Gutzeit, G.; Lorch, D.; Weber, A.; Engels, M.; Neis, U. Bioflocculent algal-bacterial biomass improves low-cost wastewater treatment. Water Sci. Technol. 2005, 52, 9–18. [Google Scholar] [PubMed]
- Chen, G.Y.; Zhao, L.; Qi, Y.; Cui, Y.L. Chitosan and its derivatives applied in harvesting microalgae for biodiesel production: An outlook. J. Nanomater. 2014, 2014, 217537. [Google Scholar] [CrossRef]
- Henderson, R.; Chips, M.; Cornwell, N.; Hitchins, P.; Holden, B.; Hurley, S.; Parsons, S.A.; Wetherill, A.; Jefferson, B. Experiences of algae in uk waters: A treatment perspective. Water Environ. J. 2008, 22, 184–192. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Breuer, G.; Evers, W.A.C.; de Vree, J.H.; Kleinegris, D.M.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Analysis of fatty acid content and composition in microalgae. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Gharami, K.; Das, M.; Das, S. Essential role of docosahexaenoic acid towards development of a smarter brain. Neurochem. Int. 2015, 89, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Letawe, C.; Boone, M.; Pierard, G.E. Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones. Clin. Exp. Dermatol. 1998, 23, 56–58. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.C. Evening primrose oil for atopic dermatitis—Time to say goodnight. BMJ 2003, 327, 1358–1359. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chaang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Sharpley, A.; Jarvie, H.P.; Buda, A.; May, L.; Spears, B.; Kleinman, P. Phosphorus legacy: Overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 2013, 42, 1308–1326. [Google Scholar] [CrossRef] [PubMed]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.M.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Krohn, B.J.; McNeff, C.V.; Yan, B.; Nowlan, D. Production of algae-based biodiesel using the continuous catalytic mcgyan® process. Bioresour. Technol. 2011, 102, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Smolinske, S.C. CRC Handbook of Food, Drug, and Cosmetic Excipients; Taylor & Francis: Didcot, UK, 1992. [Google Scholar]
- Becker, E.W. Microalgae in human and animal nutrition. Biotechnol. Adv. 2007, 25. [Google Scholar] [CrossRef]
- Paerl, H.W.; Dyble, J.; Moisander, P.H.; Noble, R.T.; Piehler, M.F.; Pinckney, J.L.; Steppe, T.F.; Twomey, L.; Valdes, L.M. Microbial indicators of aquatic ecosystem change: Current applications to eutrophication studies. FEMS Microbiol. Ecol. 2003, 46, 233–246. [Google Scholar] [CrossRef]
- Qin, B.Q.; Gao, G.; Zhu, G.W.; Zhang, Y.L.; Song, Y.Z.; Tang, X.M.; Xu, H.; Deng, J.M. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2013, 58, 961–970. [Google Scholar] [CrossRef]
- Pan, G.; Chen, J.; Anderson, D.M. Modified local sands for the mitigation of harmful algal blooms. Harmful Algae 2011, 10, 381–387. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Value | SD | Unit |
|---|---|---|---|
| Temperature | 18.1 | - | ˚C |
| pH | 7.01 | - | |
| DO | 9.86 | - | mg·L−1 |
| Conductivity | 302.1 | - | µS·cm−1 |
| Salinity | 0.17 | - | PSU |
| Dry Weight | 0.13 | 0.003 | g·L−1 |
| Chlorophyll a | 443.23 | - | µg·L−1 |
| F− | 0.00 | 0.00 | mg·L−1 |
| Cl− | 26.49 | 0.21 | mg·L−1 |
| NO2− | 9.72 | 0.02 | mg·L−1 |
| SO42− | 6.00 | 0.04 | mg·L−1 |
| Br− | 0.00 | 0.00 | mg·L−1 |
| NO3− | 2.59 | 0.04 | mg·L−1 |
| PO43− | 6.11 | 0.02 | mg·L−1 |
| Na+ | 16.85 | 0.10 | mg·L−1 |
| NH4+ | 0.48 | 0.02 | mg·L−1 |
| K+ | 4.02 | 0.06 | mg·L−1 |
| Mg2+ | 9.15 | 0.00 | mg·L−1 |
| Ca2+ | 37.42 | 0.01 | mg·L−1 |
| Item | CAPEX (£) | OPEX (£) | Application Time (min) | Full Cost for WRP (7.5 ML) |
|---|---|---|---|---|
| Compressor | 200 | 0.83 | 30 | 259 |
| Rotameter | 60 | 0 | 30 | 0 |
| Fluidic oscillator | 100 | 0 | 30 | 0 |
| eFLOAT tank | 1420 * | 0 | n/a | 0 |
| Pumps | 1000 | 0.077 | 96 | 24 |
| Skimmer | 1000 | 1.20 ** | 30 | 375 |
| Chitosan | n/a | 30 Kg−1 | 5 | 1125 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandhal, J.; Choon, W.L.; Kapoore, R.V.; Russo, D.A.; Hanotu, J.; Wilson, I.A.G.; Desai, P.; Bailey, M.; Zimmerman, W.J.; Ferguson, A.S. Harvesting Environmental Microalgal Blooms for Remediation and Resource Recovery: A Laboratory Scale Investigation with Economic and Microbial Community Impact Assessment. Biology 2018, 7, 4. https://doi.org/10.3390/biology7010004
Pandhal J, Choon WL, Kapoore RV, Russo DA, Hanotu J, Wilson IAG, Desai P, Bailey M, Zimmerman WJ, Ferguson AS. Harvesting Environmental Microalgal Blooms for Remediation and Resource Recovery: A Laboratory Scale Investigation with Economic and Microbial Community Impact Assessment. Biology. 2018; 7(1):4. https://doi.org/10.3390/biology7010004
Chicago/Turabian StylePandhal, Jagroop, Wai L. Choon, Rahul V. Kapoore, David A. Russo, James Hanotu, I. A. Grant Wilson, Pratik Desai, Malcolm Bailey, William J. Zimmerman, and Andrew S. Ferguson. 2018. "Harvesting Environmental Microalgal Blooms for Remediation and Resource Recovery: A Laboratory Scale Investigation with Economic and Microbial Community Impact Assessment" Biology 7, no. 1: 4. https://doi.org/10.3390/biology7010004
APA StylePandhal, J., Choon, W. L., Kapoore, R. V., Russo, D. A., Hanotu, J., Wilson, I. A. G., Desai, P., Bailey, M., Zimmerman, W. J., & Ferguson, A. S. (2018). Harvesting Environmental Microalgal Blooms for Remediation and Resource Recovery: A Laboratory Scale Investigation with Economic and Microbial Community Impact Assessment. Biology, 7(1), 4. https://doi.org/10.3390/biology7010004








