The Role of Oocyte Organelles in Determining Developmental Competence
Abstract
1. Introduction
2. Models of Oocyte Competence
2.1. Follicle and Oocyte Size
2.2. Glucose-6-Phosphate Dehydrogenase Activity
2.3. In Vivo Versus In Vitro Maturation
2.4. Maternal Age
3. Mitochondria and Oocyte Quality
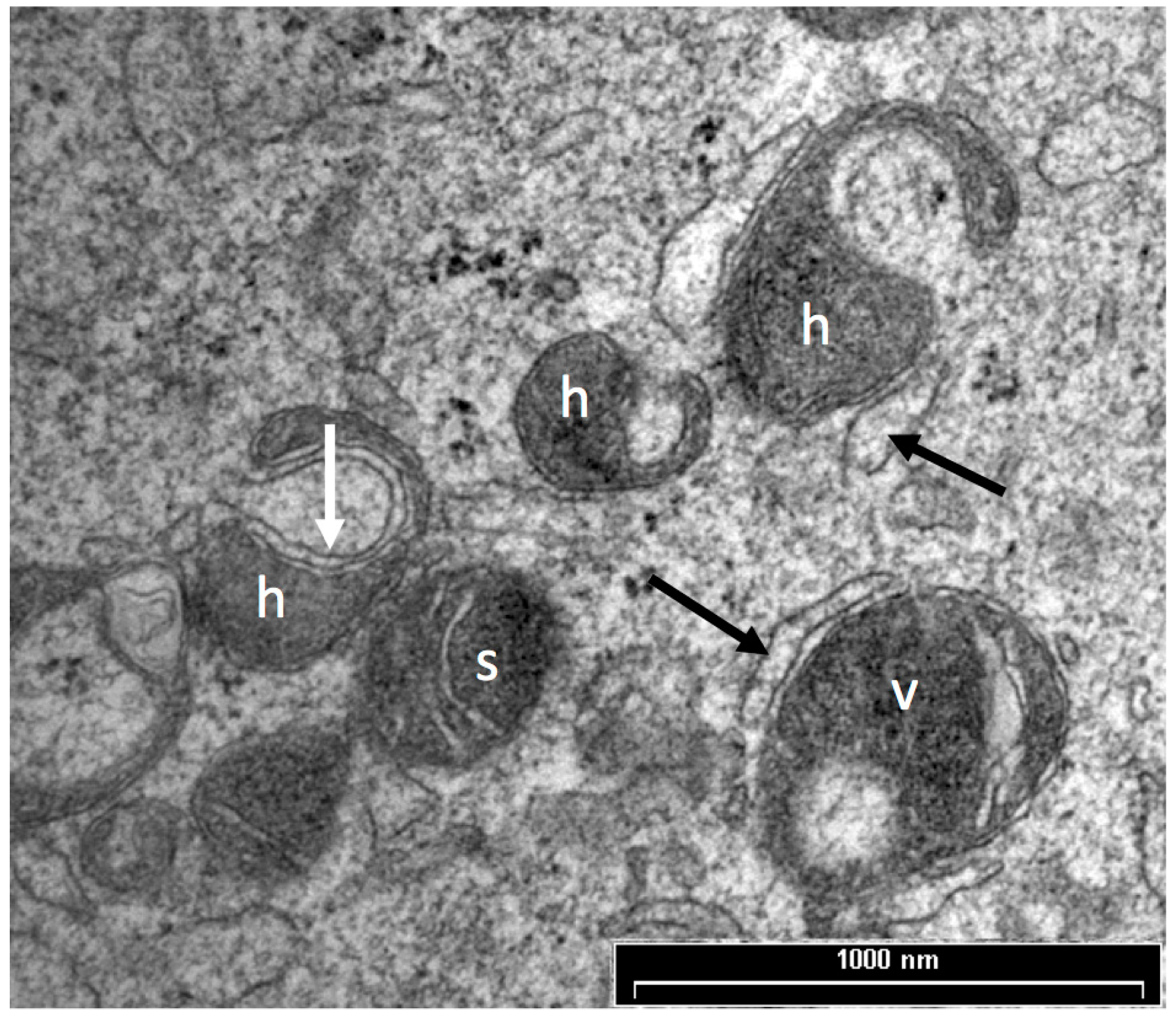
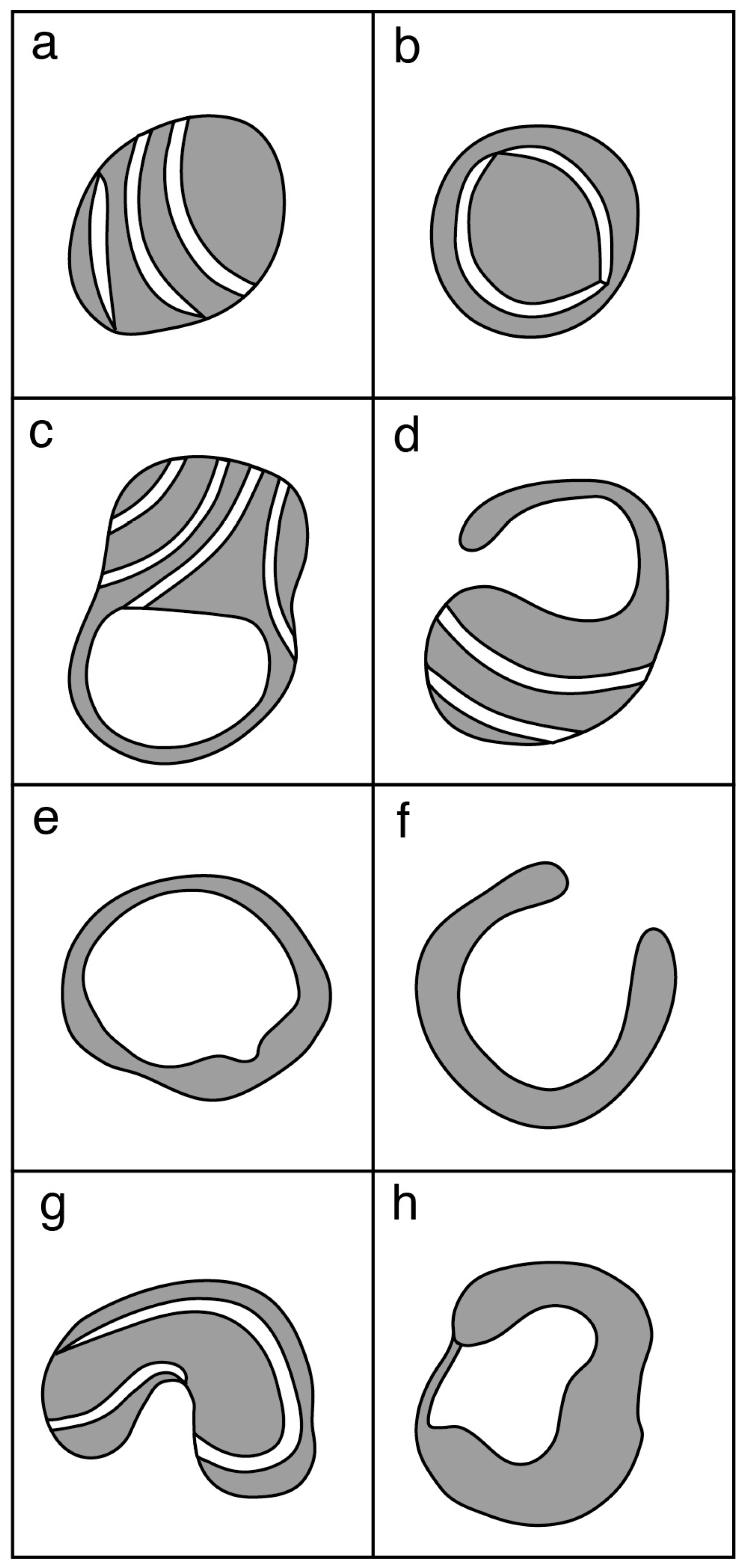
3.1. Mitochondrial Morphology
3.2. Mitochondrial Distribution
3.3. Mitochondrial Quantity
3.3.1. Mitochondrial Organelle Number and Oocyte Quality
3.3.2. Mitochondrial DNA Copy Number and Oocyte Quality
3.3.3. Mitochondrial Activity and Oocyte Quality
3.4. Summary of Mitochondria in Oocyte Quality
4. Role of Lipid Droplets in Oocyte Quality
4.1. Lipid Droplet Quantity
4.2. Lipid Droplet Distribution
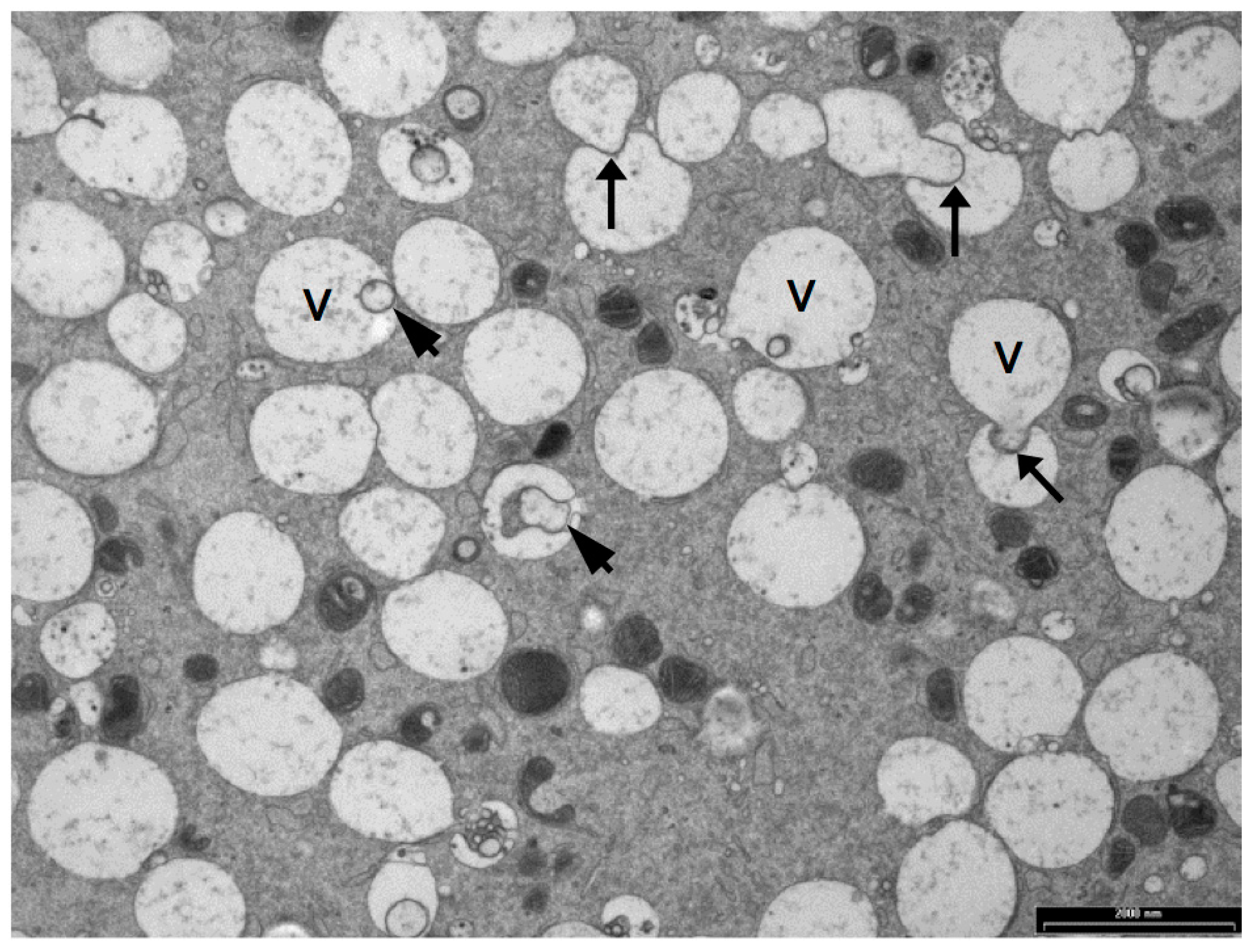
5. Vesicles and Oocyte Quality
5.1. Vesicle Quantity
5.2. Vesicle Distribution
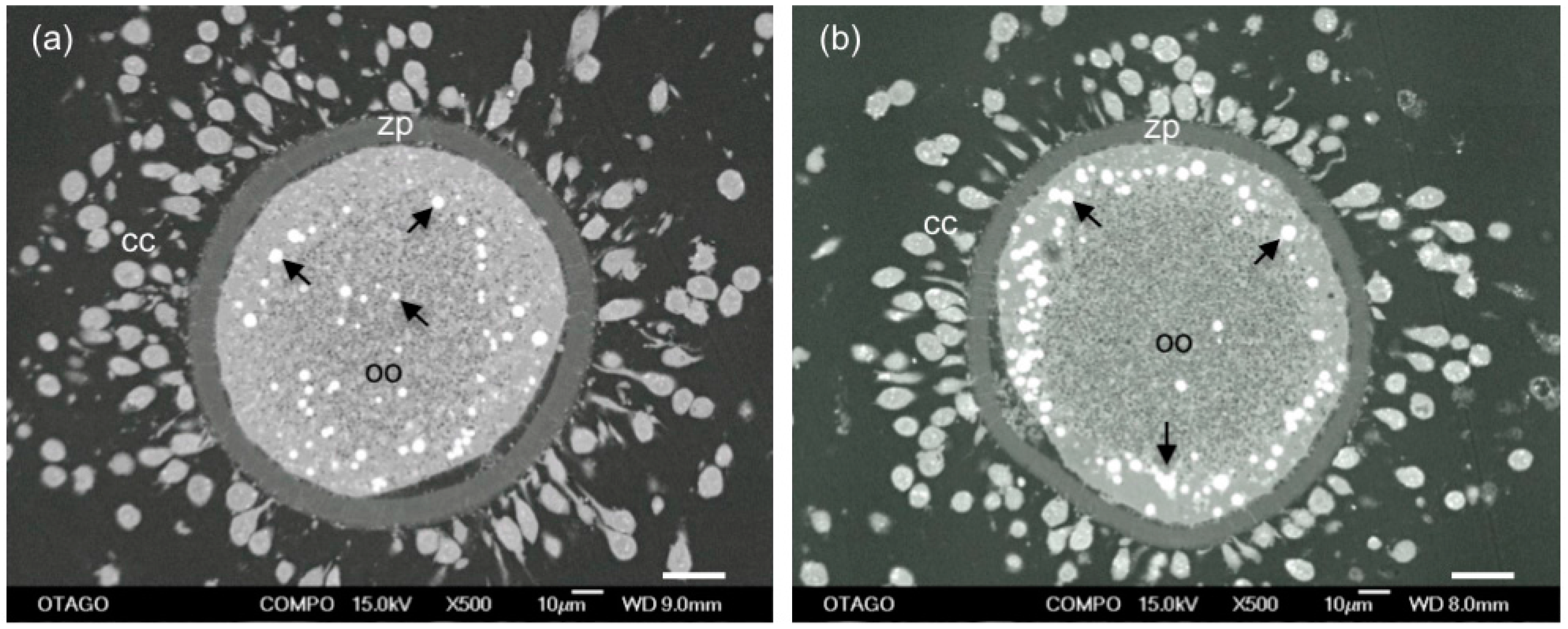
6. Role of Cortical Granules in Oocyte Quality
Cortical Granule Quantity and Distribution
7. Manipulating Oocyte Organelles to Improve Quality
8. Conclusions and Future Research
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mermillod, P.; Oussaid, B.; Cognie, Y. Aspects of follicular and oocyte maturation that affect the developmental potential of embryos. J. Reprod. Fertil. Suppl. 1999, 54, 449–460. [Google Scholar] [PubMed]
- Brevini Gandolfi, T.A.L.; Gandolfi, F. The maternal legacy to the embryo: Cytoplasmic components and their effects on early development. Theriogenology 2001, 55, 1255–1276. [Google Scholar] [CrossRef]
- Krisher, R.L. The effect of oocyte quality on development. J. Anim. Sci. 2004, 82, E14–E23. [Google Scholar] [PubMed]
- Sirard, M.A.; Richard, F.; Blondin, P.; Robert, C. Contribution of the oocyte to embryo quality. Theriogenology 2006, 65, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Fair, T.; Hulshof, S.C.; Hyttel, P.; Greve, T.; Boland, M. Oocyte ultrastructure in bovine primordial to early tertiary follicles. Anat. Embryol. 1997, 195, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Swain, J.E.; Pool, T.B. ART failure: Oocyte contributions to unsuccessful fertilization. Hum. Reprod. Update 2008, 14, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, F.; Brevini, T.A.L. RFD Award Lecture 2009: In vitro maturation of farm animal oocytes: A useful tool for investigating the mechanisms leading to full-term development. Reprod. Fertil. Dev. 2010, 22, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Diskin, M.G.; Murphy, J.J.; Sreenan, J.M. Embryo survival in dairy cows managed under pastoral conditions. Anim. Reprod. Sci. 2006, 96, 297–311. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, A.R.; Demmers, K.J.; Smaill, B.; Reader, K.L.; Juengel, J.L. Early embryo loss, morphology, and effect of previous immunization against androstenedione in the ewe. Theriogenology 2016, 86, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Ptak, G.; Matsukawa, K.; Palmieri, C.; Della Salda, L.; Scapolo, P.A.; Loi, P. Developmental and functional evidence of nuclear immaturity in prepubertal oocytes. Hum. Reprod. 2006, 21, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.T. Effects of maternal age on oocyte developmental competence. Theriogenology 2001, 55, 1303–1322. [Google Scholar] [CrossRef]
- Nicholas, F.W. Genetic improvement through reproductive technology. Anim. Reprod. Sci. 1996, 42, 205–214. [Google Scholar] [CrossRef]
- Cognie, Y.; Poulin, N.; Locatelli, Y.; Mermillod, P. State-of-the-art production, conservation and transfer of in-vitro-produced embryos in small ruminants. Reprod. Fertil. Dev. 2004, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Montag, M.; Toth, B.; Strowitzki, T. New approaches to embryo selection. Reprod. Biomed. Online 2013, 27, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M. Fertility preservation in women. Nat. Rev. Endocrinol. 2013, 9, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Elliott, T.A.; Wright, G.; Shapiro, D.B.; Toledo, A.A.; Nagy, Z.P. Prospective controlled study to evaluate laboratory and clinical outcomes of oocyte vitrification obtained in in vitro fertilization patients aged 30 to 39 years. Fertil. Steril. 2013, 99, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Schattman, G.L. Cryopreservation of Oocytes. N. Engl. J. Med. 2015, 373, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Van Wagtendonk-de Leeuw, A.M. Ovum pick up and in vitro production in the bovine after use in several generations: A 2005 status. Theriogenology 2006, 65, 914–925. [Google Scholar] [CrossRef] [PubMed]
