Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers
Abstract
:1. Introduction
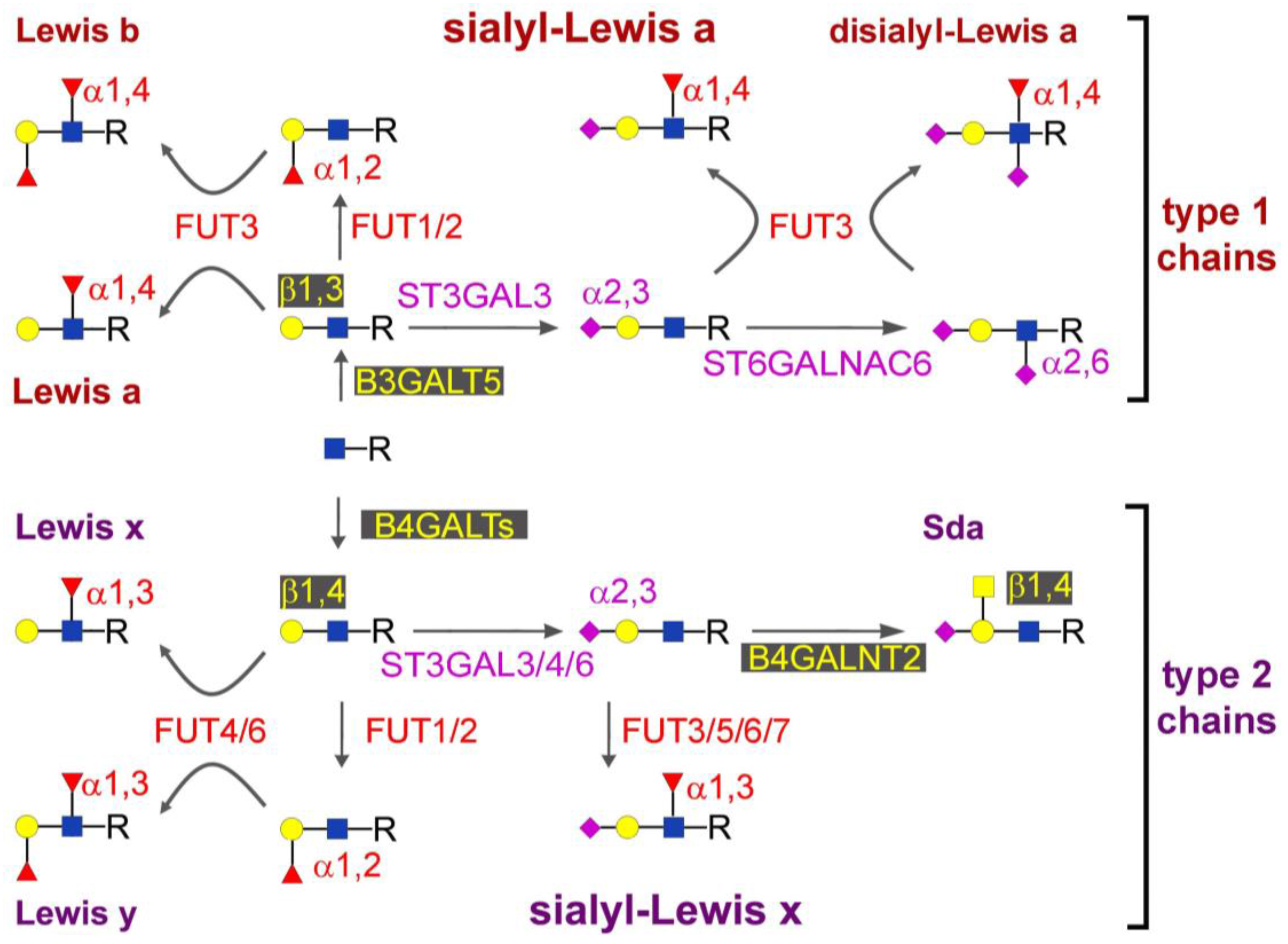
2. Biosynthesis of sLex and sLea
3. Regulation of sLea and sLex
4. Plasma Carriers of sLex or sLea
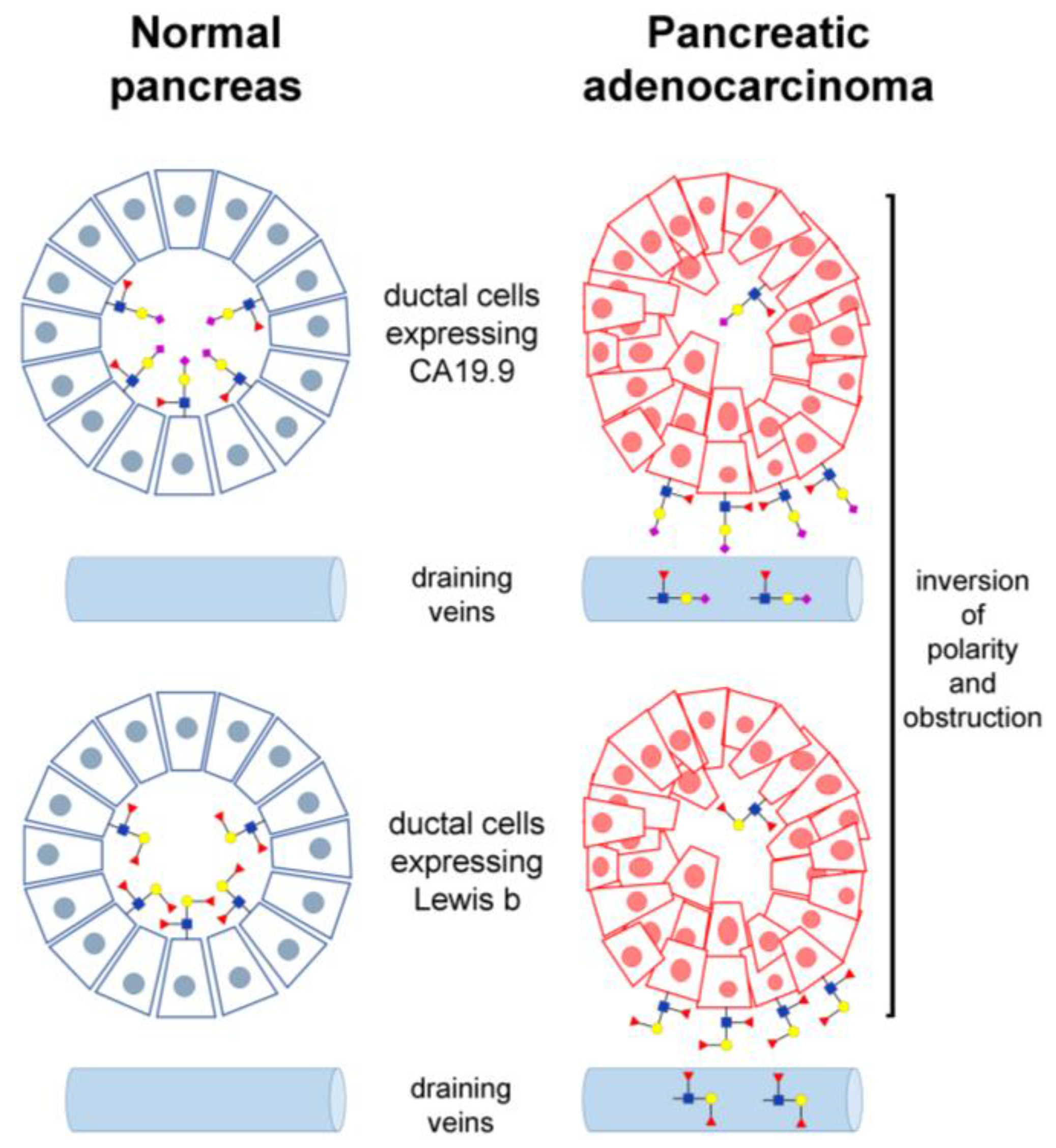
5. Role in Diagnosis
6. Role in Malignancy
7. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McEver, R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015, 107, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Petryniak, B.; Hiraoka, N.; Mitoma, J.; Huckaby, V.; Nakayama, J.; Uchimura, K.; Kadomatsu, K.; Muramatsu, T.; Lowe, J.B.; et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat. Immunol. 2005, 6, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, H.; Fukuda, M. Sulfated glycans control lymphocyte homing. Ann. N. Y. Acad. Sci. 2012, 1253, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2016. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Murayama, K.; Hanamura, T.; Okada, T.; Ito, T.; Harada, M.; Komatsu, A.; Koyama, H.; Kanai, T.; Maeno, K.; et al. CsLex (Sialyl Lewis X) is a Useful Tumor Marker for Monitoring of Breast Cancer Patients. Jpn. J. Clin. Oncol. 2011, 41, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.X.; Liang, Y.; Gao, W. Clinicopathological and prognostic significance of sialyl Lewis X overexpression in patients with cancer: A meta-analysis. OncoTargets. Ther. 2016, 9, 3113–3125. [Google Scholar]
- Lowe, J.B. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 2002, 186, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, F.; Germot, A.; Marenda, M.; Oriol, R.; Blancher, A.; Julien, R.; Maftah, A. α1,4-Fucosyltransferase Activity: A Significant Function in the Primate Lineage has Appeared Twice Independently. Mol. Biol. Evol. 2002, 19, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhaes, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of α-2,3-sialyltransferases and α-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garay, M.; Arteta, B.; Pages, L.; De Llorens, R.; de Bolos, C.; Vidal-Vanaclocha, F.; Peracaula, R. α2,3-sialyltransferase ST3Gal III modulates pancreatic cancer cell motility and adhesion in vitro and enhances its metastatic potential in vivo. PLoS ONE 2010, 5, e12524. [Google Scholar] [CrossRef] [PubMed]
- Hiller, K.M.; Mayben, J.P.; Bendt, K.M.; Manousos, G.A.; Senger, K.; Cameron, H.S.; Weston, B.W. Transfection of α1,3 fucosyltransferase antisense sequences impairs the proliferative and tumorigenic ability of human colon carcinoma cells. Mol. Carcinog. 2000, 27, 280–288. [Google Scholar] [CrossRef]
- Weston, B.W.; Hiller, K.M.; Mayben, J.P.; Manousos, G.A.; Bendt, K.M.; Liu, R.; Cusack, J.C., Jr. Expression of human α1,3 fucosyltransferase antisense sequences inhibits selectin-mediated adhesion and liver metastasis of colon carcinoma cells. Cancer Res. 1999, 59, 2127–2135. [Google Scholar] [PubMed]
- Beum, P.V.; Singh, J.; Burdick, M.; Hollingsworth, M.A.; Cheng, P.W. Expression of core 2 β1,6-N-acetylglucosaminyltransferase in a human pancreatic cancer cell line results in altered expression of MUC1 tumor-associated epitopes. J. Biol. Chem. 1999, 274, 24641–24648. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garay, M.; Arteta, B.; Llop, E.; Cobler, L.; Pages, L.; Ortiz, R.; Ferri, M.J.; de Bolos, C.; Figueras, J.; De Llorens, R.; et al. α2,3-Sialyltransferase ST3Gal IV promotes migration and metastasis in pancreatic adenocarcinoma cells and tends to be highly expressed in pancreatic adenocarcinoma tissues. Int. J. Biochem. Cell Biol. 2013, 45, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, S.; Nishihara, S.; Ikehara, Y.; Nagano, H.; Dono, K.; Sakon, M.; Narimatsu, H.; Monden, M. Molecular mechanism involved in increased expression of sialyl Lewis antigens in ductal carcinoma of the pancreas. J. Exp. Clin. Cancer Res. 1999, 18, 425–432. [Google Scholar] [PubMed]
- Kudo, T.; Ikehara, Y.; Togayachi, A.; Morozumi, K.; Watanabe, M.; Nakamura, M.; Nishihara, S.; Narimatsu, H. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab. Investig. 1998, 78, 797–811. [Google Scholar] [PubMed]
- Trinchera, M.; Malagolini, N.; Chiricolo, M.; Santini, D.; Minni, F.; Caretti, A.; Dall’Olio, F. The biosynthesis of the selectin-ligand sialyl Lewis x in colorectal cancer tissues is regulated by fucosyltransferase VI and can be inhibited by an RNA interference-based approach. Int. J. Biochem. Cell Biol. 2011, 43, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Hashiguchi, M.; Yamamoto, S.; Morita, H.; Oshima, M. Fucosyltransferase-producing sialyl Lea and sialyl Lex carbohydrate antigen in benign and malignant gastrointestinal mucosa. Cancer 1994, 73, 1552–1561. [Google Scholar] [CrossRef]
- Ito, H.; Hiraiwa, N.; Sawada-Kasugai, M.; Akamatsu, S.; Tachikawa, T.; Kasai, Y.; Akiyama, S.; Ito, K.; Takagi, H.; Kannagi, R. Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int. J. Cancer 1997, 71, 556–564. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Chiricolo, M.; Trinchera, M.; Harduin-Lepers, A. The expanding roles of the Sda/Cad carbohydrate antigen and its cognate glycosyltransferase B4GALNT2. Biochim. Biophys. Acta 2014, 1840, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, Y.I.; Kawashima, R.; Fukunaga, R.; Hirai, K.; Toyama-Sorimachi, N.; Tokuhara, M.; Shimizu, T.; Dohi, T. Introduction of Sda carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res. 2005, 65, 6220–6227. [Google Scholar] [CrossRef] [PubMed]
