Decreased STAT3 Phosphorylation Mediates Cell Swelling in Ammonia-Treated Astrocyte Cultures
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Astrocyte Cultures
2.2. Cell Volume Determination
2.3. Overexpression of STAT3
2.4. Western Blots
2.5. Statistical Analysis
3. Results
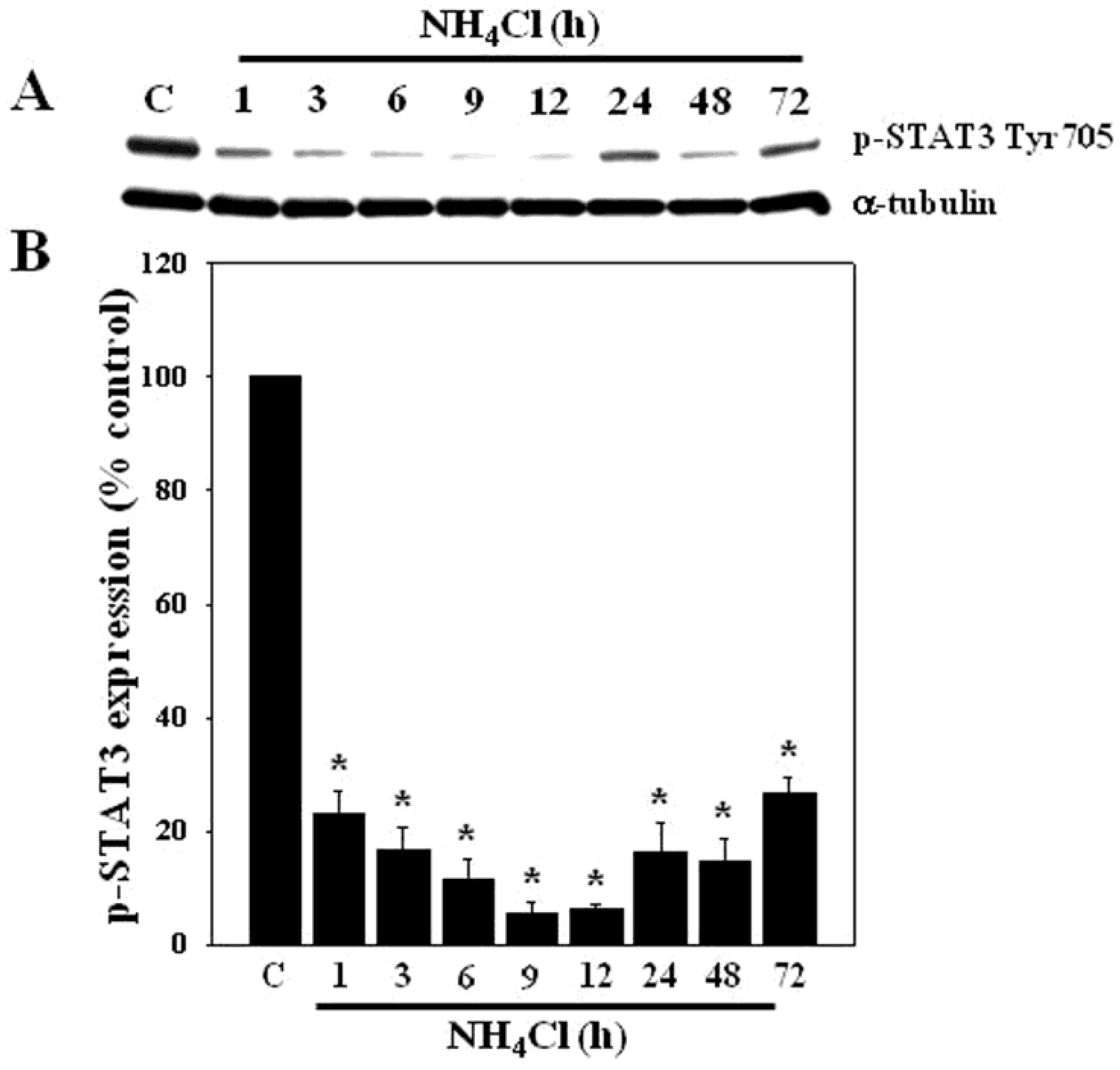
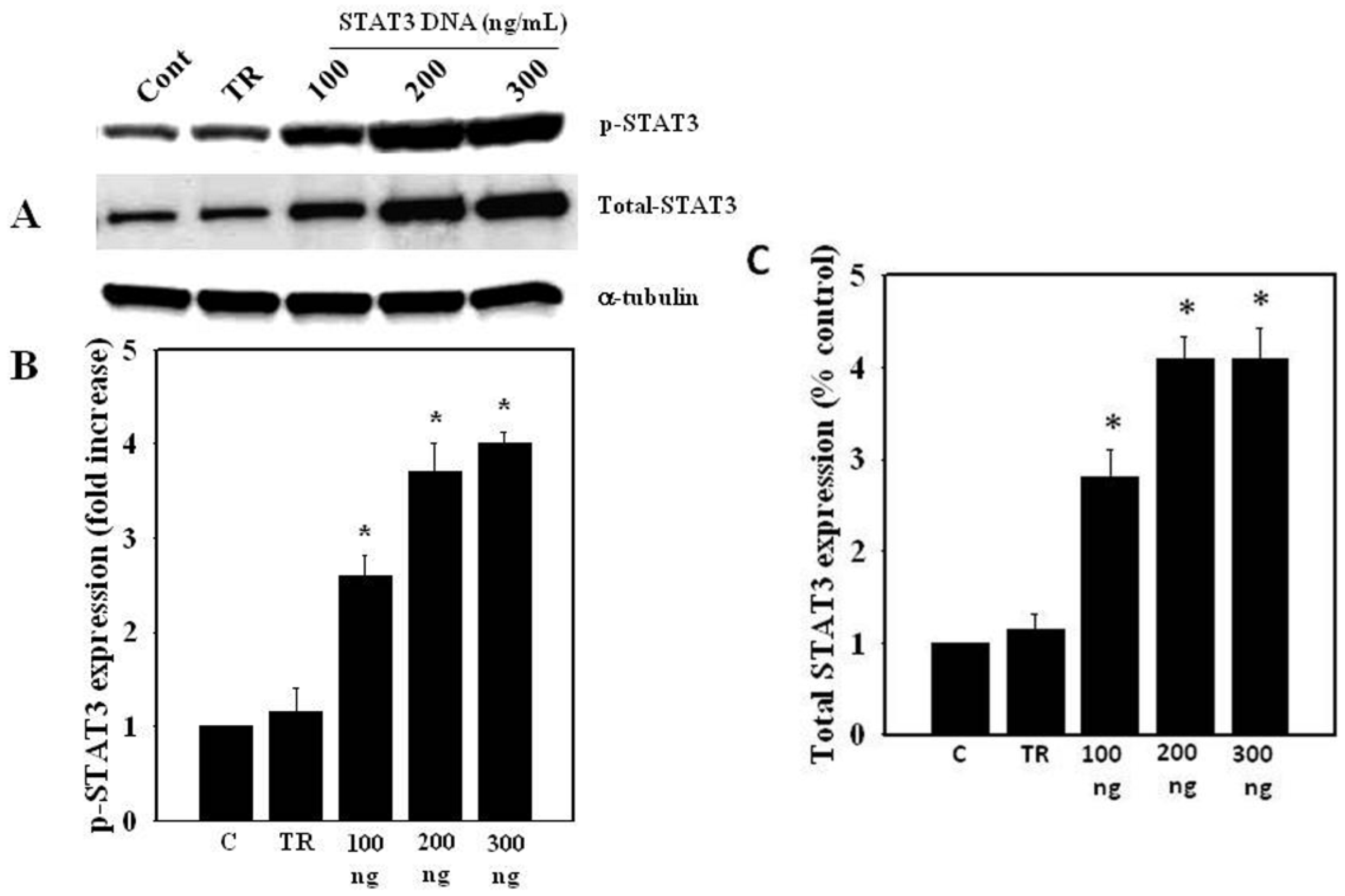
3.1. Effect of Ammonia on STAT3 Phosphorylation in Cultured Astrocytes
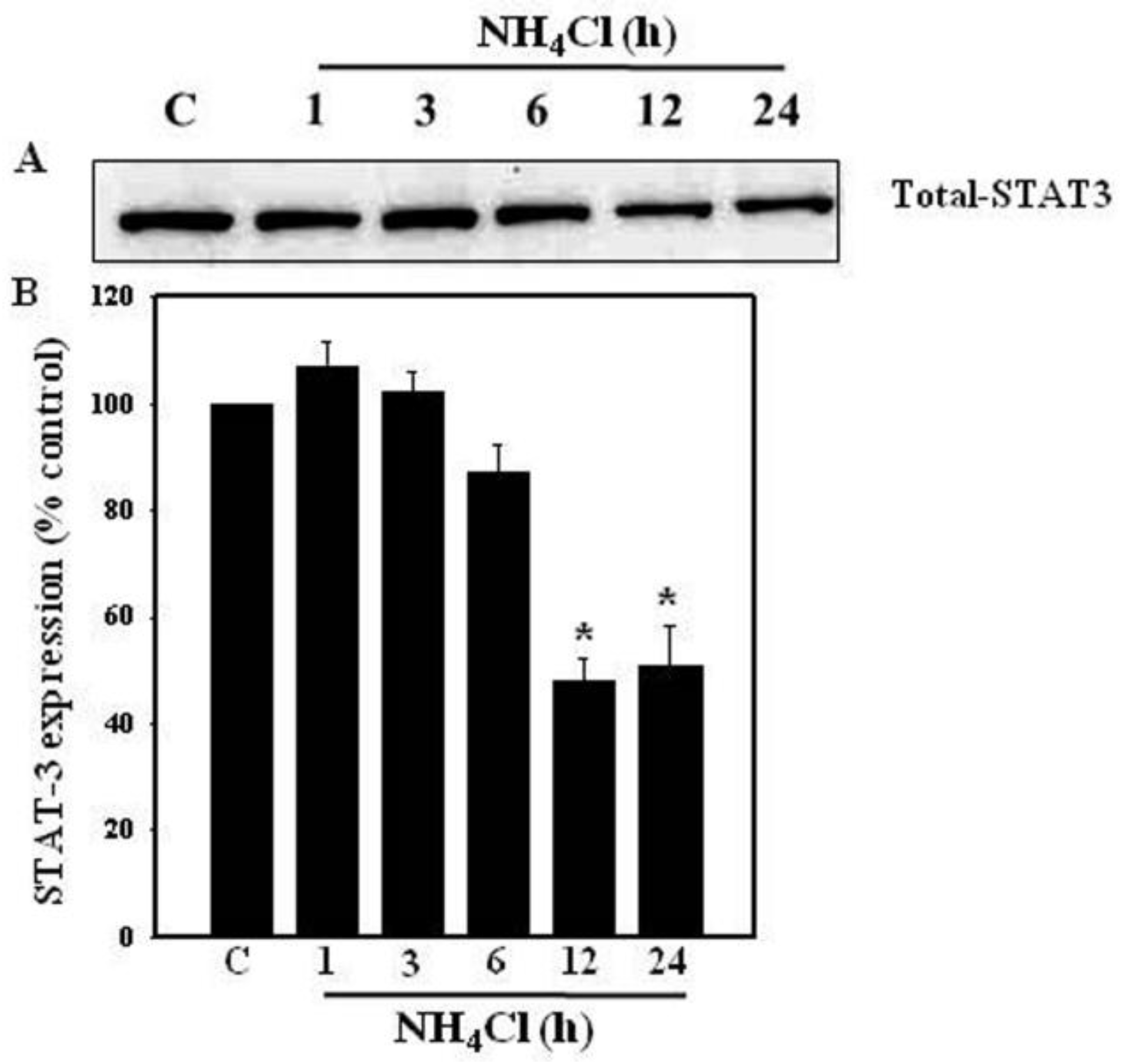
3.2. Effect of Ammonia on STAT3 Protein Levels in Astrocytes
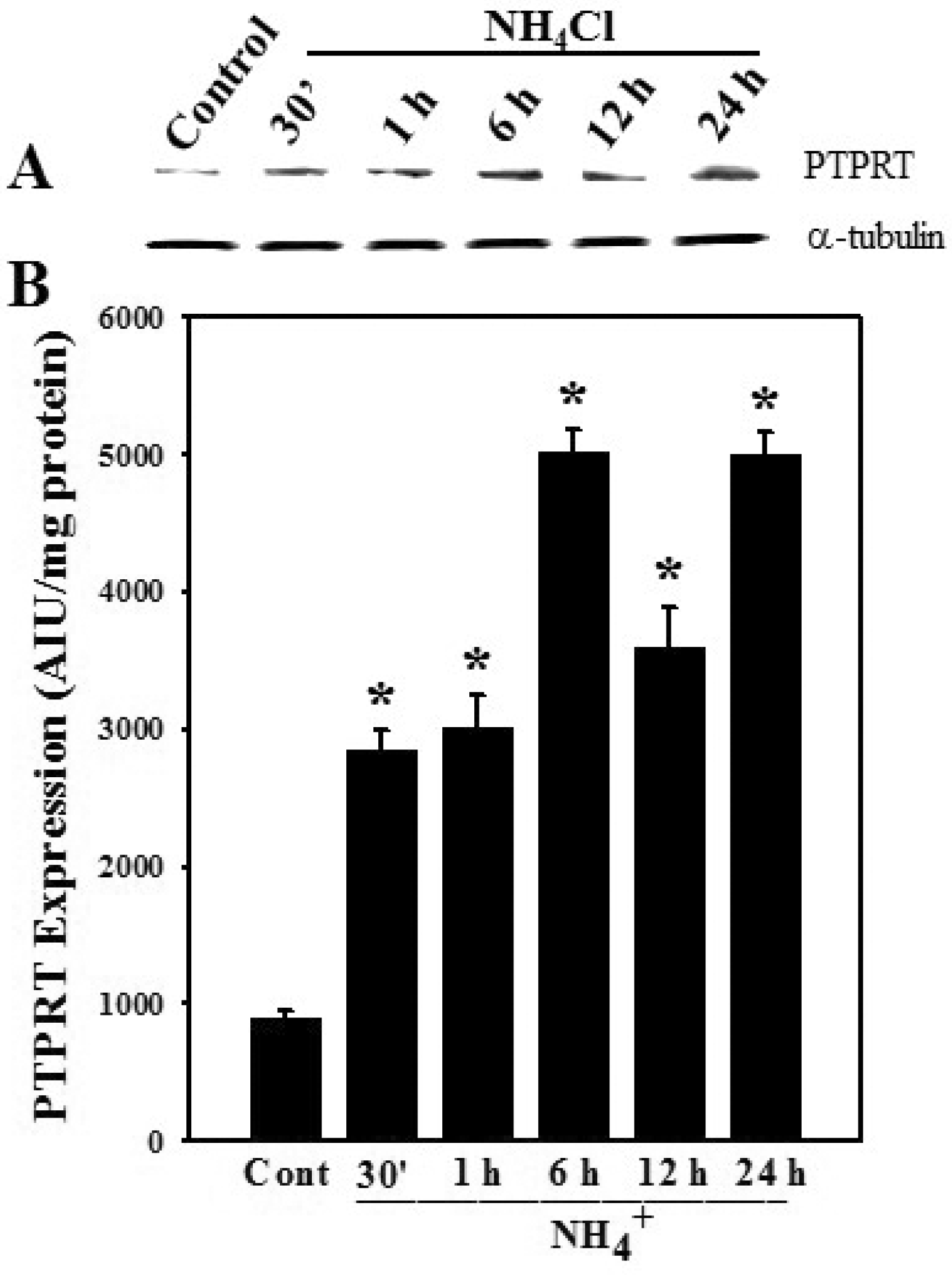
3.3. Effect of Ammonia on Protein Tyrosine Phosphatase Receptor Type-1 (PTPRT-1) Protein Expression in Astrocytes
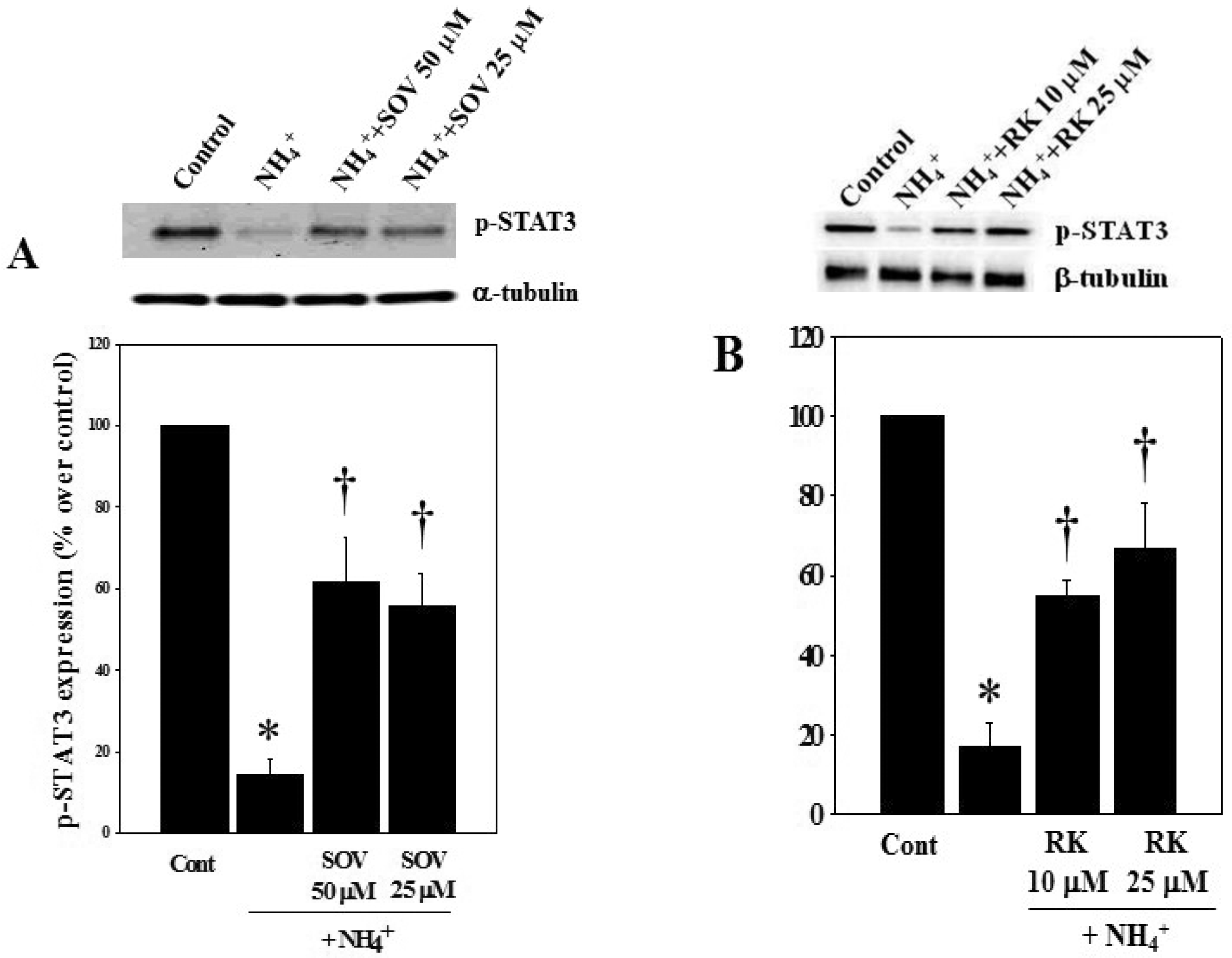
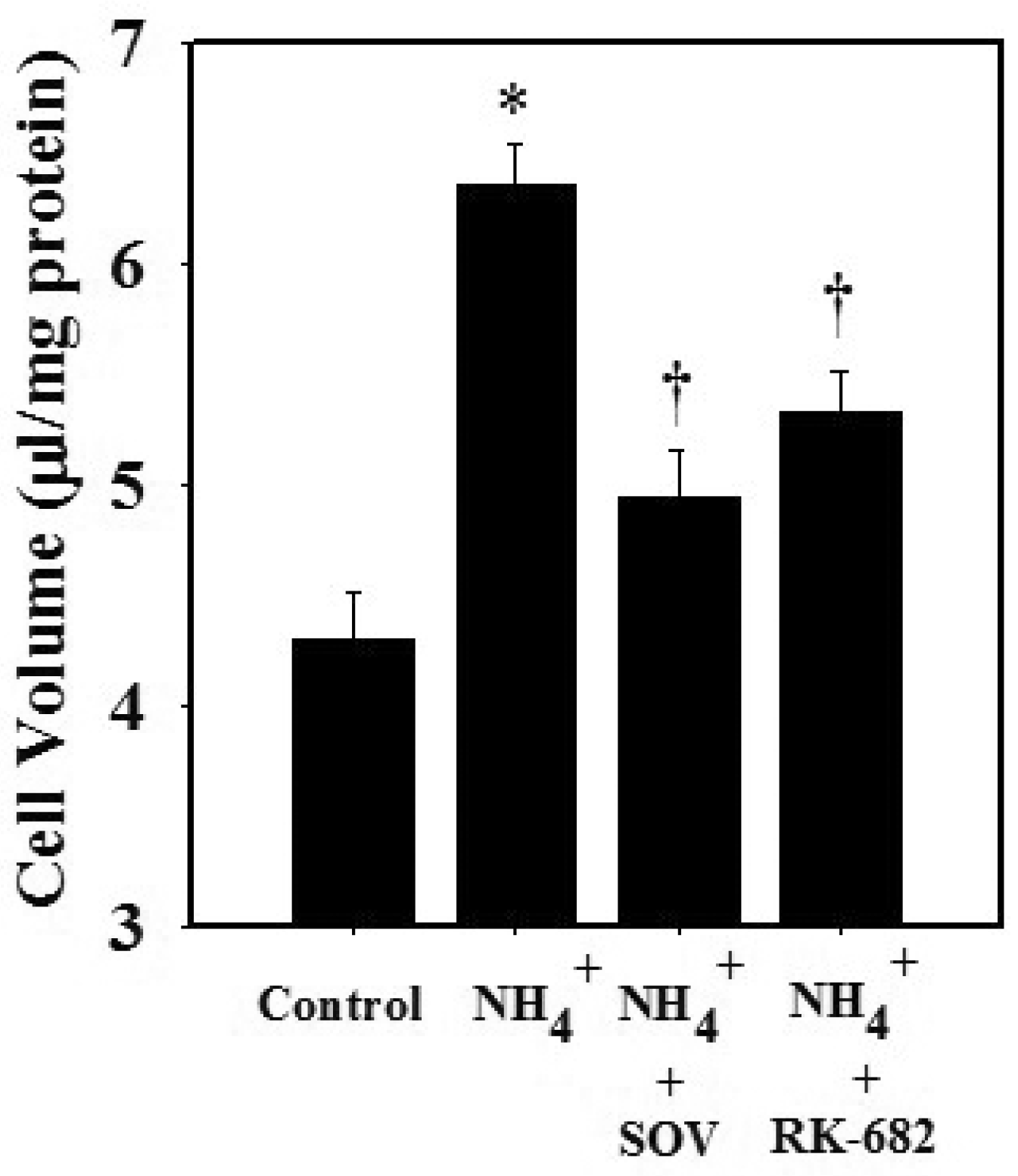
3.4. Effect of Sodium Orthovanadate (SOV) and RK-682 on Ammonia-Induced STAT3 Dephosphorylation and Astrocyte Swelling
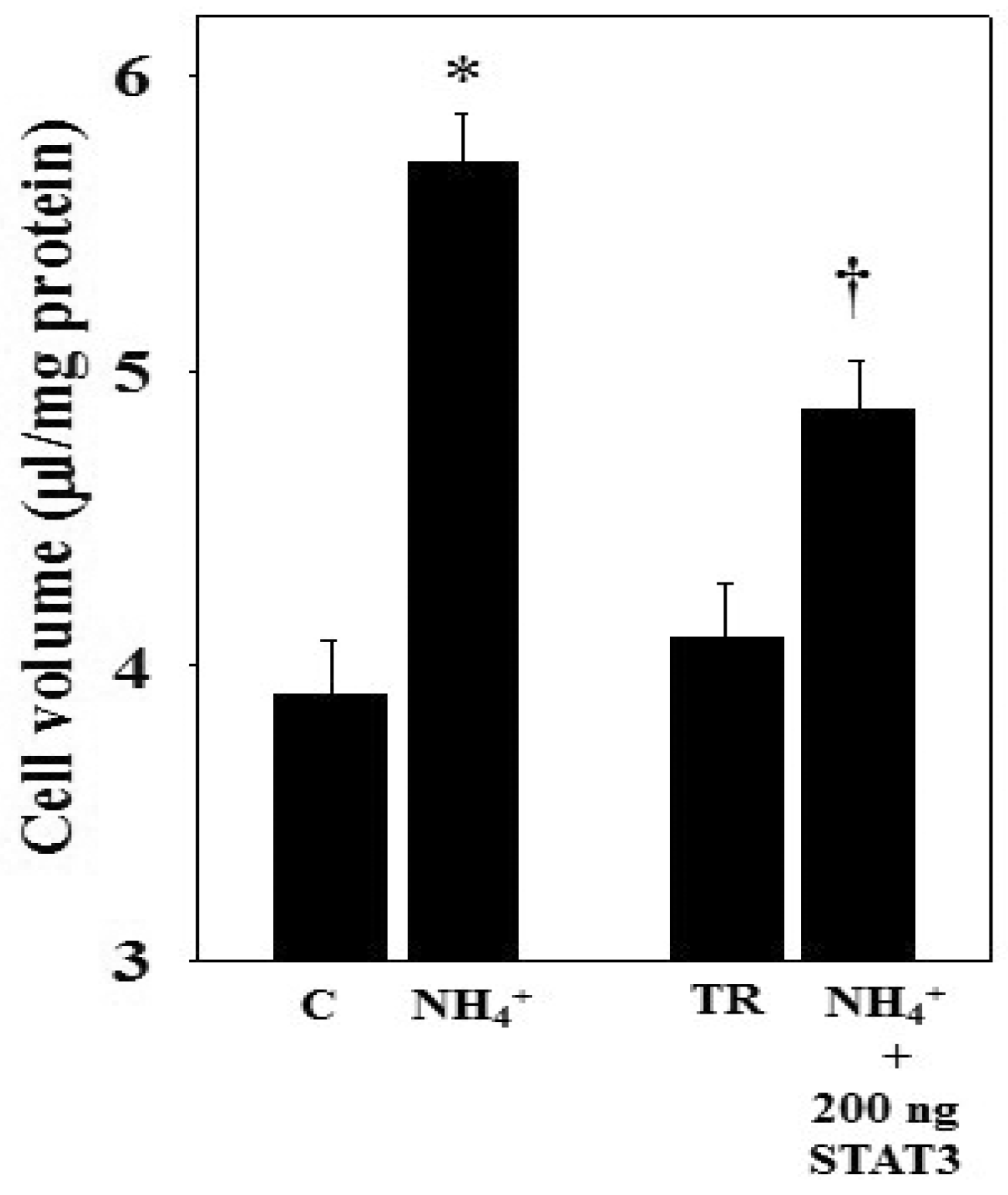
3.5. Ammonia-Induced STAT3 Dephosphorylation and Cell Swelling in STAT3 Over-Expressed Astrocytes