- De Souza-Fabjan, J.M.G.; Panneau, B.; Duffard, N.; Locatelli, Y.; de Figueiredo, J.R.; de Figueirêdo Freitas, V.J.; Mermillod, P. In Vitro production of small ruminant embryos: Late improvements and further research. Theriogenology 2014, 81, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Schatten, H.; Sun, Q.Y. Centrosome and microtubule functions and dysfunctions in meiosis: Implications for age-related infertility and developmental disorders. Reprod. Fertil. Dev. 2015, 27, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Bennabi, I.; Terret, M.E.; Verlhac, M.H. Meiotic spindle assembly and chromosome segregation in oocytes. J. Cell Biol. 2016, 215, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.M.; Vireque, A.A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.A.A.S. Cytoplasmic maturation of bovine oocytes: Structural and biochemical modifications and acquisition of developmental competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.; Smitz, J. Molecular control of oogenesis. Biochim. Biophys. Acta 2012, 1822, 1896–1912. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Lou, H.; Lou, Y.; Wang, N.; Jin, F. Behaviour of cytoplasmic organelles and cytoskeleton during oocyte maturation. Reprod. Biomed. Online 2014, 28, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, R.; Sirard, M.A. The study of mammalian oocyte competence by transcriptome analysis: Progress and challenges. Mol. Hum. Reprod. 2014, 20, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Pavlok, A.; Lucas-Hahn, A.; Niemann, H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol. Reprod. Dev. 1992, 31, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, P.; Monaghan, P.; Rizos, D.; Boland, M.P.; Gordon, I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol. Reprod. Dev. 1994, 37, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Marchal, R.; Vigneron, C.; Perreau, C.; Bali-Papp, A.; Mermillod, P. Effect of follicular size on meiotic and developmental competence of porcine oocytes. Theriogenology 2002, 57, 1523–1532. [Google Scholar] [CrossRef]
- Cognie, Y.; Benoit, F.; Poulin, N.; Khatir, H.; Driancourt, M.A. Effect of follicle size and of the FecB Booroola gene on oocyte function in sheep. J. Reprod. Fertil. 1998, 112, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Fair, T.; Hyttel, P.; Greve, T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol. Reprod. Dev. 1995, 42, 437–442. [Google Scholar] [CrossRef] [PubMed]
- El Shourbagy, S.H.; Spikings, E.C.; Freitas, M.; St John, J.C. Mitochondria directly influence fertilisation outcome in the pig. Reproduction 2006, 131, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Murakoshi, Y.; Sueoka, K.; Takahashi, K.; Sato, S.; Sakurai, T.; Tajima, H.; Yoshimura, Y. Embryo developmental capability and pregnancy outcome are related to the mitochondrial DNA copy number and ooplasmic volume. J. Assist. Reprod. Genet. 2013, 30, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Reader, K.L.; Cox, N.R.; Stanton, J.-A.L.; Juengel, J.L. Mitochondria and vesicles differ between adult and prepubertal sheep oocytes during IVM. Reprod. Fertil. Dev. 2015, 27, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, F.; Milanesi, E.; Pocar, P.; Luciano, A.M.; Brevini, T.A.; Acocella, F.; Lauria, A.; Armstrong, D.T. Comparative analysis of calf and cow oocytes during in vitro maturation. Mol. Reprod. Dev. 1998, 49, 168–175. [Google Scholar] [CrossRef]
- O’Brien, J.K.; Dwarte, D.; Ryan, J.P.; Maxwell, W.M.; Evans, G. Developmental capacity, energy metabolism and ultrastructure of mature oocytes from prepubertal and adult sheep. Reprod. Fertil. Dev. 1996, 8, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.K.; Dwarte, D.; Ryan, J.P.; Maxwell, W.M.C.; Evans, G. Comparison of in vitro maturation, in vitro fertilization, metabolism and ultrastructure of oocytes from prepubertal and adult pigs. Reprod. Domest. Anim. 2000, 35, 101–107. [Google Scholar] [CrossRef]
- Feary, E.S. Aspects of Ovarian Function in a Line of Sheep with a Novel X-Linked Maternally-Imprinted Gene that is Associated with an Increased Ovulation Rate. Ph.D. Thesis, Victoria University of Wellington, Wellington, New Zealand, 2006. [Google Scholar]