- Malagolini, N.; Santini, D.; Chiricolo, M.; Dall’Olio, F. Biosynthesis and expression of the Sda and sialyl Lewis x antigens in normal and cancer colon. Glycobiology 2007, 17, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Izawa, M.; Kumamoto, K.; Mitsuoka, C.; Kanamori, C.; Kanamori, A.; Ohmori, K.; Ishida, H.; Nakamura, S.; Kurata-Miura, K.; Sasaki, K.; et al. Expression of sialyl 6-sulfo Lewis X is inversely correlated with conventional sialyl Lewis X expression in human colorectal cancer. Cancer Res. 2000, 60, 1410–1416. [Google Scholar] [PubMed]
- Lo Presti, L.; Cabuy, E.; Chiricolo, M.; Dall’Olio, F. Molecular Cloning of the Human b1,4 N-Acetylgalactosaminyltransferase Responsible for the Biosynthesis of the Sda Histo-Blood Group Antigen: The Sequence Predicts a Very Long Cytoplasmic Domain. J. Biochem. 2003, 134, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Montiel, M.D.; Krzewinski-Recchi, M.A.; Delannoy, P.; Harduin-Lepers, A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Aca2-3Galb-R β1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: Evidence for an unusual extended cytoplasmic domain. Biochem. J. 2003, 373, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Yuyama, Y.; Natori, Y.; Smith, P.L.; Lowe, J.B.; Oshima, M. Detection of N-acetylgalactosaminyltransferase mRNA which determines expression of Sda blood group carbohydrate structure in human gastrointestinal mucosa and cancer. Int. J. Cancer 1996, 67, 626–631. [Google Scholar] [CrossRef]
- Malagolini, N.; Dall’Olio, F.; Di Stefano, G.; Minni, F.; Marrano, D.; Serafini-Cessi, F. Expression of UDP-GalNAc:NeuAc α2,3Gal b-R beta 1,4(GalNAc to Gal) N-acetylgalactosaminyltransferase involved in the synthesis of Sda antigen in human large intestine and colorectal carcinomas. Cancer Res. 1989, 49, 6466–6470. [Google Scholar] [PubMed]
- Groux-Degroote, S.; Wavelet, C.; Krzewinski-Recchi, M.A.; Portier, L.; Mortuaire, M.; Mihalache, A.; Trinchera, M.; Delannoy, P.; Malagolini, N.; Chiricolo, M.; et al. B4GALNT2 gene expression controls the biosynthesis of Sda and sialyl Lewis X antigens in healthy and cancer human gastrointestinal tract. Int. J. Biochem. Cell Biol. 2014, 53, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Sato, T. Beta-1,4-galactosylation of N-glycans is a complex process. Biochim. Biophys. Acta 1999, 1473, 54–66. [Google Scholar] [CrossRef]
- Qasba, P.K.; Ramakrishnan, B.; Boeggeman, E. Structure and function of β-1,4-galactosyltransferase. Curr. Drug Targets. 2008, 9, 292–309. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaide, M.A.; Alobydi, H.; Abdelsalam, A.G.; Zhang, R.; Srivenugopal, K.S. Multifaceted roles of 5′-regulatory region of the cancer associated gene B4GALT1 and its comparison with the gene family. Int. J. Oncol. 2015, 47, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.H.; Ostrander, G.K.; Clausen, H.; Graem, N. Oncofetal expression of Lex carbohydrate antigens in human colonic adenocarcinomas. Regulation through type 2 core chain synthesis rather than fucosylation. J. Biol. Chem. 1987, 262, 11331–11338. [Google Scholar] [PubMed]
- Ichikawa, T.; Nakayama, J.; Sakura, N.; Hashimoto, T.; Fukuda, M.; Fukuda, M.N.; Taki, T. Expression of N-acetyllactosamine and β1,4-galactosyltransferase (b4GalT-I) during adenoma-carcinoma sequence in the human colorectum. J. Histochem. Cytochem. 1999, 47, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Chang, H.Y.; Li, C.P.; Liu, J.M.; Huang, T.S. Tumor b-1,4-galactosyltransferase IV overexpression is closely associated with colorectal cancer metastasis and poor prognosis. Clin. Cancer Res. 2005, 11, 8615–8622. [Google Scholar] [CrossRef] [PubMed]
- Kolbinger, F.; Streiff, M.B.; Katopodis, A.G. Cloning of a human UDP-galactose:2-acetamido-2-deoxy-D-glucose 3b- galactosyltransferase catalyzing the formation of type 1 chains. J. Biol. Chem. 1998, 273, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, S.; Togayachi, A.; Kudo, T.; Nishihara, S.; Watanabe, M.; Kubota, T.; Kitajima, M.; Shiraishi, N.; Sasaki, K.; Andoh, T.; et al. Cloning, expression, and characterization of a novel UDP-galactose:b- N-acetylglucosamine b1,3-galactosyltransferase (b3Gal-T5) responsible for synthesis of type 1 chain in colorectal and pancreatic epithelia and tumor cells derived therefrom. J. Biol. Chem. 1999, 274, 12499–12507. [Google Scholar] [CrossRef] [PubMed]
- Salvini, R.; Bardoni, A.; Valli, M.; Trinchera, M. β1,3-Galactosyltransferase b3Gal-T5 acts on the GlcNAcb1-->3Galb1-->4GlcNAcb1-->R sugar chains of carcinoembryonic antigen and other N-linked glycoproteins and is down-regulated in colon adenocarcinomas. J. Biol. Chem. 2001, 276, 3564–3573. [Google Scholar] [CrossRef] [PubMed]