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Albrecht, J.; Jones, E.A. Hepatic encephalopathy: Molecular mechanisms underlying the clinical syndrome. J. Neurol. Sci. 1999, 170, 138–146. [Google Scholar] [CrossRef]
- Hazell, A.S.; Butterworth, R.F. Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc. Soc. Exp. Biol. Med. 1999, 222, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, M.D.; Rama Rao, K.V.; Jayakumar, A.R. Pathways and signaling in ammonia neurotoxicity. Metab. Brain Dis. 2009, 24, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Panickar, K.S.; Murthy, ChR.; Norenberg, M.D. Oxidative stress and MAPK phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J. Neurosci. 2006, 26, 4774–4784. [Google Scholar] [CrossRef] [PubMed]
- Sinke, A.P.; Jayakumar, A.R.; Panickar, K.S.; Moriyama, M.; Reddy, P.V.B.; Norenberg, M.D. NF-κB in the mechanism of ammonia-induced astrocyte swelling in culture. J. Neurochem. 2008, 106, 2302–2311. [Google Scholar] [PubMed]
- Panickar, K.S.; Jayakumar, A.R.; Rao, K.V.; Norenberg, M.D. Ammonia-induced activation of p53 in cultured astrocytes role in cell swelling and glutamate uptake. Neurochem. Int. 2009, 55, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, V.; Görg, B.; Bidmon, H.J.; Keitel, V.; Häussinger, D. Precipitants of hepatic encephalopathy induce rapid astrocyte swelling in an oxidative stress dependent manner. Arch. Biochem. Biophys. 2013, 536, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Minami, M.; Inoue, M.; Wei, S.; Takeda, K.; Matsumoto, M.; Kishimoto, T.; Akira, S. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc. Natl. Acad. Sci. USA 1996, 93, 3963–3966. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Cai-Hong, X.; Xin-Min, C.; Nai-He, J. Overexpression of STAT3 in COS7 cells causes prominent morphological changes. Acta Biochim. Biophys. Sin. 2003, 35, 717–722. [Google Scholar]
- Selvendiran, K.; Koga, H.; Ueno, T.; Yoshida, T.; Maeyama, M.; Torimura, T.; Yano, H.; Kojiro, M.; Sata, M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: An implication for the antitumor potential of flavonoids. Cancer Res. 2006, 66, 4826–4834. [Google Scholar] [CrossRef] [PubMed]
- Lui, V.W.; Wong, E.Y.; Ho, Y.; Hong, B.; Wong, S.C.; Tao, Q.; Choi, G.C.; Au, T.C.; Ho, K.; Yau, D.; et al. STAT3 activation contributes directly to Epstein-Barr virus-mediated invasiveness of nasopharyngeal cancer cells in vitro. Int. J. Cancer 2009, 125, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Nair, A.S.; Sung, B.; Pandey, M.K.; Aggarwal, B.B. Boswellic acid blocks signal transducers and activators of transcription 3 signaling, proliferation, and survival of multiple myeloma via the protein tyrosine phosphatase SHP-1. Mol. Cancer Res. 2009, 7, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Oyake, T.; Murai, K.; Ishida, Y. Deguelin suppresses cell proliferation via the inhibition of survivin expression and STAT3 phosphorylation in HTLV-1- transformed T cells. Leuk. Res. 2010, 34, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Terui, K.; Enosawa, S.; Haga, S.; Zhang, H.Q.; Kuroda, H.; Kouchi, K.; Matsunaga, T.; Yoshida, H.; Engelhardt, J.F.; Irani, K.; et al. Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. J. Hepatol. 2004, 41, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Terui, K.; Zhang, H.Q.; Enosawa, S.; Ogawa, W.; Inoue, H.; Okuyama, T.; Takeda, K.; Akira, S.; Ogino, T.; et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J. Clin. Investig. 