- Cran, D.G.; Moor, R.M.; Hay, M.F. Fine structure of the sheep oocyte during antral follicle development. J. Reprod. Fertil. 1980, 59, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Williams, M. Quantitative Methods in Biology; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherland, 1977; Volume 6. [Google Scholar]
- Rodriguez-Gonzalez, E.; Lopez-Bejar, M.; Izquierdo, D.; Paramio, M.T. Developmental competence of prepubertal goat oocytes selected with brilliant cresyl blue and matured with cysteamine supplementation. Reprod. Nutr. Dev. 2003, 43, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Torner, H.; Ghanem, N.; Ambros, C.; Holker, M.; Tomek, W.; Phatsara, C.; Alm, H.; Sirard, M.A.; Kanitz, W.; Schellander, K.; et al. Molecular and subcellular characterisation of oocytes screened for their developmental competence based on glucose-6-phosphate dehydrogenase activity. Reproduction 2008, 135, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Catala, M.G.; Izquierdo, D.; Uzbekova, S.; Morato, R.; Roura, M.; Romaguera, R.; Papillier, P.; Paramio, M.T. Brilliant Cresyl Blue stain selects largest oocytes with highest mitochondrial activity, maturation-promoting factor activity and embryo developmental competence in prepubertal sheep. Reproduction 2011, 142, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Ren, L.; Liu, D.; Ma, J.Z.; An, T.Z.; Yang, X.Q.; Ma, H.; Zhang, D.J.; Guo, Z.H.; Guo, Y.Y.; et al. Subcellular Characterization of Porcine Oocytes with Different Glucose-6-phosphate Dehydrogenase Activities. Asian-Australas. J. Anim. Sci. 2015, 28, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Rizos, D.; Ward, F.; Duffy, P.; Boland, M.P.; Lonergan, P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 2002, 61, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Bentov, Y.; Yavorska, T.; Esfandiari, N.; Jurisicova, A.; Casper, R.F. The contribution of mitochondrial function to reproductive aging. J. Assist. Reprod. Genet. 2011, 28, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Iwata, H.; Goto, H.; Tanaka, H.; Sakaguchi, Y.; Kimura, K.; Kuwayama, T.; Monji, Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod. Fertil. Dev. 2011, 23, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, H.P.S.; Wu, B.; Morris, L.H.A.; Buckrell, B.C.; Pollard, J.W.; Basrur, P.K.; King, W.A. Maturation status, protein synthesis and developmental competence of oocytes derived from lambs and ewes. Reprod. Domest. Anim. 2002, 37, 19–25. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.K.; Catt, S.L.; Ireland, K.A.; Maxwell, W.M.C.; Evans, G. In vitro and in vivo developmental capacity of oocytes from prepubertal and adult sheep. Theriogenology 1997, 47, 1433–1443. [Google Scholar] [CrossRef]
- Ledda, S.; Bogliolo, L.; Calvia, P.; Leoni, G.; Naitana, S. Meiotic progression and developmental competence of oocytes collected from juvenile and adult ewes. J. Reprod. Fertil. 1997, 109, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Leoni, G.G.; Bebbere, D.; Succu, S.; Berlinguer, F.; Mossa, F.; Galioto, M.; Bogliolo, L.; Ledda, S.; Naitana, S. Relations between relative mRNA abundance and developmental competence of ovine oocytes. Mol. Reprod. Dev. 2007, 74, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Damiani, P.; Fissore, R.A.; Cibelli, J.B.; Long, C.R.; Balise, J.J.; Robl, J.M.; Duby, R.T. Evaluation of developmental competence, nuclear and ooplasmic maturation of calf oocytes. Mol. Reprod. Dev. 1996, 45, 521–534. [Google Scholar] [CrossRef]
- Steeves, T.E.; Gardner, D.K.; Zuelke, K.A.; Squires, T.S.; Fry, R.C. In vitro development and nutrient uptake by embryos derived from oocytes of pre–pubertal and adult cows. Mol. Reprod. Dev. 1999, 54, 49–56. [Google Scholar] [CrossRef]
- Paczkowski, M.; Yuan, Y.; Fleming-Waddell, J.; Bidwell, C.A.; Spurlock, D.; Krisher, R.L. Alterations in the transcriptome of porcine oocytes derived from prepubertal and cyclic females is associated with developmental potential. J. Anim. Sci. 2011, 89, 3561–3571. [Google Scholar] [CrossRef] [PubMed]
- Khatir, H.; Lonergan, P.; Touze, J.L.; Mermillod, P. The characterization of bovine embryos obtained from prepubertal calf oocytes and their viability after non surgical embryo transfer. Theriogenology 1998, 50, 1201–1210. [Google Scholar] [CrossRef]