- Mare, L.; Trinchera, M. Suppression of β1,3galactosyltransferase β3Gal-T5 in cancer cells reduces sialyl-Lewis a and enhances poly N-acetyllactosamines and sialyl-Lewis x on O-glycans. Eur. J. Biochem. 2004, 271, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.K.; Chuang, P.K.; Huang, H.W.; Hwang-Verslues, W.W.; Cho, C.H.; Yang, W.B.; Shen, C.N.; Hsiao, M.; Hsu, T.L.; Chang, C.F.; et al. Stage-specific embryonic antigen-3 (SSEA-3) and beta3GalT5 are cancer specific and significant markers for breast cancer stem cells. Proc. Natl. Acad. Sci. USA 2016, 113, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Aronica, A.; Avagliano, L.; Caretti, A.; Tosi, D.; Bulfamante, G.P.; Trinchera, M. Unexpected distribution of CA19.9 and other type 1 chain Lewis antigens in normal and cancer tissues of colon and pancreas: Importance of the detection method and role of glycosyltransferase regulation. Biochim. Biophys. Acta 2017, 1861, 3210–3220. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, S.; Kudo, T.; Nishihara, S.; Ikehara, Y.; Togayachi, A.; Furuya, A.; Shitara, K.; Kubota, T.; Watanabe, M.; Kitajima, M.; et al. Lewis type 1 antigen synthase (b3Gal-T5) is transcriptionally regulated by homeoproteins. J. Biol. Chem. 2003, 278, 36611–36620. [Google Scholar] [CrossRef] [PubMed]
- Chachadi, V.B.; Ali, M.F.; Cheng, P.W. Prostatic cell-specific regulation of the synthesis of MUC1-associated sialyl Lewis a. PLoS ONE 2013, 8, e57416. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Taniguchi, N. Enzymes for N-Glycan Branching and Their Genetic and Nongenetic Regulation in Cancer. Biomolecules 2016. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, K.; Nakayama, J.; Nakamura, N.; Hasebe, O.; Katsuyama, T.; Fukuda, M. Carcinoma-associated expression of core 2 β1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: Role of O-glycans in tumor progression. Cancer Res. 1997, 57, 5201–5206. [Google Scholar] [PubMed]
- Holmes, E.H.; Hakomori, S.; Ostrander, G.K. Synthesis of type 1 and 2 lacto series glycolipid antigens in human colonic adenocarcinoma and derived cell lines is due to activation of a normally unexpressed b1,3N-acetylglucosaminyltransferase. J. Biol. Chem. 1987, 262, 15649–15658. [Google Scholar] [PubMed]
- Marcos, N.T.; Magalhaes, A.; Ferreira, B.; Oliveira, M.J.; Carvalho, A.S.; Mendes, N.; Gilmartin, T.; Head, S.R.; Figueiredo, C.; David, L.; et al. Helicobacter pylori induces β3GnT5 in human gastric cell lines, modulating expression of the SabA ligand sialyl-Lewis x. J. Clin. Investig. 2008, 118, 2325–2336. [Google Scholar] [CrossRef] [PubMed]
- Lauc, G.; Vojta, A.; Zoldoš, V. Epigenetic regulation of glycosylation is the quantum mechanics of biology. Biochim. Biophys. Acta 2014, 1840, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yamashita, K.; Sawaki, H.; Waraya, M.; Katoh, H.; Nakayama, N.; Kawamata, H.; Nishimiya, H.; Ema, A.; Narimatsu, H.; et al. Aberrant methylation of GCNT2 is tightly related to lymph node metastasis of primary CRC. Anticancer Res. 2015, 35, 1411–1421. [Google Scholar] [PubMed]
- Pichon, X.; Wilson, L.A.; Stoneley, M.; Bastide, A.; King, H.A.; Somers, J.; Willis, A.E. RNA binding protein/RNA element interactions and the control of translation. Curr. Protein Pept. Sci. 2012, 13, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Mardahl, M.; Schröter, M.F.; Engelbert, D.; Pink, M.; Sperandio, M.; Hamann, A.; Syrbe, U. Core 2 ß1,6-N-acetylglucosaminyltransferase-I, crucial for P-selectin ligand expression is controlled by a distal enhancer regulated by STAT4 and T-bet in CD4+ T helper cells 1. Mol. Immunol. 2016, 77, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ebel, M.E.; Kansas, G.S. Defining the Functional Boundaries of the Murine a1,3-Fucosyltransferase Fut7 Reveals a Remarkably Compact Locus. J. Biol. Chem. 2014, 289, 6341–6349. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Aziz, F.; Tian, L.L.; Wang, X.Q.; Yan, Q.; Liu, J.W. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int. J. Oncol. 2015, 46, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Bobowski, M.; Vincent, A.; Steenackers, A.; Colomb, F.; Van Seuningen, I.; Julien, S.; Delannoy, P. Estradiol represses the G(D3) synthase gene ST8SIA1 expression in human breast cancer cells by preventing NFκB binding to ST8SIA1 promoter. PLoS ONE 2013, 8, e62559. [Google Scholar] [CrossRef] [PubMed]
- Teylaert, B.; Meurice, E.; Bobowski, M.; Harduin-Lepers, A.; Gaucher, C.; Fontayne, A.; Jorieux, S.; Delannoy, P. Molecular cloning, characterization, genomic organization and promoter analysis of the α1,6-fucosyltransferase gene (fut8) expressed in the rat hybridoma cell line YB2/0. BMC Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, S.; Groux-Degroote, S.; Cazet, A.; Dhaenens, C.M.; Maurage, C.A.; Caillet-Boudin, M.L.; Delannoy, P.; Krzewinski-Recchi, M.A. Transcriptional regulation of the human ST6GAL2 gene in cerebral cortex and neuronal cells. Glycoconj. J. 2010, 27, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Chung, T.W.; Kang, N.Y.; Kim, K.S.; Lee, Y.C.; Kim, C.H. Involvement of CREB in the transcriptional regulation of the human GM3 synthase (hST3Gal V) gene during megakaryocytoid differentiation of human leukemia K562 cells. Biochem. Biophys. Res. Commun. 2004, 313, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Appenheimer, M.M.; Huang, R.Y.; Chandrasekaran, E.V.; Dalziel, M.; Hu, Y.P.; Soloway, P.D.; Wuensch, S.A.; Matta, K.L.; Lau, J.T. Biologic contribution of P1 promoter-mediated expression of ST6Gal I sialyltransferase. Glycobiology 2003, 13, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, A. Promoter structure and transcriptional regulation of human b-galactoside a2, 3-sialyltransferase genes. Curr. Drug Targets 2008, 9, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Caretti, A.; Signorelli, P.; Dall’Olio, F.; Trinchera, M. Transcriptional control of the B3GALT5 gene by a retroviral promoter and methylation of distant regulatory elements. FASEB J. 2014, 28, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, A.; Baczyńska, D.; Widerak, K.; Laskowska, A.; Ugorski, M. Promoter analysis of the human alpha1,3/4-fucosyltransferase gene (FUT III). Biochim. Biophys. Acta 2005, 1731, 66–73. [Google Scholar] [PubMed]