2003, 112, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.P.; Townsend, P.A.; McCormick, J.; Knight, R.A.; Scarabelli, T.M.; Latchman, D.S.; Stephanou, A. STAT3 deletion sensitizes cells to oxidative stress. Biochem. Biophys. Res. Commun. 2009, 385, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Perez, A.; Gary-Gouy, H.; Gaudin, F.; Maarof, G.; Marfaing-Koka, A.; de Revel, T.; Dalloul, A. IL-24 induces apoptosis of chronic lymphocytic leukemia B cells engaged into the cell cycle through dephosphorylation of STAT3 and stabilization of p53 expression. J. Immunol. 2008, 181, 6051–6060. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, W.; Kone, B.C. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappaB. Biochem. J. 2002, 367, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Sinn, D.I.; Chu, K.; Lee, S.T.; Song, E.C.; Jung, K.H.; Kim, E.H.; Park, D.K.; Kang, K.M.; Kim, M.; Roh, J.K. Pharmacological induction of heat shock protein exerts neuroprotective effects in experimental intracerebral hemorrhage. Brain Res. 2007, 1135, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ducis, I.; Norenberg, L.O.; Norenberg, M.D. The benzodiazepine receptor in cultured astrocytes from genetically epilepsy-prone rats. Brain Res. 1990, 531, 318–321. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Hertz, L. Plasticity of astrocytes in primary cultures: An experimental tool and a reason for methodological caution. Dev. Neurosci. 1985, 7, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Kletzein, R.F.; Pariza, M.W.; Becker, J.E.; Potter, V.R. A method using 3-O-methyl-d-glucose and phloretin for the determination of intracellular water space of cells in monolayer culture. Annl. Biochem. 1975, 68, 537–544. [Google Scholar] [CrossRef]
- Kimelberg, H.K. Anisotonic media and glutamate-induced ion transport and volume responses in primary astrocyte cultures. J. Physiol. 1987, 82, 294–303. [Google Scholar]
- Bender, A.S.; Norenberg, M.D. Effect of benzodiazepines and neurosteroids on ammonia-induced swelling in cultured astrocytes. J. Neurosci. Res. 1998, 54, 673–680. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Tong, X.Y.; Curtis, K.M.; Ruiz-Cordero, R.; Shamaladevi, N.; Abuzamel, M.; Johnstone, J.; Gaidosh, G.; Rama Rao, K.V.; Norenberg, M.D. Decreased astrocytic thrombospondin-1 secretion after chronic ammonia treatment reduces the level of synaptic proteins: In vitro and in vivo studies. J. Neurochem. 2014, 131, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Mans, A.M.; Saunders, S.J.; Kirsch, R.E.; Biebuyck, J.F. Correlation of plasma and brain amino acid and putative neurotransmitter alterations during acute hepatic coma in the rat. J. Neurochem. 1979, 32, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Swain, M.; Butterworth, R.F.; Blei, A.T. Ammonia and related amino acids in the pathogenesis of brain edema in acute ischemic liver failure in rats. Hepatology 1992, 15, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Valdes, V.; Norenberg, M.D. The Na-K-Cl cotransporter in the brain edema of acute liver failure. J. Hepatol. 2011, 54, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Miller School of Medicine, University of Miami, Miami, FL, USA. Unpublished observation.