- Boni, R.; Cocchia, N.; Silvestre, F.; Tortora, G.; Lorizio, R.; Tosti, E. Juvenile and adult immature and in vitro matured ovine oocytes evaluated in relation to membrane electrical properties, calcium stores, IP3 sensitivity and apoptosis occurrence in cumulus cells. Mol. Reprod. Dev. 2008, 75, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Khatir, H.; Lonergan, P.; Carolan, C.; Mermillod, P. Prepubertal bovine oocyte: A negative model for studying oocyte developmental competence. Mol. Reprod. Dev. 1996, 45, 231–239. [Google Scholar] [CrossRef]
- Dumollard, R.; Duchen, M.; Carroll, J. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar] [PubMed]
- Tarazona, A.M.; Rodriguez, J.I.; Restrepo, L.F.; Olivera-Angel, M. Mitochondrial activity, distribution and segregation in bovine oocytes and in embryos produced in vitro. Reprod. Domest. Anim. 2006, 41, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J. Mitochondria in early mammalian development. Semin. Cell Dev. Biol. 2009, 20, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Lei, A.; Qu, L.; Dou, Z. The mitochondrial ultrastructure of interspecies cloned embryos of bovine-goat. Sci. Agric. Sin. 2010, 43, 2548–2554. [Google Scholar]
- Hyttel, P. Electron microscopy of mammalian oocyte development, maturation and fertilization. In Oocyte Maturation and Fertilization: A Long History for a Short Event; Tosti, E., Boni, R., Eds.; Bentham Science Publishers: Oak Park, IL, USA, 2011; pp. 1–37. [Google Scholar]
- Sá, R.; Cunha, M.; Silva, J.; Luís, A.; Oliveira, C.; Teixeira da Silva, J.; Barros, A.; Sousa, M. Ultrastructure of tubular smooth endoplasmic reticulum aggregates in human metaphase II oocytes and clinical implications. Fertil. Steril. 2011, 96, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Shahedi, A.; Hosseini, A.; Khalili, M.A.; Norouzian, M.; Salehi, M.; Piriaei, A.; Nottola, S.A. The effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J.; Runner, M.N. Mitochondrial reorganization during resumption of arrested meiosis in the mouse oocyte. Am. J. Anat. 1984, 171, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Simsek-Duran, F.; Li, F.; Ford, W.; Swanson, R.J.; Jones, H.W., Jr.; Castora, F.J. Age-associated metabolic and morphologic changes in mitochondria of individual mouse and hamster oocytes. PLoS ONE 2013, 8, e64955. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, L. Ultrastructure of mammalian oocytes and ova. Biol. Reprod. 1970, 2, 44–63. [Google Scholar] [CrossRef]
- Cran, D.G. Qualitative and quantitative structural changes during pig oocyte maturation. J. Reprod. Fertil. 1985, 74, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.S.; Callesen, H.; Lovendahl, P.; Chen, F.; Nyengaard, J.R.; Nikolaisen, N.K.; Holm, P.; Hyttel, P. Ultrastructure and mitochondrial numbers in pre- and postpubertal pig oocytes. Reprod. Fertil. Dev. 2016, 28, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Galloway, C.A.; Lee, H.; Yoon, Y. Mitochondrial morphology-emerging role in bioenergetics. Free Radic. Biol. Med. 2012, 53, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Benard, G.; Bellance, N.; James, D.; Parrone, P.; Fernandez, H.; Letellier, T.; Rossignol, R. Mitochondrial bioenergetics and structural network organization. J. Cell Sci. 2007, 120, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Giedt, R.J.; Pfeiffer, D.R.; Matzavinos, A.; Kao, C.Y.; Alevriadou, B.R. Mitochondrial dynamics and motility inside living vascular endothelial cells: Role of bioenergetics. Ann. Biomed. Eng. 2012, 40, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hajnoczky, G. Altered fusion dynamics underlie unique morphological changes in mitochondria during hypoxia-reoxygenation stress. Cell Death Differ. 2011, 18, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Markova, O.V.; Mokhova, E.N.; Tarakanova, A.N. The abnormal-shaped mitochondria in thymus lymphocytes treated with inhibitors of mitochondrial energetics. J. Bioenerg. Biomembr. 1990, 22, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.X.; Li, M.; Biazik, J.M.; Morgan, D.G.; Guo, F.; Ni, H.M.; Goheen, M.; Eskelinen, E.L.; Yin, X.M. Electron microscopic analysis of a spherical mitochondrial structure. J. Biol. Chem. 2012, 287, 42373–42378. [Google Scholar] [CrossRef] [PubMed]
- Crocomo, L.F.; Filho, W.C.M.; Sudano, M.J.; Paschoal, D.M.; Alvarenga, F.d.C.L.; Bicudo, S.D. Effect of roscovitine and cycloheximide on ultrastructure of sheep oocytes. Small Rumin. Res. 2013, 109, 156–162. [Google Scholar] [CrossRef]