- Taniuchi, F.; Higai, K.; Tanaka, T.; Azuma, Y.; Matsumoto, K. Transcriptional regulation of fucosyltransferase 1 gene expression in colon cancer cells. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.L.; Guo, P.; Zhang, Y.; Chen, H.L. Regulation of metastasis-suppressive gene Nm23-H1 on glycosyl-transferases involved in the synthesis of sialyl Lewis antigens. J. Cell Biochem. 2005, 94, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, M.; Takimoto, R.; Tamura, F.; Yoshida, M.; Ono, M.; Murase, K.; Sato, Y.; Osuga, T.; Sato, T.; Iyama, S.; et al. Fucosylated TGF-beta receptors transduces a signal for epithelial-mesenchymal transition in colorectal cancer cells. Br. J. Cancer 2014, 110, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Isaji, T.; Im, S.; Fukuda, T.; Hashii, N.; Takakura, D.; Kawasaki, N.; Gu, J. β-Galactoside α2,6-Sialyltranferase 1 Promotes Transforming Growth Factor-beta-mediated Epithelial-Mesenchymal Transition. J. Biol. Chem. 2014, 289, 34627–34641. [Google Scholar] [CrossRef] [PubMed]
- Buckhaults, P.; Chen, L.; Fregien, N.; Pierce, M. Transcriptional regulation of N-acetylglucosaminyltransferase V by the src oncogene. J. Biol. Chem. 1997, 272, 19575–19581. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, W.; Fregien, N.; Pierce, M. The her-2/neu oncogene stimulates the transcription of N-acetylglucosaminyltransferase V and expression of its cell surface oligosaccharide products. Oncogene 1998, 17, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.B.; Zhang, Q.S.; Chen, H.L. Effects of H-ras and v-sis overexpression on N-acetylglucosaminyltransferase V and metastasis-related phenotypes in human hepatocarcinoma cells. J. Cancer Res. Clin. Oncol. 2000, 126, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chaney, W. Induction of N-acetylglucosaminyltransferase V by elevated expression of activated or proto-Ha-ras oncogenes. Mol. Cell Biochem. 1993, 122, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowicz, D.C.; Park, P.Y.; Datta, R.V.; Paty, P.B. CEA is the major PHA-L-reactive glycoprotein in colon carcinoma cell lines and tumors: Relationship between K-ras activation and β1-6 branching of N-linked carbohydrate on CEA. Biochem. Biophys. Res. Commun. 2000, 273, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Saito, H.; Ihara, Y.; Miyoshi, E.; Koyama, N.; Sheng, Y.; Taniguchi, N. Transcriptional regulation of the N-acetylglucosaminyltransferase V gene in human bile duct carcinoma cells (HuCC-T1) is mediated by Ets-1. J. Biol. Chem. 1996, 271, 26706–26712. [Google Scholar] [PubMed]
- Ko, J.H.; Miyoshi, E.; Noda, K.; Ekuni, A.; Kang, R.; Ikeda, Y.; Taniguchi, N. Regulation of the GnT-V promoter by transcription factor Ets-1 in various cancer cell lines. J. Biol. Chem. 1999, 274, 22941–22948. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, M.; Dall’Olio, F.; Mungul, A.; Piller, V.; Piller, F. Ras oncogene induces b-galactoside α2,6-sialyltransferase (ST6Gal I) via a RalGEF-mediated signal to its housekeeping promoter. Eur. J. Biochem. 2004, 271, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, P.; Pelczar, H.; Vandamme, V.; Verbert, A. Sialyltransferase activity in FR3T3 cells transformed with ras oncogene: Decreased CMP-Neu5Ac:Gal b1-3GalNAc α-2,3-sialyltransferase. Glycoconj. J. 1993, 10, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Easton, E.W.; Bolscher, J.G.; van den Eijnden, D.H. Enzymatic amplification involving glycosyltransferases forms the basis for the increased size of asparagine-linked glycans at the surface of NIH 3T3 cells expressing the N-ras proto-oncogene. J. Biol. Chem. 1991, 266, 21674–21680. [Google Scholar] [PubMed]
- Le Marer, N.; Laudet, V.; Svensson, E.C.; Cazlaris, H.; van Hille, B.; Lagrou, C.; Stehelin, D.; Montreuil, J.; Verbert, A.; Delannoy, P. The c-Ha-ras oncogene induces increased expression of b-galactoside α-2,6-sialyltransferase in rat fibroblast (FR3T3) cells. Glycobiology 1992, 2, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Seales, E.C.; Jurado, G.A.; Singhal, A.; Bellis, S.L. Ras oncogene directs expression of a differentially sialylated, functionally altered b1 integrin. Oncogene 2003, 22, 7137–7145. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Osorio, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3GAL4 leads to sLex expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.S.; Liu, S.; Liu, Y.J.; Liu, J.W.; Liu, T.J.; Wang, X.Q.; Yan, Q. Overexpression of fucosyltransferase IV promotes A431 cell proliferation through activating MAPK and PI3K/Akt signaling pathways. J. Cell Physiol. 2010, 225, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Shaper, N.L.; Mann, P.L.; Shaper, J.H. Cell surface galactosyltransferase: Immunochemical localization. J. Cell Biochem. 1985, 28, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Lin, B.; Hao, Y.Y.; Li, F.F.; Liu, D.W.; Qi, Y.; Zhu, L.C.; Zhang, S.L.; Iwamori, M. Lewis(y) antigen stimulates the growth of ovarian cancer cells via regulation of the epidermal growth factor receptor pathway. Oncol. Rep. 2010, 23, 833–841. [Google Scholar] [PubMed]