- Woetmann, A.; Nielsen, M.; Christensen, S.T.; Brockdorff, J.; Kaltoft, K.; Engel, A.M.; Skov, S.; Brender, C.; Geisler, C.; Svejgaard, A.; et al. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc. Natl. Acad. Sci. USA 1999, 96, 10620–10625. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Steinberg, B.M. PTEN is a negative regulator of STAT3 activation in human papillomavirus-infected cells. J. Gen. Virol. 2002, 83, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Sasin, J.; Bottini, N.; Friedberg, I.; Friedberg, I.; Osterman, A.; Godzik, A.; Hunter, T.; Dixon, J.; Mustelin, T. Protein tyrosine phosphatases in the human genome. Cell 2004, 117, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Akasaki, Y.; Liu, G.; Matundan, H.H.; Ng, H.; Yuan, X.; Zeng, Z.; Black, K.L.; Yu, J.S. A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and -9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J. Biol. Chem. 2005, 281, 6165–6174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, A.; Yu, J.; Possemato, A.; Chen, Y.; Zheng, W.; Polakiewicz, R.D.; Kinzler, K.W.; Vogelstein, B.; Velculescu, V.E.; et al. Identification of STAT3 as a substrate of receptor protein tyrosine phosphatase T. Proc. Natl. Acad. Sci. USA 2007, 104, 4060–4064. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Sudo, T.; Osada, H. RK-682, a potent inhibitor of tyrosine phosphatase, arrested the mammalian cell cycle progression at G1phase. FEBS Lett. 1995, 372, 54–58. [Google Scholar] [CrossRef]
- Gao, B. Cytokines, STATs and liver disease. Cell. Mol. Immunol. 2005, 2, 92–100. [Google Scholar] [PubMed]
- Reich, N.C.; Liu, L. Tracking STAT nuclear traffic. Nat. Rev. Immunol. 2006, 6, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.P.; Cao, X. Structure, function and regulation of STAT proteins. Mol. BioSyst. 2006, 2, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Brantley, E.C.; Nabors, L.B.; Gillespie, G.Y.; Choi, Y.H.; Palmer, C.A.; Harrison, K.; Roarty, K.; Benveniste, E.N. Loss of protein inhibitors of activated STAT-3 expression in glioblastoma multiforme tumors: Implications for STAT-3 activation and gene expression. Clin. Cancer Res. 2008, 14, 4694–4704. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef]
- Liang, H.; Venema, V.J.; Wang, X.; Ju, H.; Venem, R.C.; Marrero, M.B. Regulation of angiotensin II-induced phosphorylation of STAT3 in vascular smooth muscle cells. J. Biol. Chem. 1999, 274, 19846–19851. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Hwa, V.; Rosenfeld, R.G. Interferon-gamma-induced dephosphorylation of STAT3 and apoptosis are dependent on the mTOR pathway. Exp. Cell Res. 2006, 312, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Barnea, M.; Olender, T.; Bedford, M.T.; Elson, A. Regulation of receptor-type protein tyrosine phosphatases by their C-terminal tail domains. Biochem. Soc. Trans. 2016, 44, 1295–1303. [Google Scholar] [CrossRef]
- Görg, B.; Karababa, A.; Shafigullina, A.; Bidmon, H.J.; Häussinger, D. Ammonia-induced senescence in cultured rat astrocytes and in human cerebral cortex in hepatic encephalopathy. Glia 2015, 63, 37–50. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayakumar, A.R.; Curtis, K.M.; Panickar, K.S.; Shamaladevi, N.; Norenberg, M.D. Decreased STAT3 Phosphorylation Mediates Cell Swelling in Ammonia-Treated Astrocyte Cultures. Biology 2016, 5, 48. https://doi.org/10.3390/biology5040048
Jayakumar AR, Curtis KM, Panickar KS, Shamaladevi N, Norenberg MD. Decreased STAT3 Phosphorylation Mediates Cell Swelling in Ammonia-Treated Astrocyte Cultures. Biology. 2016; 5(4):48. https://doi.org/10.3390/biology5040048
Chicago/Turabian StyleJayakumar, Arumugam R., Kevin M. Curtis, Kiran S. Panickar, Nagarajarao Shamaladevi, and Michael D. Norenberg. 2016. "Decreased STAT3 Phosphorylation Mediates Cell Swelling in Ammonia-Treated Astrocyte Cultures" Biology 5, no. 4: 48. https://doi.org/10.3390/biology5040048
APA StyleJayakumar, A. R., Curtis, K. M., Panickar, K. S., Shamaladevi, N., & Norenberg, M. D. (2016). Decreased STAT3 Phosphorylation Mediates Cell Swelling in Ammonia-Treated Astrocyte Cultures. Biology, 5(4), 48. https://doi.org/10.3390/biology5040048