- Hyttel, P.; Callesen, H.; Greve, T. Ultrastructural features of preovulatory oocyte maturation in superovulated cattle. J. Reprod. Fertil. 1986, 76, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dumollard, R.; Rossbach, A.; Lai, F.A.; Swann, K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J. Cell Physiol. 2010, 224, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Sohn, I.P.; Kwon, H.C.; Jo, D.H.; Park, Y.D.; Min, C.K. Characteristics of the cell membrane fluidity, actin fibers, and mitochondrial dysfunctions of frozen-thawed two-cell mouse embryos. Mol. Reprod. Dev. 2002, 61, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Stojkovic, M.; Machado, S.A.; Stojkovic, P.; Zakhartchenko, V.; Hutzler, P.; Gonçalves, P.B.; Wolf, E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: Correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol. Reprod. 2001, 64, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Brevini, T.A.L.; Vassena, R.; Francisci, C.; Gandolfi, F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol. Reprod. 2005, 72, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Machatkova, M.; Jeseta, M.; Hulinska, P.; Knitlova, D.; Nemcova, L.; Kanka, J. Characteristics of Bovine Oocytes with Different Meiotic Competence in Terms of Their Mitochondrial Status and Expression of Nuclear-Encoded Factors. Reprod. Domest. Anim. 2012, 47, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Campello, S.; Lacalle, R.A.; Bettella, M.; Manes, S.; Scorrano, L.; Viola, A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J. Exp. Med. 2006, 203, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J.; Davis, P.; Alexander, S. Differential mitochondrial distribution in human pronuclear embryos leads to disproportionate inheritance between blastomeres: Relationship to microtubular organization, ATP content and competence. Hum. Reprod. 2000, 15, 2621–2633. [Google Scholar] [CrossRef] [PubMed]
- Pikó, L.; Matsumoto, L. Number of mitochondria and some properties of mitochondrial DNA in the mouse egg. Dev. Biol. 1976, 49, 1–10. [Google Scholar] [CrossRef]
- Piko, L.; Taylor, K.D. Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev. Biol. 1987, 123, 364–374. [Google Scholar] [CrossRef]
- Reader, K.L.; Cox, N.R.; Stanton, J.-A.L.; Juengel, J.L. Effects of acetyl-L-carnitine on lamb oocyte blastocyst rate, ultrastructure, and mitochondrial DNA copy number. Theriogenology 2015, 83, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Pendergrass, W.; Wolf, N.; Poot, M. Efficacy of MitoTracker Green and CMXrosamine to measure changes in mitochondrial membrane potentials in living cells and tissues. Cytom. Part A J. Int. Soc. Anal. Cytol. 2004, 61, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Pawley, J.B. Handbook of Biological Confocal Microscopy, 2nd ed.; Plenum Press: New York, NY, USA, 1989. [Google Scholar]
- May-Panloup, P.; Vignon, X.; Chretien, M.F.; Heyman, Y.; Tamassia, M.; Malthiery, Y.; Reynier, P. Increase of mitochondrial DNA content and transcripts in early bovine embryogenesis associated with upregulation of mtTFA and NRF1 transcription factors. Reprod. Biol. Endocrinol. 2005, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Thundathil, J.; Filion, F.; Smith, L.C. Molecular control of mitochondrial function in preimplantation mouse embryos. Mol. Reprod. Dev. 2005, 71, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Scott, R.; Schimmel, T.; Levron, J.; Willadsen, S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet 1997, 350, 186–187. [Google Scholar] [CrossRef]
- Lanzendorf, S.E.; Mayer, J.F.; Toner, J.; Oehninger, S.; Saffan, D.S.; Muasher, S. Pregnancy following transfer of ooplasm from cryopreserved-thawed donor oocytes into recipient oocytes. Fertil. Steril. 1999, 71, 575–577. [Google Scholar] [CrossRef]
- De Paz, P.; Sánchez, A.J.; De la Fuente, J.; Chamorro, C.A.; Alvarez, M.; Anel, E.; Anel, L. Ultrastructural and cytochemical comparison between calf and cow oocytes. Theriogenology 2001, 55, 1107–1116. [Google Scholar] [CrossRef]
- May-Panloup, P.; Chretien, M.F.; Jacques, C.; Vasseur, C.; Malthiery, Y.; Reynier, P. Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum. Reprod. 2005, 20, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, M.; Harris, S.E.; Collado Fernandez, E.; Lu, J.; Huntriss, J.D.; Campbell, B.K.; Picton, H.M. The activity and copy number of mitochondrial DNA in ovine oocytes throughout oogenesis in vivo and during oocyte maturation in vitro. Mol. Hum. Reprod. 2013, 19, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Barritt, J.A.; Kokot, M.; Cohen, J.; Steuerwald, N.; Brenner, C.A. Quantification of human ooplasmic mitochondria. Reprod. Biomed. Online 2002, 4, 243–247. [Google Scholar] [CrossRef]