- Sakuma, K.; Aoki, M.; Kannagi, R. Transcription factors c-Myc and CDX2 mediate E-selectin ligand expression in colon cancer cells undergoing EGF/bFGF-induced epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. USA 2012, 109, 7776–7781. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, K.; Goto, Y.; Sekikawa, K.; Takenoshita, S.; Ishida, N.; Kawakita, M.; Kannagi, R. Increased expression of UDP-galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen- Friedenreich antigen and sialyl Lewis A/X determinants. Cancer Res. 2001, 61, 4620–4627. [Google Scholar] [PubMed]
- Yin, J.; Hashimoto, A.; Izawa, M.; Miyazaki, K.; Chen, G.Y.; Takematsu, H.; Kozutsumi, Y.; Suzuki, A.; Furuhata, K.; Cheng, F.L.; et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006, 66, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Yusa, A.; Miyazaki, K.; Kimura, N.; Izawa, M.; Kannagi, R. Epigenetic silencing of the sulfate transporter gene DTDST induces sialyl Lewisx expression and accelerates proliferation of colon cancer cells. Cancer Res. 2010, 70, 4064–4073. [Google Scholar] [CrossRef] [PubMed]
- Koike, T.; Kimura, N.; Miyazaki, K.; Yabuta, T.; Kumamoto, K.; Takenoshita, S.; Chen, J.; Kobayashi, M.; Hosokawa, M.; Taniguchi, A.; et al. Hypoxia induces adhesion molecules on cancer cells: A missing link between Warburg effect and induction of selectin-ligand carbohydrates. Proc. Natl. Acad. Sci. USA 2004, 101, 8132–8137. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Noda, K.; Furukawa, Y.; Ohshima, K.; Uchiyama, A.; Nakagawa, T.; Taniguchi, N.; Daigo, Y.; Nakamura, Y.; Hayashi, N.; et al. Deficiency of GMDS leads to escape from NK cell-mediated tumor surveillance through modulation of TRAIL signaling. Gastroenterology 2009, 137, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Moriwaki, K.; Imai, T.; Shinzaki, S.; Kamada, Y.; Murata, K.; Miyoshi, E. Mutation of GDP-Mannose-4,6-Dehydratase in Colorectal Cancer Metastasis. PLoS ONE 2013, 8, e70298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, D.; Chen, K.Y.; Cui, M.; Wang, W.; Huang, X.; Awadellah, A.; Li, Q.; Friedman, A.; Xin, W.W.; et al. Fucosylation Deficiency in Mice Leads to Colitis and Adenocarcinoma. Gastroenterology 2017, 152, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Osuga, T.; Takimoto, R.; Ono, M.; Hirakawa, M.; Yoshida, M.; Okagawa, Y.; Uemura, N.; Arihara, Y.; Sato, Y.; Tamura, F.; et al. Relationship Between Increased Fucosylation and Metastatic Potential in Colorectal Cancer. J. Natl. Cancer Inst. 2016. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.R.; Mackay, A.; Vong, M.; Desa, J.; Lamontagne, A.; Comunale, M.A.; Hafner, J.; Block, T.; Lec, R.; Mehta, A. Development of recombinant Aleuria aurantia lectins with altered binding specificities to fucosylated glycans. Biochem. Biophys. Res. Commun. 2011, 414, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Sawanobori, A.; Moriwaki, K.; Takamatsu, S.; Kamada, Y.; Miyoshi, E. A glycoproteomic approach to identify novel glycomarkers for cancer stem cells. Proteomics 2016, 16, 3073–3080. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Maupin, K.A.; Fallon, B.; Li, L.; Partyka, K.; Anderson, M.A.; Brenner, D.E.; Kaul, K.; Zeh, H.; Moser, A.J.; et al. Enhanced discrimination of malignant from benign pancreatic disease by measuring the CA 19-9 antigen on specific protein carriers. PLoS ONE 2011, 6, e29180. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Goldstein, I.J.; Hollingsworth, M.A.; Kaul, K.; Brand, R.E.; Haab, B.B. The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol. Cell Proteom. 2009, 8, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Partyka, K.; Maupin, K.A.; Hurley, M.; Andrews, P.; Kaul, K.; Moser, A.J.; Zeh, H.; Brand, R.E.; Haab, B.B. Identification of blood-protein carriers of the CA 19-9 antigen and characterization of prevalence in pancreatic diseases. Proteomics 2011, 11, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Sarrats, A.; Saldova, R.; Pla, E.; Fort, E.; Harvey, D.J.; Struwe, W.B.; De Llorens, R.; Rudd, P.M.; Peracaula, R. Glycosylation of liver acute-phase proteins in pancreatic cancer and chronic pancreatitis. Proteom. Clin. Appl. 2010, 4, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Balmana, M.; Sarrats, A.; Llop, E.; Barrabes, S.; Saldova, R.; Ferri, M.J.; Figueras, J.; Fort, E.; De Llorens, R.; Rudd, P.M.; et al. Identification of potential pancreatic cancer serum markers: Increased sialyl-Lewis X on ceruloplasmin. Clin. Chim. Acta 2015, 442C, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Uozumi, N.; Gao, C.; Yoshioka, T.; Nakano, M.; Moriwaki, K.; Nakagawa, T.; Masuda, T.; Tanabe, M.; Miyoshi, E. Identification of a novel type of CA19-9 carrier in human bile and sera of cancer patients: An implication of the involvement in nonsecretory exocytosis. J. Proteome Res. 2010, 9, 6345–6353. [Google Scholar] [CrossRef] [PubMed]
- Croce, M.V.; Salice, V.C.; Lacunza, E.; Segal-Eiras, A. Alpha 1-acid glycoprotein (AGP): A possible carrier of sialyl lewis X (slewis X) antigen in colorectal carcinoma. Histol. Histopathol. 2005, 20, 91–97. [Google Scholar] [PubMed]
- Rho, J.H.; Mead, J.R.; Wright, W.S.; Brenner, D.E.; Stave, J.W.; Gildersleeve, J.C.; Lampe, P.D. Discovery of sialyl Lewis A and Lewis X modified protein cancer biomarkers using high density antibody arrays. J. Proteom. 2014, 96, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Burdick, M.D.; Harris, A.; Reid, C.J.; Iwamura, T.; Hollingsworth, M.A. Oligosaccharides expressed on MUC1 produced by pancreatic and colon tumor cell lines. J. Biol. Chem. 1997, 272, 24198–24202. [Google Scholar] [CrossRef] [PubMed]