- Mahrous, E.; Yang, Q.; Clarke, H.J. Regulation of mitochondrial DNA accumulation during oocyte growth and meiotic maturation in the mouse. Reproduction 2012, 144, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Duran, H.E.; Simsek-Duran, F.; Oehninger, S.C.; Jones, H.W., Jr.; Castora, F.J. The association of reproductive senescence with mitochondrial quantity, function, and DNA integrity in human oocytes at different stages of maturation. Fertil. Steril. 2011, 96, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, P.; Cieslak, A.; Warzych, E.; Zejden, Z.; Szumacher-Strabel, M.; Molinska-Glura, M.; Lechniak, D. No single way to explain cytoplasmic maturation of oocytes from prepubertal and cyclic gilts. Theriogenology 2012, 78, 2020–2030. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.A.; El Shourbagy, S.; St John, J.C. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil. Steril. 2006, 85, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.S.; Lovendahl, P.; Larsen, K.; Madsen, L.B.; Callesen, H. Porcine oocyte mtDNA copy number is high or low depending on the donor. Zygote 2016, 24, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.T.; Yeung, W.S.; Cheung, M.P.; Ho, P.C.; Lee, C.K.; Zhuang, G.L.; Liang, X.Y.; O, W.S. In vitro-matured rat oocytes have low mitochondrial deoxyribonucleic acid and adenosine triphosphate contents and have abnormal mitochondrial redistribution. Fertil. Steril. 2009, 91, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tollner, T.L.; Hu, Z.; Da, M.; Li, X.; Guan, H.; Shan, D.; Lu, J.; Huang, C.; Dong, Q. Impaired mitochondrial function in murine oocytes is associated with controlled ovarian hyperstimulationand in vitro maturation. Reprod. Fertil. Dev. 2012, 24, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Tollner, T.L.; Hu, Z.; Dai, M.; Li, X.; Guan, H.; Shan, D.; Zhang, X.; Lv, J.; Huang, C.; et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol. Reprod. Dev. 2012, 79, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Gutnisky, C.; Morado, S.; Dalvit, G.C.; Thompson, J.G.; Cetica, P.D. Glycolytic pathway activity: Effect on IVM and oxidative metabolism of bovine oocytes. Reprod. Fertil. Dev. 2013, 25, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, C.A.; Kaye, P.; Pantaleon, M.; Phillips, N.; Norman, S.; Fry, R.; D’Occhio, M.J. Lipid content, active mitochondria and brilliant cresyl blue staining in bovine oocytes. Theriogenology 2013, 79, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.J.; Cho, S.J.; Lee, H.S.; Deb, G.K.; Lee, Y.S.; Kwon, T.H.; Kong, I.K. Effect of cytoplasmic lipid content on in vitro developmental efficiency of bovine IVP embryos. Theriogenology 2009, 72, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Kruip, T.A.M.; Cran, D.G.; van Beneden, T.H.; Dieleman, S.J. Structural changes in bovine oocytes during final maturation in vivo. Gamete Res. 1983, 8, 29–47. [Google Scholar] [CrossRef]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and beta-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.M.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; Leese, H.J. Energy metabolism in pig oocytes and early embryos. Reproduction 2003, 126, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.M.; Leese, H.J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2006, 73, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Cashman, K.; Russell, D.L.; Thompson, J.G.; Norman, R.J.; Robker, R.L. Beta-Oxidation Is Essential for Mouse Oocyte Developmental Competence and Early Embryo Development. Biol. Reprod. 2010, 83, 909–918. [Google Scholar] [CrossRef] [PubMed]
- McKeegan, P.J.; Sturmey, R.G. The role of fatty acids in oocyte and early embryo development. Reprod. Fertil. Dev. 2011, 24, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Homa, S.T.; Racowsky, C.; McGaughey, R.W. Lipid analysis of immature pig oocytes. J. Reprod. Fertil. 1986, 77, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Auclair, S.; Uzbekov, R.; Elis, S.; Sanchez, L.; Kireev, I.; Lardic, L.; Dalbies-Tran, R.; Uzbekova, S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E599–E613. [Google Scholar] [CrossRef] [PubMed]