- Bones, J.; Byrne, J.C.; O’Donoghue, N.; McManus, C.; Scaife, C.; Boissin, H.; Nastase, A.; Rudd, P.M. Glycomic and glycoproteomic analysis of serum from patients with stomach cancer reveals potential markers arising from host defense response mechanisms. J. Proteome Res. 2011, 10, 1246–1265. [Google Scholar] [CrossRef] [PubMed]
- Balmana, M.; Gimenez, E.; Puerta, A.; Llop, E.; Figueras, J.; Fort, E.; Sanz-Nebot, V.; de, B.C.; Rizzi, A.; Barrabes, S.; et al. Increased a1-3 fucosylation of a-1-acid glycoprotein (AGP) in pancreatic cancer. J. Proteom. 2016, 132, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, F.; Theodoratou, E.; Thaci, K.; Timofeeva, M.; Vojta, A.; Stambuk, J.; Pucic-Bakovic, M.; Derek, L.; Servis, D.; Rudd, P.; et al. IgG glycome in colorectal cancer. Clin. Cancer Res. 2016, 22, 3078–3086. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Vanhooren, V.; Chen, C.C.; Slagboom, P.E.; Wuhrer, M.; Franceschi, C. N-glycomic biomarkers of biological aging and longevity: A link with inflammaging. Ageing Res. Rev. 2013, 12, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Dewald, J.H.; Colomb, F.; Bobowski-Gerard, M.; Groux-Degroote, S.; Delannoy, P. Role of Cytokine-Induced Glycosylation Changes in Regulating Cell Interactions and Cell Signaling in Inflammatory Diseases and Cancer. Cells 2016. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ishida, H.; Galustian, C.; Feizi, T.; Kiso, M. Synthesis and selectin-binding activity of N-deacetylsialyl Lewis X ganglioside. Carbohydr. Res. 2002, 337, 2111–2117. [Google Scholar] [CrossRef]
- Lopez, P.H.; Schnaar, R.L. Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 2009, 19, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Laskowska, A.; Dolińiska-Krajewska, B.; Zabel, M.; Ugorski, M. Sialosyl Le(a)-carrying gangliosides present on the surface of colon carcinoma cells are not directly involved in adhesion to E-selectin. Eur. J. Cell Biol. 2001, 80, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Dall’Olio, F.; Malagolini, N.; Trinchera, M.; Chiricolo, M. Mechanisms of cancer-associated glycosylation changes. Front. Biosci. 2012, 17, 670–699. [Google Scholar] [CrossRef]
- Accordino, M.K.; Wright, J.D.; Vasan, S.; Neugut, A.I.; Tergas, A.; Hu, J.C.; Hershman, D.L.; Accordino, M.K.; Wright, J.D.; Vasan, S.; et al. Serum Tumor Marker Use in Patients With Advanced Solid Tumors. J. Oncol. Pract. 2016, 12, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Gion, M.; Peloso, L.; Trevisiol, C.; Squarcina, E.; Zappa, M.; Fabricio, A.S. An epidemiology-based model as a tool to monitor the outbreak of inappropriateness in tumor marker requests: A national scale study. Clin. Chem. Lab. Med. 2016, 54, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Locker, G.Y.; Hamilton, S.; Harris, J.; Jessup, J.M.; Kemeny, N.; Macdonald, J.S.; Somerfield, M.R.; Hayes, D.F.; Bast, R.C., Jr. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J. Clin. Oncol. 2006, 24, 5313–5327. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Sturgeon, C.; Lamerz, R.; Haglund, C.; Holubec, V.L.; Klapdor, R.; Nicolini, A.; Topolcan, O.; Heinemann, V. Tumor markers in pancreatic cancer: A European Group on Tumor Markers (EGTM) status report. Ann. Oncol. 2010, 21, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Liu, F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: A meta-analysis. Tumour. Biol. 2014, 35, 7459–7465. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.; Hackert, T.; Hinz, U.; Hartwig, W.; Buchler, M.W.; Werner, J. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br. J. Surg. 2011, 98, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Fujii, T.; Takami, H.; Suenaga, M.; Inokawa, Y.; Yamada, S.; Nakayama, G.; Sugimoto, H.; Koike, M.; Nomoto, S.; et al. The combination of the serum carbohydrate antigen 19-9 and carcinoembryonic antigen is a simple and accurate predictor of mortality in pancreatic cancer patients. Surg. Today 2014, 44, 1692–1701. [Google Scholar] [CrossRef] [PubMed]
- Kaprio, T.; Satomaa, T.; Heiskanen, A.; Hokke, C.H.; Deelder, A.M.; Mustonen, H.; Hagstrom, J.; Carpen, O.; Saarinen, J.; Haglund, C. N-glycomic Profiling as a Tool to Separate Rectal Adenomas from Carcinomas. Mol. Cell Proteom. 2015, 14, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.R.; Park, J.C.; Kim, J.H.; Shin, S.K.; Lee, S.K.; Lee, Y.C.; Chung, J.B. High level of preoperative carbohydrate antigen 19-9 is a poor survival predictor in gastric cancer. World J. Gastroenterol. 2013, 19, 5302–5308. [Google Scholar] [CrossRef] [PubMed]
- Han, E.S.; Lee, H.H.; Lee, J.S.; Song, K.Y.; Park, C.H.; Jeon, H.M. At which stage of gastric cancer progression do levels of carcinoembryonic antigen and carbohydrate antigen 19-9 increase? Application in advanced gastric cancer treatment. J. Gastric. Cancer 2014, 14, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.S.; Huang, J.X.; Yu, H. Elevated serum level of carbohydrate antigen 19-9 in benign biliary stricture diseases can reduce its value as a tumor marker. Int. J. Clin. Exp. Med. 2014, 7, 744–750. [Google Scholar] [PubMed]