- Warzych, E.; Pawlak, P.; Pszczola, M.; Cieslak, A.; Lechniak, D. Prepubertal heifers versus cows-The differences in the follicular environment. Theriogenology 2017, 87, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, S.; Van Soom, A.; Nauwynck, H.; Coryn, M.; de Kruif, A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol. Reprod. Dev. 2002, 61, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Angermuller, S.; Fahimi, H.D. Imidazole-buffered osmium tetroxide: An excellent stain for visualization of lipids in transmission electron microscopy. Histochem. J. 1982, 14, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.D.; Brown, W.J.; Warfel, J.; Greenspan, P. Use of nile red for the rapid in situ quantitation of lipids on thin-layer chromatograms. J. Lipid Res. 1987, 28, 1225–1232. [Google Scholar] [PubMed]
- Kim, J.Y.; Kinoshita, M.; Ohnishi, M.; Fukui, Y. Lipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytes. Reproduction 2001, 122, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Aardema, H.; Vos, P.L.A.M.; Lolicato, F.; Roelen, B.A.J.; Knijn, H.M.; Vaandrager, A.B.; Helms, J.B.; Gadella, B.M. Oleic Acid Prevents Detrimental Effects of Saturated Fatty Acids on Bovine Oocyte Developmental Competence. Biol. Reprod. 2011, 85, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dunning, K.R.; Wu, L.L.; Hickey, T.E.; Norman, R.J.; Russell, D.L.; Liang, X.; Robker, R.L. Identification of Perilipin-2 as a lipid droplet protein regulated in oocytes during maturation. Reprod. Fertil. Dev. 2010, 22, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; O’Toole, P.J.; Leese, H.J. Fluorescence resonance energy transfer analysis of mitochondrial:lipid association in the porcine oocyte. Reproduction 2006, 132, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Reader, K.L. Quantitative Ultrastructural Differences in the Cytoplasm of Prepubertal Lamb and Adult Ewe Oocytes. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2014. [Google Scholar]
- Gualtieri, R.; Mollo, V.; Barbato, V.; Fiorentino, I.; Iaccarino, M.; Talevi, R. Ultrastructure and intracellular calcium response during activation in vitrified and slow-frozen human oocytes. Hum. Reprod. 2011, 26, 2452–2460. [Google Scholar] [CrossRef] [PubMed]
- Falconnier, C.; Kress, A. Ultrastructural aspects of oocyte growth in the marsupial Monodelphis domestica (grey short-tailed opossum). J. Anat. 1992, 181, 481–498. [Google Scholar] [PubMed]
- Ducibella, T.; Rangarajan, S.; Anderson, E. The development of mouse oocyte cortical reaction competence is accompanied by major changes in cortical vesicles and not cortical granule depth. Dev. Biol. 1988, 130, 789–792. [Google Scholar] [CrossRef]
- Petr, J.; Rozinek, J.; Hruban, V.; Jílek, F.; Sedmíková, M.; Vaňourková, Z.; Němeček, Z. Ultrastructural localization of calcium deposits during in vitro culture of pig oocytes. Mol. Reprod. Dev. 2001, 58, 196–204. [Google Scholar] [CrossRef]
- Máximo, D.M.; Martins da Silva, I.G.; Mondadori, R.G.; Neves, J.P.; Lucci, C.M. Ultrastructural characteristics of sheep oocytes during in vitro maturation (IVM). Small Rumin. Res. 2012, 105, 210–215. [Google Scholar] [CrossRef]
- Hyttel, P.; Xu, K.P.; Smith, S.; Greve, T. Ultrastructure of in-vitro oocyte maturation in cattle. J. Reprod. Fertil. 1986, 78, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. The biology and dynamics of mammalian cortical granules. Reprod. Biol. Endocrinol. 2011, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Szollosi, D.; Desmedt, V.; Crozet, N.; Brender, C. In vitro maturation of sheep ovarian oocytes. Reprod. Nutr. Dev. 1988, 28, 1047–1080. [Google Scholar] [CrossRef] [PubMed]
- Ducibella, T. The cortical reaction and development of activation competence in mammalian oocytes. Hum. Reprod. Update 1996, 2, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cran, D.G.; Moor, R.M.; Irvine, R.F. Initiation of the cortical reaction in hamster and sheep oocytes in response to inositol trisphosphate. J. Cell Sci. 1988, 91, 139–144. [Google Scholar] [PubMed]
- Kline, D.; Kline, J.T. Repetitive calcium transients and the role of calcium in exocytosis and cell cycle activation in the mouse egg. Dev. Biol. 1992, 149, 80–89. [Google Scholar] [CrossRef]
- Velilla, E.; Izquierdo, D.; Rodriguez–Gonzalez, E.; Lopez-Bejar, M.; Vidal, F.; Paramio, M.T. Distribution of prepubertal and adult goat oocyte cortical granules during meiotic maturation and fertilisation: Ultrastructural and cytochemical study. Mol. Reprod. Dev. 2004, 68, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Barritt, J.; Willadsen, S.; Brenner, C.; Cohen, J. Cytoplasmic transfer in assisted reproduction. Hum. Reprod. Update 2001, 7, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Ben-Meir, A.; Burstein, E.; Borrego-Alvarez, A.; Chong, J.; Wong, E.; Yavorska, T.; Naranian, T.; Chi, M.; Wang, Y.; Bentov, Y.; et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell 2015, 14, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.S.; Jeong, S.A.; Jeon, Y.B.; Hyun, S.H. The effects of resveratrol on porcine oocytes in vitro maturation and subsequent development after parthenogenetic activation and in vitro fertilization. Reprod. Fertil. Dev. 2011, 24, 207. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; He, C.; Ji, P.; Li, Y.; Tan, D.; Liu, G. Beneficial effect of resveratrol on bovine oocyte maturation and subsequent embryonic development after in vitro fertilization. Fertil. Steril. 2014, 101, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Somfai, T.; Kaneda, M.; Akagi, S.; Watanabe, S.; Haraguchi, S.; Mizutani, E.; Dang-Nguyen, T.Q.; Geshi, M.; Kikuchi, K.; Nagai, T. Enhancement of lipid metabolism with L-carnitine during in vitro maturation improves nuclear maturation and cleavage ability of follicular porcine oocytes. Reprod. Fertil. Dev. 2011, 23, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Imai, H.; Yamada, M. Beneficial effects of acetyl-L-carnitine treatment during IVM on post-fertilzation development of bovine oocytes. Reprod. Fertil. Dev. 2006, 18, 280–281. [Google Scholar] [CrossRef]
- Wu, G.Q.; Jia, B.Y.; Li, J.J.; Fu, X.W.; Zhou, G.B.; Hou, Y.P.; Zhu, S.E. L-carnitine enhances oocyte maturation and development of parthenogenetic embryos in pigs. Theriogenology 2011, 76, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Dunning, K.R.; Robker, R.L. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim. Reprod. Sci. 2012, 134, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Labas, V.; Teixeira-Gomes, A.P.; Bouguereau, L.; Gargaros, A.; Spina, L.; Marestaing, A.; Uzbekova, S. Intact cell MALDI-TOF mass spectrometry on single bovine oocyte and follicular cells combined with top-down proteomics: A novel approach to characterise markers of oocyte maturation. J. Proteom. 2017. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Serrano, A.F.; Pirro, V.; Ferreira, C.R.; Oliveri, P.; Eberlin, L.S.; Heinzmann, J.; Lucas-Hahn, A.; Niemann, H.; Cooks, R.G. Desorption electrospray ionization mass spectrometry reveals lipid metabolism of individual oocytes and embryos. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Ziyyat, A.; Naoura, I.; Chabbert-Buffet, N.; Aractingi, S.; Darai, E.; Lefevre, B. Effect of induced peritoneal endometriosis on oocyte and embryo quality in a mouse model. J. Assist. Reprod. Genet. 2015, 32, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.; Lippens, S.; Bartunkova, S.; Asselbergh, B.; Blanpain, C.; Fendrych, M.; Goossens, A.; Holt, M.; Janssens, S.; Krols, M.; et al. Developing 3D SEM in a broad biological context. J. Microsc. 2015, 259, 80–96. [Google Scholar] [CrossRef] [PubMed]
| Organelle | Good Quality | Poor Quality |
|---|---|---|
| mito morphology | lighter matrix; hooded | denser matrix; vacuoles and granules; altered timing of hooded form |
| mito distribution | Even | Peripheral |
| mito number | Higher | Lower |
| mito activity | ? | ? |
| lipid volume | Greater | Smaller |
| lipid distribution | Even | Peripheral |
| vesicle volume | Greater | Smaller |
| CG distribution | Peripheral | clustered, even |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reader, K.L.; Stanton, J.-A.L.; Juengel, J.L. The Role of Oocyte Organelles in Determining Developmental Competence. Biology 2017, 6, 35. https://doi.org/10.3390/biology6030035
Reader KL, Stanton J-AL, Juengel JL. The Role of Oocyte Organelles in Determining Developmental Competence. Biology. 2017; 6(3):35. https://doi.org/10.3390/biology6030035
Chicago/Turabian StyleReader, Karen L., Jo-Ann L. Stanton, and Jennifer L. Juengel. 2017. "The Role of Oocyte Organelles in Determining Developmental Competence" Biology 6, no. 3: 35. https://doi.org/10.3390/biology6030035
APA StyleReader, K. L., Stanton, J.-A. L., & Juengel, J. L. (2017). The Role of Oocyte Organelles in Determining Developmental Competence. Biology, 6(3), 35. https://doi.org/10.3390/biology6030035