- Kalthoff, H.; Kreiker, C.; Schmiegel, W.H.; Greten, H.; Thiele, H.G. Characterization of CA 19-9 bearing mucins as physiological exocrine pancreatic secretion products. Cancer Res. 1986, 46, 3605–3607. [Google Scholar] [PubMed]
- Baeckstrom, D.; Karlsson, N.; Hansson, G.C. Purification and characterization of sialyl-Le(a)-carrying mucins of human bile; evidence for the presence of MUC1 and MUC3 apoproteins. J. Biol. Chem. 1994, 269, 14430–14437. [Google Scholar] [PubMed]
- La Greca, G.; Sofia, M.; Lombardo, R.; Latteri, S.; Ricotta, A.; Puleo, S.; Russello, D. Adjusting CA19-9 values to predict malignancy in obstructive jaundice: Influence of bilirubin and C-reactive protein. World J. Gastroenterol. 2012, 18, 4150–4155. [Google Scholar] [CrossRef] [PubMed]
- Furuya, N.; Kawa, S.; Hasebe, O.; Tokoo, M.; Mukawa, K.; Maejima, S.; Oguchi, H. Comparative study of CA242 and CA19-9 in chronic pancreatitis. Br. J. Cancer 1996, 73, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Kloppel, G.; Lingenthal, G.; von, B.M.; Kern, H.F. Histological and fine structural features of pancreatic ductal adenocarcinomas in relation to growth and prognosis: Studies in xenografted tumours and clinico-histopathological correlation in a series of 75 cases. Histopathology 1985, 9, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Itai, S.; Nishikata, J.; Yoneda, T.; Ohmori, K.; Yamabe, H.; Arii, S.; Tobe, T.; Kannagi, R. Tissue distribution of 2-3 and 2-6 sialyl Lewis A antigens and significance of the ratio of two antigens for the differential diagnosis of malignant and benign disorders of the digestive tract. Cancer 1991, 67, 1576–1587. [Google Scholar] [CrossRef]
- Ohshio, G.; Ogawa, K.; Kudo, H.; Yamabe, H.; Nakashima, Y.; Kim, Y.C.; Endo, K.; Watanabe, Y.; Manabe, T.; Tobe, T. Immunohistochemical studies on the localization of cancer associated antigens DU-PAN-2 and CA19-9 in carcinomas of the digestive tract. J. Gastroenterol. Hepatol. 1990, 5, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Portela, S.V.; Martin, C.V.; Romay, L.M.; Cuevas, E.; Martin, E.G.; Briera, A.F. sLea and sLex expression in colorectal cancer: Implications for tumourigenesis and disease prognosis. Histol. Histopathol. 2011, 26, 1305–1316. [Google Scholar] [PubMed]
- Wolf, B.C.; Salem, R.R.; Sears, H.F.; Horst, D.A.; Lavin, P.T.; Herlyn, M.; Itzkowitz, S.H.; Schlom, J.; Steele, G.D., Jr. The expression of colorectal carcinoma-associated antigens in the normal colonic mucosa. An immunohistochemical analysis of regional distribution. Am. J. Pathol. 1989, 135, 111–119. [Google Scholar] [PubMed]
- Miyazaki, K.; Ohmori, K.; Izawa, M.; Koike, T.; Kumamoto, K.; Furukawa, K.; Ando, T.; Kiso, M.; Yamaji, T.; Hashimoto, Y.; et al. Loss of disialyl Lewisa the ligand for lymphocyte inhibitory receptor sialic acid-binding immunoglobulin-like lectin-7 (Siglec-7) associated with increased sialyl Lewisa expression on human colon cancers. Cancer Res. 2004, 64, 4498–4505. [Google Scholar] [CrossRef] [PubMed]
- Caretti, A.; Sirchia, S.M.; Tabano, S.; Zulueta, A.; Dall’Olio, F.; Trinchera, M. DNA methylation and histone modifications modulate the b1,3 galactosyltransferase b3Gal-T5 native promoter in cancer cells. Int. J. Biochem. Cell Biol. 2012, 44, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Mare, L.; Caretti, A.; Albertini, R.; Trinchera, M. CA19.9 antigen circulating in the serum of colon cancer patients: Where is it from? Int. J. Biochem. Cell Biol. 2013, 45, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Terraneo, L.; Avagliano, L.; Caretti, A.; Bianciardi, P.; Tosi, D.; Bulfamante, G.P.; Samaja, M.; Trinchera, M. Expression of carbohydrate-antigen sialyl-Lewis a on colon cancer cells promotes xenograft growth and angiogenesis in nude mice. Int. J. Biochem. Cell Biol. 2013, 45, 2796–2800. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Singh, S.; Partyka, K.; Kletter, D.; Hsueh, P.; Yadav, J.; Ensink, E.; Bern, M.; Hostetter, G.; Hartman, D.; et al. Glycan motif profiling reveals plasma sialyl-Lewis X elevations in pancreatic cancers that are negative for CA 19-9. Mol. Cell Proteom. 2015, 14, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Partyka, K.; Hsueh, P.; Sinha, J.Y.; Kletter, D.; Zeh, H.; Huang, Y.; Brand, R.E.; Haab, B.B. Glycans related to the CA19-9 antigen are elevated in distinct subsets of pancreatic cancers and improve diagnostic accuracy over CA19-9. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Laubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Paschos, K.A.; Canovas, D.; Bird, N.C. The engagement of selectins and their ligands in colorectal cancer liver metastases. J. Cell Mol. Med. 2010, 14, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Witz, I.P. The selectin-selectin ligand axis in tumor progression. Cancer Metastasis Rev. 2008, 27, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Renkonen, R.; Mattila, P.; Majuri, M.L.; Rabina, J.; Toppila, S.; Renkonen, J.; Hirvas, L.; Niittymaki, J.; Turunen, J.P.; Renkonen, O.; et al. In vitro experimental studies of sialyl Lewis x and sialyl Lewis a on endothelial and carcinoma cells: Crucial glycans on selectin ligands. Glycoconj. J. 1997, 14, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, D.; Kitamura, K.; Tani, N.; Nishida, S.; Tsurutome, H.; Hakomori, S.I.; Ikeda, E.; Mutoh, F.; Kurioka, H.; Yamagishi, H. Molecular detection of disseminated cancer cells in the peripheral blood and expression of sialylated antigens in colon cancers. J. Surg. Oncol. 2000, 75, 98–102. [Google Scholar] [CrossRef]
- Nakamori, S.; Kameyama, M.; Imaoka, S.; Furukawa, H.; Ishikawa, O.; Sasaki, Y.; Kabuto, T.; Iwanaga, T.; Matsushita, Y.; Irimura, T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: Clinicopathological and immunohistochemical study. Cancer Res. 1993, 53, 3632–3637. [Google Scholar] [PubMed]
- Nakamori, S.; Kameyama, M.; Imaoka, S.; Furukawa, H.; Ishikawa, O.; Sasaki, Y.; Izumi, Y.; Irimura, T. Involvement of carbohydrate antigen sialyl Lewisx in colorectal cancer metastasis. Dis. Colon Rectum 1997, 40, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Chung, Y.S.; Maeda, K.; Sawada, T.; Ikehara, T.; Nishino, H.; Okuno, M.; Sowa, M. Increased expression of sialyl Lewis A and sialyl Lewis X in liver metastases of human colorectal carcinoma. Invasion Metastasis 1995, 15, 95–102. [Google Scholar] [PubMed]
- Opolski, A.; Laskowska, A.; Madej, J.; Wietrzyk, J.; Klopocki, A.; Radzikowski, C.; Ugorski, M. Metastatic potential of human CX-1 colon adenocarcinoma cells is dependent on the expression of sialosyl Lea antigen. Clin. Exp. Metastasis 1998, 16, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Thurin, M.; Kieber-Emmons, T. SA-Lea and Tumor Metastasis: The Old Prediction and Recent Findings. Hybrid Hybridomics 2002, 21, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, F.; Wicklein, D.; Stubke, K.; Nehmann, N.; Schmidt, A.; Salamon, J.; Peldschus, K.; Nentwich, M.F.; Adam, G.; Tolstonog, G.; et al. Selectin binding is essential for peritoneal carcinomatosis in a xenograft model of human pancreatic adenocarcinoma in pfp--/rag2-- mice. Gut 2012, 62, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Tei, K.; Kawakami-Kimura, N.; Taguchi, O.; Kumamoto, K.; Higashiyama, S.; Taniguchi, N.; Toda, K.; Kawata, R.; Hisa, Y.; Kannagi, R. Roles of cell adhesion molecules in tumor angiogenesis induced by cotransplantation of cancer and endothelial cells to nude rats. Cancer Res. 2002, 62, 6289–6296. [Google Scholar] [PubMed]
- Mathieu, S.; Gerolami, R.; Luis, J.; Carmona, S.; Kol, O.; Crescence, L.; Garcia, S.; Borentain, P.; El Battari, A. Introducing a1,2-linked fucose into hepatocarcinoma cells inhibits vasculogenesis and tumor growth. Int. J. Cancer 2007, 121, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, C.; Tsuboi, S.; Fukuda, M. Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 1999, 18, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, C.; Kanto, S.; Kato, K.; Nakano, O.; Arai, Y.; Kato, T.; Chen, S.; Fukuda, M.N.; Fukuda, M. Natural killer cells attack tumor cells expressing high levels of sialyl Lewis x oligosaccharides. Proc. Natl. Acad. Sci. USA 2002, 99, 13789–13794. [Google Scholar] [CrossRef] [PubMed]
- Doekhie, F.S.; Morreau, H.; de Bock, G.H.; Speetjens, F.M.; Dekker-Ensink, N.G.; Putter, H.; van de Velde, C.J.; Tollenaar, R.A.; Kuppen, P.J. Sialyl Lewis X expression and lymphatic microvessel density in primary tumors of node-negative colorectal cancer patients predict disease recurrence. Cancer Microenviron. 2008, 1, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Futamura, N.; Nakamura, S.; Tatematsu, M.; Yamamura, Y.; Kannagi, R.; Hirose, H. Clinicopathologic significance of sialyl le(x)expression in advanced gastric carcinoma. Br. J. Cancer 2000, 83, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Vukobrat-Bijedic, Z.; Husic-Selimovic, A.; Sofic, A.; Bijedic, N.; Bjelogrlic, I.; Gogov, B.; Mehmedovic, A. Cancer Antigens (CEA and CA 19-9) as Markers of Advanced Stage of Colorectal Carcinoma. Med. Arch. 2013, 67, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Watanabe, M. Clinic al significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009, 100, 195–199. [Google Scholar] [CrossRef] [PubMed]
| Selectin Ligand | Carrier Molecule | Disease Involved | Reference |
|---|---|---|---|
| sLea | MUC1 | cancer > chronic pancreatitis | [92,93] |
| sLea | MUC5AC | cancer > chronic pancreatitis | [92,93] |
| sLea | MUC16 | chronic pancreatitis > cancer | [92,93] |
| sLea | Apo-B-100 | cancer = chronic pancreatitis | [94] |
| sLea | Apo-E | cancer = chronic pancreatitis | [94] |
| sLea | Kininogen | cancer = chronic pancreatitis | [94] |
| sLex | α1-acid glycoprotein | cancer > chronic pancreatitis | [95] |
| sLex | Ceruloplasmin | cancer > chronic pancreatitis | [95] |
| sLex | Haptoglobin | chronic pancreatitis > cancer | [95] |
| sLex | Fetuin | chronic pancreatitis > cancer | [95] |
| sLex | Antitrypsin | chronic pancreatitis > cancer | [95] |
| sLex | Transferrin | chronic pancreatitis > cancer | [95] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinchera, M.; Aronica, A.; Dall’Olio, F. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology 2017, 6, 16. https://doi.org/10.3390/biology6010016
Trinchera M, Aronica A, Dall’Olio F. Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology. 2017; 6(1):16. https://doi.org/10.3390/biology6010016
Chicago/Turabian StyleTrinchera, Marco, Adele Aronica, and Fabio Dall’Olio. 2017. "Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers" Biology 6, no. 1: 16. https://doi.org/10.3390/biology6010016
APA StyleTrinchera, M., Aronica, A., & Dall’Olio, F. (2017). Selectin Ligands Sialyl-Lewis a and Sialyl-Lewis x in Gastrointestinal Cancers. Biology, 6(1), 16. https://doi.org/10.3390/biology6010016








